Abstract
Objective
The objective of this article is to compare the effectiveness of primary angioplasty and/or stenting with stent retriever thrombectomy in acute anterior large-vessel occlusion due to atherosclerotic disease.
Methods
Patients were retrospectively reviewed from the endovascular treatment for acute anterior circulation ischemic stroke registry. Patients with large-vessel occlusions due to atherosclerosis were selected. We evaluated modified Rankin Scale (mRS) score at 90 days, modified thrombolysis in cerebral infarction (mTICI) score immediately post-procedure, and symptomatic and asymptomatic intracranial hemorrhage within 72 hours.
Results
Of 302 patients with acute anterior circulation occlusion due to atherosclerotic disease, 269 were treated with stent retriever thrombectomy as first-line therapy and 33 with angioplasty and/or stenting. Patients who received primary angioplasty treatment showed favorable independent outcome at 90 days (69.7% (23/33) vs 47.6% (128/269), p = 0.02) and lower rate of asymptomatic intracranial hemorrhage (9.1% (3/23) vs 30.5% (82/269), p = 0.01). Recanalization immediately post procedure did not differ (78.8%% (26/33) vs 86.2% (232/269), p = 0.29). Primary angioplasty therapy (OR, 0.27; 95% confidence interval (CI): 0.08–0.90; p = 0.03) and small baseline infarct (OR 0.36: 0.16–0.82; p = 0.02) were protective factors against poor functional outcome, while old age (OR 1.04:1.01–1.07; p = 0.006), severe neurological deficits (OR 3.76: 2.00–7.07; p < 0.001), and high glucose (OR 1.11: 1.01–1.23; p = 0.03) were associated with poor prognosis.
Conclusions
Patients with acute anterior circulation large-vessel occlusion due to atherosclerosis may benefit from urgent angioplasty and/or stenting as first-line therapy. Randomized controlled trials are warranted.
Keywords: Stroke, angioplasty, stents, thrombectomy, atherosclerosis
Introduction
Seven recent randomized controlled trials mainly using stent retrievers demonstrated that endovascular therapy was superior to standard medical treatment in acute anterior circulation large-vessel occlusion (AC-LVO).1–7 Consequently, guidelines have recommended endovascular therapy with a stent retriever as first-line treatment for acute AC-LVO.8
However, these guidelines were established on the basis of a Western population, and lacking evidence from a Chinese population. In China, intracranial atherosclerotic disease-related stroke is more common than embolism, which is different from Western countries.9 On the other hand, stent retrievers were primarily designed for embolic occlusion rather than in situ thrombus occlusion.10,11 Mechanical thrombectomy with stent retrievers often leads to re-occlusion at the site of the atherosclerotic lesions,12,13 and risk of vessel ruptures as well as intracranial hemorrhage (ICH).14,15 Hence, treatment strategy for acute LVO due to atherosclerosis may be different from that resulting from embolism.
Besides mechanical thrombectomy, urgent intracranial angioplasty and/or stenting (AS) has increasingly been utilized for acute stroke, but the effectiveness has not been well established.8 As we know, primary percutaneous transluminal coronary angioplasty has been widely performed in patients with acute coronary syndrome with favorable outcomes, leading to the highest-level recommendation (IA evidence) in the guideline on the management of acute myocardial infarction.16 Considering coronary artery occlusions usually caused by local thrombosis of atherosclerotic vessels, with similar mechanism of atherosclerotic-related occlusions, we believe that angioplasty as first-line therapy might be safe and effective for acute ischemic stroke as well. In this study, we aimed to test the hypothesis that primary AS may be superior to stent retriever thrombectomy (ST) for acute AC-LVO due to atherosclerosis in a multicenter retrospective registry.
Methods
Study design and participants
This is a retrospective observational study. Patients were those registered in the endovAsCular Treatment for acUte Anterior circuLation ischemic stroke registry (ACTUAL) from January 2014 to June 2016. The ACTUAL was a multicenter registry of acute ischemic stroke patients who underwent intra-arterial treatment in 21 comprehensive stroke centers across 10 provinces in China (S1 in online supplement). This study was approved by the ethics committees of the participating centers.
The details of the ACTUAL registry can be found in our previous study.17 For the current study, we retrieved patients with atherosclerotic occlusion by ST or primary AS from the ACTUAL. For the AS group, the determination of atherosclerosis as etiology of given occlusion was based on the criteria of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification18 with minor modification. An atherosclerotic etiology was made if: (a) cardiogenic embolism, artery-to-artery embolism and arterial dissection were excluded; (b) the imaging findings, such as magnetic resonance imaging (MRI) vessel wall imaging, hyperdense (computed tomography (CT)) or gradient hypointense (MRI) calcific lesions in the arteries warranted; (c) the presence of intracranial stenoses consistent with atherosclerosis elsewhere was noted; (d) the patient was of advanced age and had a medical history such as smoking, cardio-cerebrovascular disease, diabetes, hypertension, hyperlipidemia, etc. For patients in the ST group, in addition to the criteria in the AS group, post-procedure digital subtraction angiography (DSA) was also considered. Atherosclerotic occlusion was considered if there was significant fixed focal stenosis in the occlusion site with evidence of an intra-arterial treatment procedure or in the final angiography.19 To differentiate the residual thrombus and vasospasm from atherosclerotic stenosis, the postoperative images (computed tomography angiography (CTA), magnetic resonance angiography (MRA) and/or DSA) were utilized if necessary. We excluded patients treated with aspiration devices or microguidewire alone from the present study because of the very small sample.
Images were evaluated by two neurointerventionists independently and consensus was reached by a third neurointerventionist in case of disagreement in the core lab. Collateral flow was assessed by an American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology (ASITN/SIR)20 grade ranging from 0 to 4. Vessel recanalization was defined as a modified thrombolysis in cerebral infarction (mTICI)21 score of 2b–3 immediately post-procedure.
Patients
We retrospectively enrolled 309 consecutive patients of AC-LVO due to atherosclerosis who underwent endovascular treatment from the ACTUAL. Of these patients, five underwent thrombus aspiration alone and two were treated with a microguidewire to disrupt clots alone. Finally, 302 patients were analyzed.
Procedures
The procedure protocol has been standardized in each center. All the devices have received China Food and Drug Administration approval. The interventional procedures were performed via the femoral artery approach. Each patient received heparin for systemic anticoagulation with a bolus dose of 2000 IU at the beginning of the procedure.
ST group
The stent retriever was delivered through a microcatheter and deployed inside the thrombus, unsheathed and allowed full expansion through the thrombus to restore flow immediately. The fully deployed stent-like retriever was then partially resheathed and gently pulled back together with the microcatheter. If this failed or recanalization was insufficient, the sequence was repeated. Rescue therapy was also attempted including local delivery of thrombolytic agents, thrombus aspiration, angioplasty with balloon or stenting, or a combination of all these approaches, irrespective of the use of intravenous thrombolytic agent.
AS group
A microguidewire-guided monorail balloon was advanced into the occlusion site and then inflated. Several inflations of 30 seconds each were performed. The balloon angioplasty was performed once to three times. If it failed to recanalize, a stent was deployed. Before stent deployment, an additional 3000 IU of intravenous heparin was given. The type of stent was selected according to the vessel characteristics and lesion morphology, as well as the operator’s preference. A balloon-mounted stent was utilized if the vessel was straight, and a self-expandable stent was used if the occluded segment was tortuous. Rescue therapy following local delivery of thrombolytic agents was also considered. After stenting, for the patients without prior intravenous alteplase, intravenous tirofiban infusion was bolus-injected with a low dose and maintained for at least 24 hours, or 300 mg oral dose of aspirin and clopidogrel were administered separately, while for patients with prior intravenous alteplase, clopidogrel (75 mg) and aspirin (100 mg) were given after 24 hours of alteplase administration. All the patients were given clopidogrel (75 mg/d) and aspirin (100 mg/d) for one to three months.
In the ST group, the thrombectomy was used, and other rescue therapy including but not limited to AS was also utilized. In the AS group, AS was first utilized but no thrombectomy could be used. Figures 1 and 2 illustrate the processes of primary ST and primary AS.
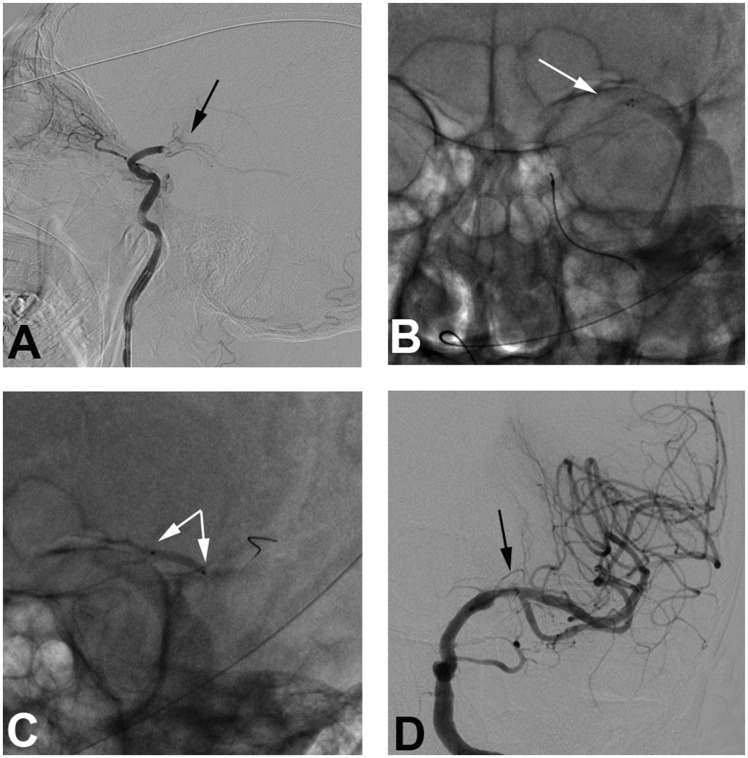
Figure 1.
A 64-year-old male patient with hypertension for 10 years and smoking history for 40 years presented with progressive weakness of the right limb for three hours. (a) Digital subtraction angiography confirms occlusion of the left middle cerebral artery (MCA) segment (black arrow). (b) Angiogram shows deployment of the Solitaire 4 × 20 mm stent retriever (white arrow). (c) A 2 × 20 mm balloon (Gateway) is deployed and inflates at the occluded segment as rescue therapy (white arrows). (d) Final angiography shows good perfusion with fixed focal stenosis at the left MCA M1 segment (black arrow).
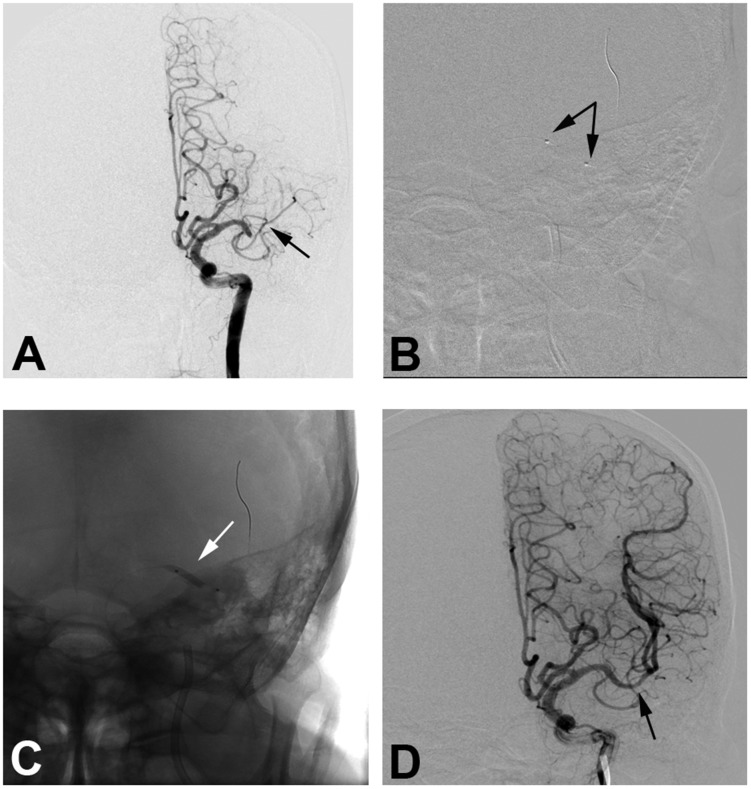
Figure 2.
An 81-year-old male patient with hypertension and coronary heart disease for 10 years presented with paralysis of the right upper limb for six hours. (a) Digital subtraction angiography confirms occlusion of the left middle cerebral artery (MCA) M1 segment (black arrow). (b) A 2 × 15 mm balloon (Gateway) is deployed at the occluded segment (black arrows). (c) The balloon inflates at the occluded segment (white arrow). (d) After three rounds of inflation, final angiography shows good perfusion with 10% fixed focal stenosis at the joint site between the MCA M1 and M2 segment with good distal perfusion (black arrow).
Outcomes
Functional outcome was evaluated at 90 days using the dichotomized modified Rankin Scale (mRS) score (0–2 as favorable functional outcome, and 3–6 as unfavorable functional outcome). Safety outcomes included mortality in hospital and at 90 days, and rate of symptomatic intracranial hemorrhage (sICH) within 72 hours after endovascular treatment. sICH was diagnosed according to the Heidelberg Bleeding Classification.22 Technical complications with devices such as vessel perforation, subarachnoid hemorrhage, and stent failure to deploy were also recorded. We recorded the follow-up information based on clinical records in each center. The follow-ups on mRS were performed at 90 days in outpatient clinics or by telephone in clinical routines in these centers. For survivor patients without mRS at 90 days, mRS at discharge was a substitute using last observation carried forward method, if the patient was also without mRS at discharge, he or she was recorded as missing a value and then was excluded.
Statistical analysis
Univariable analysis of baseline characteristics was compared between the ST and AS groups using the Student t test (normal distribution) or Mann–Whitney test (skewed distribution) for continuous variables and the Chi-squared test for categorical variables. Multivariable logistic regression analyses (step-wise method, with entry and removal limits set at 0.05 and 0.1 separately, and factors significant at p < 0.1 included) were performed to determine factors associated with unfavorable functional outcome at 90 days. Data were analyzed by Statistical Product and Service Solutions 22.0 (SPSS 22.0, IBM Corporation, Armonk, NY, USA).
Results
Study population
Of 302 patients, 89.1% (269/302) underwent primary ST therapy and 10.9% (33/302) accepted primary AS separately. Stent retriever devices included the Solitaire AB/FR (99.3%, 267/269) and the TREVO retriever (0.7%, 2/269). The AS group included intracranial occlusion (51.5%, 17/33) and extracranial occlusion (48.5%, 16/33). Twenty patients (60.6%, 20/33) underwent stent placement in the angioplasty group. Stent devices used for the AS group included the self-expanding stent (80%, 16/20) and balloon-mounted stent (20%, 4/20) (Table 1 in online supplement). A total of 118 (39.1%) patients underwent CTA or MRA post-procedure, of whom 109 (92.4%) patients presented with significant fixed focal stenosis for occluded arteries.
Table 1.
Clinical characteristics of the study patients.
| Variable | All (n = 302) | ST (n = 269) | AS (n = 33) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), years | 64 (55–73) | 64 (54–73) | 62 (58–71) | 0.83 |
| Male, no. (%) | 225 (74.5%) | 193 (71.7%) | 32 (97.0%) | 0.002 |
| Medical history | ||||
| Hypertension, no. (%) | 211 (69.9%) | 183 (68.0%) | 28 (84.8%) | 0.047 |
| Diabetes mellitus, no. (%) | 58 (19.2%) | 53 (19.7%) | 5 (15.2%) | 0.53 |
| Hyperlipidemia, no. (%) | 30 (9.9%) | 26 (9.7%) | 4 (12.1%) | 0.55 |
| Current smoker, no. (%) | 108 (35.8%) | 90 (33.5%) | 18 (54.5%) | 0.02 |
| Laboratory measures | ||||
| TG, median (IQR), mmol/l | 1.11 (0.78–1.65) | 1.10 (0.76–1.60) | 1.35 (0.91–1.96) | 0.12 |
| TC, mean (SD), mmol/l | 4.46 (1.18) | 4.46 (1.21) | 4.45 (0.95) | 0.96 |
| HDL, median (IQR), mmol/l | 1.13 (0.94–1.34) | 1.14 (0.95–1.35) | 0.99 (0.87–1.18) | 0.02 |
| LDL, median (IQR), mmol/l | 2.55 (2.03–3.26) | 2.55 (2.03–3.25) | 2.66 (2.00–3.33) | 0.75 |
| Glu, median (IQR), mmol/l | 6.60 (5.46–8.50) | 6.70 (5.58–8.55) | 5.51 (4.93–8.28) | 0.04 |
| ALB, median (IQR), g/l | 39.0 (36.2–42.0) | 39.2 (36.4–42.1) | 37.5 (34.6–42.0) | 0.32 |
| GLOB, mean (SD), g/l | 26.4 (4.9) | 26.6 (5.0) | 24.9 (4.3) | 0.09 |
| Clinical characteristics | ||||
| SBP, median (IQR), mmHg | 148 (132–162) | 148 (132–161) | 150 (130–170) | 0.38 |
| Baseline NIHSS score | ||||
| Median (IQR) | 15 (11–19) | 16 (11–19) | 12 (8–14) | <0.001 |
| ≤16, no. (%) | 176 (58.3%) | 148 (55.0%) | 28 (84.8%) | 0.001 |
| >16, no. (%) | 126 (41.7%) | 121 (45.0%) | 5 (15.2%) | |
| Baseline ASPECTS | ||||
| Median (IQR) | 9 (8–10) | 9 (8–10) | 10 (8–10) | 0.07 |
| 0–7, no. (%) | 57 (18.9%) | 56 (20.8%) | 1 (3.0%) | 0.01 |
| 8–10, no. (%) | 245 (81.1%) | 213 (79.2%) | 32 (97.0%) | |
ST: stent retriever thrombectomy; AS: angioplasty and/or stenting; SD: standard deviation; IQR: interquartile range; TG: triglyceride; TC: total cholesterol; HDL: high-density lipoprotein; LDL: low-density lipoprotein; Glu: glucose; ALB: albumin; GLOB: globulin; SBP: systolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta Stroke Program Early Computed Tomography Score.
Table 1 shows the clinical characteristics in all patients in the ST group and the AS group. Male sex, hypertension, current smoker, serum glucose, serum high-density lipoprotein, dichotomized National Institutes of Health Stroke Scale (NIHSS) score 0–16 on admission, and dichotomized Alberta Stroke Program Early Computed Tomography Score (ASPECTS) 8–10 on admission were significantly different between the ST and the AS groups.
Endovascular characteristics
Table 2 presents endovascular characteristics. There was no difference between the two groups except for favorable collateral flow (ASITN/SIR 2–3) (ST 56.9% (152/269) vs AS 75.8% (25/33); p = 0.04), occluded vessel of the internal carotid artery (ICA) (ST 33.1% (89/269) vs AS 63.6% (21/33); p = 0.001) and occluded vessel of the MCA M1 (ST 59.9% (161/269) vs AS 30.3% (10/33); p = 0.001).
Table 2.
Characteristics of endovascular procedures.
| Variables | All (n = 302) | ST (n = 269) | AS (n = 33) | p value |
|---|---|---|---|---|
| Collateral (ASITN/SIR), no. (%) | 0.04 | |||
| 0–1 | 123 (40.7%) | 115 (43.1%) | 8 (24.2%) | |
| 2–3 | 177 (58.6%) | 152 (56.9%) | 25 (75.8%) | |
| Occlusion site, no. (%) | ||||
| ICA | 110 (36.4%) | 89 (33.1%) | 21 (63.6%) | 0.001 |
| MCA M1 | 171 (56.6%) | 161 (59.9%) | 10 (30.3%) | 0.001 |
| MCA M2 | 16 (5.3%) | 16 (5.9%) | 0 | 0.23 |
| ACA | 5 (1.7%) | 3 (1.1%) | 2 (6.1%) | 0.09 |
| Intravenous thrombolysis, no. (%) | 105 (34.8%) | 91 (33.8%) | 14 (42.4%) | 0.33 |
| Time from onset to treatment, median (IQR), minutes | 285 (213–358) | 285 (220–359) | 250 (180–355) | 0.24 |
| ≤285 minutes | 154 (51.0%) | 135 (50.2%) | 19 (57.6%) | 0.42 |
| >285 minutes | 149 (49.0%) | 134 (49.8%) | 14 (42.4%) | |
| Time from groin puncture to reperfusion, median (IQR), minutes | 110 (78–154) | 110 (78–155) | 104 (64–144) | 0.28 |
| ≤110 minutes | 154 (51.0%) | 135 (50.4%) | 19 (59.4%) | 0.34 |
| >110 minutes | 146 (48.3%) | 133 (49.6%) | 13 (40.6%) | |
| Time from onset to reperfusion, median (IQR), minutes | 398 (321–503) | 405 (325–506) | 340 (282–437) | 0.06 |
| ≤398 minutes | 150 (49.7%) | 129 (48.1%) | 21 (65.6%) | 0.06 |
| >398 minutes | 150 (49.7%) | 139 (51.9%) | 11 (34.4%) | |
ASITN/SIR: American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology; ICA: internal carotid artery; MCA M1: middle cerebral artery first segment; MCA M2: middle cerebral artery second segment; ACA: anterior cerebral artery; IQR: interquartile range; ST: stent retriever thrombectomy; AS: angioplasty and/or stenting.
Outcomes
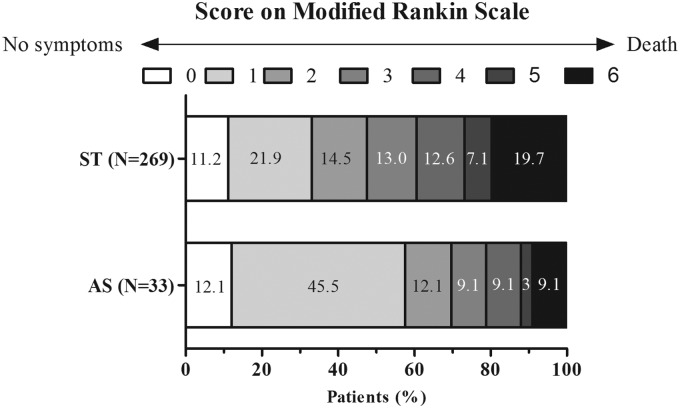
Table 3 shows the outcomes comparisons. Patients in the AS group presented with better mRS outcome at 90 days (69.7% (23/33) vs 47.6% (128/269), p = 0.02) than that in the ST group (Figure 3). Outcomes of recanalization immediately post-procedure, sICH rate, and mortality rate did not differ between both groups. A lower asymptomatic intracranial hemorrhage (aICH) rate (9.1% (3/23) vs 30.5% (82/269), p = 0.01) was observed in the AS group. However, sICH rate (9.1% (3/23) vs 11.5% (31/269), p = 1.00) did not differ between both groups. Correlation analyses suggested that collateral flow was significantly associated with NIHSS score (Spearman’s rho = –0.36, p < 0.001) and ASPECTS (Spearman’s rho = 0.189, p = 0.01).
Table 3.
Comparisons of outcomes.
| Variables | All (n = 302) | ST (n = 269) | AS (n = 33) | p value |
|---|---|---|---|---|
| mTICI, no. (%) | ||||
| 0–2a | 44 (14.6%) | 37 (13.8%) | 7 (21.2%) | 0.29 |
| 2b–3 | 258 (85.4%) | 232 (86.2%) | 26 (78.8%) | |
| mRS 0–2 at 90 days, no. (%) | 151 (50.0%) | 128 (47.6%) | 23 (69.7%) | 0.02 |
| Mortality, no. (%) | ||||
| At 90 days | 56 (18.5%) | 53 (19.7%) | 3 (9.1%) | 0.14 |
| In hospital or at discharge | 52 (17.2%) | 49 (18.2%) | 3 (9.1%) | 0.19 |
| ICH, no. (%) | ||||
| sICH | 34 (11.3%) | 31 (11.5%) | 3 (9.1%) | 1.00 |
| aICH | 85 (28.1%) | 82 (30.5%) | 3 (9.1%) | 0.01 |
| Technical complications, no. (%) | ||||
| Arterial perforation | 4 (1.3%) | 4 (1.5%) | 0 | 1.00 |
| SAH alone | 6 (2.0%) | 6 (2.2%) | 0 | 1.00 |
| Stent failure to deploy | 1 (0.3%) | 1 (0.4%) | NA | |
mTICI: modified thrombolysis in cerebral infarction; mRS: modified Rankin Scale; ICH: intracranial hemorrhage; sICH: symptomatic intracranial hemorrhage; aICH: asymptomatic intracranial hemorrhage; SAH: subarachnoid hemorrhage; ST: stent retriever thrombectomy; AS: angioplasty and/or stenting; NA: not available.
Figure 3.
Distribution of mRS at 90 days in patients with atherosclerosis-related large-vessel occlusion.
mRS: modified Rankin Scale; ST: stent retriever thrombectomy; AS: angioplasty and/or stenting.
Logistic regression analysis using mRS 3–6 as a dependent variable revealed that primary AS (OR 0.27; 95% confidence interval (CI) 0.08–0.90; p = 0.03) and ASPECT 8–10 (OR 0.36: 0.16–0.82; p = 0.02) were protective factors against poor functional outcome, while old age (OR 1.04: 1.01–1.07; p = 0.006), severe neurological deficits (OR 3.76: 2.00–7.07; p < 0.001) and high glucose (OR 1.11: 1.01–1.23; p = 0.03) were associated with poor functional outcomes with sex, hypertension, current smoker, high-density lipoprotein, globulin, time from onset to reperfusion, site of vessel occlusion, collateral flow and mTICI as covariates (Table 4).
Table 4.
Multivariable analyses of functional outcome.
| Variables | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age | 1.04 | 1.01–1.07 | 0.006 |
| Glucose | 1.11 | 1.01–1.23 | 0.03 |
| ASPECTS (8–10) | 0.36 | 0.16–0.82 | 0.02 |
| NIHSS score (>16) | 3.76 | 2.00–7.07 | <0.001 |
| Primary angioplasty and/or stenting | 0.27 | 0.08–0.90 | 0.03 |
The logistic regression model for mRS at 90 days included the following factors (step-wise method): age, sex, hypertension, current smoker, glucose, HDL, GLOB, dichotomized time from onset to reperfusion, NIHSS score, ASPECTS, site of vessel occlusion, collateral flow, mTICI and primary angioplasty and/or stenting.
ASPECTS: Alberta Stroke Program Early Computed Tomography Score; GLOB: globulin; HDL: high-density lipoprotein; mTICI: modified thrombolysis in cerebral infarction; NIHSS: National Institutes of Health Stroke Scale.
Subgroup analysis of occlusion site also suggested that primary angioplasty treatment was associated with functional outcomes in the MCA (OR 0.11: 0.01–0.89; p = 0.04) and ICA (OR 0.31: 0.11–0.86; p = 0.02) models separately with age as a covariate (Tables 5 and 6).
Table 5.
Multivariable analyses of functional outcome in the middle cerebral artery occlusion model.
| Variables | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age | 1.05 | 1.02–1.08 | <0.001 |
| Primary angioplasty and/or stenting | 0.11 | 0.01–0.89 | 0.04 |
The logistic regression model for modified Rankin Scale at 90 days included the following factors (step-wise method): age, primary angioplasty and/or stenting.
Table 6.
Multivariable analyses of functional outcome in the internal carotid artery occlusion model.
| Variables | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Age | 1.04 | 1.01–1.08 | 0.02 |
| Primary angioplasty and/or stenting | 0.31 | 0.11–0.86 | 0.02 |
The logistic regression model for modified Rankin Scale at 90 days included the following factors (step-wise method): age, primary angioplasty and/or stenting.
Discussion
As the primary report to investigate the differences on effect of first-line therapy between ST and AS for AC-LVO due to atherosclerotic disease, we found patients obtain more favorable functional outcome and lower aICH rate when AS is used. Good functional outcome was associated with AS therapy, age, lower NIHSS score, small infarct, and lower glucose level.
Many previous studies proved angioplasty in the endovascular treatment of acute stroke to be safe and effective,23,24 but these studies mostly regarded angioplasty as a “rescue” technique after mechanical thrombectomy but not the “first-line” treatment. The Stent-Assisted Recanalization in Acute Ischemic Stroke study was a prospective, non-randomized and single-center study that enrolled 20 patients to evaluate the safety and efficacy of primary stent deployment for revascularization in acute stroke patients.25 The study suggested that direct stenting of the occluded culprit vessel was technically effective with recanalization 100% (thrombolysis in myocardial infarction, TIMI 2/3), sICH rate 5%, mRS 0–3 at one month 60%, and mortality at one month 25%. Guo et al.26 reported recanalization at 100% (TIMI 2/3), no sICH incidence, mortality at 90 days 9.1% and mRS 0–2 at 90 days 70% in 11 retrospectively included patients with acute MCA occlusions who received emergent AS. Another retrospective study enrolled 22 patients with acute intracranial artery occlusions who underwent self-expandable stent deployment for treatment, and found recanalization 90.9% (TICI 2b/3), mRS 0–2 at 90 days 50%, sICH rate 9.1%, and mortality at 90 days 9.1%.27 Our study indicated comparable recanalization, mRS 0–2, and a similar sICH rate and mortality rate. Our result suggested that for acute AC-LVO due to atherosclerosis, emergent AS was safe and effective. Also, patients who received direct AS gained similar outcomes of reperfusion, sICH and mortality compared with ST in our study, indicating this first-choice method is reasonable and acceptable.
Our result validated the hypothesis that primary AS results in a more favorable outcome than ST in patients with atherosclerosis (Figure 3). As we know, ST has been the first-choice method for intra-arterial therapy.8 Although encouraging efficacy and safety, when encountered with vessels occlusion related to atherosclerosis due to subsequent platelet aggregation, mechanical thrombectomy via a stent retriever would damage the endothelium or the atherosclerotic plaque, increasing risk of acute thrombosis and reocclusion,12–14 leading to refractory occlusion, which would make the procedure more complex and more time consuming.19,28 In spite of no statistical significance, longer procedure time was observed in the ST group in our result. It has been known that functional outcome is better the sooner that endovascular reperfusion is achieved.29 Another major concern was risk of vessel rupture or tearing of perforator vessels and ICH due to more attempts of pass following ST.14,15 Unlike retrievers, AS increased the safety of the procedure by not requiring repeat maneuvers to gain access to the target site.30 Our study showed a higher aICH rate in patients receiving ST therapy.
In accordance with previous studies, our study revealed that small baseline infarct (ASPECTS 8–10)31 was a protective factor against poor functional outcome, while older age,32 severe neurological deficits32 and high glucose level33 were associated with poor functional outcome. However, we did not find that good collateral status was an independent predictor of favorable prognosis in this study, which was inconsistent with the current viewpoint that robust collateral flow was associated with better clinical outcomes.34 This may be explained by our controlling covariates in the multivariable analyses such as ASPECTS and NIHSS score, which may mediate the association between collateral flow and functional outcome.
The most intriguing finding was that primary angioplasty was associated with favorable prognosis, and the association was robust in ICA and MCA subgroups. Emergent AS has been used for acute ischemic stroke progressively, but the effectiveness remains unclear.8 Our results suggested this first-line therapy was an independent predictor of favorable outcome in patients due to atherosclerosis. This is also the first study to compare primary emergent methods for AC-LVO between AS and ST. Our study was retrieved from a multicenter registry (ACTUAL) with larger representative samples in China, reflecting the current situation of endovascular therapy practice in the real world. From this point of view, our finding may pave the way for future investigations on AS in AC-LVO due to atherosclerotic disease. However, further randomized controlled trials are warranted to explore the efficacy and safety on primary AS vs primary ST in AC-LVO due to atherosclerosis.
We modified the criteria of the TOAST classification to define atherosclerotic occlusions due to no currently established guidelines. We tried to diagnose atherosclerosis as far as possible; moreover, the post-procedure vessel images also confirmed that the majority of our initial assessments were correct, suggesting our criteria were feasible and reliable.
We also have some limitations. First, the sample size of the AS group was limited, which did not allow more powerful analysis (e.g. propensity score matching) or more covariates in the regression models for the ICA and MCA subgroups; further studies with larger sample sizes are needed to confirm or refute our findings. Second, the definition of atherosclerosis was extremely difficult; consequently, we may have misclassified a proportion of patients to have or not to have atherosclerosis. However, after checking our post-procedure CTA or MRA images, we believe the misclassification rate to be very low. Third, most patients treated with primary AS were attributed to ICA occlusion, which may have selection bias in our study based on the retrospective nature itself. In this study, 63.6% (21/33) of occlusions in the primary AS group were ICA occlusion, which can be regarded as a different lesion location when compared with intracranial occlusions. Therefore, it is possible that the lesion location influenced the effects of interventional therapy. Fourth, recanalization represented only immediate reperfusion post-procedure, thus a long-term follow-up study is needed to evaluate vessel restenosis after endovascular treatment.
Conclusions
In conclusion, our study suggests that patients with AC-LVO due to atherosclerosis may benefit from urgent AS as first-line therapy. Further studies with a larger sample size of AS patients and future randomized trials are warranted.
Supplemental Material
Supplemental material for Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion by Dong Yang, Min Lin, Shuiping Wang, Huaiming Wang, Yonggang Hao, Wenjie Zi, Penghua Lv, Dequan Zheng, Guodong Xiao, Gelin Xu, Yunyun Xiong and Xinfeng Liu; on behalf of the ACTUAL Investigators in Interventional Neuroradiology
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Natural Science Foundation of China (No. 81400898, 81400993, 81571143 and 81671172) and the Chinese Postdoctoral Science Fund (No. 2015M572815).
References
- 1.Muir KW, Ford GA, Messow CM, et al. Endovascular therapy for acute ischaemic stroke: The Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry 2017; 88: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafé A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 9.Wong L. Global burden of intracranial atherosclerosis. Int J Stroke 2006; 1: 158–159. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 11.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang DH, Kim YW, Hwang YH, et al. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis 2014; 37: 350–355. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Hong JM, Lee KS, et al. Primary stent retrieval for acute intracranial large artery occlusion due to atherosclerotic disease. J Stroke 2016; 18: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Lo WT, Sun X, et al. Combined use of mechanical thrombectomy with angioplasty and stenting for acute basilar occlusions with underlying severe intracranial vertebrobasilar stenosis: Preliminary experience from a single Chinese center. AJNR Am J Neuroradiol 2015; 36: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim TK, Rhim JK, Lee CJ, et al. The limitations of thrombectomy with Solitaire AB as first-line treatment in acute ischemic stroke: A single center experience. J Cerebrovasc Endovascr Neurosurg 2012; 14: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: An Update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2016; 67: 1235–1250. [DOI] [PubMed] [Google Scholar]
- 17.Zi W, Wang H, Yang D, et al. Clinical effectiveness and safety outcomes of endovascular treatment for acute anterior circulation ischemic stroke in China. Cerebrovasc Dis 2017; 44: 248–258. [DOI] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 19.Lee JS, Hong JM, Lee KS, et al. Endovascular therapy of cerebral arterial occlusions: Intracranial atherosclerosis versus embolism. J Stroke Cerebrovasc Dis 2015; 24: 2074–2080. [DOI] [PubMed] [Google Scholar]
- 20.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 21.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 2008; 29: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 23.Gupta R, Vora NA, Horowitz MB, et al. Multimodal reperfusion therapy for acute ischemic stroke: Factors predicting vessel recanalization. Stroke 2006; 37: 986–990. [DOI] [PubMed] [Google Scholar]
- 24.Baek JH, Kim BM, Kim DJ, et al. Stenting as a rescue treatment after failure of mechanical thrombectomy for anterior circulation large artery occlusion. Stroke 2016; 47: 2360–2363. [DOI] [PubMed] [Google Scholar]
- 25.Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (Stent-Assisted Recanalization in Acute Ischemic Stroke). Stroke 2009; 40: 3552–3556. [DOI] [PubMed] [Google Scholar]
- 26.Guo XB, Song LJ, Guan S. Emergent angioplasty and stent placement recanalization without thrombolysis in acute middle cerebral artery occlusions. J Stroke Cerebrovasc Dis 2013; 22: 694–699. [DOI] [PubMed] [Google Scholar]
- 27.Roth C, Papanagiotou P, Behnke S, et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 2010; 41: 2559–2567. [DOI] [PubMed] [Google Scholar]
- 28.Al Kasab S, Almadidy Z, Spiotta AM, et al. Endovascular treatment for AIS with underlying ICAD. J Neurointerv Surg 2017; 9: 948–951. [DOI] [PubMed] [Google Scholar]
- 29.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016; 316: 1279. [DOI] [PubMed] [Google Scholar]
- 30.Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol 2007; 28: 816–822. [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal M, Menon BK, Coutts SB, et al. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke 2011; 42: 93–97. [DOI] [PubMed] [Google Scholar]
- 32.Shi ZS, Liebeskind DS, Xiang B, et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014; 45: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JT, Jahan R, Saver JL, et al. Impact of glucose on outcomes in patients treated with mechanical thrombectomy: A post hoc analysis of the Solitaire Flow Restoration With the Intention for Thrombectomy Study. Stroke 2016; 47: 120–127. [DOI] [PubMed] [Google Scholar]
- 34.Liebeskind DS, Tomsick TA, Foster LD, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014; 45: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Primary angioplasty and stenting may be superior to thrombectomy for acute atherosclerotic large-artery occlusion by Dong Yang, Min Lin, Shuiping Wang, Huaiming Wang, Yonggang Hao, Wenjie Zi, Penghua Lv, Dequan Zheng, Guodong Xiao, Gelin Xu, Yunyun Xiong and Xinfeng Liu; on behalf of the ACTUAL Investigators in Interventional Neuroradiology