Abstract
Lung cancer remains the leading cause of cancer deaths in the United States (US) and worldwide. Radiation therapy is a mainstay in the treatment of locally advanced non-small cell lung cancer (NSCLC) and serves as an excellent alternative for early stage patients who are medically inoperable or who decline surgery. Proton therapy has been shown to offer a significant dosimetric advantage in NSCLC patients over photon therapy, with a decrease in dose to vital organs at risk (OARs) including the heart, lungs and esophagus. This in turn, can lead to a decrease in acute and late toxicities in a population already predisposed to lung and cardiac injury. Here, we present a review on proton treatment techniques, studies, clinical outcomes and toxicities associated with treating both early stage and locally advanced NSCLC.
Keywords: proton therapy, non-small cell lung cancer, pencil beam scanning, toxicities, radiation therapy
Introduction
Lung cancer is the second most common cancer in the United States (US), with 234,030 new cases predicted to be diagnosed in 2018 alone.1 Despite advancements in treatment paradigms, lung cancer remains the leading cause of cancer deaths worldwide. Over 85% of lung cancer diagnosed in the US is non-small cell lung cancer (NSCLC), which currently has a 5-year overall survival (OS) rate of 44% across all stages.1,2 However, 58% of patients will present with metastatic (stage IV) disease at the time of diagnosis and these patients have an especially poor prognosis with a 5% OS at 5 years.1,2
In early stage disease, radiation therapy is typically limited to patients who are medically inoperable or who decline surgical resection,3 although there are some prospective and institutional data to suggest that radiation treatment for stage I NSCLC has similar oncological outcomes to surgery in operable patients.4–6 In stage III or locally advanced disease, radiation is given in approximately 60% of all patients7 and is often delivered with chemotherapy, either concurrently or sequentially, as definitive bimodality treatment. There is a 4.5% absolute OS benefit at 5 years to receiving concurrent chemotherapy and radiation therapy as opposed to a sequential treatment paradigm at the expense of increased toxicities, most notably esophagitis,8–10 and the ability to deliver concurrent therapy is frequently contingent on a patient’s performance status. Chemoradiation can also be given as a neoadjuvant treatment before surgical resection (trimodality therapy) in patients who have operable disease in an attempt to further improve local control and progression-free survival in the most fit patients with more limited nodal disease.11
Despite the improved precision of modern-day radiotherapy with techniques like intensity-modulated radiation therapy (IMRT), the advent of daily image guidance, and the increased use of advanced techniques to account for and mitigate tumor motion, delivery of definitive photon therapy for NSCLC remains quite challenging. The dose of radiotherapy needed for potential cure regularly exceeds the tolerance of surrounding normal organs at risk (OARs) such as the lung parenchyma, heart and spinal cord.12 Toxicity at these doses is not trivial, especially with concurrent chemotherapy in a patient population highly susceptible to treatment side effects. The majority of NSCLC patients present at an advanced age and have pre-existing cardiopulmonary disease secondary to a smoking history, leaving them prone to significant treatment toxicity.13,14 Proton therapy may allow for a reduction in such toxicities from thoracic radiation therapy and is the subject of this review.
Modalities of proton treatment
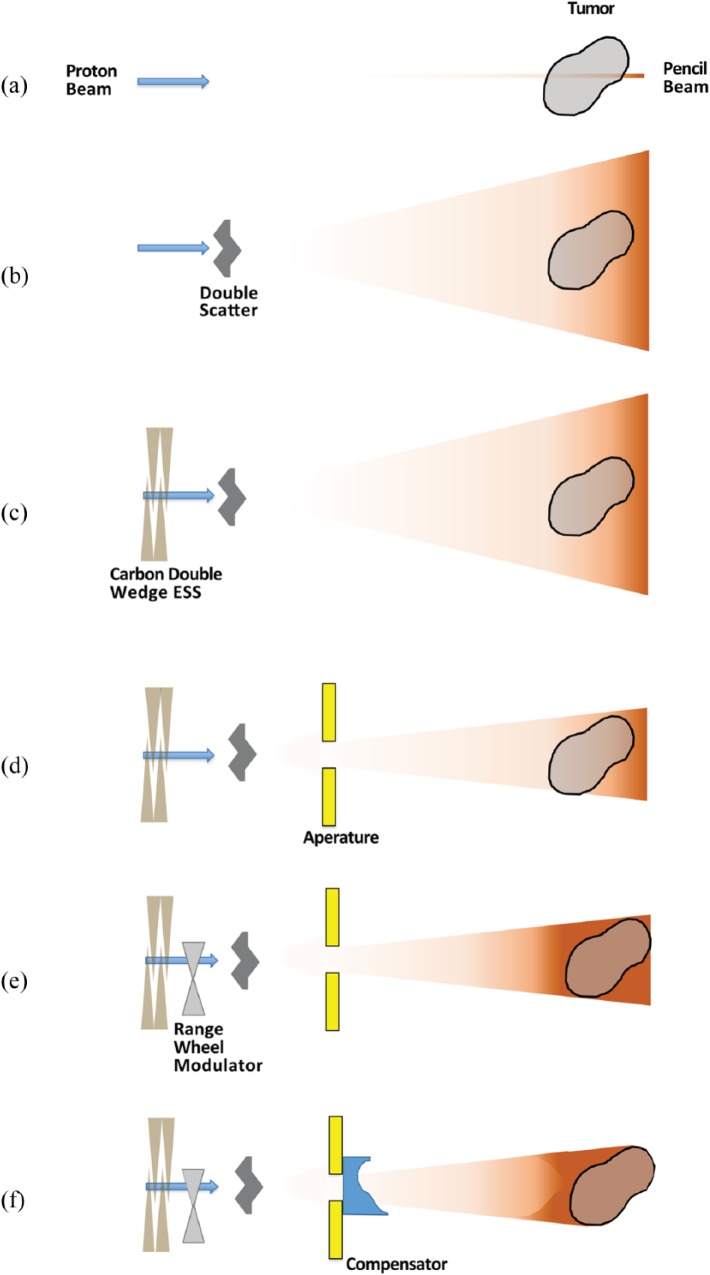
Proton therapy has become an enticing treatment modality for NSCLC, largely based on the physical property of the Bragg peak, where the majority of the proton dose is deposited across a very narrow range, with very little to no ‘exit dose’ to normal structures in the thorax. The planning and delivery of proton therapy can be broadly divided into two main categories: passively scattered proton therapy (often called passive scattering; PS) and active scanning proton therapy (often referred to as pencil beam scanning proton therapy; PBS).1 Both techniques take a single, monoenergetic (high energy ~250 MeV) proton beam, typically with approximately a 4 mm-cross sectional beam diameter (or spot size) and disperse the protons to deliver a dose to targets that are usually large and irregularly shaped [i.e. not perfect spheres; Figure 1(a)]. This complex task can be further subdivided into two elementary components: dispersal of particles in a plane that is orthogonal to the entry of the beam and dispersal of particles in a plane that is parallel to the entry of the beam.2,3
Figure 1.
Components involved in PS proton therapy.
ESS, energy selection system; PS, passive scatter.
PS has been used for several decades and is an effective solution for tumors with relatively simple treatment geometry (shape, position, proximity to OARs). PS uses physical scatterers (typically two scatterers) that cause, as the name suggests, the protons to be scattered in a plane that is orthogonal to the entry of the beam. For small tumors (i.e. eye malignancies), a single scatterer is sufficient for tumor coverage. For any tumor with significant size, such as a bulky NSCLC primary tumor, a double scatterer with high scattering power is needed. This allows for the 4 mm spot size proton beam to cover a much broader surface area. Assuming that there is no energy loss during this step, scatterers would merely allow the coverage of a flat circular target [Figure 1(b)]. The beam energy exited from the cyclotron/synchrotron can be adjusted by a carbon double wedge energy selection system (ESS) to reduce the high proton energy and give a maximum energy such that protons stop at the distal edge of a tumor [Figure 1(c)].3 Since most targets are not perfectly spherical, an additional physical device called an aperture (typically made of brass) is used to shape the beam in that plane [Figure 1(d)].
Most tumors are also not flat, and this is why the second elementary task, dispersing protons in a plane parallel to the entry of the beam, must be accomplished to adequately treat the thickness of the tumor. PS uses a similar two step approach as the previous task to achieve this goal. The depth at which a proton beam deposits the bulk of its energy (i.e. the Bragg peak) is related to the initial energy of the proton beam. Protons are continually being slowed down as they lose their kinetic energy through various interactions in matter and eventually stop once all their energy is deposited. In order to disperse the dose in the plane of the beam, the protons would need to stop at different depths instead of all stopping at the same depth. This means the monoenergetic proton beam must be replaced with a beam containing protons of many different energies. This is accomplished by using a range-modulator wheel [Figure 1(e)].3,4 Like the scatterer, the range-modulator wheel, spreads out the distance over which the protons stop uniformly across the entire field (called the Spread-Out Bragg Peak; SOBP), and, therefore, can allow for an otherwise flat circular field to be modified to a field that can adequately cover the tumor [Figure 1(e)]. However, one last step is required to address any differences in the deepest portion of the tumor across the plane, and this is accomplished by a device known as a compensator [Figure 1(f)]. As evident from the figure, PS is unable to conform the proximal edge of the dose to the target volume, and this is one of the important distinctions between PS and PBS.
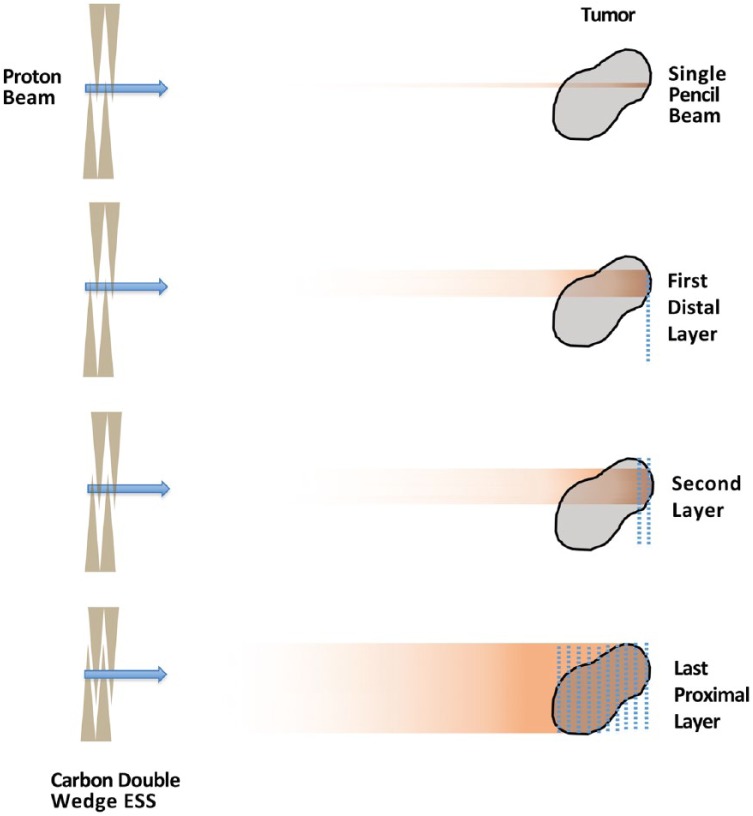
PBS is a newer approach to creating a broader target coverage from the narrow, monoenergetic proton beam. PBS uses a system of magnets near the exit of the beam to deflect the beam in order to deliver protons across the entire cross-section of the target that is orthogonal to the proton beam. As the beam is shifted through the tumor, ‘spots’ of protons are left throughout the target; therefore, this technique is also referred to as ‘spot-scanning’ (Figure 2). The distribution of protons in the plane parallel to the direction of the beam is attained in a very similar fashion to PS, except instead of the range-modulator wheel, a series of wedges, known collectively as the ESS, are used to degrade the energy in a step-wise fashion.3 This allows the radiation dose to be painted in a layer-by-layer approach. Because each layer can have an independent set of spots where a dose is delivered, both the distal and proximal edges of the dose can now be conformed to the target (Figure 2), which can further allow for OAR sparing compared with PS.5
Figure 2.
PBS proton therapy.
ESS, energy selection system; PBS, pencil beam scanning.
Some additional advantages of PBS over PS include the omission of beam-specific apertures and compensators, which are physical pieces that must be individually manufactured for each field, adding time, labor, and cost to treatments. This in turn reduces the burden of treating with multiple different fields from multiple different angles and has led to the development of intensity-modulated proton therapy (IMPT).6,7 Simply put, IMPT allows the use of multiple fields with a very heterogeneous dose in each field to add together to provide a more homogenously distributed final target dose (Figure 2).
The one potential caveat to this advancement has been the theoretical increase in uncertainty. Because PBS does not treat the entire target volume simultaneously, changes in density along the path of the proton beam and changes in the target location, such as due to respiration, could alter the actual delivered dose from the initial planned dose, thereby potentially over-treating some areas of adjacent normal tissues or undertreating areas of the tumor volume.3,8–11 This phenomenon has been termed the ‘interplay effect’, as it represents the ‘interplay’ between the motion of the target and the motion of the proton beam as it cycles through the various ‘spots’. Multiple methods are being developed to address these uncertainties, including turning the beam on and off at particular points during the respiratory cycle (termed gating) and mitigating the total excursion of tumor motion via external techniques such as abdominal compression.12–15 Alternatively, because the effects of the interplay can be relatively stochastic, techniques that increase frequency of delivery can lead to a regression toward the mean, and thereby abrogate the effect. This can be accomplished through dose fractionation, as radiation doses, particularly in more advanced disease, are usually delivered in small fractions over multiple weeks, or by specifically introducing the analogous effect in shorter courses of radiation via repainting.16 Repainting can be done in several different ways, with volumetric and layered techniques being the two most common strategies. In general, either strategy employs delivering a portion of the dose for that fraction while the target is scanned multiple times, rather than delivering the full fraction dose to each spot with one round of scanning.3,17
Proton therapy allows for the opportunity to reduce dose to normal structures relative to the target dose. While there are multiple studies ongoing that will help elucidate the magnitude and utility of this benefit, preliminary clinical results are encouraging for early stage and locally advanced lung cancers and are increasingly being reported in the literature.
Rationale for proton therapy in NSCLC
Photon therapy is a form of ionizing radiation treatment that delivers dose though out the course of its beam path, which differs from proton therapy, where dose deposition happens across a very narrow range, allowing proton therapy to better spare normal tissues and organs beyond the tumor. This property allows for improved dose distributions with proton treatment by decreasing irradiation to critical structures (Table 1). In lung cancer, proton therapy, when compared with photon treatment, has significant dosimetric advantages, with reduced dose to normal lung, esophagus, heart and other OARs.18 The dosimetric gain with proton therapy can potentially decrease treatment-related toxicities that may attenuate survival19 and can allow for safer delivery of dose-escalated therapy that may improve local control.18,20 Furthermore, sparing normal tissues might also increase the safe utility of multimodality therapy21 along with the feasibility of safely giving reirradiation in the setting of locoregional recurrence.22,23
Table 1.
Rationale and potential benefits of proton therapy for non-small cell lung cancer.a
| Rationale | Potential benefit |
|---|---|
| Reduce dose to normal tissue | Reduce treatment toxicities |
| Safer delivery of high-dose radiation to tumors close to critical organs (i.e. spinal cord or heart) | Increased chance of cure not attainable with photon treatment or chemotherapy alone, without attenuation of survival secondary to treatment toxicities (i.e. cardiovascular events) |
| Safer delivery of dose escalation | Improvement in local tumor control and survival |
| Allows for a safer combination of radiation therapy with chemotherapy and surgery for trimodality treatment | Improvement in local tumor control and progression-free survival compared with definitive radiation alone or bimodality chemoradiation |
| Safer treatment of locoregionally recurrent tumors with radiation in patients who previously had radiotherapy | Chance of cure not attainable with photon therapy or chemotherapy alone |
Table adapted from Simone CB II and Rengan R.
A recent National Cancer Database (NCDB) analysis was done comparing outcomes in NSCLC patients (stages I–IV) using photon (n = 243, 822) versus proton (n = 348) radiation treatment. Proton utilization was associated with a significant OS advantage using a propensity-matched analysis at 5 years (22% versus 16%, p = 0.025), especially in more advanced disease (stage II–III).24 The physical attributes of proton therapy makes it an appealing choice for the treatment of NSCLC, with quite possibly a survival benefit when compared with conventional radiation therapy.
Proton therapy in early stage NSCLC
Surgical resection has been the primary treatment modality for patients with early stage disease and achieves an OS rate of approximately 60–80% at 5 years.25–27 However, in patients who are medically inoperable or decline surgery, radiation therapy is an excellent alternative. Radiation for early stage disease is typically delivered using larger doses of per fraction (i.e. hypofractionation), also known as stereotactic body radiation therapy (SBRT) or stereotactic ablative radiation therapy, which has been shown to have equivalent clinical outcomes when compared with surgical resection.22,27–30 SBRT also has a more favorable toxicity and OS profile when compared with conventional fractionation (i.e. smaller doses of radiation that ranges from 1.8 to 2 Gy per fraction).31–33 Various studies have shown a strong correlation between oncological outcomes and total dose given in early stage disease, where a biological effective dose of ⩾100–105 Gy is associated with improved local control and OS.34,35 However, multiple arcs or beams are needed to deliver these hypofractionated doses, often passing through large amounts of normal lung parenchyma, exposing these areas to low doses of radiation, which can potentiate treatment toxicities in an already susceptible population.31,35
Furthermore, studies have shown a significant increase in treatment-related toxicities associated with SBRT-treated lesions proximal to the bronchial tree (typically within 2 cm), vasculature and other critical organs.36–38 In addition, larger early stage lesions (⩾5 cm) are a challenge to treat. Recent retrospective studies have shown good local control with SBRT, although toxicity is still of great concern, especially since these patients may be considered for systemic therapy along with SBRT.39–41 proton beam therapy (PBT) may provide an optimal means to spare important OARs, particularly for large or centrally located lesions, without compromising on the dose necessary for desirable clinical outcomes. PBT may also allow for the safe utility of multimodality treatment (i.e. chemotherapy or even immunotherapy after SBRT for large lesions).
There have been a number of dosimetric analyses showing the benefit of proton therapy over photon treatment for early stage NSCLC,42,43 as well as several clinical studies portraying improvement in target coverage while decreasing dose to the lungs, esophagus, cord and heart (Table 2). One of the earliest prospective studies was conducted at Loma Linda and published by Bush and colleagues in 1999.44 There was a total of 37 patients (n = 27 with stage I NSCLC) in the study, and those with good cardiopulmonary function (n = 18) were given a total of 73.8 CGEs (cobalt gray equivalents), using photon radiation to deliver the initial 45 Gy in 25 fractions to the gross disease/mediastinum and proton therapy for the additional 28.8 CGEs ‘boost’ to just gross disease in 16 fractions. Those with poor cardiopulmonary function (n = 19) received a total of 51 CGEs given solely with protons in 10 fractions over 2 weeks. The 2-year disease-free survival (DFS) for the early stage patients was 86%, with good local control (LC) at the primary site (87%) and minimal toxicity, with only two patients developing symptomatic radiation pneumonitis with the mixed proton–photon treatment.
Table 2.
Proton therapy studies for early stage non-small cell lung cancer.
| First author | Year published | No. patients | Stage | Fractionation regimen | Median follow up | Overall survival | Local control | Toxicity |
|---|---|---|---|---|---|---|---|---|
| Bush and colleagues44 | 1999 | 37 | I (n = 27), II (n = 2), IIIA (n = 8) | 51 CGEs in 10 fractions or 45 Gy in 25 fractions + 28.8 CGEs in 16 fractions = 73.8 CGEs to GTV | 14 months | 2 years 31% (39% stage I) | 2 years 87% | 6% grade 2 pneumonitis (0% for protons alone) |
| Shioyama and colleagues45 | 2003 | 51 | IA (n = 9), IB (n = 19), II (n = 9), III (n = 8), IV (n = 1), recurrent (n = 5) | Median 76 CGEs in median 3.0 CGEs fractions with protons alone (n = 33) or protons + photons (n = 18) | 30 months | 5 years 29% (70% IA, 16% IB) | 5 years 57% (89% IA, 39% IB) | 8% grade ⩾2 lung |
| Bush and colleagues46 | 2004 | 68 | IA (n = 29), IB (n = 39) | 51 CGEs in 10 fractions (n = 22) or 60 CGEs in 10 fraction (n = 46) | 30 months | 3 years 44% (27% for 51 CGEs, 55% for 60 CGEs) | 3 years 74% (87% IA 49% IB) | 0% symptomatic pneumonitis |
| Nihei and colleagues47 | 2006 | 37 | IA (n = 17), IB (n = 20) | 70 CGEs in 20 fractions (n = 3), 80 CGEs in 20 fractions (n = 17), 88 CGEs in 20 fractions (n = 16), 94 CGEs in 20 fractions (n = 1) | 24 months | 2 years 84% | 2 years 80% | 16% grade ⩾2 pneumonitis/pleural effusion |
| Hata and colleagues48 | 2007 | 21 | IA (n = 11), IB (n = 10) | 50 CGEs in 10 fractions (n = 3) or 60 CGEs in 10 fractions (n = 18) | 25 months | 2 years 74% (100% IA, 47% IB) | 2 years 95% (100% IA, 90% IB) | 5% grade 2 pneumonitis, 10% late grade 2 subcutaneous induration/myositis |
| Nakayama and colleagues49 | 2010 | 55 (58 tumors) | IA (n = 30), IB (n = 28) | 66 CGEs in 10 fractions (peripheral, n = 41) or 72.6 CGEs in 22 fractions (central, n = 17) | 17.7 months | 2 years 97.8% | 2 years 97% | 7% grade ⩾2 pneumonitis, 2% rib fracture |
| Iwata and colleagues50 | 2010 | 80 | IA (n = 42), IB (n = 38) | Proton: 80 CGEs in 20 fractions (n = 20) or 60 CGEs in 10 fractions (n = 37); carbon ion: 52.8 CGEs in 4 fractions (n = 23) | 30.5 months | 3 years 75% (74% IA, 76% IB) | 3 years 82% (87% IA, 77% IB) | 12% grade ⩾2 lung, 16% grade ⩾2 skin, 23% rib fracture |
| Busch and colleagues | 2013 | 111 | IA (n = 47), IB (n = 64) | 51 CGEs in 10 fractions (n = 29), 60 CGEs in 10 fractions (n = 56), or 70 CGEs in 10 fractions (n = 26) | 30.5 months | 4 years 18% for 51 CGEs, 32% for 60 CGEs, 51% for 70 CGEs | 4 years 45% for 60 CGEs, 74% for 70 CGEs | 0% grade ⩾2 pneumonitis, 4% rib fractures |
| Iwata and colleagues52 | 2013 | 70 | IA (n = 47), IB (n = 23) | Proton: 80 CGEs in 20 fractions (n = 14), 60 CGEs in 10 fractions (n = 20), 66 CGEs in 10 fractions (n = 8), or 70.2 CGEs in 26 fractions (n = 1); carbon ion: 52.8 CGEs in 4 fractions (n = 16), 66 CGEs in 10 fractions (n = 8), or 68.4 CGEs in 9 fractions (n = 3) | 51 months | 4 years 58% (72% for operable patient, n = 30) | 4 years 75% | 3% grade ⩾2 pneumonitis, 7% grade ⩾2 skin, 27% rib fracture |
| Kanemoto and colleagues53 | 2014 | 74 (80 lesions) | IA (n = 59), IB (n = 21) | 66 CGEs in 10–12 fractions (peripheral, n = 59) or 72.6 CGEs in 22 fractions (central, n = 21) | 31 months | 5 years 65.8% | 5 years 81.8% | 3% grade 2 skin, 1% grade 2 esophagitis, 1% grade 3 pneumonitis, 14% rib fracture |
| Makita and colleagues54 | 2015 | 56 | IA (n = 43), IB (n = 13) | 66 CGEs in 10 fractions (peripheral, n = 32) or 80 CGEs in 25 fractions (central, n = 24) | 33.7 months | 3 years 81.3% | 3 years 96% | 16.1% grade 2, 1.8% grade 3 pneumonitis, 17.9% grade 1 and 2 rib fractures |
| Chang and colleagues55 | 2017 | 35 | IA (n = 12), IB (n = 16), IIA (n = 4), IIB (n = 3) | 87.5 Gy at 2.5 Gy/fraction | 83.1 months | 5 years 28.1 % |
5 years 85% | 51.4% grade 2, 2.9% grade 3 dermatitis, 2.9% grade 3 pneumonitis |
CGE, cobalt gray equivalents; GTV, gross tumor volume.
Loma Linda then opened a phase II trial looking at proton-only hypofractionated radiotherapy for stage I NSCLC in patients who were medically inoperable or who declined surgery (n = 68).46 As studies showing suboptimal LC with conventional fractionation in early stage disease accumulated, the first 22 patients received 51 CGEs in 10 fractions over 2 weeks, but then the remaining 46 patients received 60 CGEs in 10 fractions over 2 weeks. The 3-year LC and disease-specific survival (DSS) were 74% and 72%, respectively. There was a statistically significant improvement in OS at 3 years for those patients who received 60 CGEs when compared with the lower dose (55% versus 27%, p = 0.03). There was no clinical evidence of acute radiation pneumonitis, acute/chronic esophagitis or cardiac toxicity in this cohort.46 In 2013, the Loma Linda experience was updated to then include 111 NSCLC patients with stage I disease, and a third dose-escalated arm of 70 Gy in 10 fractions delivered over 2 weeks was employed. The entire group showed a 4-year OS benefit with dose escalation at 18%, 32% and 51% using 51 Gy, 60 Gy and 70 Gy, respectively (p = 0.006). There were especially good outcomes with peripheral T1 tumors, with LC of 96%, DSS of 88% and OS of 60% at 4 years with 70 Gy. There were no treatment-related adverse events of grade 2 or higher in this cohort.51
There have been multiple Japanese studies evaluating the use of PBT in early stage disease. Yoshiuki and colleagues from the University of Tsukuba published their retrospective experience of 28 patients with stage I disease in 2003. Total doses ranged from 60 to 81 Gy (median 70 Gy) for stage IA patients, whereas stage IB patients were treated to 60 to 93 Gy (median 78 Gy), and all patients received a median of 3 Gy per fraction (range 2–6 Gy). Clinical outcomes for stage IA patients were significantly better when compared with those with stage IB, where the 5-year OS was 70% versus 16% (p = 0.015), DFS was 89% versus 17% (p = 0.005), and LC was 89% versus 39% (p = 0.10).45 The same institution performed a prospective study of stage I patients (n = 21) treated with hypofractionated proton therapy initially using 50 Gy in 10 fractions 5 days a week for the first 3 patients, and then the dose was escalated to 60 Gy in 10 fractions for the subsequent 18 patients.48 The 2-year OS, cause-specific survival (CSS) and local progression-free survival were 74%, 86% and 95%, respectively, for the entire cohort. For stage IA patients, the OS and CSS were 100%, 47%, while for the stage IB cohort, OS and CSS were 100%, 70% at 2 years, respectively. There were no grade ⩾3 toxicities. Overall, two patients developed a grade 1 hematologic toxicity and only one patient developed a grade 2 pneumonitis. In 2010, Nakayama and colleagues published an extended prospective analysis on 55 medically inoperable stage I patients, where a total of 66 CGEs in 10 fractions was given to peripheral lesions (n = 41) and 72.6 CGEs were given to more centrally located tumors (n = 17) in 22 fractions. OS at 2 years was 97.8%, with no significant difference between stage IA and stage IB patients. The 2-year progression-free survival (PFS) and LC were also excellent at 88.7% and 97%, respectively.49 An update from the prospective University of Tsukuba early stage proton trial was published in 2014,53 including 80 patients with centrally (n = 21) or peripherally (n = 59) located stage I NSCLC. Again, the hypofractionated dose used depended on location (peripheral versus central). The OS, DSS and PFS at 5 years were 65.8%, 73.8%, and 52.5%, respectively, across the entire cohort. LC for stage IA was 86.2% and 67% for stage IB patients at 3 years. The incidence of grade 3 pneumonitis was 1.3% (n = 1), and the only grade 4 events were rib fractures, which encompassed 13.8% of the cohort (n = 11).
Another Japanese study from the National Cancer Center Hospital East was reported in 2006. In this experience, a total of 37 patients (stage IA = 17, stage IB = 20) were treated initially on a phase I dose escalation trial (70 CGEs up to 98 CGEs; n = 10 patients) that was terminated early due to symptomatic radiation pneumonitis seen at 94 CGEs. After closure of the study, an additional 27 patients were treated to either 88 CGEs (4.4 CGE/fraction) if they had good cardiopulmonary function or 80 CGEs (4 CGE/fraction) with a poor pulmonary status. PFS at 2 years was 80%, with an OS of 84% for the entire cohort. In stage IA and IB disease, local-regional relapse-free survival rates were 79% and 60%, respectively.47
In 2010, Iwata and colleagues reported on a series of 80 patients with stage I NSCLC treated with either proton (n = 57) or carbon ion (n = 23) radiation treatment. In the proton cohort, 80 CGEs given in 20 fractions or 60 CGEs in 10 fractions were administered. The LC at 3 years for the 80 and 60 CGE cohorts were 83% and 81%, respectively, whereas the OS were 90% and 61%.50 Another study was reported by the same group in 2013, then focusing on larger (T2a/T2b) early stage NSCLC treated with either proton (n = 43) or carbon ion (n = 27) therapy. A total of three fractionation schemes were used as shown in Table 2 to treat 47 patients with T2a disease and 23 patients with T2b disease. For the entire cohort, the 4-year OS was 58% and LC was 46%.52 Makita and colleagues published their study on 56 stage I NSCLC patients in 2015. In this study, patients with peripheral tumors were treated with 66 CGEs in 10 fractions while more centrally located lesions were treated with 80 CGEs in 25 fractions. The OS, PFS and LC for the entire cohort were 81.3%, 73.4%, and 96%, respectively, at 3 years, with no grade 4 or 5 toxicities reported, and grade 3 toxicities limited to two patients; one with dermatitis and the other with pneumonitis.54
More recently, investigators from the MD Anderson Cancer Center and Massachusetts General Hospital, USA reported their long-term outcomes of a phase I/II prospective dose-escalated proton study in early stage patients that used a relative biologic equivalent dose of 87.5 Gy in 35 fractions.55 The majority of the patients had stage I disease (n = 28) and primaries less than 5 cm in size (n = 29). At 5 years, the OS and LC were 28% and 85% for the entire cohort, with no grade 4 or 5 toxicities. Grade 3 dermatitis and radiation pneumonitis were observed in one patient each. Furthermore, a meta-analysis was just published comparing proton beam therapy and SBRT with photons in early stage NSCLC using 72 SBRT and 9 hypofractionated proton studies in early stage disease.56 The 5-year OS was significantly improved with proton therapy when compared with photon treatment (60% versus 41.3%, p = 0.005), along with an observed, but statistically not significant, improvement in LC (87.2% versus 80.8%). PBT was associated with a significantly decreased overall rate of grade 3–5 toxicities (4.8% versus 6.9%, p = 0.05), including a lower risk of grade ⩾3 radiation pneumonitis (0.9% versus 3.4%, p = 0.001). PBT did, however, have a higher rate of grade ⩾3 chest wall toxicity (1.9% versus 0.9%, p = 0.03).
Proton therapy in locally advanced NSCLC
The current standard of care for inoperable locally advanced NSCLC is concurrent chemotherapy and radiation to a dose of 60–70 Gy.57–60 Previous efforts to dose-escalate with photon therapy based on prior promising phase II trials failed to demonstrate a survival benefit in the phase III trial, Radiation Therapy Oncology Group (RTOG) 0617.58,61,62 While disappointing, the results led to enlightening studies exploring radiation doses to normal tissues such as the heart and the effects on patient outcomes in the context of locally advanced disease.19,63 Speirs and colleagues examined the population of patients in RTOG 0617 treated at Washington University, USA and found that cardiac dose (V50, or the proportion of normal heart tissue that that receives at least 50 Gy) was the strongest independent prognostic factor on multivariate analysis for OS. In addition, heart volume and dose to the lungs (V5, or the proportion of normal lung tissue that is not in the target volume that receives at least 5 Gy) were also found to correlate with outcomes. Collectively, the findings suggest that dose escalation may, in fact, be beneficial if specific dosimetric parameters to surrounding normal tissues can be achieved. Herein lies the appeal of PBT in the setting of locally advanced NSCLC.
Data regarding the dosimetric benefits of PBT have been well documented. In locally advanced NSCLC, PBT has consistently demonstrated promising results regarding dose reduction to normal tissues.64–66 In a dose–volume histogram (DVH) analysis, Chang and colleagues found that patients with stage III NSCLC demonstrated a significant improvement in the dose to the lungs, spinal cord, heart, esophagus, and overall integral body dose compared with photon therapy (3D-CRT or IMRT).64
Moreover, a growing preponderance of recent evidence suggests a significant clinical benefit of proton beam radiotherapy for locally advanced NSCLC patients. In 2011, a phase II study from the MD Anderson Cancer Center, USA examined outcomes of PBT (74 Gy) with concurrent chemotherapy in stage III NSCLC patients. The median follow up was 19.7 months, with a median OS of 29.4 months achieved. There were no grade 4 or 5 proton-related toxicities.67 At 1 year, the OS and PFS was 86% and 63%, respectively. Additionally, 9.1% of patients experienced an isolated local recurrence, whereas the rate of distant metastases was 43.2%. These findings were corroborated by Hoppe and colleagues who examined 19 patients with stage III disease treated with PBT to a median dose of 74 Gy with concurrent chemotherapy.68 At the median follow up of 15 months, only one patient demonstrated acute nonhematologic grade ⩾3 toxicity (grade 4 esophagitis), while three patients developed late grade ⩾3 toxicities.
Reporting on the results from two separate phase II trials with a median follow up of 15.2 months, Sejpal and colleagues examined the toxicities of 62 patients with stage III NSCLC treated with PBT and compared outcomes to case-matched controls treated with 3D-CRT or IMRT.69 Treatment with PBT resulted in a 2% and 5% rate of grade ⩾3 pneumonitis and esophagitis, respectively. Despite higher doses of radiation therapy (RT), the incidence of toxicities (grade ⩾3 pneumonitis and esophagitis) in those patients treated with PBT was comparably lower relative to that of patients treated with 3D-CRT (30% and 18%) and IMRT (9% and 44%).
More recently, the University of Florida, USA reported the results of a phase II trial of PBT (74–80 Gy) and concurrent chemotherapy treatment in stage III NSCLC patients.69 The 2-year OS and PFS were 57% and 25%, respectively. Median OS and PFS were 33 months and 14 months, correspondingly. There were no grade 3 toxicities. Notably, this trial closed early due to poor accrual, highlighting the difficulty in successfully enrolling on to single institutional studies.
Long-term outcomes after PBT and concurrent chemotherapy have also been reported.70,71 In 2015, Nguyen and colleagues reported the results of a non-randomized observational study that included 134 patients with stage II/III NSCLC (113 stage III patients) treated with PBT and concurrent chemotherapy.70 The median follow up was 4.7 years. The OS was 30.4 months for stage III patients, and the 5-year DFS was 18%. Only one patient experienced grade 4 esophagitis, while six experienced grade 3 esophagitis, and 2 experienced grade 3 pneumonitis. MD Anderson Cancer Center investigators recently published the long-term results of their prospective phase II study with a median follow up of 27.3 months for all patients and 79.6 months for survivors. The median OS was 26.5 months, with a 5-year PFS of 22%. Local recurrences occurred in 16% of patients, whereas distant metastases occurred in 48%. Acute grade 3 esophagitis occurred in 12% of patients, and there was no acute grade ⩾3 pneumonitis reported. Acute cardiac arrhythmia or ischemia occurred in 3% of patients. Long-term toxicities included grade 3 esophagitis (12%); grade 4 esophagitis (2%); grade 3 pneumonitis (16%); and grade 4 bronchial fistula (2%).
Even in patients that do not receive concurrent chemotherapy, proton beam radiotherapy has demonstrated compelling outcomes with regard to LC and toxicity.72,73 In 2010, a Japanese retrospective analysis of 35 patients with stage II/III NSCLC treated with proton beam radiotherapy alone (median dose 72.6 Gy) demonstrated an OS/PFS of 81.8/56.9% and 58.9/29.2% at 1 and 2 years, respectively.72 There were no grade 3 toxicities noted. In a 2015 retrospective analysis, Hatayama and colleagues examined 27 patients with stage III disease treated to a median dose of 77 Gy with neoadjuvant chemotherapy administered to 11 patients.74 The 1-year OS for the entire cohort was 92.3% and 52% at 2 years. There was a significant improvement in survival in those patients that received chemotherapy compared with those patients that did not. Overall, two patients developed grade 3 pneumonitis and one developed grade 3 esophagitis. In 2014, a phase II study by Oshiro and colleagues examining 15 stage III patients demonstrated a median survival of 26.7 months, with three cases of grade 3 pneumonitis, one case of grade 3 esophagitis, and no other grade 4/5 toxicities.75
While the literature has generally demonstrated the benefit of PBT compared with conventional RT, a recently reported Bayesian randomized trial was reported where IMRT was compared with PBT (all received PS treatment) and showed no clinical difference between the two techniques.76 The study included a number of inoperable NSCLC patients ranging from stage IIB to oligometastatic disease or patients with recurrent disease after surgery, but all had to be candidates for concurrent chemoradiation. Both IMRT and PBT treatment plans were generated for each patient, yet patients were only randomized if both plans satisfied predefined OAR constraints. The primary outcome of the study examined treatment failure defined as either grade 3 pneumonitis or local recurrence within 12 months and found that there was no significant difference between the two modalities. The rate of grade 3+ radiation pneumonitis was 8.1% for the entire cohort (IMRT: 6.5%; passively scattered proton therapy (PSPT): 10.5%) and local failure was 10.7% for all patients (IMRT: 10.9%; PSPT: 10.5%). There could be several reasons as to why this phase II study showed no difference in outcomes between the two techniques. First, only patients who had a pair of acceptable IMRT and PBT treatment plans, to the same tumor dose, were randomized. This method could have excluded patients who would have potentially preferentially benefitted from PBT, thus favoring the IMRT arm. Furthermore, this trial required the same tumor dose for both IMRT and PBT, thereby hindering the proton arm from depicting its true potential of higher dose to the tumor, possibly increasing local control, while sparing nearby organs-at-risk. Additionally, this trial used a fairly heterogeneous population of patients and allowed neoadjuvant chemotherapy, which can increase the risk of radiation pneumonitis in patients who then go on to receive concurrent chemoradiation, which could have confounded the results. Lastly, the trial demonstrated a steep learning curve with proton therapy and underscored the importance of experience when using this advanced radiation modality, as the rate of grade ≥ 3 radiation pneumonitis and local failure at 12 months was 31.0% for patients enrolled before the study midpoint versus only 13.1% for patients enrolled after the midpoint. Nonetheless, despite several affirming studies regarding PBT, these sobering findings does underscore the need for more randomized trials.
Discussion and future directions
NSCLC remains a challenging disease to treat with radiotherapy given the close proximity to critical organs such as the heart, spinal cord and normal lung parenchyma. The physical properties of proton therapy allow for dose escalation and hypofractionation that can potentially improve LC and survival,20,77,78 while minimizing dose to normal structures, especially in the setting of concurrent chemotherapy, trimodality therapy with surgery for locally advanced disease, and for reirradiation.79 There have been a number of recently published institutional experiences using proton therapy as a means to deliver definitive, high doses of reirradiation therapy to patients with recurrent NSCLC, providing them a second chance of cure.23,80–82
Although proton therapy is precise, this same precision can lead to delivery uncertainty, where intrafractional tumor motion (i.e. motion during beam delivery) can potentially lead to underdosing the gross disease and giving normal tissues higher doses of irradiation. Therefore, motion mitigation techniques, 4D image guidance and adaptive replanning, as described previously, maybe even more critical for proton treatment when compared with photons.83,84 Though studies have consistently shown the dosimetric superiority of protons radiation over conventional photon treatment in NSCLC, the clinical outcomes data indicating that these benefits translate to a clinical advantage are currently still limited but emerging. Randomized clinical trials are necessary to ultimately measure the superiority of proton versus photon therapy in NSCLC.
The RTOG 1308 trial [ClinicalTrials.gov identifier: NCT01993810] is a multi-institutional phase III randomized trial comparing OS in stage II–IIIB, nonoperable NSCLC patients being treated to a total dose of 70 Gy (2 Gy per fraction) with concurrent chemotherapy using image-guided, motion-managed proton or photon radiation techniques. In this study, the total dose can be decreased to as low as 60 Gy if OAR dose constraints are not met. Secondary end points also include PFS, quality of life measures, adverse events, cost-effectiveness, and pulmonary function before and after radiation treatment.85 The Proton Collaborative Group is conducting a phase I/II study (LUN005; ClinicalTrials.gov identifier: NCT0177041) that is assessing the feasibility of hypofractionated proton therapy with concurrent chemotherapy in stage II/III NSCLC patients. The phase I portion of the study is looking to determine the maximum tolerated dose per fraction to a total of 60 Gy, starting at 2.5 Gy per fraction and escalating to 4 Gy. In the phase II portion, the trial is evaluating survival at 12 months, adverse events and tumor control. Future studies also include the German, phase II, randomized PROTOX trial, which aims to compare pneumonitis and esophagitis rates in locally advanced NSCLC patients treated with PBT or IMRT.86 The trial employs an accelerated approach informed by the previous CHARTWEL that demonstrated a LC benefit of hyperfractionation in locally advanced NSCLC.87
For early stage disease, University of Florida investigators are assessing the safety of image-guided hypofractionated proton therapy for inoperable stage I NSCLC patients using 48 Gy (12 Gy per fraction) for peripheral lesions and 60 Gy (6 Gy per fraction) for centrally located tumors (LU03; ClinicalTrials.gov identifier: NCT00875901). The primary outcomes measure is the rate of grade 3 or higher toxicities, with secondary endpoints assessing OS, tumor control, dosimetric parameters of OARs, and quality of life metrics.
The majority of the clinical data published to date on proton therapy for NSCLC have utilized PS proton technology. PBS is the latest form of proton radiation treatment, and although the clinical data in NSCLC are limited, early data demonstrate its dosimetric superiority over PS PBT91,92 and clinical experiences, especially in the reirradiation setting,93 express its efficacy and safety. As additional new proton centers arise and established centers continue to mature in their experience, new data promise to further elucidate the dynamic landscape of NSCLC and the role of PBT.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Charles B. Simone  https://orcid.org/0000-0002-0867-3694
https://orcid.org/0000-0002-0867-3694
Contributor Information
Melissa A.L. Vyfhuis, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA
Nasarachi Onyeuku, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Tejan Diwanji, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Sina Mossahebi, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Neha P. Amin, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA
Shahed N. Badiyan, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA
Pranshu Mohindra, Maryland Proton Treatment Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Charles B. Simone, II, Maryland Proton Treatment Center, University of Maryland School of Medicine, 850 West Baltimore Street, Baltimore, MD 21201, USA.
References
- 1. Wink KC, Roelofs E, Solberg T, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Front Oncol 2014; 4: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slater JM, Archambeau JO, Miller DW, et al. The proton treatment center at Loma Linda University Medical Center: rationale for and description of its development. Int J Radiat Oncol 1992; 22: 383–389. [DOI] [PubMed] [Google Scholar]
- 3. Liu H, Chang JY. Proton therapy in clinical practice. Chin J Cancer 2011; 30: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelsman M, Lu HM, Herrup D, et al. Commissioning a passive-scattering proton therapy nozzle for accurate SOBP delivery. Med Phys 2009; 36: 2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin L, Kang M, Huang S, et al. Beam specific planning target volumes incorporating 4DCT for pencil beam scanning proton therapy of thoracic tumors. J Appl Clin Med Phys 2015; 16: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys 2006; 64: 1589–1595. [DOI] [PubMed] [Google Scholar]
- 7. Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2016; 95: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori S, Dong L, Starkschall G, et al. A serial 4DCT study to quantify range variations in charged particle radiotherapy of thoracic cancers. J Radiat Res 2014; 55: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albertini F, Bolsi A, Lomax AJ, et al. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother Oncol 2008; 86: 187–194. [DOI] [PubMed] [Google Scholar]
- 10. Dowdell S, Grassberger C, Sharp G, et al. Fractionated lung IMPT treatments. Technol Cancer Res Treat 2016; 15: 689–696. [DOI] [PubMed] [Google Scholar]
- 11. Szeto YZ, Witte MG, van Kranen SR, et al. Effects of anatomical changes on pencil beam scanning proton plans in locally advanced NSCLC patients. Radiother Oncol 2016; 120: 286–292. [DOI] [PubMed] [Google Scholar]
- 12. Furukawa T, Inaniwa T, Sato S, et al. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 2010; 37: 4874–4879. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Zhu XR, Zhang X. Reducing dose uncertainty for spot-scanning proton beam therapy of moving tumors by optimizing the spot delivery sequence. Int J Radiat Oncol Biol Phys 2015; 93: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang JY. and JDC. Improving radiation conformality in the treatment of non-small cell lung cancer. Semin Radiat Oncol 2010; 20: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang M, Huang S, Solberg TD, et al. A study of the beam-specific interplay effect in proton pencil beam scanning delivery in lung cancer. Acta Oncol (Madr) 2017; 56: 531–540. [DOI] [PubMed] [Google Scholar]
- 16. Poulsen PR, Eley J, Langner U, et al. Efficient interplay effect mitigation for proton pencil beam scanning by spot-adapted layered repainting evenly spread out over the full breathing cycle. Int J Radiat Oncol Biol Phys 2018; 100: 226–234. [DOI] [PubMed] [Google Scholar]
- 17. Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res 2017; 6: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kesarwala AH, Ko CJ, Ning H, et al. Intensity-modulated proton therapy for elective nodal irradiation and involved-field radiation in the definitive treatment of locally advanced non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer. 2015;16(3):237–244. DOI: 10.1016/j.cllc.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Speirs CK, Dewees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non – small cell lung cancer. J Thorac Oncol 2016; 12: 293–301. [DOI] [PubMed] [Google Scholar]
- 20. Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys 2012; 82: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Remick JS, Schonewolf C, Gabriel P, et al. First clinical report of proton beam therapy for postoperative radiotherapy for non-small-cell lung cancer. Clin Lung Cancer 2017; 18: 364–371. [DOI] [PubMed] [Google Scholar]
- 22. Simone CB, 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014; 20: 427–432. [DOI] [PubMed] [Google Scholar]
- 23. Chao HH, Berman AT, Simone CB, 2nd, et al. Multi-institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non-small cell lung cancer. J Thorac Oncol 2016; 12: 281–292. [DOI] [PubMed] [Google Scholar]
- 24. Higgins KA, O’Connell K, Liu Y, et al. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol 2017; 97: 128–137. [DOI] [PubMed] [Google Scholar]
- 25. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995; 60: 615–623. [DOI] [PubMed] [Google Scholar]
- 26. Dziedzic R, Żurek W, Marjański T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the polish national lung cancer registry†. Eur J Cardio-Thoracic Surg 2017; 46: 1–7. [DOI] [PubMed] [Google Scholar]
- 27. Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2016; 16: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010; 28: 928–935. [DOI] [PubMed] [Google Scholar]
- 29. Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011; 81: 1352–1358. [DOI] [PubMed] [Google Scholar]
- 30. Simone CB, 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015; 3: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyman J, Hallqvist A, Lund J, et al. SPACE - a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016; 121: 1–8. [DOI] [PubMed] [Google Scholar]
- 32. Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012; 84: 1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010; 28: 5153–5159. [DOI] [PubMed] [Google Scholar]
- 34. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004; 101: 1623–1631. [DOI] [PubMed] [Google Scholar]
- 35. Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. J Thorac Oncol 2012; 7: 1382–1393. [DOI] [PubMed] [Google Scholar]
- 36. Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24: 4833–4839. [DOI] [PubMed] [Google Scholar]
- 37. Senthi S, Haasbeek CJA, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013; 106: 276–282. [DOI] [PubMed] [Google Scholar]
- 38. Song SY, Choi W, Shin SS, et al. Fractionated stereotactic body radiation therapy for medically inoperable stage I lung cancer adjacent to central large bronchus. Lung Cancer 2009; 66: 89–93. [DOI] [PubMed] [Google Scholar]
- 39. Verma V, Shostrom VK, Kumar SS, et al. Multi-institutional experience of stereotactic body radiotherapy for large (⩾5 centimeters) non-small cell lung tumors. Cancer 2017; 123: 688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verma V, Shostrom VK, Zhen W, et al. Influence of fractionation scheme and tumor location on toxicities after stereotactic body radiation therapy for large (⩾5 cm) non-small cell lung cancer: a multi-institutional analysis. Int J Radiat Oncol 2017; 97: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Speicher PJ, Gu L, Wang X, et al. Adjuvant chemotherapy after lobectomy For T1–2N0 non-small cell lung cancer: are the guidelines supported? J Natl Compr Canc Netw 2015; 13: 755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang C, Nakayama H, Sugahara S, et al. Comparisons of dose-volume histograms for proton-beam versus 3-D conformal X-ray therapy in patients with stage I non-small cell lung cancer. Strahlentherapie und Onkol 2009; 185: 231–234. [DOI] [PubMed] [Google Scholar]
- 43. Macdonald OK, Kruse JJ, Miller JM, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: a comparative dosimetric analysis. Int J Radiat Oncol 2009; 75: 950–958. [DOI] [PubMed] [Google Scholar]
- 44. Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999; 116: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 45. Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003; 56: 7–13. [DOI] [PubMed] [Google Scholar]
- 46. Bush DA, Slater JD, Shin BB, et al. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 2004; 126: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 47. Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006; 65: 107–111. [DOI] [PubMed] [Google Scholar]
- 48. Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007; 68: 786–793. [DOI] [PubMed] [Google Scholar]
- 49. Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys 2010; 78: 467–471. [DOI] [PubMed] [Google Scholar]
- 50. Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer. 2010; 116: 2476–2485. [DOI] [PubMed] [Google Scholar]
- 51. Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys. 2013; 86(5):964–948. [DOI] [PubMed] [Google Scholar]
- 52. Iwata H, Demizu Y, Fujii O, et al. Long-term outcome of proton therapy and carbon-ion therapy for large (T2a-T2bN0M0) non-small-cell lung cancer. J Thorac Oncol 2013; 8: 726–735. [DOI] [PubMed] [Google Scholar]
- 53. Kanemoto A, Okumura T, Ishikawa H, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non-small-cell lung cancer. Clin Lung Cancer 2014; 15: e7–e12. [DOI] [PubMed] [Google Scholar]
- 54. Makita C, Nakamura T, Takada A, et al. High-dose proton beam therapy for stage I non-small cell lung cancer: clinical outcomes and prognostic factors. Acta Oncol (Madr) 2015; 54: 307–314. [DOI] [PubMed] [Google Scholar]
- 55. Chang JY, Zhang W, Komaki R, et al. Long-term outcome of phase I/II prospective study of dose-escalated proton therapy for early-stage non-small cell lung cancer. Radiother Oncol 2017; 122: 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chi A, Chen H, Wen S, et al. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: a systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017; 123: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Curran WJ, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103: 1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16: 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perez CA, Stanley K, Grundy G, et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer 1982; 50: 1091–1099. [DOI] [PubMed] [Google Scholar]
- 60. National Comprehensive Cancer Network. Non-small cell lung cancer version 3.2018. Access March, 2018 www.nccn.org
- 61. Bradley JD, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 2010; 77: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 2010; 28: 2475–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dess RT, Sun Y, Matuszak MM, et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol 2017; 35: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1087–1096. [DOI] [PubMed] [Google Scholar]
- 65. Nichols RC, Huh S, Henderson R, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage III non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer 2011; 12: 252–257. [DOI] [PubMed] [Google Scholar]
- 66. Roelofs E, Engelsman M, Rasch C, et al. Results of a multicentric in silico clinical trial (ROCOCO). J Thorac Oncol 2012; 7: 165–176. [DOI] [PubMed] [Google Scholar]
- 67. Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 2011; 117: 4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hoppe BS, Henderson R, Pham D, et al. A phase 2 trial of concurrent chemotherapy and proton therapy for stage III non-small cell lung cancer: results and reflections following early closure of a single-institution study. Int J Radiat Oncol Biol Phys 2016; 95: 517–522. [DOI] [PubMed] [Google Scholar]
- 69. Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer 2011; 117: 3004–3013. [DOI] [PubMed] [Google Scholar]
- 70. Nguyen QN, Ly NB, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol 2015; 115: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chang JY, Verma V, Li MZW, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer final results of a phase 2 study. JAMA Oncol 2017; 3: e172032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakayama H, Satoh H, Sugahara S, et al. Proton beam therapy of stage II and III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 81: 979–984. [DOI] [PubMed] [Google Scholar]
- 73. Oshiro Y, Mizumoto M, Okumura T, et al. Results of proton beam therapy without concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer. J Thorac Oncol 2012; 7: 370–375. [DOI] [PubMed] [Google Scholar]
- 74. Hatayama Y, Nakamura T, Suzuki M, et al. Preliminary results of proton-beam therapy for stage III non-small-cell lung cancer. Curr Oncol 2015; 22: 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Oshiro Y, Okumura T, Kurishima K, et al. High-dose concurrent chemo-proton therapy for stage III NSCLC: preliminary results of a phase II study. J Radiat Res 2014; 55: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liao Z, Lee JJ, Komaki R, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. Epub ahead of print 2 January 2018. DOI: 10.1200/JCO.2017.74.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kong F-MS, Zhao J, Wang J, et al. Radiation dose effect in locally advanced non-small cell lung cancer. J Thorac Dis 2014; 6:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maguire J, Khan I, McMenemin R, et al. SOCCAR: a randomised phase II trial comparing sequential versus concurrent chemotherapy and radical hypofractionated radiotherapy in patients with inoperable stage III non-small cell lung cancer and good performance status. Eur J Cancer 2014; 50: 2939–2949. [DOI] [PubMed] [Google Scholar]
- 79. Vyfhuis M, Rice S, Remick J, et al. Reirradiation for locoregionally recurrent non-small cell lung cancer. J Thorac Dis 2017. DOI: 10.21037/jtd.2017.12.50. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 2013; 109: 38–44. [DOI] [PubMed] [Google Scholar]
- 81. McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014; 90: 819–827. [DOI] [PubMed] [Google Scholar]
- 82. Ho J, Nguyen Q, Li H, et al. Reirradiation of thoracic cancers with intensity modulated proton therapy. Pract Radiat Oncol. 2018; 8(1):58–65. DOI: 10.1016/J.PRRO.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 83. Koay EJ, Lege D, Mohan R, et al. Adaptive/non-adaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012; 84: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Veiga C, Janssens G, Teng C-L, et al. First clinical investigation of cone beam computed tomography and deformable registration for adaptive proton therapy for lung cancer. Int J Radiat Oncol 2016; 95: 549–559. [DOI] [PubMed] [Google Scholar]
- 85. Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II- IIIB NSCLC. Radiat Oncol 2016; 11: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zschaeck S, Simon M, Löck S, et al. PRONTOX – proton therapy to reduce acute normal tissue toxicity in locally advanced non-small-cell lung carcinomas (NSCLC): study protocol for a randomised controlled trial. Trials 2016; 17: 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baumann M, Herrmann T, Koch R, et al. Final results of the randomized phase III CHARTWEL-trial (ARO 97–1) comparing hyperfractionated-accelerated versus conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC). Radiother Oncol 2011; 100: 76–85. [DOI] [PubMed] [Google Scholar]