Abstract
Background:
The excessive retention of sputum in the airways, leading to pulmonary infections, is a common consequence of bronchiectasis. Although inhalation of 7% hypertonic saline (HS) has proven an effective method to help remove the mucus, many patients are intolerant of this treatment. The addition of 0.1% hyaluronic acid to HS (HS+HA) could increase tolerance to HS in these patients. The main objective of this study was to evaluate the tolerability of HS+HA in bronchiectasis patients who are intolerant to HS.
Methods:
This prospective, observational, open-label study analysed the outcomes of two groups of bronchiectasis patients previously scheduled to start HS therapy. Patients were assessed for tolerance to HS by a questionnaire, spirometry and clinical evaluation. Patients who were intolerant were evaluated for tolerance to HS+HA approximately one week later. All patients were evaluated for their tolerance to HS or HS+HA 4 weeks after the start of their treatment. Patients were also assessed with quality-of-life and adherence questionnaires, and all adverse events were registered.
Results:
A total of 137 bronchiectasis patients were enrolled in the study (age = 63.0 ± 14.7 years; 63.5% women). Of these, 92 patients (67.1%) were tolerant and 45 patients (32.9%) were intolerant to HS. Of the 45 patients intolerant to HS, 31 patients (68.9%) were tolerant and 14 patients (31.1%) intolerant to HS+HA. Of these 31 tolerant patients, 26 (83.9%) could complete the 4-week treatment with HS+HA.
Conclusions:
Two-thirds of bronchiectasis patients that presented intolerance to inhaled HS alone are tolerant to inhaled HS+HA, suggesting that HA improves tolerance to HS therapy.
Keywords: bronchiectasis, hypertonic saline, hyaluronic acid, mucoactive agents, inhaled hyperosmolar agents
Introduction
Bronchiectasis can be caused by diverse disease processes and mechanisms, resulting in the characteristic chronic cough, purulent sputum production and airway dilation.1 The excessive retention of sputum in bronchiectasis contributes to the vicious circle of chronic respiratory infection with inflammation and more sputum production.2 There are several factors that contribute to the abnormal mucociliary clearance characteristic of bronchiectasis, including pulmonary infection, increased mucus production, anomalous mucus composition, slowing of cilia and loss of ciliated cells.3
The inhalation of hypertonic saline (HS) has been shown to be an effective method of promoting the removal of mucus from the airways, and a clinically accepted technique of physiotherapy in the treatment of various pulmonary diseases, including bronchiectasis and cystic fibrosis.4,5 Several studies have shown that long-term inhalation of HS in patients with bronchiectasis is well-tolerated, improved quality of life (QoL) and decreased sputum viscosity; however, the comparison of HS to isotonic saline solutions gave disparate results.5–7
Although a majority of bronchiectasis patients tolerate HS well, some report adverse events (AEs) that prevent longer-term treatment, including intense cough, bronchospasm, dyspnoea, throat irritation, chest tightness and salty taste. In these cases a possible treatment alternative is the combination of HS and hyaluronic acid (HA). Inhaled HA hydrates the airways, attenuates bronchial hyper-responsiveness, reduces inflammation and disrupts the biofilm associated with chronic infection.8–10 Additionally, HA improves tolerability of HS by reducing its salty taste.11
Although previous studies have demonstrated the potential safety and efficacy of the use of HS in patients with bronchiectasis,4,6,7,12 there are limited data on the tolerability of HS in combination with HA and its impact specifically on these patients.13 We hypothesized that addition of HA to HS could improve tolerability for those patients intolerant to HS alone. Therefore, the objective of this study was to evaluate the tolerability and effectiveness of the combination of 7% HS supplemented with 0.1% HA in patients diagnosed with bronchiectasis who were intolerant to treatment with 7% HS. Additionally, we assessed changes in QoL and compliance after four weeks of treatment, as well as any possible emergent AEs.
Methods
This prospective, observational, open-label study was carried out by 11 pulmonologists in public teaching hospitals throughout Spain, and took place between November 2015 (inclusion of the first patient) and May 2017 (last visit of the last patient). The study protocol was approved by the ethical review committee of each participating centre. All patients were informed by the investigator about the research procedure and gave informed consent in accordance with good clinical practice guidelines and the Declaration of Helsinki.
Patient selection
Consecutive patients were recruited by pulmonologists at their health centres. Inclusion criteria were: >18 years old; previous diagnosis of bronchiectasis by high-resolution computed tomography; decision of the physician to start treatment with inhaled HS solution; clinically active sputum production greater than 30 ml every 24 h (semi-quantitative measurement); mean baseline forced expiratory volume in one second (FEV1) post-bronchodilator ⩾35% or ⩾1 L; and written informed consent to participate in this clinical research. The decision to start treatment with inhaled HS was made based on the physician’s judgement according to routine clinical practice and was independent from the inclusion of the patient into the study.
Exclusion criteria were: exacerbations in the 4 weeks previous to inclusion in the study; treatment with oral antibiotics or systemic corticoids during the 4 weeks previous to inclusion in the study; previous events of haemoptysis caused by inhaled drugs; current treatment with inhaled HS; diagnosis of allergic bronchopulmonary aspergillosis14 or cystic fibrosis, following guidelines;15 patients unable, because of their medical or psychological condition, to perform necessary tests (e.g. spirometry) of the study; pregnant women; patients enrolled in other clinical studies; or patients with treated but uncontrolled arterial hypertension.
Main outcome
The main variable of the study was the percentage of patients intolerant to HS but tolerant to HS+HA. Tolerance to HS+HA was assessed based on the patient’s report on symptoms after inhalation (cough, pharyngeal irritation, salty taste, chest tightness, dyspnoea, wheezing and nausea), change in lung function (FEV1) after inhalation and the physician’s judgement.
Study design
This study was carried out in real-life conditions at the participating centres, imposing no restrictions on the participating physician who prescribed the treatment nor influencing any medical decisions. With the exception of the scales or questionnaires used as measurement instruments in this clinical investigation, the included patients were not given any diagnostic or follow-up intervention other than those followed in usual clinical practice.
Both solutions for inhalation were used with a nebulizer supplied by the health centre at the request of the doctor treating the patient, as is the usual practice in nebulized therapies. The use of jet or mesh electronic devices was allowed. The nebulizer nozzle was placed in the mouth, and the patient was asked to breathe normally. In all cases the recommendations of the instructions of use of the medical device were followed and the dose was adjusted to the requirements of each patient and to the clinical judgement of the doctor.
Spirometry measurements were obtained prior to the intervention, after the administration of a bronchodilator (20 min after two puffs of salbutamol), and after the administration of HS or HS+HA (30 min after inhalation).
Data were collected during three visits to determine tolerability of the treatment (Figure 1):
Figure 1.
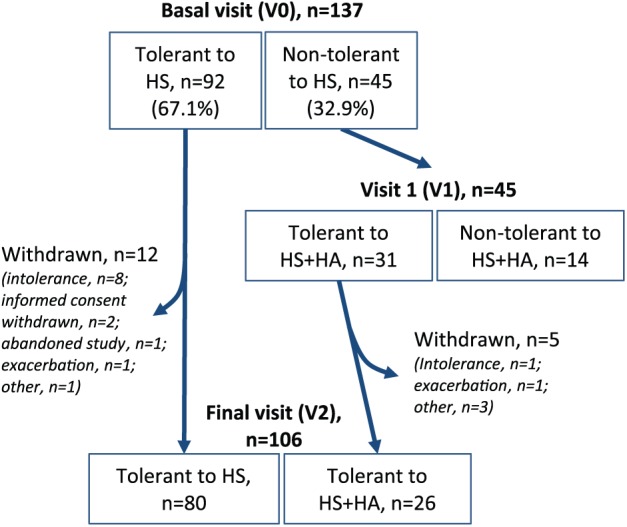
Flow chart of the study.
In the basal visit (V0) the patients were administered HS and evaluated (see below). Tolerant patients continued with this treatment for 4 weeks, while intolerant patients were offered to start treatment with HS+HA one week later (visit 1, V1) to allow the patient to recover from symptoms caused by HS.
In visit 1 (V1), those patients intolerant to HS in V0 were evaluated for their tolerance to HS+HA. Tolerant patients were asked to remain on this treatment for 4 weeks.
In the final visit (V2), after 4 weeks of treatment with either HS or HS+HA, patients were evaluated for their tolerability of either treatment.
Criteria of tolerability
We assessed tolerability to HS or HS+HA in each of the visits according to three parameters. The patient was asked to complete a seven-question, Likert-type questionnaire to assess tolerability to the treatment within 15 min after inhaling HS for the first time. The questionnaire evaluated cough, pharyngeal irritation, salty taste, chest tightness, dyspnoea, wheezing and nausea. The responses to each question ranged from “0” (nothing) to “3” (very much). The patient was scored as intolerant if, first, they answered “2” or “3” to any of the questions in the questionnaire; and/or, second, if in post-treatment spirometry the mean baseline FEV1 decreased by at least 15%; and/or, third, as judged by the physician supervising the test.
Quality of life and therapeutic compliance
QoL was self-evaluated by the patients in each of the visits by the Spanish-validated versions of the Quality of Life Questionnaire Bronchiectasis (QOL-B-Sp-V3.0) and the Leicester Questionnaire.16,17 The QOL-B-Sp-V3.0 consists of two sections that evaluate QoL (28 items) and respiratory symptoms (9 items). The Leicester Questionnaire assigns a score based on the physical, social and psychological impact of the cough, and assesses its frequency and severity. It consists of 19 items with seven-point, Likert-type responses.
In addition, data on the patient’s therapeutic compliance were collected on the final visit through the Morisky–Green (completed by the patient) and the Haynes–Sackett (completed by the doctor) tests.18,19
AEs were documented by the investigator at each visit, and their relationship to the treatment was evaluated.
Statistical analysis
Sample size calculation
The sample size was calculated based on a previous study of cystic fibrosis patients, in which it was observed that the percentage of patients intolerant to HS but tolerant to HS+HA was 21.0%.13 Assuming the expected proportion of non-tolerant patients to be 21%, and with a 95% confidence interval calculated with the exact (Clopper–Pearson) formula, we found that a sample size of 125 patients would provide an accuracy of ±7.5% in the estimation of the percentage of patients who do not tolerate HS+HA. Allowing for 10% patient loss because of invalid data or drop-out, we estimated that the target number of patients to be recruited was N = 139.
Data analysis
Categorical variables were described by absolute and relative frequencies, including the 95% confidence interval. Variables were tabulated as mean [± standard deviation (SD)] or median (interquartile range) depending on their distribution. Normality of the variable was assessed by the Kolmogorov–Smirnov test. Parametric [Student’s t test or ANOVA (analysis of variance)] or non-parametric (Mann–Whitney U test or Kruskal–Wallis test) tests were used for the comparative analysis of subgroups of patients, according to the distribution of the variables under study. For comparison of data between visits, parametric tests (Student’s t test for dependent data) or non-parametric tests (Wilcoxon or Friedman tests) were used, depending on the distribution of the variables under study. For the qualitative variables, the Chi-square test or the Fisher exact test were applied to compare subgroups of patients, or the McNemar test for comparisons between visits. A statistical significance level of 0.05 was applied on all statistical tests.
All statistical analyses were performed using the statistical package SAS version 9.4 or later.
Results
The sociodemographic characteristics of the 137 patients included in this study are shown in Table 1. Most patients were female (63.5%) and had developed bronchiectasis as a result of an infectious disease (42.3%). However, for 35% of the patients the aetiology of the disease was unknown. The mean (±SD) time since diagnosis of bronchiectasis was 6.5 ± 7.0 years (range, 0.0–43.7 years). Jet nebulizers were used most frequently (91.2%) for a median of 12.0 min (10.0–15.0) (range: 4.0–50.0).
Table 1.
Baseline characteristics of the patients, n = 137.
| Age, years, mean ± SD | 63.0 ± 14.7 |
| Sex, female, n (%) | 87 (63.5) |
| BMI, kg/m2, mean ± SD | 24.8 ± 4.1 |
| Time since diagnosis, mean ± SD | 6.5 ± 7.0 |
| Relevant cardiopulmonary background, n (%)1 | |
| Smoking | 47 (34.3) |
| Hypertension | 30 (21.9) |
| Asthma | 21 (15.3) |
| COPD | 19 (13.9) |
| Ischaemic heart disease | 5 (3.6) |
| Atrial fibrillation | 5 (3.6) |
| Other | 21 (15.3) |
| Cause of bronchiectasis, n (%)1 | |
| Bacterial pneumonia | 31 (22.6) |
| Tuberculosis | 20 (14.6) |
| Viral infection | 7 (5.1) |
| COPD | 7 (5.1) |
| Ciliary dyskinesia | 6 (4.4) |
| Asthma | 4 (2.9) |
| Other | 19 (13.8) |
| Unknown | 48 (35.0) |
| Pulmonary lobes affected, n (%) | |
| Localized | 66 (48.2) |
| Generalized | 71 (51.8) |
| Basal FEV1, ml, mean ± SD | 1810.1 ± 639.6 |
| %, mean ± SD | 75.8 ± 24.5 |
| Bronchodilation test (V0)2, n (%) | |
| Positive | 8 (6.2) |
| Negative | 122 (93.8) |
| Symptoms, n (%)1 | |
| Chronic cough | 135 (98.5) |
| Daily mucopurulent expectoration | 133 (97.1) |
| Two or more yearly exacerbations | 91 (66.4) |
| Dyspnoea | 72 (52.6) |
| Haemoptoic sputum | 16 (11.7) |
| Bronchorrhea (ml/day) | |
| 30–50 | 95 (69.3) |
| 51–100 | 38 (27.7) |
| >100 | 4 (2.9) |
Patients could specify more than one category; percentages calculated over n = 137.
The test was considered positive if post-bronchodilation FEV1 values (%) ⩾12% and ⩾200 ml (absolute over basal levels).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; SD, standard deviation; V0, visit 0 (basal).
Tolerability to saline solutions
In the basal visit (V0) the patients were evaluated for their tolerability to HS. According to the tolerability criteria, of the 137 patients enrolled in the study, 92 patients (67.1%) were tolerant and 45 patients (32.9%) were intolerant to HS. The most common complaints by the intolerant patients, as revealed by the questionnaire, were cough, pharyngeal irritation and salty taste. Basal, post-bronchodilator and post-HS FEV1 values were significantly higher (p < 0.01) in tolerant patients than in intolerant patients (Table 2). The patients tolerant to HS at the baseline visit were then advised to remain on this treatment for 4 weeks. The HS-intolerant patients were cited approximately one week later for administration of HS+HA in visit 1 (V1).
Table 2.
Spirometry measurements, FEV1 (%).
| Basal visit (V0) | Tolerant to HS (n = 92) | Non-tolerant to HS (n = 45) | p value1 |
|---|---|---|---|
| Basal | 79.7 (25.2) | 67.9 (21.2) | 0.0095 |
| Post-bronchodilator | 83.7 (25.9) | 70.9 (20.9) | 0.0065 |
| Post-HS | 82.4 (26.4) | 67.1 (22.8) | 0.0025 |
| Visit 1 (V1) | Tolerant to HS+HA (n = 31) | Non-tolerant to HS+HA (n = 14) | |
| Basal | 70.1 (21.2) | 63.9 (22.0) | 0.3913 |
| Post-bronchodilator | 73.4 (20.8) | 65.6 (20.6) | 0.3027 |
| Post-HS+HA | 70.5 (22.4) | 62.2 (25.3) | 0.3032 |
| Final visit (V2) | Tolerant to HS (n = 80) | Tolerant to HS+HA (n = 26) | |
| Basal | 81.4 (24.2) | 75.2 (22.0) | 0.2976 |
| Post-bronchodilator | 84.5 (24.5) | 78.2 (21.8) | 0.2934 |
| Post-HS or HS+HA | 82.9 (25.2) | 75.9 (21.6) | 0.2407 |
Mann–Whitney U test.
HA, hyaluronic acid; HS, hypertonic saline.
In V1 the 45 patients intolerant to HS were evaluated for their tolerance to HS+HA. We found that, of the 45 patients, 31 patients (68.9%) were tolerant and 14 patients (31.1%) intolerant to the HS+HA solution. The spirometry measurements revealed no significant differences in basal, post-bronchodilator or post-HS+HA for tolerant versus intolerant patients (Table 2). The 31 patients tolerant to HS+HA at V1 were instructed to continue treatment for an additional 4 weeks. The 14 patients not tolerant to HS+HA did not continue treatment.
After four weeks of self-administration of HS or HS+HA, the patients were evaluated for their tolerance to either treatment in a final visit. Of the 92 patients initially tolerant to HS in the basal visit, 12 patients ended the treatment before the end of the 4 weeks due to decreased tolerance (8 patients), withdrawal of informed consent (2 patients), or other reasons. Similarly, of the 31 patients tolerant to HS+HA in V1, 5 patients interrupted treatment before the end of the 4 weeks. Of the 45 patients intolerant to HS in the basal visit, 26 patients (57.8%) could complete the 4-week treatment with HS+HA.
Spirometry measurements revealed that the changes in FEV1 (baseline, post-bronchodilator and post-saline solution) observed between the start of the treatment (V0 for HS and V1 for HS+HA) compared to the visit after 4 weeks of treatment were similar in both groups. Although in the group of patients tolerant to HS+HA the percentage improvements of FEV1 were higher (baseline, 1.3%; post-bronchodilator, 1.5%; post-HS+HA inhalation, 1.7%) than the improvements observed in the HS group (baseline, 1.3%; post-bronchodilator, 1.0%; post-HS inhalation, 1.0%), these differences were not statistically significant (baseline, p = 0.8528; post-bronchodilator, p = 0.6717; post-HS+HA inhalation, p = 0.3880, Mann–Whitney U test).
Quality of life
The effectiveness of saline solutions (HS and HS+HA) in terms of QoL was evaluated by comparing the difference between the scores obtained in the QoL-B-Sp-V3.0 and Leicester Questionnaires in the final visit and in the first administration of the saline solution, V0 for HS or V1 for HS+HA. Both treatment groups behaved similarly in seven of the eight dimensions assessed by the QoL-B-Sp-V3.0 Questionnaire (Table 3). For the QoL measured by the Leicester Questionnaire, both groups behaved in a similar manner.
Table 3.
Percentage of change in quality of life from visits 1 (HS) or 2 (HS+HA) to visit 2, mean (SD).
| Patients tolerant to HS |
Patients tolerant to HS+HA |
p-value1 | |
|---|---|---|---|
| QoL-B-Sp-V3.0 | |||
| Physical functioning | 11.4 (52.3) | 16.8 (40.3) | 0.7199 |
| Role functioning | 16.1 (135.4) | 9.7 (24.5) | 0.1727 |
| Vitality | 18.7 (86.1) | 10.8 (37.6) | 0.9243 |
| Emotional functioning | 8.9 (32.8) | 5.6 (23.2) | 0.7272 |
| Social functioning | 9.8 (59.6) | −5.5 (21.0) | 0.4589 |
| Treatment burden | −16.1 (52.3) | −9.4 (24.9) | 0.1447 |
| Health perceptions | 17.9 (47.9) | 15.9 (42.8) | 0.7783 |
| Respiratory symptoms | 4.4 (20.9) | 10.8 (23.2) | 0.1270 |
| Leicester Cough Questionnaire | |||
| Physical domain | 11.8 (31.0) | 12.1 (18.5) | 0.1149 |
| Psychological domain | 7.6 (31.2) | 6.9 (25.0) | 0.4213 |
| Social domain | 10.7 (39.0) | 10.8 (23.9) | 0.2787 |
| Global | 8.3 (25.2) | 11.0 (20.5) | 0.1136 |
Mann–Whitney U test.
Therapeutic adherence
Therapeutic adherence after 4 weeks was evaluated with two tests. First, the Morisky–Green test showed that the patients had a good adherence to the treatment (52.5% of patients tolerant to HS, 61.5% of patients tolerant to HS+HA), and no statistically significant differences were found between the groups (p = 0.4212, Chi-square test). Second, the Haynes–Sackett test indicated that 99.1% of the patients presented good treatment compliance, with no statistically significant differences between groups.
Adverse events
Twenty AEs were reported (Table 4): 9 AEs in the group of patients tolerant to HS (7 with confirmed or probable relationship to treatment) that affected 8 patients; 10 AEs in the group of patients tolerant to HS+HA (7 with confirmed or probable relationship to treatment) that affected 8 patients; and 1 AE in a patient intolerant to saline solutions. Overall, 87.5% of patients who experienced AEs in the group of patients treated with HS had to withdraw from treatment. This percentage was 62.5% in the group of patients treated with HS+HA. Thus, HS+HA generated fewer reactions leading to treatment withdrawal. There were five serious AEs reported by five patients (3.6% of total number of patients included in the study) (Table 4).
Table 4.
AEs reported during the study, n = 137.
| AE | N (patients) | N (AEs) | Percentage (AEs) | Serious AE |
|---|---|---|---|---|
| Decreased FEV1 | 1 | 1 | 5 | |
| Infection | 2 | 2 | 10 | 2 |
| Shaking | 1 | 1 | 5 | |
| Abdominal pain | 1 | 1 | 5 | 1 |
| Nausea | 2 | 2 | 10 | |
| Dry mouth | 1 | 1 | 5 | |
| Exacerbation of bronchiectasis | 1 | 1 | 5 | |
| Dyspnoea | 2 | 4 | 20 | 2 |
| COPD | 1 | 1 | 5 | |
| Haemoptysis | 1 | 1 | 5 | |
| Pharyngeal inflammation | 2 | 2 | 10 | |
| Cough | 3 | 3 | 15 | |
| Total | 18 | 20 | 100 | 5 |
AE, adverse event; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second.
Discussion
In this study we set out to evaluate the tolerability of an inhaled HS solution supplemented with HA 0.1% on patients intolerant to HS 7% alone. We found that 68.9% of patients intolerant to HS 7% could tolerate HS+HA, and 57.8% could continue the treatment for at least four weeks. In this regard, our study achieved its objective and suggests that HA improves tolerability of inhaled HS therapy. In considering the validity and applicability of this finding, several points should be noted.
The FEV1 values at baseline indicated that those patients intolerant to HS alone had worse lung function than those tolerant to HS, suggesting that FEV1 could be a potential predictor of good tolerance to inhaled saline solutions. In this regard, our study shows a similar pattern of a previous work with cystic fibrosis patients, which showed worse tolerance in patients with lower FEV1.13 However, despite their worse lung condition, a large fraction of these patients (68%) could tolerate HS+HA.
Regarding the specific reasons for better tolerance of HS+HA versus HS, a previous study of cystic fibrosis patients had shown a statistically significant lower incidence of bronchoconstriction, cough and throat irritation, resulting in a decrease in the use of β2 bronchodilators.20 Our study did not detect specific reasons that could explain the higher tolerance of HS+HA in bronchiectasis patients, and this should remain the subject of future investigations. The precise mechanism by which HA reduces the side effects of HS inhalation remains unknown, although HA has been shown to hydrate the airways, attenuate bronchial hyper-responsiveness and reduce inflammation.8–10
The goal of our open-label study was to demonstrate, in standard clinical practice, that HS+HA can be tolerated by bronchiectasis patients intolerant to HS. In this regard, our study complements a previous study that showed that up to 81% of cystic fibrosis patients intolerant to HS were tolerant to HS+HA.13
This study has a number of limitations worth mentioning. First, since this was a non-controlled, open-label study, it was subjected to possible biases from physicians and patients, who had to evaluate and quantify parameters which entail a considerable degree of subjectivity. Second, the seven-question, Likert-type questionnaire used to assess tolerability was not validated as a tool for the study of tolerance of inhaled saline solutions, although it was based on the most common AEs of HS reported in previous studies. To our knowledge, no such validated questionnaire has been described. Third, there was no control for possible biases derived from repeated tests of HS solutions: the results of the second visit (to test HS+HA) could be influenced by the experience of the patient in the first (to test HS). However, our study aimed to reflect real-world clinical practice, which is certainly influenced by subjective judgements. Further, our study did not consider population heterogeneity, comorbidities or concomitant medication. Also, the small number of patients analysed limited the statistical analysis of some of the secondary objectives. Finally, our study was limited by the time frame of 4 weeks, which could be insufficient to demonstrate the long-term tolerance of the saline solutions investigated, although it has been observed that most AEs are typically resolved in the first 15 days of treatment.5 We observed that HS+HA treatment generated fewer adverse reactions leading to treatment withdrawal than HS, suggesting a better long-term tolerance. Future randomized, blinded and long-term trials should be carried out to support our hypothesis.
The efficacy of long-term HS therapy in the treatment of bronchiectasis patients is still the subject of debate.4,6,7,21 A 12-month, randomized, double-blind study of bronchiectasis patients in therapy with HS versus isotonic saline did not find differences in exacerbations, QoL, sputum colonization or respiratory function.7 However, after study completion, more patients (73%) in this study chose to continue with HS therapy than with isotonic saline therapy. Recent reviews of the available evidence are still inconclusive as to the benefits of inhaled hyperosmolar versus isotonic therapies, especially in patients with milder disease, and suggest that future studies are needed in patients with severe disease.21,22 Since initial tolerability is a major factor that limits inhaled HS treatment for many patients, our study shows that HS+HA could greatly increase the number of bronchiectasis patients benefiting from this therapy.
Conclusion
Our study shows that addition of HA to HS could benefit a large fraction of patients that are initially intolerant to HS alone, improving tolerability. The addition of HA could also enhance QoL and adherence to the treatment for bronchiectasis patients.
Acknowledgments
The authors thank Francisco López de Saro (Trialance SCCL) for medical writing support.
Footnotes
Funding: This study was sponsored by Chiesi España SAU.
Conflict of interest statement: Luis Máiz reports participation in teaching and research activities by Chiesi SAU, Grifols SA, TEVA Pharma SL and Zambon SAU.
Rosa M. Girón reports receiving speaker fees from Chiesi SAU, Laboratorios Esteve, Vertex Pharmaceuticals Inc. and Zambon SAU.
Eva Polverino reports receiving speaker fees from Bayer Hispania SL, Chiesi SAU, Grifols SA, Laboratorios Menarini SA and Zambon SAU; a research grant from Chiesi SAU; and participation in advisory boards by Bayer Hispania SL and Insmed Inc.
Silvia Caño was a full-time employee of Chiesi España SAU.
Miguel A. Martínez-García reports participation in teaching and research activities by Chiesi SAU, Grifols SA, TEVA Pharma SL and Zambon SAU.
Marta G. Clemente, Rosa Cordovilla, Jordi Dorca Carlos Peñalver, Eva Prats and Félix Baranda report no relevant conflicts of interest.
ORCID iD: Luis Máiz  https://orcid.org/0000-0003-0547-6238
https://orcid.org/0000-0003-0547-6238
Contributor Information
Luis Máiz, Chronic Bronchial Infection, Cystic Fibrosis and Bronchiectasis Unit, Ramón y Cajal University Hospital, Ctra. Colmenar Viejo, km. 9,100, Madrid 28034, Spain.
Rosa M. Girón, La Princesa University Hospital, Madrid, Spain
Eva Prats, Fuenlabrada University Hospital, Madrid, Spain.
Marta G. Clemente, Central Asturias University Hospital, Asturias, Spain
Eva Polverino, Clínico y Provincial Hospital and Vall d’Hebron University Hospital, Barcelona, Spain.
Silvia Caño, Chiesi España SAU, Barcelona, Spain.
Rosa Cordovilla, Salamanca University Hospital, Salamanca, Spain.
Jordi Dorca, Bellvitge University Hospital, Hospitalet, Barcelona, Spain.
Carlos Peñalver, Virgen de la Arrixaca Hospital, Murcia, Spain.
Félix Baranda, de Cruces Hospital, Baracaldo, Vizcaya, Spain.
Miguel A. Martínez-García, La Fe University Hospital, Valencia, Spain
References
- 1. McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013; 188: 647–656. [DOI] [PubMed] [Google Scholar]
- 2. Martínez-García M, Máiz Carro L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol 2018; 54: 88–98. [DOI] [PubMed] [Google Scholar]
- 3. Wilson R, Cole PJ. The effect of bacterial products on ciliary function. Am Rev Respir Dis 1998; 138: S49–S53. [DOI] [PubMed] [Google Scholar]
- 4. Kellett F, Redfern J, Niven RM. Evaluation of nebulised hypertonic saline (7%) as an adjunct to physiotherapy in patients with stable bronchiectasis. Respir Med 2005; 99: 27–31. [DOI] [PubMed] [Google Scholar]
- 5. Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006; 354: 229–240. [DOI] [PubMed] [Google Scholar]
- 6. Kellett F, Robert NM. Nebulised 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med 2011; 105: 1831–1835. [DOI] [PubMed] [Google Scholar]
- 7. Nicolson CH, Stirling RG, Borg BM, et al. The long term effect of inhaled hypertonic saline 6% in non-cystic fibrosis bronchiectasis. Respir Med 2012; 106: 661–667. [DOI] [PubMed] [Google Scholar]
- 8. Garantziotis S, Brezina M, Castelnuovo P, et al. The role of hyaluronan in the pathobiology and treatment of respiratory disease. Am J Physiol Lung Cell Mol Physiol 2016; 310: L785–L795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gavina M, Luciani A, Villella VR, et al. Nebulized hyaluronan ameliorates lung inflammation in cystic fibrosis mice. Pediatr Pulmonol 2013; 48: 761–771. [DOI] [PubMed] [Google Scholar]
- 10. Lamas A, Marshburn J, Stober VP, et al. Effects of inhaled high-molecular weight hyaluronan in inflammatory airway disease. Respir Res 2016; 17: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buonpensiero P, De Gregorio F, Sepe A, et al. Hyaluronic acid improves “pleasantness” and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Adv Ther 2010; 27: 870–878. [DOI] [PubMed] [Google Scholar]
- 12. Koskela H, Purokivi M. Inhalation of hypertonic saline: a promising therapy in bronchiectasis. Duodecim 2011; 127: 2653–2659. [PubMed] [Google Scholar]
- 13. Máiz Carro L, Lamas Ferreiro A, Ruiz de Valbuena Máiz M, et al. Tolerance of two inhaled hypertonic saline solutions in patients with cystic fibrosis. Med Clin (Barc) 2012; 138: 57–59. [DOI] [PubMed] [Google Scholar]
- 14. Greenberger PA, Bush RK, Demain JG, et al. Allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2014; 2: 703–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warwick WJ, Huang NN, Waring WW, et al. Evaluation of a cystic fibrosis screening system incorporating a miniature sweat stimulator and disposable chloride sensor. Clin Chem 1986; 32: 850–853. [PubMed] [Google Scholar]
- 16. Olveira C, Olveira G, Espildora F, et al. Validation of a quality of life questionnaire for bronchiectasis: psychometric analyses of the Spanish QOL-B-V3.0. Qual Life Res 2014; 23: 1279–1292. [DOI] [PubMed] [Google Scholar]
- 17. Muñoz G, Buxo M, de Gracia J, et al. Validation of a Spanish version of the Leicester Cough Questionnaire in non-cystic fibrosis bronchiectasis. Chron Respir Dis 2016; 13: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Val Jiménez A, Amorós G, Martínez P, et al. Estudio descriptivo del cumplimiento del tratamiento farmacológico antihipertensivo y validación del test Morisky y Green. Aten Primaria 1992; 10: 767–770. [PubMed] [Google Scholar]
- 19. Sackett DL, Haynes RB, Gibson ES, et al. Randomised clinical trial of strategies for improving medication compliance in primary hypertension. Lancet 1975; 1: 1205–1207. [DOI] [PubMed] [Google Scholar]
- 20. Furnari ML, Termini L, Traverso G, et al. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: a prospective, randomized, double-blind, controlled study. Ther Adv Respir Dis 2012; 6: 315–322. [DOI] [PubMed] [Google Scholar]
- 21. Hart A, Sugumar K, Milan SJ, et al. Inhaled hyperosmolar agents for bronchiectasis. Cochrane Database Syst Rev 2014: CD002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tarrant BJ, Le Maitre C, Romero L, et al. Mucoactive agents for chronic, non-cystic fibrosis lung disease: a systematic review and meta-analysis. Respirology 2017; 22: 1084–1092. [DOI] [PubMed] [Google Scholar]


