Short abstract
Objectives
We investigated the clinical characteristics and treatment response in myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-associated disease and looked for evidence of subclinical disease.
Methods
We prospectively evaluated the frequency and pattern of relapse, tested afferent visual function and monitored treatment response in 42 south Asian patients from a single centre.
Results
Eighteen patients (42.9%) had monophasic and 24 (57.1%) a relapsing course. Disease duration was longer (P<0.02) in those with a relapsing course. Median time to the second attack was prolonged (P<0.04) in patients with recurrent transverse myelitis when compared with neuromyelitis optica spectrum disorder and recurrent optic neuritis. Thirteen out of 17 patients (76.5%) initially presenting with optic neuritis developed recurrent optic neuritis later. After the first attack of transverse myelitis, 17 out of 22 (77.3%) had disease confined to the spinal cord. Optical coherence tomography detected peripapillary retinal nerve fibre layer thickness (P<0.05) and macular ganglion cell complex volume (P<0.005) abnormalities in seven out of 10 (70.0%) patients without clinical optic neuritis. Immunosuppressants induced remission in 17 out of 22 (77.3%) patients during a median follow-up of 48 months and the median Expanded Disability Status Score was 1 (range 1–10).
Conclusion
Our study highlighted the tendency for stereotypical attacks in MOG-IgG-associated disease, heterogeneity in clinical course among subtypes, subclinical visual impairment and the need for early and sustained immunosuppressive therapy.
Keywords: Demyelinating disorders, anti-MOG-IgG, disease heterogeneity, subclinical visual loss, South India
Introduction
The clinical spectrum of myelin oligodendrocyte glycoprotein antibody (MOG-IgG)-associated disease has been well characterised. Earlier reports included paediatric patients with acute disseminated encephalomyelitis (ADEM),1 multiple sclerosis (MS)2 and neuromyelitis optica spectrum disorder (NMOSD).3 Subsequently several studies were published4–11 that included adult patients from single or multicentre collaborative efforts. It is now known that a proportion of MOG-IgG-positive patients may have a predominantly relapsing course12 and significant visual system damage after clinical optic neuritis (ON).12,13
In previous studies from India we have shown that 20% of patients in our registry with non-MS demyelinating disorders were MOG-IgG positive,14,15 and highlighted the clinical subtypes and imaging characteristics. In the present study we have evaluated a larger cohort of 42 patients who were uniformly investigated and prospectively followed up at our centre. The natural history of untreated patients with a monophasic course and that of relapsing patients who discontinued therapy were documented. In addition, we looked for evidence supporting heterogeneity in the disease course, subclinical neurological deterioration and documented the treatment response over time.
Materials and methods
Patient selection
Forty-two MOG-IgG-positive patients were identified from among 235 consecutive patients (see Supplementary Table 1) with non-MS demyelinating disorders, who were prospectively enrolled in our registry.16 They included (Table 1) NMOSDs,17 transverse myelitis (TM), recurrent transverse myelitis (RTM), ON, recurrent optic neuritis (RON) and ADEM. Patients who were diagnosed with MS (McDonald criteria)18 were excluded from the study. All patients with monophasic/recurrent myelitis had longitudinally extensive TM confirmed by magnetic resonance imaging. They had been investigated using a standard in-house protocol (see Supplementary Table 2) and the decision to treat with an immunosuppressant was taken before the MOG-IgG status was known.
Table 1.
Clinical and demographic features of anti-MOG-IgG-positive patients (N=42).
| Number | M/F | Age of onset (median years) | Disease duration (median months) | Duration of follow-up (median months) | |
|---|---|---|---|---|---|
| Total | 42 | 24/18 | 21.0 (6–53) | 57.6 (12.0–336) | 36.0 (12.0–84) |
| Monophasic disease | |||||
| Total | 18 | 10/8 | 22.0 (10–53) | 36.0 (12.0–84) | 36.0 (12.0–84) |
| ATM | 14 | 10/4 | 27.5 (16–53) | 36.0 (12.0–108) | 36.0 (12.0–108) |
| ADEM | 2 | 0/2 | 17.5 (15–20) | 69.6 (67.2–72) | 69.6 (67.2–72) |
| ON | 2 | 0/2 | 12.0 (10–14) | 30.0 (24.0–36) | 30.0 (24.0–36) |
| Recurrent disease | |||||
| Total | 24 | 14/10 | 19.0 (5–42) | 72.0 (12.0–336) | 24.0 (12.0–84) |
| NMOSD | 8 | 5/3 | 22.5 (7–49) | 33.6 (12.0–120) | 18.0 (12.0–72) |
| RTM | 3 | 3/0 | 6.0 (7–12) | 144.0 (72.0–336) | 24.0 (24.0–36) |
| RON | 13 | 6/7 | 21.0 (5–42) | 72.0 (20.4–240) | 48.0 (19.2–84) |
ATM: acute transverse myelitis; ADEM: acute disseminated encephalomyelitis; ON: optic neuritis: NMOSD: neuromyelitis optica spectrum disorder; RTM: recurrent transverse myelitis; RON: recurrent optic neuritis.
Biomarker assay
Serum samples were collected at the onset of relapse and prior to steroids, aliquoted and stored at –20° prior to testing. Samples were shipped in bulk in dry ice to Tohoku University, Japan, where investigators (IN, KK and TT) were blinded to the clinical details of patients and aquaporin-4 antibody (AQP4-IgG) status. The live cell-based assay for MOG and AQP4-IgG was performed using protocols described earlier.8,19 The antibody titre for anti-MOG-IgG was measured twice by consecutive serial dilutions. Samples were determined to be positive for a titre of 1:128 or greater. Forty-two samples (17.9%) were positive for MOG-IgG. All 51 (21.7%) samples that had tested positive previously at our laboratory in India (commercial fixed-cell based assay, Euroimmun, Lübeck, Germany) were also positive for AQP4-IgG by live cell-based assay.
Visual function analysis
At study entry, tests for afferent visual functions were performed in all 42 patients. High contrast visual acuity of both eyes was performed in all patients using ETDRS charts and values converted into Snellen equivalents. Visual evoked potentials (VEPs) were recorded with checkerboard pattern stimulation (Nihon Kohden model MEB – 2300 k) in all 42 patients. Results were considered abnormal when P100 latency was prolonged (normal ≤111.8 milliseconds) or the waveform was not elicited. We were able to recall 27 patients who were MOG-IgG positive for spectral domain optical coherence tomography (OCT; Heidelberg Engineering, Heidelberg, Germany, version 9.10.0). Scans were performed at least 3 months following the last incidence of ON and after a median interval of 36 months (12–228 months) from disease onset. Eighteen patients had clinical ON (NMOSD 6, RON 8, ADEM 1, ON 3) and nine had either a single attack of TM (8) or RTM (1) in the past. Peripapillary retinal nerve fibre layer thickness (pRNFL) was derived from a standard ring scan around the optic nerve head (scan angle 12°, 1536 A scans per B scan, ART 100). Macula volume scans were performed in high resolution mode and vertical orientation centring on the fovea (25 vertical B scans, scanning angle 15° × 15°, ART 100, 1024 A scans per B scan). A single trained technician performed all tests under standard ambient lighting without pupillary dilatation after correcting for spherical errors. Intraretinal layer segmentation was performed in a semi-automated way with manual corrections wherever applicable, using a software provided by Heidelberg (Eye Explorer software version – 1.9.14.0). An operator blinded to patient data analysed the mean thickness of the following: macular ganglion cell complex (mGCC), which includes retinal nerve fibre, ganglion cell and inner plexiform layer; inner nuclear layer (INL); outer plexiform layer; outer nuclear layer and photoreceptors consisting of retinal pigmentary epithelium and Bruchs membrane. All scans were observed for microcystic macular oedema (MMO). The latter was diagnosed when two adjacent B scans showed a cystic lesion in the INL. Data were compared with that of 17 healthy volunteers and 18 AQP4-IgG-positive NMOSD who had clinical ON.
Clinical follow-up and evaluation of response to therapy
All 42 patients were under dedicated follow-up prior to the detection of MOG-IgG status and continued to be monitored. Decision to treat was uniform for all patients and was not altered after the result of MOG-IgG testing was known except in one patient (detailed under Results). Attacks were treated initially with intravenous methyl prednisolone (1 g daily for 5 days) followed by an oral taper with prednisolone in a starting dose of 0.5 mg/kg body weight over 6 months. Patients who had a well-defined disease relapse were started on non-steroidal immunosuppressants concurrently. The choice was between azathioprine or mycophenolate mofetil (MMF), both of which were made available through a subsidised programme at the first author’s centre. One patient with NMOSD received rituximab every 6 months. Patients were seen initially at 3-month intervals and subsequently twice a year. A trained nurse coordinator kept an additional telephone follow-up of patients, and treatment compliance was monitored.
Statistical analysis
Statistical analysis was performed on SPSS 20.0 software (IBM SPSS Statistics for Windows, version 20.0; IBM Corp., Armonk, NY, USA). For OCT data analysis we included both eyes separately (n = 54) in all 27 patients. The Mann–Whitney U test was used for comparison of data. A P value less than 0.05 was considered significant.
Institutional ethics committee permissions and signed informed consents were obtained from all participants.
Results
Demographics
Clinical data from 42 MOG-IgG-positive patients obtained from a single centre were analysed. Thirteen patients (31%) had their first attack when under 18 years of age. Overall, the median age of onset was 21 years (6–53). Eighteen patients (42.9%) were women/girls, corresponding to a sex ratio (F:M) of 1:1.3. Thirteen out of 17 (76.5%) patients who had attacks confined to the spinal cord were men/boys (Table 1). Gender distribution was even among patients with NMOSD and RON subtypes. The majority (28/42) were seen from disease onset and included all patients with monophasic disease (18), NMOSD (3), RTM (3) and RON (4).
Clinical course
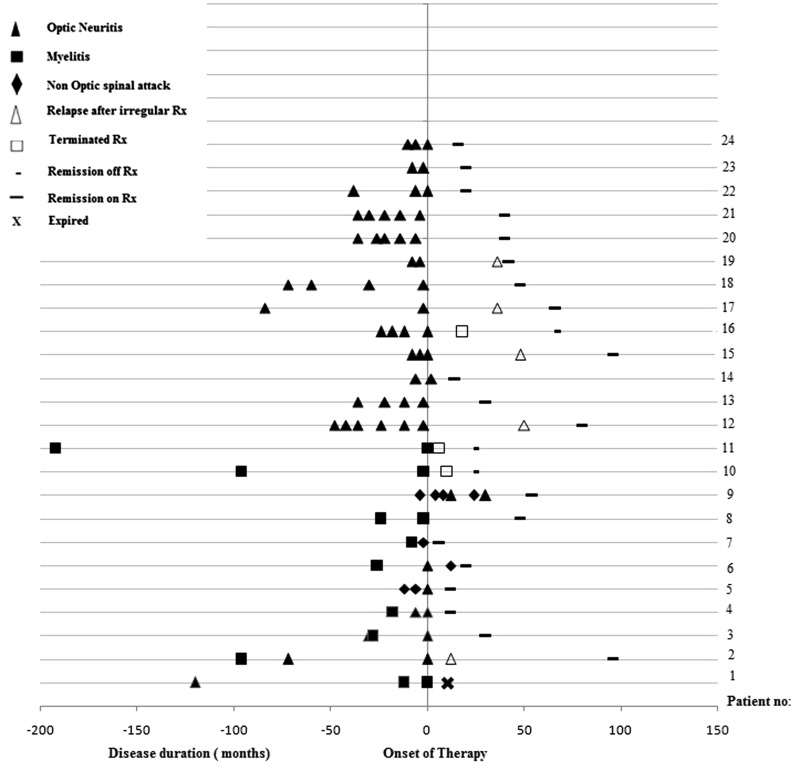
The site of first attack involved the optic nerve in 17 out of 42 (40.5%), spinal cord in 22 out of 42 (52.4%) and brainstem one out of 42 (2.4%). In addition, two out of 42 (4.8%) had an encephalitic presentation. A relapsing course was observed in 24 (57.1%) patients. The median disease duration was significantly longer (P<0.02) among those with a relapsing course (72 months, range 12–336) when compared with patients having a single clinical attack (36 months, range 12–84). A total of 82 attacks (Figure 1) were documented during the course of disease in the latter. ON constituted 73.2% (60/82), acute transverse myelitis (ATM) 15.9% (13/82), brainstem attacks 4.9% (4/82), encephalitis 3.7% (3/82) and simultaneous ON and ATM in 2.4% (2/82).
Figure 1.
Graph showing the clinical course in 24 anti-MOG-IgG-positive patients before and after immunosuppressant therapy.
Time to second attack
Among patients with relapsing disease, the median time between the first and second attack was 12 months (3–204 months). When patients were evaluated based on their disease subtypes, the median time to the second attack was significantly prolonged (P<0.04) for the RTM group (96 months, range 24–192) when compared with NMOSD (10 months, range 3–96) and RON (12 months, range 3–204).
Annualised relapse rate
The mean annualised relapse rate (ARR) (number of attacks per duration of disease) for the whole group (n = 24) was 1.04 ± 0.03. Patients with RTM had the lowest ARR (0.03 ± 0.01; P<0.01) when compared with NMOSD (1.03 ± 0.06) and RON (1.09 ± 0.05).
Attack pattern
Among relapsing MOG-IgG-positive patients, 13 out of 17 (76.5%) who had an initial attack of ON developed RON. Among patients who had myelitis as the first attack, 17 out of 22 (77%) had disease confined to the spinal cord (ATM 14, RTM 3).
Visual function testing
Visual acuity and VEP analysis
Eleven out of 23 patients (47.8%) with a history of ON were functionally blind at the peak of the attack. Two patients had permanent loss of vision in the worst affected eye. VEP was abnormal at least once during the course of the disease in 16 out of 23 (69.6%) patients with a history of ON. Visual acuity and VEP results were normal initially in the remaining 19 patients. Ten of the latter could be retested at the time of their OCT evaluation, after a median interval of 29 months (range 24–72 months). The results were abnormal in six out of 10 (60.0%) patients. These were patients with ATM (5) and RTM (1).
OCT results
Twenty-seven patients (54 eyes) positive for MOG-IgG were evaluated, including 10 who had no history of ON (Table 2). MMO was seen in seven out of 34 (21%) eyes clinically affected by ON. mGCC volume (Figure 2(a)) was significantly reduced (P = 0.001). In contrast, thickness of the INL was increased and persisted (P<0.01) after MMO-affected eyes were removed from the analysis. There was a significant reduction in mean pRNFL thickness (Table 2) in patients when compared with healthy controls (P = 0.001) and was more prominent in the temporal pRNFL.
Table 2.
Optical coherence tomography study.
| Retinal OCT | MOG-IgG+ (n=27) | Normal (n=17) | P value | MOG-IgG+ (n=27) | AQP4-IgG+ (n=18) | P value |
|---|---|---|---|---|---|---|
| mGCC (mm3) | 2.65 (2.17–2.82) | 2.88 (2.76–3.21) | 0.001 | 2.65 (2.17–2.82) | 2.34 (1.83–2.70) | 0.03 |
| INL (mm3) | 0.97 (0.95–1.02) | 0.94 (0.89–1.0) | 0.01 | 0.97 (0.95–1.02) | 0.95 (0.93–1.02) | 0.17 |
| OPL (mm3) | 0.78 (0.76–0.84) | 0.77 (0.75–0.82) | 0.15 | 0.78 (0.76–0.84) | 0.77 (0.74–0.82) | 0.21 |
| ONL (mm3) | 1.72 (1.53–1.84) | 1.65 (1.55–1.82) | 0.73 | 1.72 (1.53–1.84) | 1.58 (1.44–1.80) | 0.14 |
| PRs (mm3) | 0.41 (0.37–0.43) | 0.41 (0.38–0.42) | 0.44 | 0.41 (0.37–0.43) | 0.41 (0.390.45) | 0.10 |
| Mean pRNFL (μm) (global) | 89.5 (72.0–101.7) | 102.5 (96.5–108) | 0.001 | 89.5 (72.0–101.7) | 82 (50.2–96.7) | 0.11 |
| pRNFL (μm) (nasal) | 77.0 (54.75–95.5) | 78 (70.0–85.7) | 0.59 | 77.0 (54.75–95.5) | 57.5 (36.5–75.2) | 0.001 |
| pRNFL (μm) (temporal) | 61.0 (48.7–73.7) | 72.5 (65.7–80.0) | 0.001 | 61.0 (48.7–73.7) | 50.0 (30.0–67.0) | 0.02 |
mGCC: macular ganglion cell complex; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; PRs: photoreceptors; pRNFL: peripapillary retinal nerve fibre layer.
Figure 2.
Optical coherence tomography in anti-MOG-IgG-positive patients. (a) Significant thinning of macular ganglion cell complex (mGCC) in anti-MOG-IgG-positive patients compared to healthy controls, which persists after removal of patients with microcystic macular oedema (MMO). (b) Significant thickening of IPL in patients compared to controls, which persists after controlling for MMO. (c) and (d) Anti-MOG-IgG-positive patients showed significant thinning of the peripapillary retinal nerve fibre layer and mGCC in all and when patients with no clinical optic neuritis were separately analysed when compared to controls.
We have separately evaluated OCT data of the 10 anti-MOG-IgG-positive patients who had no prior ON. The results were abnormal in seven out of 10 (70%) patients (Figure 2(c and d)) and showed a reduction of pRFNL thickness (P = 0.05) and mGCC volume (P = 0.005) when compared with healthy controls.
In addition, we compared our patient results with OCT data from 18 patients with AQP4-IgG-positive ON. The mGCC volume (P = 0.03) and temporal pRNFL (P = 0.001) thickness was significantly reduced in AQP4-IgG-positive patients when compared to our patient cohort. MMO was seen in four out of 36 (11%) eyes that were tested.
Treatment response
Twenty-two patients who had a relapsing disease were started on immunosuppressants, and the clinical response was evaluated over time (Figure 1). Treatment was started within a median period of 8 months (6–24 months) in NMOSD and 36 months (6–204 months) in RON subtypes. A single patient who had RTM started immunosuppressants 30 months after the first attack of myelitis. Nine patients (40.9%) were on azathioprine, 12 (54.5%) were on MMF and a single patient received rituximab. During a median follow-up of 48 months (12–240 months), 17 (77.3%) patients remained in clinical remission. The remaining five (22.7%) relapsed when they discontinued therapy. These patients were on monotherapy with a non-steroidal immunosuppressant at the time and had received treatment for a median period of 24 months (range 7–36 months). The median time to relapse after stopping therapy was 18 months (5–25 months).
Two patients in the RTM group were unwilling to start immunosuppressive therapy and remained well 24 and 35 months after the second attack of myelitis. One patient with NMOSD (patient 1, Figure 1) died within months of starting azathioprine, due to complications related to urosepsis. Another patient (patient 9, Figure 1) had been misdiagnosed with MS and had multiple relapses after being treated with interferon beta. This patient had a relapsing course, ‘MS typical’ brain lesions and the cerebrospinal fluid was positive for oligoclonal bands. Her treatment was revised when the MOG-IgG status became known and she responded well to MMF. The median period of follow-up was 42 months (12–84 months). At the last visit, the median Expanded Disability Status Score (EDSS) was 1 (0–10). Among the various clinical subtypes, EDSS was as follows: NMOSD 1.5 (0–10), RTM 2 (0–2.5), longitudinally extensive TM 1 (1–3.5) and RON 1 (0–3).
Discussion
In this study 42 consecutive patients with non-MS demyelinating disease (AQP4-IgG negative) were seropositive for MOG-IgG. They were unique in several aspects. They were of non-Caucasian origin and were the largest cohort reported from a single centre. Even prior to the knowledge of MOG-IgG seropositivity, they had been uniformly investigated and treated in the same manner as other AQP4-IgG-seronegative patients. The majority of patients were seen from onset and were prospectively monitored, including patients who were untreated or discontinued treatment.
In the present study MOG-IgG-positive patients had overall clinical features in line with earlier reports, including male predominance,7,8,10 selective predilection for optic nerve involvement,12,20,21 good recovery of visual functions after RON22 and complete resolution of longitudinally extensive TM in the majority of patients.23 Treatment response was good for all compliant patients irrespective of the immunosuppressant prescribed. Several factors may have contributed. Our data and those of others suggest that the risk of relapse is reduced with slow taper of oral steroids.22 All of our patients had received 6 months of oral steroids, alongside non-steroidal immunosuppressants. We initiated therapy early in the disease course in the majority of relapsing patients and well before the diagnosis was known. Recent observations suggest that long-term immunosuppression reduces the risk of relapse and disability in these patients.22,24 In a recent multicentre study of MOG-IgG-seropositive patients24 (n = 75), after a median interval of 28 months, 47% were left with permanent disability in vision/mobility corresponding to EDSS of 4.5 or greater and another 5% had EDSS of 6 or greater. Only 15% had been treated for more than 6 months and 40% had received no long-term immunosuppressant therapy. These observations underscore the fact that while MOG-IgG-associated disease is responsive to therapy, long-term immunosuppression is necessary to limit disability. Although there are anecdotal reports of poor response to rituximab therapy,21,25 our patient remained well after more than 1 year of treatment. Knowledge of MOG-IgG status later in the disease course did not change treatment decisions in 41 out of 42 patients.
In addition to the above observations, our patient data also revealed additional ‘disease-specific’ characteristics. After the first attack of ON, 13 out of 17 (76.5%) patients subsequently developed RON. Among patients who had myelitis as the first attack, 17 out of 22 (77%) had disease confined to the spinal cord. This tendency for stereotypical attacks has previously been noted by Jarius et al.,12 who found that 75% of ON and ATM relapsed at the same site during subsequent attacks. Patients with myelitis (ATM and RTM) were predominantly men and had an indolent disease course characterised by long intervals between attacks and low ARR. During a median follow-up of 45 months, only three out of 22 (13.6%) patients with an initial attack of myelitis converted to RTM. During this period 13 out of 17 (76.5%) patients who had an initial attack of ON had developed multiple relapses restricted to the optic nerves. Patients fulfilling the current diagnostic criteria for NMOSD17 were few (19%).
Our study for the first time showed evidence of subclinical visual impairment in MOG-IgG-seropositive patients with myelitis. It is notable that VEP was initially normal in 60% of them and suggests that some of these patients may have developed optic nerve dysfunction later in the disease course. In an earlier study, Havla et al.26 reported subclinical retinal atrophy involving the non-ON eye in MOG-IgG-positive patients. This is consistent with the OCT results from our study and supports the notion that there may be silent disease progression in MOG-IgG-associated disease, similar to the subclinical optic atrophy reported among MS patients.27
In clinically affected eyes we detected MMO in one in five eyes tested, which was twice the number seen in AQP4-IgG-positive patients in our comparator arm. The high frequency of MMO has previously been reported in both AQP4-IgG and MOG-IgG-seropositive patients,26 particularly in the latter.27 In addition, a significant thickening of the INL was seen, which could not be accounted for by MMO alone and may be indicative of trans-syntactic degeneration reported earlier with both AQP4-positive NMOSD and MS.28 The comparison of OCT data from MOG-IgG and AQP4-IgG-positive patients in our study highlighted significant and very similar retinal pathology in both. Neuroaxonal injury in the retina may be severe for both AQP4 and MOG-IgG-associated disease,29,30 and present with common OCT abnormalities that suggest similarity in the underlying pathophysiology. Yet early intervention with immunosuppressant therapy and treatment compliance may ensure the preservation of vision in anti-MOG-IgG-associated disease as opposed to AQP4-IgG-related ON.26This may be due in part to steroid responsiveness,20 and partly due to the transient nature of injury induced by MOG-IgG.31
In conclusion, our study highlighted heterogeneity in the clinical course of MOG-IgG-associated disorders. The initial attack appears to have a significant influence on disease phenotype and the ARR. The present study supports previous observations12,21 that long-term follow-up is essential to reveal the relapsing nature of this disease. In a resource-poor set-up such as ours, it was not cost effective to recheck anti-MOG-IgG titres, the practical relevance of which is unclear.12,21 Our inclusion criteria restricted the testing of patients with myelitis and short cord lesions, and we may have missed patients presenting with the same.12 Our study design overcame some of the shortcomings of previously published multicentre studies, including patient selection bias and retrospective data accrual. It was also a good model for evaluating treatment response. However, a larger prospective study is warranted to replicate our findings. In particular, MOG-IgG-associated monophasic TM may need careful scrutiny and lengthy follow-up. It is also important to understand the significance of subclinical visual dysfunction and its impact on decisions for early treatment in this subset of MOG-IgG-positive patients.
Supplemental Material
Supplemental material for MOG-IgG-associated disease has a stereotypical clinical course, asymptomatic visual impairment and good treatment response by Lekha Pandit, Sharik Mustafa, Ichiro Nakashima, Toshyuki Takahashi and Kimhiko Kaneko in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
The authors acknowledge the contributions of their clinical coordinator Ms Akshatha Sudhir and senior neurophysiology technician Mr Srinivas Rao in this study.
Author contribution
LP: study concept, design, acquisition of data, analysis and interpretation; SM: study design and concept, acquisition of data, critical revision of draft; IN: analysis and interpretation; TT: analysis and interpretation; KK: analysis and interpretation.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material
Supplementary material is available for this article online.
References
- 1.O’Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 2007; 13: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol 2009; 66: 833–842. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin KA, Chitnis T, Newcombe J, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol 2009; 183: 4067–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 2007; 13: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mader S, Gredler V, Schanda K, et al. Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 2011; 8: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitley J, Woodhall M, Waters P, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012; 79: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 7.Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014; 71: 276–283. [DOI] [PubMed] [Google Scholar]
- 8.Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody- positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014; 82: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Probstel AK, Rudolf G, Dornmair K, et al. Anti-MOG antibodies are present in a subgroup of patients with a neuromyelitis optica phenotype. J Neuroinflammation 2015; 12: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler 2015; 21: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Pelt ED, Wong YY, Ketelslegers IA, et al. Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur J Neurol 2016; 23: 580–507. [DOI] [PubMed] [Google Scholar]
- 12.Jarius S, Ruprecht K, Kleiter I, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016; 13: 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima H, Motomura M, Tanaka K, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open 2015; 5, e007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandit L, Sato D, Mustafa S, et al. Relapsing optic neuritis and isolated transverse myelitis are the predominant clinical phenotypes for patients with antibodies to myelin oligodendrocyte glycoprotein in India. Mult Scler J Exp Trans Clinic 2016; 2: 2055217316675634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandit L, Sato D, Mustafa S, et al. Serological markers associated with NMO spectrum disorders in south India. Ann Indian Acad Neurol 2016; 19: 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandit L, Mustafa S. Optimizing the management of neuromyelitis optica and spectrum disorders in resource poor settings: experience from the Mangalore demyelinating disease registry. Ann Ind Academy Neurol 2013; 16: 572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingerchuck DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato DK, Nakashima I, Takahashi T, et al. Aquaporin-4 antibody-positive cases beyond current diagnostic criteria for NMO spectrum disorders. Neurology 2013; 80: 2210–2216. [DOI] [PubMed] [Google Scholar]
- 20.Sepúlveda M, Armangue T, Martinez-Hernandez E, et al. Clinical spectrum associated with MOG autoimmunity in adults: significance of sharing rodent MOG epitopes. J Neurol 2016; 263: 1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanathan S, Reddel S, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm 2014; 1: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramanathan S, Mohammad S, Tantsis E, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody associated demyelination. J Neurol Neurosurg Psychiatry 2018; 89: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobo-Calvo A, Sepúlveda M, Bernard-Valnet R, et al. Antibodies to myelin oligodendrocyte glycoprotein in aquaporin 4 antibody seronegative longitudinally extensive transverse myelitis: clinical and prognostic implications. Mult Scler 2016; 22: 312–319. [DOI] [PubMed] [Google Scholar]
- 24.Jurynczyk M, Messina S, Woodhall MR, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017; 140: 3128–3138. [DOI] [PubMed] [Google Scholar]
- 25.Hyun JW, Woodhall MR, Kim SH, et al. Longitudinal analysis of myelin oligodendrocyte glycoprotein antibodies in CNS inflammatory diseases. J Neurol Neurosurg Psychiatry 2017; 88: 811–817. [DOI] [PubMed] [Google Scholar]
- 26.Havla J, Kumpfel T, Schinner R, et al. Myelin-oligodendrocyte-glycoprotein (MOG) autoantibodies as potential markers of severe optic neuritis and subclinical retinal axonal degeneration. J Neurol 2017; 264: 139–151. [DOI] [PubMed] [Google Scholar]
- 27.Schneider E, Zimmerman H, Oberwahren brock T, et al. Optical coherence tomography reveals distinct pattern of retinal damage in neuromyelitis optica and multiple sclerosis. PLOS one 2013; 6e66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saidha S, Sotirchos E, Ibrahim M, et al. Microcystic macular oedema, thickness of inner nuclear layer of the retina and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol 2012; 11: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pache F, Zimmermann H, Mikolajczak J, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J NeuroInflammation 2016; 13: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akaishi T, Sato DK, Nakashima I, et al. MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry 2016; 87: 446–448. [DOI] [PubMed] [Google Scholar]
- 31.Saadoun S, Waters P, Owens GP, et al. Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun 2014; 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for MOG-IgG-associated disease has a stereotypical clinical course, asymptomatic visual impairment and good treatment response by Lekha Pandit, Sharik Mustafa, Ichiro Nakashima, Toshyuki Takahashi and Kimhiko Kaneko in Multiple Sclerosis Journal – Experimental, Translational and Clinical