Abstract
MicroRNAs (miRNAs) as small non-coding RNAs act as either tumor suppressors or oncogenes in human cancers, of which miR-149-5p (miR-149) is involved in tumor growth and metastasis, but its role and molecular mechanisms underlying osteosarcoma growth are poorly understood. The correlation of miR-149 expression with clinicopathological characteristics and prognosis in patients with sarcoma was analyzed by The Cancer Genome Atlas (TCGA) RNA-sequencing data. Osteosarcoma cell growth affected by miR-149 was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and colony formation assays. As a result, we found that the expression level of miR-149 was markedly downregulated in human sarcoma samples and were negatively associated with tumor size, acting as an independent prognostic factor for overall survival of the sarcoma patients. Restoration of miR-149 expression suppressed osteosarcoma cell growth, while its knockdown reversed these effects. Furthermore, we identified TNFRSF12A (TNF receptor superfamily member 12A), also called fibroblast growth factor–inducible 14 (Fn14) as a direct target of miR-149, and TNFRSF12A and its ligand TNFSF12 (TNF superfamily member 12), also called tumor necrosis factor–related weak inducer of apoptosis (TWEAK), were both negatively correlated with miR-149 expression in sarcoma samples. Knockdown of TNFRSF12A suppressed cell growth, but its overexpression weakened the antiproliferative effects of miR-149 via the PI3K/AKT (AKT serine/threonine kinase) signaling pathway. Altogether, our findings show that miR-149 functions as a tumor suppressor in osteosarcoma via inhibition of the TWEAK–Fn14 axis and represents a potential therapeutic target in patients with osteosarcoma.
Keywords: growth, miR-149, osteosarcoma, TNFRSF12A, TWEAK
Introduction
Osteosarcoma (OS), the most common malignant bone tumor, is an aggressive neoplasm, occurring primarily in children and young adults.1 In spite of the advances in multidrug chemotherapy and surgical removal of the primary tumors, the 5-year overall survival rate and recurrence of OS patients have not been improved owing to the tumor metastases.2 A variety of molecular alterations related with critical signal transduction pathways are implicated in multiple processes of OS pathobiology, such as proliferation, invasion, survival, and metastasis.3 Therefore, identifying these important molecular targets may shed light on the novel approaches for OS diagnosis and therapy.
MicroRNAs (miRNAs) play key roles in the biological processes, such as cell proliferation and invasion, by negatively regulating the expression of target genes, of which miR-149 is reported to be downregulated in breast cancer4,5 and glioma,6 correlates with advanced stages, lymph node metastasis, and early recurrence, and mediates the inhibition of cell proliferation, migration, and invasion via integrin and Akt signaling.4–6 In addition, miR-149 induces cell apoptosis by repressing Akt1 and E2F1 expressions in neuroblastoma,7 represses cell proliferation and invasion in bladder cancer,8 and blocks cycle progression in gastric cancer.9 Given the downregulation of miR-149 in some cancers, it is subject to DNA hypermethylation that mediates transcriptional repression in cervical carcinogenesis10 and colorectal cancer.11 However, several studies show that miR-149 is highly expressed in metastatic melanoma12 and accelerates cell proliferation by reducing sex-determining region Y-box 2 (SOX2), NANOG (Nanog homeobox), and octamer-binding transcription factor 4 (OCT4) expressions that are required for syndecan-1-mediated androgen-refractory prostate cancer13 and decreases cell apoptosis in T-cell acute lymphoblastic leukemia,14 suggesting that miR-149 exhibits a critical role in tumor progression.
Tumor necrosis factor–related weak inducer of apoptosis (TWEAK), a member of the tumor necrosis factor superfamily, acts by binding to its exclusive receptor fibroblast growth factor–inducible 14 (Fn14). Activation of the TWEAK/Fn14 axis is implicated in the tumorigenesis of multiple malignancies.15 Increasing evidence shows that the ectopic expression of the TWEAK/Fn14 axis is associated with poor prognosis and promotes cell survival and invasion in prostate cancer,16 neuroblastoma,17 and non-small-cell lung cancer (NSCLC).18 But targeting of the TWEAK/Fn14 axis reduces cancer-induced cachexia and prolongs survival.19 To clarify the functions and the underlying mechanisms of miR-149 in human OS, in this study, we discovered that miR-149 inhibited OS growth via inhibition of the TWEAK–Fn14 axis and indicated a potential therapeutic target for OS patients.
Materials and methods
Clinical data
The clinicopathological and prognostic data for 191 sarcoma patients and 66 adjacent normal samples as well as the relative expression levels of miR-149, TNFRSF12A, and TWEAK were downloaded from The Cancer Genome Atlas 2015 RNA sequencing database (https://genome-cancer.ucsc.edu). The protocols used in our study were approved by the Ethics Committee of Renji Hospital. The information on clinicopathological characteristics of sarcoma patients is summarized in Supplementary Table S1.
Materials
OS cell lines (U-2 OS, Saos-2, MG-63, SW-1353, HOS, and 143B) used in our experiments were from Chinese Academy of Science Shanghai Cell Bank (Shanghai, P.R. China). Lentivirus-mediated miR-149 overexpression and miR-149 shRNA and TNFRSF12A overexpression vectors and the negative control (NC) vector were from GeneChem (Shanghai, P.R. China); the primary antibodies of TWEAK (rabbit polyclonal antibody, ab37170), Fn14 (also called TWEAKR, mouse monoclonal antibody, ab21127), p-PI3K p85 (rabbit polyclonal antibody, ab182651), anti-PI3K (mouse monoclonal antibody, BYK-0128R), AKT (rabbit polyclonal antibody, ab126811), p-AKT (rabbit polyclonal antibody, ab18206), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; rabbit polyclonal antibody, ab70699) were from Abcam (Cambridge, MA, USA).
Drugs and reagents
Specific details for the sources of drugs and reagents were described in Supplementary Table S2.
Construction of vectors
The detailed description was referred from the previous study.20
Cell culture
OS cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells in this medium were placed in a humidified atmosphere containing 5% CO2 at 37°C.
Quantitative real-time polymerase chain reaction
To quantitatively confirm the expression levels of miR-149, real-time polymerase chain reaction (RT-PCR) was performed. Total RNA was extracted from each clone using TRIzol according to the manufacturer’s protocol. Reverse transcription was carried out using M-MLV and cDNA amplification was performed using the SYBR Green Master Mix kit according to the manufacturer’s guidelines. miR-149 was amplified using a specific primer: forward, 5’-GGCTCTGGCTCCGTGTCTT-3’, and reverse, 5’-CAGTGCAGGGT CCGAGGTATT-3’; U6 forward, 5’-CAAATTCGTGAAGCGTTCCATA-3’, and reverse, 5’-AGTGCAGGGTCCGAGGTA TTC-3’; and GAPDH forward, CCTGTACGCC AA CACAGTGC, and reverse, ATACTCCTGCTTG CTGATCC. GAPDH gene or U6 was used as an endogenous control. Data were analyzed using the comparative Ct method. Three separate experiments were performed for each clone.
Western blot analysis
The cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Thermo Scientific, Rockford, IL, USA) with protease inhibitors. The proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA), which were probed with anti-TWEAK (1:1000), anti-Fn14 (1:1000), anti-p-PI3K p85 (1:1000), anti-PI3K (1:1000), anti-AKT (1:1000), anti-p-AKT (1:1000), and anti-GAPDH (1:1000), followed by horseradish peroxidase (HRP)-conjugated secondary antibody. The proteins of interest were visualized using enhanced chemiluminescence (ECL) substrate. GAPDH was used as the loading control.
Cell viability assay
Cell proliferation was analyzed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After the OS cells were transfected with miR-149 vector (20 μL, multiplicity of infection (MOI) = 50) for 48 h and incubated in 96-well plates at a density of 4 × 103 cells per well with DMEM medium supplemented with 10% FBS. Then the cells were treated with 20 μL of MTT and subsequently incubated with 150 μL of dimethyl sulfoxide (DMSO) for 10 min. The color reaction was measured at 570 nm using an Enzyme Immunoassay Analyzer (Bio-Rad Laboratories, Hercules, CA, USA).
Colony formation assay
2× DMEM containing 20% FBS and 2 × 103 cells was mixed with an equal volume of 0.7% agarose and immediately plated in 6-well plates containing an underlayer of 0.5% agarose made in 1× DMEM supplemented with 10% FBS. The plates were cultured at 37°C under 5% CO2 for 7 days.
Dual-Luciferase Reporter Assay
OS cells were seeded into 24-well plates. After 24-h incubation, pmirGLO report vector carrying wild-type (WT) 3’-UTR or mutated 3’-UTR of miR-149 targets was co-transfected with miR-149 (10 μL, MOI = 50) or NC into the OS cells. At 48 h after transfection, luciferase activities were examined with a Dual-Luciferase Reporter Assay System (Promega Corporation, Beijing, China).
Statistical analysis
SPSS 20.0 was used for the statistical analysis. All of the values were recorded as the mean ± standard error of the mean (SEM) from at least three independent experiments. Two-tailed Student’s t-test was used to evaluate the differences between each group. The Pearson’s correlation coefficient analysis was used to analyze the correlations. Overall survival (OS) was defined as the interval between the dates of surgery and death and the Overall survival and disease-free survival (DFS; or recurrence) curves were analyzed with the Kaplan–Meier method and log-rank test. Statistical significance was set at P < 0.05.
Results
miR-149 expression is downregulated in human OS samples
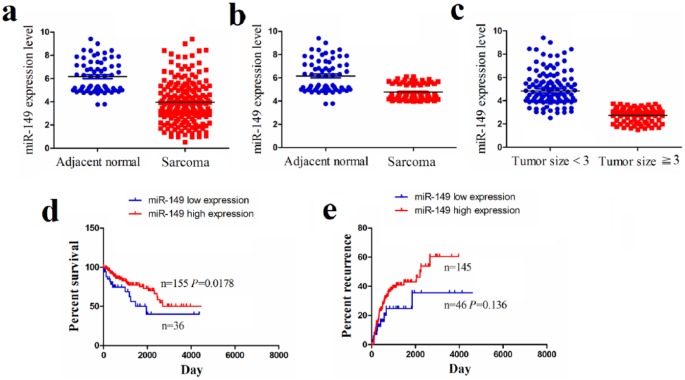
The expression levels of miR-149 in human OS samples (n = 191) and adjacent normal tissues (n = 66) were analyzed using The Cancer Genome Atlas (TCGA) sequencing data, which indicated a decreased expression level of miR-149 in OS samples (P < 0.0001; Figure 1(a)) as well as in the pair-matched OS tissues (n = 66, P < 0.0001; Figure 1(b)) in comparison to the adjacent normal tissues. Then, we examined the expression level of miR-149 in OS samples with tumor size ⩾3 cm (n = 69) or <3 cm (n = 122), suggesting that miR-149 expression was markedly downregulated in patients with tumor size ⩾ 3 cm compared to those with tumor size < 3 cm (P < 0.0001; Figure 1(c)).
Figure 1.
The expression of miR-149 in human sarcoma samples. (a) TCGA cohort analysis of miR-149 expression level in sarcoma samples and the adjacent normal tissues. (b) TCGA cohort analysis of miR-149 expression level in pair-matched sarcoma samples. (c) TCGA cohort analysis of miR-149 expression level in sarcoma samples with tumor size <3 cm and ⩾3 cm. (d), (e) Overall survival and recurrence curves demonstrated the link of the high or low expression of miR-149 with the percent survival and recurrence of OS patients.
The expression of miR-149 was correlated with tumor size and overall survival in OS patients
To confirm the correlation of miR-149 expression with OS patients, we analyzed the association between its expression and clinicopathological characteristics and prognosis in OS patients. As shown in Table 1, miR-149 expression was negatively correlated with age (P = 0.033) and tumor size (P = 0.001), but had no link with gender, lymph node infiltration, and distant metastasis in OS patients (each P > 0.05). We further analyzed the correlation of miR-149 expression with overall survival and recurrence in OS patients y using Kaplan–Meier and multivariate analysis, which demonstrated that miR-149 expression was an independent prognostic factor for overall survival (P = 0.024; Supplementary Table S3) in OS patients. Overall survival curve showed that the patients with high miR-149 expression harbored longer survival time (P = 0.0178; Figure 1(d)) compared to those with low miR-149 expression, but the tumor recurrence curve showed no difference between the patients with high miR-149 expression and those with low miR-149 expression (P = 0.136; Figure 1(e)).
Table 1.
The correlation of miR-149 expression with clinicopathological factors of sarcoma patients.
| Variables | Cases (n) | miR-149
expression |
P value | |
|---|---|---|---|---|
| Low | High | |||
| Total | 191 | 36 | 155 | |
| Age (years) | ||||
| ⩾20 | 39 | 12 | 27 | |
| <20 | 152 | 24 | 128 | 0.033 |
| Gender | ||||
| Male | 89 | 13 | 76 | |
| Female | 102 | 23 | 79 | 0.163 |
| Tumor size (cm) | ||||
| ⩾3 | 69 | 22 | 47 | |
| <3 | 122 | 14 | 108 | 0.001 |
| Lymph node infiltration | ||||
| No | 95 | 22 | 73 | |
| Yes | 96 | 14 | 82 | 0.131 |
| Distant metastases | ||||
| No | 171 | 29 | 142 | |
| Yes | 20 | 7 | 13 | 0.052 |
Ectopic expression of miR-149 inhibited cell growth
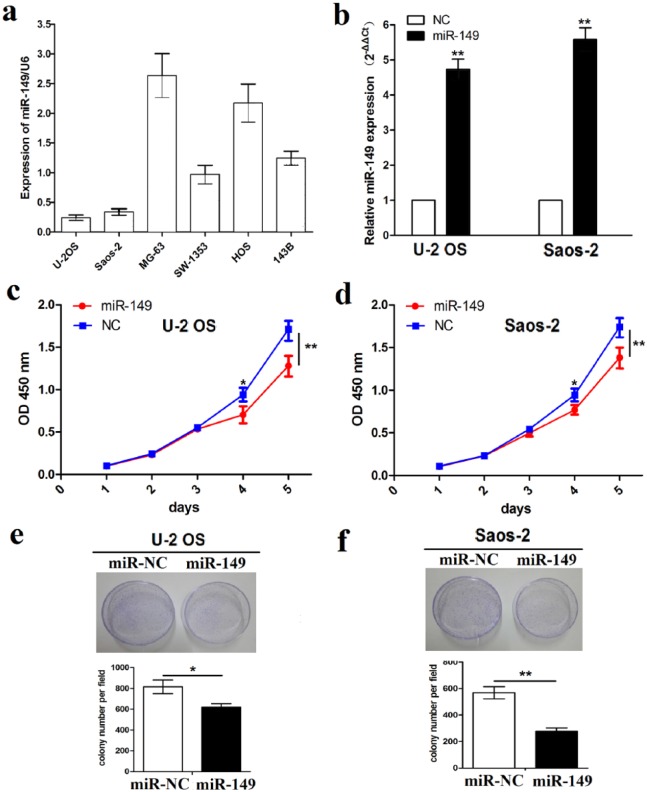
Having confirmed the negative correlation of miR-149 expression with tumor size in OS patients (Figure 1(c) and Table 1), we explored the functions of miR-149 in OS cell growth. The expression levels of miR-149 were examined in different OS cell lines by quantitative real-time polymerase chain reaction (qRT-PCR) analysis, which indicated that miR-149 displayed high expression in MG-63 and HOS cell lines but low expression in U-2 OS and Saos-2 cell lines (Figure 2(a)). Thus, miR-149 overexpression vector was used to transfect into U-2 OS and Saos-2 cell lines with low miR-149 expression. After transfection for 48 h, miR-149 expression levels were verified by qRT-PCR analysis (**P < 0.01; Figure 2(b)). Then, MTT assay was performed to investigate cell proliferation capacity, indicating that the overexpression of miR-149 significantly inhibited the cell proliferation activity (Figure 2(c) and (d)) and colony formation ability (Figure 2(e) and (f)) in U-2OS and Saos-2 cells.
Figure 2.
The effects of miR-149 on cell proliferation and colony formation. (a) Expression levels of miR-149 in different OS cell lines indicated by qRT-PCR analysis. (b) Expression level of miR-149 after miR-149 overexpression vector transfection into U-2 OS and Saos-2 cell lines for 48 h. (c), (d) The effects of miR-149 overexpression on cell proliferation by MTT assay. (e), (f) The effects of miR-149 overexpression on cell colony formation. Data are the means ± SEM of three experiments.
*P < 0.05, **P < 0.01.
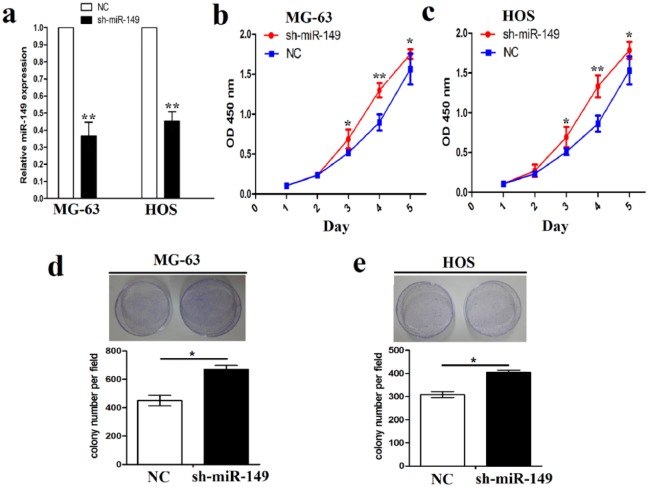
Furthermore, miR-149 shRNA vector was transfected into MG-63 and HOS cell lines with high miR-149 expression. After transfection for 48 h, miR-149 expression levels were confirmed by qRT-PCR analysis (Figure 3(a)). Cell growth assays showed that the knockdown of miR-149 accelerated cell proliferation and colony formation capability in MG-63 and HOS cell lines, respectively, indicated by MTT (Figure 3(b) and (c)) and colony formation assays (Figure 3(d) and (e)).
Figure 3.
The effects of miR-149 knockdown on cell proliferation and colony formation. (a) Expression level of miR-149 after miR-149 shRNA vector transfection into MG-63 and HOS cell lines for 48 h. (b), (c) The effects of miR-149 knockdown on cell proliferation by MTT assay. (d), (e) The effects of miR-149 knockdown on cell colony formation. Data are the means ± SEM of three experiments.
*P < 0.05, **P < 0.01.
TNFRSF12A is a direct target of miR-149
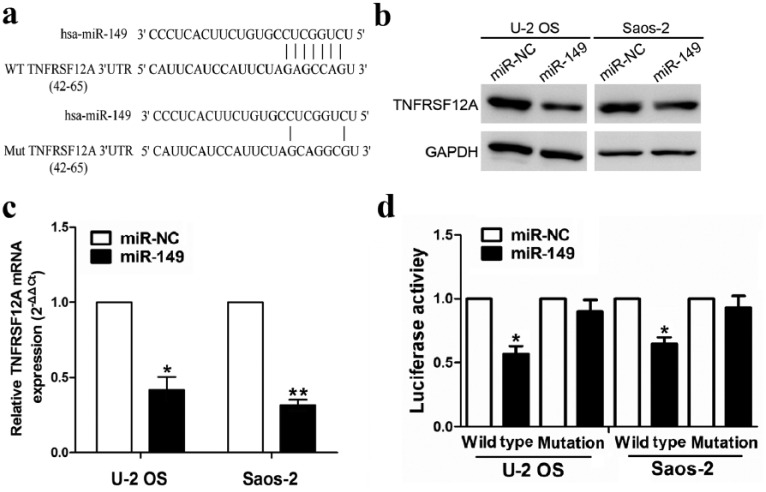
To expound the molecular mechanisms by which miR-149 suppresses OS cell growth, we attempted to identify publicly available miR-149 targets by prediction websites (http://starbase.sysu.edu.cn/targetSite.php). Among hundreds of target genes, TNFRSF12A was considered as the most suitable candidate target gene of miR-149 due to its low mirSVR score and high PhastCons score. To confirm whether miR-149 directly binds to the 3’-UTR of TNFRSF12A, the WT 3’-UTR or mutant 3’-UTR target sequences of TNFRSF12A (Figure 4(a)) were cloned into the luciferase reporter vector and transfected into U-2 OS and Saos-2 cells. The results showed that miR-149 decreased both the mRNA (Figure 4(b)) and protein expression levels of TNFRSF12A (Figure 4(c)) in U-2OS and Saos-2 cells, indicated by qRT-PCR and Western blot analysis. Moreover, miR-149 decreased the luciferase activity of WT 3’-UTR of TNFRSF12A in U-2 OS and Saos-2 cells, but had no effect on that of mutant 3’-UTR of TNFRSF12A (Figure 4(d)).
Figure 4.
TNFRSF12A is a novel target of miR-149. (a) The miR-149 putative binding sites and the corresponding mutant sites of TNFRSF12A. (b), (c) Expression level of TNFRSF12A was examined by qRT-PCR and Western blot analysis in the miR-149 and miR-NC groups. (d) Luciferase activity of TNFRSF12A 3’-UTR was detected after miR-149 vector transfection. Data were normalized to the luciferase activity after transfection with miR-NC. Data are the means ± SEM of three experiments.
**P < 0.01.
Knockdown of TNFRSF12A represses cell proliferation and colony formation
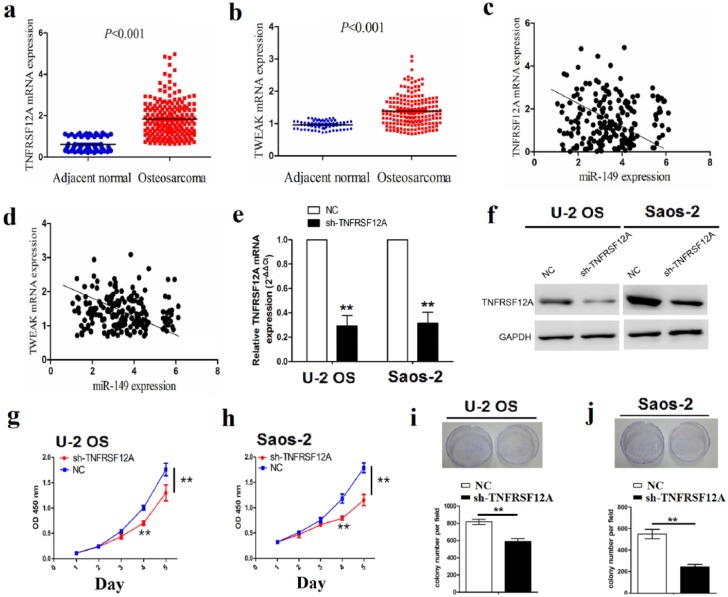
To address the correlation of miR-149 with TNFRSF12A and TWEAK expression in OS samples, the expression levels of TNFRSF12A and TWEAK in sarcoma tissues (n = 191) were analyzed by TCGA cohort, which showed that both TNFRSF12A and TWEAK expression levels were remarkably upregulated in sarcoma samples compared to the adjacent normal tissues (n = 66; Figure 5(a) and (b)), and the correlation analysis indicated that miR-149 had the negative correlation with TNFRSF12A (r = –4.613, P < 0.01) and TWEAK expression (r = –3.475, P < 0.01) in sarcoma tissues (Figure 5(c) and (d)). Furthermore, to figure out the functions of TNFRSF12A in OS cells, we constructed the TNFRSF12A shRNA stably transfected U-2 OS and Saos-2 cell lines and detected its mRNA and protein expression levels by qRT-PCR and Western blot analysis (Figure 5(e) and (f)). Then, the knockdown of TNFRSF12A significantly repressed cell proliferation (Figure 5(g) and (h)) and the colony formation abilities in OS cells (Figure 5(i) and (j)).
Figure 5.
The effect of TNFRSF12A knockdown on cell proliferation and colony formation. (a), (b) qRT-PCR analysis was used to detect the expression of TNFRSF12A and TWEAK in sarcoma and adjacent normal tissues. (c), (d) The correlation analysis of the association between miR-149 and TNFRSF12A and TWEAK expression in sarcoma tissues. (e), (f) Expression level of TNFRSF12A was determined by qRT-PCR and Western blot analysis in TNFRSF12A shRNA–transfected U-2 OS and Saos-2 cell lines. (g), (h) The effects of TNFRSF12A knockdown on cell proliferation by MTT assay. (i), (j) The effects of TNFRSF12A knockdown on cell colony formation. Data are the means ± SEM of three experiments.
**P < 0.01.
miR-149 mimic undermines TNFRSF12A-induced activation of TWEAK/EGFR signaling
To gain insight into the molecular mechanisms by which TNFRSF12A mediated miR-149 to modulate cell proliferation, TNFRSF12A overexpression vector was transfected into miR-149-pretreated U-2 OS and Saos-2 cells, indicating that TNFRSF12A overexpression promoted cell viability and miR-149 impaired TNFRSF12-induced cell proliferation (Figure 6(a) and (b)). The protein expression levels of TNFRSF12A, TWEAK, and epidermal growth factor receptor (EGFR), PI3K/p-PI3K and AKT/p-AKT as the potential downstream factors of TNFRSF12A were detected by Western blot analysis, referring that miR-149 inactivated the TWEAK/EGFR pathway, and TNFRSF12A attenuated the inhibitory effects of miR-149 on TWEAK/EGFR signaling (Figure 6(c)).
Figure 6.
TNFRSF12A attenuates miR-149-induced inactivation of TWEAK/EGFR signaling. (a), (b) The effects of TNFRSF12A overexpression on cell proliferation in miR-149-transfected U-2 OS and Saos-2 cells indicated by MTT assay. (c) The effects of TNFRSF12A overexpression on the activity of the TWEAK/EGFR/PI3K/Akt signaling pathway in miR-149-transfected U-2 OS and Saos-2 cells indicated by Western blot analysis.
Discussion
Increasing evidence shows that miR-149 exerts a pivotal role in multiple types of cancers and is associated with distant metastasis and poor prognosis in hepatocellular carcinoma (HCC).20,21 Also, miR-149 gene rs2292832 polymorphism results in the risk and susceptibility of patients with cancers.22–25 However, some studies illustrate the reduced risk and susceptibility of miR-149 gene rs2292832 polymorphism for overall survival and recurrence in patients with digestive cancers.26 In this study, we found that the decreased expression of miR-149 was closely associated with the age and tumor size, and Kaplan–Meier analysis uncovered that miR-149 was an independent prognostic factor for overall survival in sarcoma patients, suggesting that miR-149 might be a potential prognostic biomarker for sarcoma patients.
Recent studies have documented the indispensable role of miR-149 in various diseases including cancer. miR-149 overexpression represses cell migration and invasion in colorectal cancer27 and epithelial-to-mesenchymal transition (EMT) in non-small-cell lung cancer,28 and reverses cancer-associated fibroblast-induced EMT and stem-like properties in gastric cancer.29 miR-149 decreases cell proliferation and migration in renal cell carcinoma30 and attenuates chemoresistance in breast cancer.31 However, miR-149 promotes glioma cell growth by targeting caspase-2/p53/p21 pathways.32 To further clarify the functions of miR-149 in OS cells, we found that miR-149 suppressed cell proliferation and colony formation, but the knockdown of miR-149 reversed these effects, indicating the tumor suppressor role of miR-149 in OS cells.
TWEAK can bind with its receptor Fn14 and the TWEAK/Fn14 axis is implicated in multiple biological responses including oncogenesis.33,34 High expression of TWEAK/Fn14, related with EGFR activation, promotes tumor invasion and metastasis.35–37 TWEAK–Fn14 interaction contributes to cell growth, angiogenesis, and drug resistance in gastric cancer.38 Activation of the TWEAK–Fn14 axis leads to the activation of the PI3K/AKT signaling pathway, thus promoting the tumorigenesis.39 In addition, TWEAK induces cardiomyocyte apoptosis and cell cycle activation in a PI3K/AKT pathway–dependent manner.40,41 Intriguingly, we first confirmed that TNFRSF12A was a target of miR-149 and had a negative correlation with miR-149 expression in OS tissues; knockdown of TNFRSF12A inhibited cell growth and attenuated miR-149-mediated inhibition of cell proliferation in OS cells. Then, we found that miR-149 overexpression downregulated the expression of the TWEAK–Fn14 axis and inactivated the PI3K/AKT pathway, but TNFRSF12A overexpression reversed the inhibitory effects of miR-149 on the TWEAK–Fn14 axis and the PI3K/AKT pathway. The TWEAK receptor TNFRSF12 is a potential biomarker for glioblastoma,42 and the antibodies against it curb tumor growth.43 These findings demonstrated that TNFRSF12A might mediate the tumor suppressor role of miR-149 in OS cells via the PI3K/AKT pathway.
In conclusion, decreased expression of miR-149 represents an independent prognostic factor for overall survival in sarcoma patients, and miR-149 suppresses tumor growth of OS cells via inhibition of the TWEAK–Fn14 axis.
Acknowledgments
R.-D.X. and F.F. contributed equally to this article. X.-S.Y. and Z.-D.L. helped to analyze the data. L.-F.L. and Z.-D.L. provided the guidance for this work.
Supplementary Tables
Table S1.
Clinicopathologic data of sarcoma patients.
| Variables | Number of cases (%) |
|---|---|
| 191 (100%) | |
| Age (years) | |
| >20 | 39 (79.58%) |
| <20 | 152 (20.42%) |
| Gender | |
| Male | 89 (46.60%) |
| Female | 102 (53.40%) |
| Tumor size (cm) | |
| >3 | 69 (36.13%) |
| <3 | 122 (63.87%) |
| Lymph node infiltration | |
| No | 99 (51.83%) |
| Yes | 92 (48.17%) |
| Distant metastases | |
| No | 171 (89.53%) |
| Yes | 20 (10.47%) |
Table S2.
List of drugs and reagents.
| Drugs and reagents | Manufacturer |
|---|---|
| Dulbecco’s Modified Eagle medium (DMEM) | Thermo Fisher Scientific Inc (Waltham, MA, USA) |
| fetal bovine serum (FBS) | Thermo Fisher Scientific Inc (Waltham, MA, USA) |
| 3-(4,5)-dimethylthiahiazo(-z-yl)- 3,5-di-phenytetrazoliumromide (MTT) | Dingguo biology (Shanghai, PR, China) |
| TRIzol Reagent | Invitrogen (Carlsbad, CA, USA) |
| M-MLV Reverse Transcriptase | Promega (Madison, WI, USA) |
| SYBR Green Master Mixture was from; | Takara (Otsu, Japan) |
| RNase A | KeyGEN biology (Nanjing, PR, China) |
Table S3.
Univariate and multivariate analyses of factors associated with overall survival.
| overall
survival |
||||
|---|---|---|---|---|
| Multivariate |
||||
| Factors | Univariate P | HR | 95% CI | P value |
| Age (years) (⩾20 vs. <20) | 0.959 | NA | ||
| Gender (male vs. female) | 0.970 | NA | ||
| Tumor size (cm) (⩾3 vs. <3) | 0.012 | 0.573 | 0.368-1.215 | NS |
| Lymph node infiltration (Yes vs. No) | 0.113 | NA | ||
| Distant metastasis (Yes vs. No) | 0.073 | NA | ||
| miR-30e expression (high vs. low) | 0.021 | 0.469 | 0.247-0.890 | 0.024 |
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was partly supported by grants from the National Natural Science Foundation (No. 81101394), Shanghai Pujiang Program (No. 15PJD026), Shanghai Science and Technology Fund (17411964200), Incubating Program for Clinical Research and Innovation of Renji Hospital (PYXJS16-006), and Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ030).
ORCID iD: Li-Feng Lao  https://orcid.org/0000-0002-8161-2388
https://orcid.org/0000-0002-8161-2388
References
- 1. Ando K, Mori K, Verrecchia F, et al. (2012) Molecular alterations associated with osteosarcoma development. Sarcoma 2012: 523432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu D, Kahen E, Cubitt CL, et al. (2015) Identification of synergistic, clinically achievable, combination therapies for osteosarcoma. Scientific Reports 5: 16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adamopoulos C, Gargalionis AN, Basdra EK, et al. (2016) Deciphering signaling networks in osteosarcoma pathobiology. Experimental Biology and Medicine (Maywood) 241: 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan SH, Huang WC, Chang JW, et al. (2014) MicroRNA-149 targets GIT1 to suppress integrin signaling and breast cancer metastasis. Oncogene 33: 4496–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pérez-Rivas LG, Jerez JM, Carmona R, et al. (2014) microRNA signature associated with early recurrence in breast cancer. PLoS ONE 9: e91884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan SJ, Zhan SK, Pei BG, et al. (2012) MicroRNA-149 inhibits proliferation and invasion of glioma cells via blockade of AKT1 signaling. International Journal of Immunopathology and Pharmacology 25: 871–881. [DOI] [PubMed] [Google Scholar]
- 7. Lin RJ, Lin YC, Yu AL. (2010) miR-149* induces apoptosis by inhibiting Akt1 and E2F1 in human cancer cells. Molecular Carcinogenesis 49: 719–727. [DOI] [PubMed] [Google Scholar]
- 8. Yang D, Du G, Xu A, et al. (2017) Expression of miR-149-3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. American Journal of Cancer Research 7: 2209–2219. [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Zheng X, Zhang Z, et al. (2012) MicroRNA-149 inhibits proliferation and cell cycle progression through the targeting of ZBTB2 in human gastric cancer. PLoS ONE 7: e41693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilting SM, Verlaat W, Jaspers A, et al. (2013) Methylation-mediated transcriptional repression of microRNAs during cervical carcinogenesis. Epigenetics 8: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang F, Ma YL, Zhang P, et al. (2013) SP1 mediates the link between methylation of the tumour suppressor miR-149 and outcome in colorectal cancer. Journal of Pathology 229: 12–24. [DOI] [PubMed] [Google Scholar]
- 12. Pfeffer SR, Grossmann KF, Cassidy PB, et al. (2015) Detection of exosomal miRNAs in the plasma of melanoma patients. Journal of Clinical Medicine 4: 2012–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujii T, Shimada K, Tatsumi Y, et al. (2015) Syndecan-1 responsive microRNA-126 and 149 regulate cell proliferation in prostate cancer. Biochemical and Biophysical Research Communications 456: 183–189. [DOI] [PubMed] [Google Scholar]
- 14. Fan SJ, Li HB, Cui G, et al. (2016) miRNA-149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T-cell acute lymphoblastic leukemia. Leukemia Research 41: 62–70. [DOI] [PubMed] [Google Scholar]
- 15. Hu G, Zeng W, Xia Y. (2017) TWEAK/Fn14 signaling in tumors. Tumour Biology. Epub ahead of print 22 June DOI: 10.1177/1010428317714624. [DOI] [PubMed] [Google Scholar]
- 16. Huang M, Narita S, Tsuchiya N, et al. (2011) Overexpression of Fn14 promotes androgen-independent prostate cancer progression through MMP-9 and correlates with poor treatment outcome. Carcinogenesis 32: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 17. Pettersen I, Baryawno N, Abel F, et al. (2013) Expression of TWEAK/Fn14 in neuroblastoma: Implications in tumorigenesis. International Journal of Oncology 42: 1239–1248. [DOI] [PubMed] [Google Scholar]
- 18. Cheng E, Whitsett TG, Tran NL, et al. (2015) The TWEAK receptor Fn14 is an Src-inducible protein and a positive regulator of Src-driven cell invasion. Molecular Cancer Research 13: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston AJ, Murphy KT, Jenkinson L, et al. (2015) Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell 162: 1365–1378. [DOI] [PubMed] [Google Scholar]
- 20. Luo G, Chao YL, Tang B, et al. (2015) miR-149 represses metastasis of hepatocellular carcinoma by targeting actin-regulatory proteins PPM1F. Oncotarget 6: 37808–37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin L, Zhang YD, Chen ZY, et al. (2016) The clinicopathological significance of miR-149 and PARP-2 in hepatocellular carcinoma and their roles in chemo/radiotherapy. Tumour Biology 37: 12339–12346. [DOI] [PubMed] [Google Scholar]
- 22. Jia H, Yu H, Liu Q. (2016) Single nucleotide polymorphisms of MIR-149 gene rs2292832 contributes to the risk of hepatocellular carcinoma, but not overall cancer: A meta-analysis. Minerva Medica 107: 259–269. [PubMed] [Google Scholar]
- 23. Feng Y, Duan F, Song C, et al. (2016) Systematic evaluation of cancer risk associated with rs2292832 in miR-149 and rs895819 in miR-27a: A comprehensive and updated meta-analysis. Oncotarget 7: 22368–22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Tang W. (2015) The associations of nucleotide polymorphisms in mir-196a2, mir-146a, mir-149 with lung cancer risk. Cancer Biomarkers 15: 57–63. [DOI] [PubMed] [Google Scholar]
- 25. Li L, Sheng Y, Lv L, et al. (2013) The association between two microRNA variants (miR-499, miR-149) and gastrointestinal cancer risk: A meta-analysis. PLoS ONE 8: e81967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Liu T, Li Z, et al. (2015) The miR-149 rs2292832 T/C polymorphism may decrease digestive cancer susceptibility: An updated meta-analysis. International Journal of Clinical and Experimental Medicine 8: 15351–15361. [PMC free article] [PubMed] [Google Scholar]
- 27. Xu K, Liu X, Mao X, et al. (2015) MicroRNA-149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cellular Physiology and Biochemistry 35: 499–515. [DOI] [PubMed] [Google Scholar]
- 28. Ke Y, Zhao W, Xiong J, et al. (2013) miR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochemistry Research International 2013: 506731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li P, Shan JX, Chen XH, et al. (2015) Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Research 25: 588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin L, Li Y, Liu J, et al. (2016) Tumor suppressor miR-149-5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Molecular Medicine Reports 13: 5386–5392. [DOI] [PubMed] [Google Scholar]
- 31. He DX, Gu XT, Li YR, et al. (2014) Methylation-regulated miR-149 modulates chemoresistance by targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast cancer. FEBS Journal 281: 4718–4730. [DOI] [PubMed] [Google Scholar]
- 32. Shen X, Li J, Liao W, et al. (2016) microRNA-149 targets caspase-2 in glioma progression. Oncotarget 7: 26388–26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sabour Alaoui S, Dessirier V, de Araujo E, et al. (2012) TWEAK affects keratinocyte G2/M growth arrest and induces apoptosis through the translocation of the AIF protein to the nucleus. PLoS ONE 7: e33609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Winkles JA, Tran NL, Berens ME. (2006) TWEAK and Fn14: New molecular targets for cancer therapy? Cancer Letters 235: 11–17. [DOI] [PubMed] [Google Scholar]
- 35. Whitsett TG, Cheng E, Inge L, et al. (2012) Elevated expression of Fn14 in non-small cell lung cancer correlates with activated EGFR and promotes tumor cell migration and invasion. American Journal of Pathology 181: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jandova J, Mason CJ, Pawar SC, et al. (2015) Fn14 receptor promotes invasive potential and metastatic capacity of non-small lung adenocarcinoma cells through the up-regulation of integrin α6. Neoplasma 62: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watts GS, Tran NL, Berens ME, et al. (2007) Identification of Fn14/TWEAK receptor as a potential therapeutic target in esophageal adenocarcinoma. International Journal of Cancer 121: 2132–2139. [DOI] [PubMed] [Google Scholar]
- 38. Kwon OH, Kim JH, Kim SY, et al. (2014) TWEAK/Fn14 signaling mediates gastric cancer cell resistance to 5-fluorouracil via NF-κB activation. International Journal of Oncology 44: 583–590. [DOI] [PubMed] [Google Scholar]
- 39. Wajant H. (2013) The TWEAK-Fn14 system as a potential drug target. British Journal of Pharmacology 170: 748–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang B, Yan P, Gong H, et al. (2016) TWEAK protects cardiomyocyte against apoptosis in a PI3K/AKT pathway dependent manner. American Journal of Translational Research 8: 3848–3860. [PMC free article] [PubMed] [Google Scholar]
- 41. Novoyatleva T, Sajjad A, Pogoryelov D, et al. (2014) FGF1-mediated cardiomyocyte cell cycle reentry depends on the interaction of FGFR-1 and Fn14. FASEB Journal 28: 2492–2503. [DOI] [PubMed] [Google Scholar]
- 42. Perez JG, Tran NL, Rosenblum MG, et al. (2016) The TWEAK receptor Fn14 is a potential cell surface portal for targeted delivery of glioblastoma therapeutics. Oncogene 35: 2145–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Culp PA, Choi D, Zhang Y, et al. (2010) Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clinical Cancer Research 16: 497–508. [DOI] [PubMed] [Google Scholar]