Abstract
Background
Use of flow-diversion technology in the treatment of incidental and recanalized posterior communicating artery (PComA) aneurysms.
Methods
Patients treated with the Pipeline embolization device (PED) for PComA aneurysms were identified and included in our retrospective analysis. We evaluated aneurysm characteristics, modified Rankin Scale score (mRS) on admission, angiography follow-up, and patient clinical outcome at discharge, at three to nine months, and at 12–18 months.
Results
We included 56 patients with a mean age of 56 years. Median mRS on admission was 0. All aneurysms involved the PComA and were either new findings or found to have shown recanalization at angiography follow-up from previous coil embolization or surgical clipping. Intraprocedural device foreshortening was observed in one case requiring additional placement of a self-expanding stent. One intraprocedural aneurysm rupture occurred because of a broken distal wire. This patient had an mRS of 4 after the procedure. Three- to nine-month and 12- to 18-month follow-up angiography showed near complete or complete aneurysm occlusion in most cases. Minimal to mild intimal hyperplasia was seen in five cases at three to nine months. PComA patency over time showed 29 of 46 initially patent vessels still patent at six months. Thirteen and seven PComAs showed progressive decrease in flow at three to nine months and 12–18 months, respectively. Median mRS remained 0 for all patients at three- to nine-month and 12- to 18-month follow-up.
Conclusions
Our preliminary results show that flow-diversion technology is an effective and safe treatment option. Larger studies with long-term follow-up are needed to validate our promising results.
Keywords: Intracranial aneurysm, flow diverter, stent, posterior communicating artery
Introduction
The posterior communicating artery (PComA) is one of the most common locations for Circle of Willis aneurysms.1 Incidence of PComA aneurysms and ipsilateral oculomotor nerve palsy has been reported in the literature of up to 34%–56%.2–9
Treatment options of cerebral aneurysms include surgical clipping and endovascular methods such as coil embolization, stent-assisted coiling and, most recently, the use of flow diverters, which are designed for reconstruction of the parent artery and aneurysm occlusion.
The use of flow diverters promises a less-invasive and safe approach without the need for surgical clip placement or intra-saccular coil delivery and the potential risk of ischemic complications or intraprocedural aneurysm rupture. At the same time, flow diversion achieves alternation of flow velocities within the parent artery, thereby protecting the friable aneurysm from high-pressure inflow but meanwhile allowing sufficient blood flow to small perforators.
Here we present our data on patients with either incidental or recanalized (non-fetal) PComA aneurysms that were endovascularly managed with the use of flow-diverter stents. The goal of our study is to determine the safety, efficacy, and patient outcome after use of the Pipeline embolization device (PED) for treatment of non-fetal PComA aneurysms.
Methods
Each hospital’s institutional review board approved this retrospective study.
Patients
We retrospectively included all patients who were treated with the PED for PComA aneurysms between July 2011 and June 2017 at the University of Massachusetts Medical Center (n = 17), Miami Cardiac & Vascular Institute and Baptist Neuroscience Center (n = 19), and Baylor College of Medicine (n = 20). Patients were treated by five interventionalists with 5–30 years of experience.
For each patient, demographic data including age, gender, and aneurysm characteristics were collected.
Additionally, recorded relevant clinical data included neurological examination at baseline, and neurological outcome based on modified Rankin Scale score (mRS) at discharge, at three- to nine-month and 12- to 18-month follow-up. Pre- and post-treatment imaging was evaluated for the occurrence of thromboembolic events, vessel patency, and aneurysm occlusion.
Management options such as conservative treatment (observation), surgical clipping, and endovascular treatment were discussed with the patients. Risks and benefits were balanced for all management options. Flow-diversion technology was chosen for patients in whom, owing to vascular and aneurysm anatomy, a higher technical success was expected when compared to other endovascular management options.
Diagnostic approach and interventional procedure
All patients underwent computed tomography (CT) scanning of the head and received digital subtraction angiography (DSA) prior to intervention.
Aspirin 81 mg and clopidogrel 75 mg were administered daily for at least five days prior to the elective procedure. Dual-antiplatelet therapy was continued for a minimum of six months. Aspirin is to be continued lifelong.
Diagnostic and interventional procedures were performed on a state-of the-art biplane digital angiography system. All procedures were performed with the patients under general anesthesia. Devices used included the PED classic and PED Flex. Angiography control was obtained immediately after placement of the device, at three to nine months and at 12–18 months. Exclusion of the aneurysm and patency of the parent vessel was assessed. Any residual aneurysm was recorded. Both pre- and postoperative angiograms were reviewed. We also determined PComA patency in each case immediately after the initial procedure and at follow-up angiography. PComA patency was scored according to a previously described three-point grading scale by Brinjikji et al.:10 Grade 1 (patent): similar filling of the PComA compared to pre- and post-treatment angiograms, Grade 2 (diminished flow but patent): decreased or delayed filling of the PComA compared to pre- and post-treatment angiograms, Grade 3 (occluded): no filling of the PComA on post-treatment images.
Postoperatively, dual-antiplatelet therapy was continued for six months in order to prevent thromboembolism and stent occlusion. Beyond the six-month follow-up time point, patients were maintained on lifelong aspirin treatment.
Noninvasive imaging (magnetic resonance imaging (MRI) or CT/CT angiography (CTA)) was obtained only in symptomatic patients after the procedure. No routine cross-sectional imaging follow-up was performed.
Results
Fifty-six patients, nine males and 47 females, with mean age of 56 years ranging from 19 to 82 years, were identified. All aneurysms involved a non-fetal PComA, defined as the PComA smaller than or equal in size when compared to the ipsilateral P1 segment of the posterior cerebral artery (PCA) and with the PCA supplying the majority of blood flow to the PCA territory. Mean PComA diameter was 0.95 mm (calculated from 35 of 56 cases) with a range of 0.2–2.0 mm.
Thirty-two aneurysms were incidental findings. Five previously ruptured and 15 previously unruptured aneurysms demonstrated recanalization on angiography follow-up after prior coil embolization. Three patients with previously unruptured and one patient with a previously ruptured aneurysm presented with recanalization on follow-up angiography after initial clipping (Figure 1). The majority of our incidentally found aneurysms were small (<10 mm) and it was decided to proceed with treatment (Figure 2) as these patients either had a strong family history for intracranial aneurysms or suffered a prior aneurysm rupture in a different location. Also, PComA arteries are known to have a higher five-year cumulative rupture rate compared to other anterior circulation aneurysms at the same size in patients without prior history of subarachnoid hemorrhage; less than 7 mm and at 7–12 mm PComA aneurysm rupture risk of 2.5% and 14.5% vs. anterior circulation aneurysm rupture risk of 0% and 2.5%, respectively.11
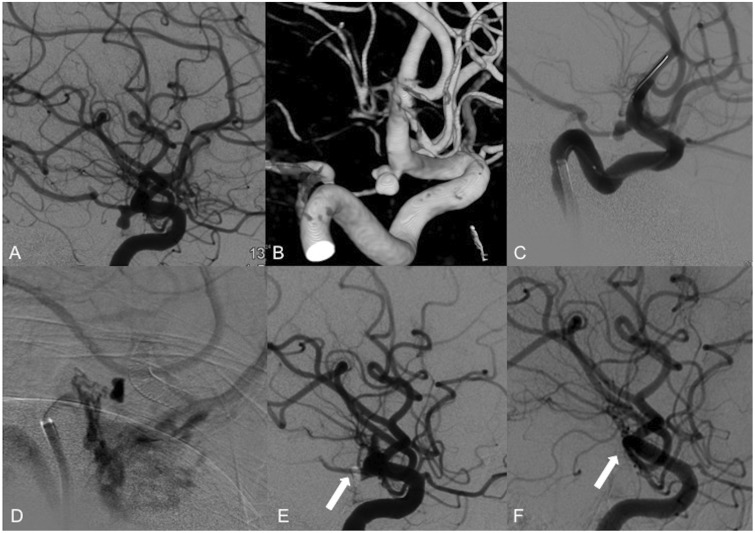
Figure 1.
Use of the PED in a case of coil compaction and recanalization. (a) Right ICA angiography shows coil compaction with recanalization of the aneurysm neck. (b) A 4.25 × 14 mm Pipeline classic was successfully placed across the aneurysm neck. (c) Follow-up angiography showed proper device placement with coverage of the aneurysm neck and patency of the PComA. (d) Contrast stagnation was seen within the aneurysm lumen during the venous phase. (e) Six-month follow-up angiography demonstrates residual filling of the aneurysm neck. (f) One-year follow-up angiography shows stable residual filling of the aneurysm neck. PED: Pipeline embolization device; ICA: internal carotid artery; PComA: posterior communicating artery.
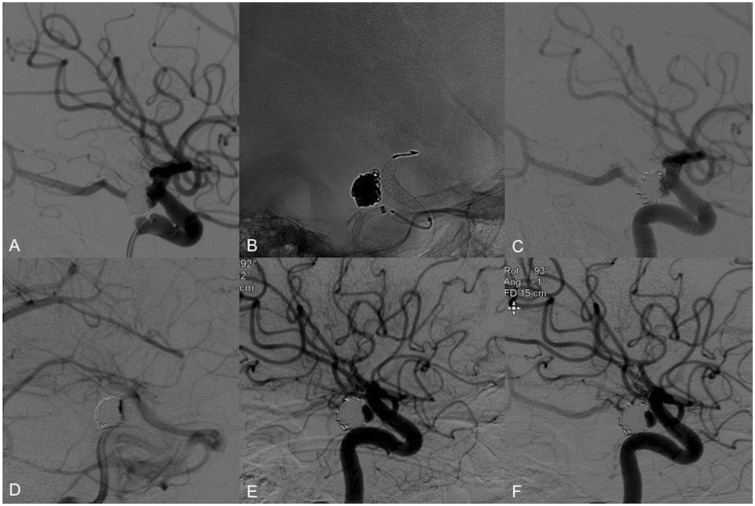
Figure 2.
Use of the PED in an incidentally found PComA aneurysm. (a) Selective right ICA contrast injection shows an incidental 6 × 4 × 3 mm PComA aneurysm. (b) Acquisition of cone-beam CT 3D reconstruction images for procedure planning and better visualization of the vessel anatomy. (c) A 3.75 × 18 mm PED was placed across the right PcomA. (d) Flow stagnation is immediately seen within the aneurysm lumen after placement of the device. ((e), arrow) There is also decreased flow across the right PComA post-device placement upon completion of the procedure. ((f), arrow) Six-month follow-up angiography shows complete occlusion of the aneurysm and PcomA. PED: Pipeline embolization device; PComA: posterior communicating artery; ICA: internal carotid artery; CT: computed tomography; 3D: three-dimensional.
Two patients with unruptured PComA aneurysms presented with oculomotor nerve palsy.
On admission, median mRS was 0. (Table 1). The PED was successfully placed in 54 of 56 cases. One patient required additional placement of a 3.0 × 15 Neuroform EZ stent (Stryker Neurovascular, Fremont, CA) owing to foreshortening of the distal end of the device, partially uncovering the aneurysm neck, necessitating pinning of the PED with a laser-cut nitinol self-expanding stent (Figure 3). As there was uncovering only of the distal part of the aneurysm but the PED still protected the proximal portion and given the presence of coils in the aneurysm lumen, it was decided that the flow-diverter treatment effect would still allow for aneurysm occlusion over time. The main goal was to simply anchor the device and provide a scaffold to avoid coil protrusion, which was achieved using a self-expanding stent. Follow-up angiography in this case showed proper vessel wall apposition of the devices with complete aneurysm occlusion and no hemodynamically significant intimal hyperplasia. All other devices were successfully placed over the aneurysm neck, providing complete coverage.
Table 1.
Patient data and aneurysm characteristics.
| Pt # | Gender | Age | Aneurysm location | Aneurysm size (mm) | Aneurysm characteristics | mRS on admission |
|---|---|---|---|---|---|---|
| 1 | F | 48 | Right PComA | 6 × 4 × 3 | Incidental finding | 0 |
| 2 | F | 49 | Left PComA | 12 × 6 × 6 | Incidental finding | 0 |
| 3 | F | 68 | Right PComA | 1.2 × 1.7 × 1.3 20 mm dysplastic segment | Ruptured, recanalized post-CE | 0 |
| 4 | F | 51 | Right PComA | 4.3 × 4.5 × 3.0 | Unruptured, recanalized post-CE | 0 |
| 5 | F | 52 | Left PComA | 8 × 4 × 6 | Ruptured, recanalized post-CE | 0 |
| 6 | F | 55 | Right PComA | 5.6 × 4.8 × 5.6 | Ruptured, recanalized post-CE | 0 |
| 7 | F | 61 | Right PComA | 4 × 2 × 3.5 | Incidental finding | 0 |
| 8 | M | 75 | Right PComA | 3.0 × 3.0 × 3.5 | Incidental finding | 0 |
| 9 | F | 43 | Left PComA | 1.7 × 1.3 × 1.7 | Ruptured, recanalized post-clipping | 0 |
| 10 | F | 61 | Right PComA | 3.6 × 2.75 × 5 | Incidental finding | 0 |
| 11 | F | 60 | Left PComA | 2.3 × 2.2 × 3.1 2.5 × 2.1 × 2.0 | Incidental finding | 3 |
| 12 | F | 55 | Right PComA | 2.4 × 1.4 × 2.4 | Incidental finding | 0 |
| 13 | M | 57 | Left PComA | 4.9 × 2.8 × 2.2 | Unruptured, recanalized post-clipping | 0 |
| 14 | F | 35 | Right PComA | 2.5 × 2.9 × 2.2 | Ruptured, recanalized post-SACE | 0 |
| 15 | M | 68 | Right PComA | 2.5 × 2.6 × 2.5 | Ruptured, recanalized post-CE | 0 |
| 16 | M | 68 | Right PComA | 2.5 × 2.64 × 2.52 | Incidental finding | 0 |
| 17 | F | 63 | Left PComA | 4.6 × 3.6 | Incidental finding | 0 |
| 18 | F | 62 | Right PComA | 4.8 × 3.2 | Incidental finding | 0 |
| 19 | F | 58 | Right PComA | 16 × 12.4 | Incidental finding | 2 |
| 20 | F | 58 | Left PComA | 3.2 × 2.9 | Incidental finding | 1 |
| 21 | F | 78 | Right PComA | 9.1 × 6.4 | Incidental finding | 0 |
| 22 | F | 82 | Right PComA | 11 × 6 | Incidental finding | 0 |
| 23 | F | 47 | Right PComA | 3.5 × 2 | Ruptured, recanalized post-CE | 1 |
| 24 | F | 72 | Right PCom | 14 × 12 | Incidental finding | 1 |
| 25 | F | 44 | Left PComA | 4 × 3.5 | Ruptured, recanalized post-CE | 0 |
| 26 | F | 61 | Right PComA | 8 × 6 | Incidental finding | 2 |
| 27 | F | 56 | Right PComA | 2 × 2 | Ruptured, recanalized post-CE | 0 |
| 28 | F | 48 | Left PComA | 6.8 × 5.6 | Ruptured, recanalized post-CE | 1 |
| 29 | M | 60 | Right PCom | 6.4 × 3.4 | Incidental finding | 2 |
| 30 | F | 59 | Left PComA | 3 × 2.2 | Ruptured, recanalized post-CE | 0 |
| 31 | F | 38 | Left PComA | 7.2 × 6.6 | Incidental finding | 0 |
| 32 | F | 58 | Left PComA | 9.8 × 5.5 | Ruptured, recanalized post-CE | 1 |
| 33 | F | 76 | Left PComA | 6 × 6 | Incidental finding | 0 |
| 34 | F | 38 | Right PComA | 3 × 3 | Ruptured, recanalized post CE | 1 |
| 35 | F | 70 | Right PComA | 4.5 × 4.9 | Ruptured, recanalized post-CE | 0 |
| 36 | F | 63 | Left PComA | 3 × 2 | Unruptured, recanalized post-CE | 0 |
| 37 | F | 54 | Left PComA | 3.5 × 2.5 | Unruptured, recanalized post-CE | 0 |
| 38 | M | 57 | Right PComA | 3.5 × 4.0 | Incidental finding | 0 |
| 39 | F | 51 | Right PComA | 5.0 × 4.0 | Unruptured, recanalized post-clipping | 0 |
| 40 | F | 67 | Right PComA | 7.0 × 5.0 | Incidental finding | 0 |
| 41 | M | 66 | Left PComA | 5.0 × 3.5 | Incidental finding | |
| 42 | F | 40 | Left PComA | 3.0 × 2.2 × 3.2 | Unruptured, recanalized post-clipping | 0 |
| 43 | F | 76 | Right PComA | 4.0 × 2.8 | Incidental finding | 0 |
| 44 | F | 82 | Left PComA | 7.0 × 3.2 | Incidental finding | 0 |
| 45 | F | 49 | Right PComA | 4 × 2.5 | Incidental finding | 0 |
| 46 | F | 76 | Right PComA | 4.7 × 2.3 | Incidental finding | 0 |
| 47 | F | 51 | Right and Left PComA | 5.0 × 6.0 × 6.0 | Incidental finding | 0 |
| 48 | M | 19 | Right PComA | 3.2 × 4.8 | Incidental finding | 0 |
| 49 | F | 54 | Right PComA | 2.0 × 3.0 | Ruptured, recanalized post-CE | 0 |
| 50 | F | 57 | Left PComA | 2.8 × 2.0 | Ruptured, recanalized post-CE | 0 |
| 51 | F | 58 | Right PComA | 6.0 × 4.0 | Unruptured, recanalized post-CE | 0 |
| 52 | F | 61 | Right PComA | 3.0 × 3.0 | Incidental finding | 0 |
| 53 | M | 59 | Right PComA | 3.1 × 2.9 | Unruptured, recanalized post-CE | 0 |
| 54 | F | 76 | Right PComA | 10.0 × 6 .0 | Incidental finding | 0 |
| 55 | F | 52 | Left PComA | 10.0 × 4.0 | Incidental finding | 0 |
| 56 | F | 69 | Left PComA | 3.4 × 2.4 | Incidental finding | 0 |
F: female; M: male; Pt#: patient number; Hx: history; Y: yes; N: no; HTN: hypertension; DL: dyslipidemia; PComA: posterior communicating artery; CE: coil embolization; mRS: modified Rankin Scale score.
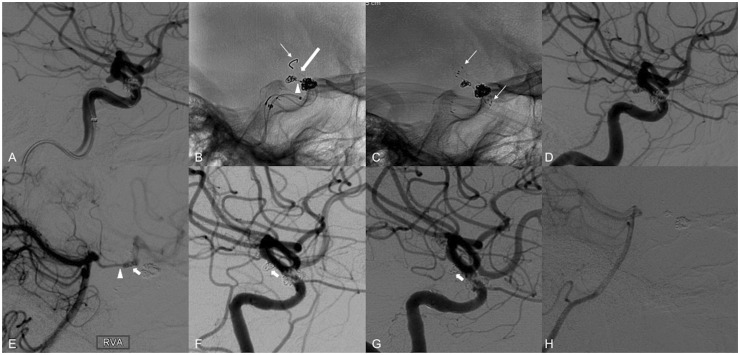
Figure 3.
Use of the PED in conjunction with a self-expanding laser-cut stent. (a) Right ICA angiography reveals a 2.5 × 2.9 × 2.2 mm PComA aneurysm with the branch arising from the dome of the aneurysm. The patient underwent previous stent-assisted coiling of a right paraophthalmic aneurysm. (b) A 3 mm × 4 cm coil was placed within the aneurysm lumen via a microcatheter (arrow head) which was jailed by the 3.0 × 14 mm Pipeline Flex device (thin arrow on tip of delivery wire). Unfortunately, the PED foreshortened and covered only the proximal portion of the aneurysm neck (thick arrow). ((c), arrows on distal and proximal markers of the stent) A 3 × 15 mm Neuroform EZ stent (Stryker Neurovascular, Fremont, CA) was then telescopically placed across the distal end of the PED, successfully covering the aneurysm lumen and anchoring the PED against the vessel wall. (d) Follow-up angiogram demonstrating patency of the PComA. (e) Right vertebral artery angiogram post-treatment also demonstrates patency of the PComA (arrow head) and flow through the aneurysm (arrow) into the anterior circulation. (f) Six-month follow-up angiography demonstrates complete occlusion of the PComA and aneurysms. (g) One-year follow-up angiography shows unchanged complete occlusion of the PComA and aneurysm. (h) Similar to at six months (image not shown), the right vertebral artery angiogram at one year reveals no flow through the PComA. PED: Pipeline embolization device; PComA: posterior communicating artery; ICA: internal carotid artery.
Except for three cases, all patients were treated with a single PED. Two cases required telescopic placement of two PEDs for treatment of the aneurysm and an adjacent dysplastic vessel segment. Another case required two PEDs to treat a second superior hypophyseal aneurysm.
One intra-procedural aneurysm rupture with subsequent internal carotid artery sacrifice occurred because of a broken distal wire, which did not allow the interventionalist to release the device. This patient unfortunately had an mRS of 4 at discharge but improved to an mRS of 3 at three-year clinical follow-up. There was no change in mRS from admission to discharge for the remaining patients (n = 55). Median mRS score at discharge was 0. Three- to nine-month median mRS scores remained 0. Twelve- to 18-month follow-up was available for 23 patients. Median mRS remained 0. Both patients who initially presented with oculomotor nerve palsy showed complete recovery.
Angiography results
Control angiographies immediately after PED placement showed significantly reduced flow or flow stasis within the aneurysms. Grade 1 (patent) flow in the PComA and confirmed good flow within the parent artery was observed in 49 cases (87.5%). Diminished flow (Grade 2) or an occluded PComA (Grade 3) was seen in four and three cases, respectively. No vasospasm or distal emboli were noted.
Three- to nine-month follow-up angiography was available for 47 patients (83.9%) and 12- to 18-month follow-up was available for 24 patients (42.9%). Three- to nine-month follow-up angiography showed partial aneurysm occlusion in five cases, near complete occlusion in 11 cases, and complete aneurysm occlusion in 32 cases. Persistent patency of the PComA (Grade 1) was seen in 29 cases (61.7%), stable diminished flow (Grade 2) was seen in three cases, and stable PComA occlusion (Grade 3) in one case.
Progression from Grade 1 to Grade 2 flow was seen in eight cases. Four cases showed complete occlusion of a previously patent PComA. One patient demonstrated progression from diminished PComA flow (Grade 2) to complete occlusion (Grade 3). Another patient demonstrated new Grade 2 flow in a previously occluded PComA post intervention. Development of non-hemodynamically significant mild intimal hyperplasia was noted in five cases. No in-stent stenosis was observed.
Control angiography at 12–18 months (n = 24) demonstrated progression from near complete to complete aneurysm occlusion in two cases. The remaining patients showed stable aneurysm occlusion. Two cases of minimal to mild intimal hyperplasia at six months remained stable. One patient presented with new minimal intimal hyperplasia at one-year follow-up. There was no evidence of in-stent thrombosis or stenosis (Table 2).
Table 2.
Summary of treatment, outcome, and follow-up results.
| Pt # | PED size (mm) | Coils used | Periprocedural complications | PComA patency (Brinjikji et al.) | mRS at discharge | Angiography FU at three to nine months | PComA patency at three to nine months (Brinjikji et al.) | mRS at three to nine months | Angiography FU at 12–18 months | PComA patency at 12–18 months (Brinjikji et al.) | mRS at 12–18 months | Angio- graphy FU at 24–36 months | PComA patency at 24–36 months (Brinjikji et al.) | mRS at 24–36 months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.75 × 18 | No | None | 2 | 0 | Complete occlusion, no IH | 3 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 2 | 4.5 × 20 | No | None | 1 | 0 | Partial occlusion, no IH | 1 | 0 | Partial occlusion, no IH | 2 | 0 | Partial occlusion, no IH | 2 | 0 |
| 3 | 4.0 × 18 and 4.0 × 16 | No | None | 1 | 0 | Complete occlusion, mild IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 |
| 4 | 4.25 × 14 | No | None | 2 | 0 | Near complete occlusion, no IH | 2 | 0 | Near complete occlusion, no IH | 2 | 0 | Complete occlusion, no IH | 2 | 0 |
| 5 | 3.5 × 20 | No | None | 1 | 1 | Near complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 2 | 0 | N/A | N/A | N/A |
| 6 | 4.0 × 20 | Yes | None | 1 | 0 | Complete occlusion, mild IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 7 | 3.5 × 18 | No | None | 2 | 0 | Partial occlusion, no IH | 2 | 0 | Partial occlusion, no IH | 1 | 0 | Partial occlusion, no IH | 1 | 0 |
| 8 | 4.5 × 16 | No | None | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 9 | 4.0 × 18 | No | None | 1 | 0 | Complete occlusion, mild IH | 1 | 0 | Complete occlusion, minimal IH | 1 | 0 | Complete occlusion, no IH | N/A | N/A |
| 10 | 4.5 × 16 | No | None | 1 | 0 | Complete occlusion, mild IH | 1 | 0 | Complete occlusion, mild IH | 3 | 0 | Complete occlusion, no IH | N/A | N/A |
| 11 | 4.0 × 14 | No | None | 1 | 3 | Near complete occlusion, no IH | 1 | 3 | Near complete occlusion, minimal IH | 1 | 3 | Near complete occlusion, minimal IH | a | a |
| 12 | 4.25 × 16 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 13 | 4 × 14 | No | None | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | Near complete occlusion, no IH | 1 | 0 |
| 14 | 3 × 14 | Yes | PED migration requiring stent placement | 1 | 0 | Complete occlusion, mild IH | 1 | 0 | Complete occlusion, no IH | 3 | 0 | a | a | a |
| 15 | 3.5 × 14 | No | None | 1 | 0 | – | – | – | Complete occlusion, no IH | 3 | 0 | N/A | N/A | N/A |
| 16 | 3.5 × 14 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 17 | 3.75 × 12 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | – | – | – | a | a | a |
| 18 | 3.75 × 12 | No | None | 1 | 0 | Complete occlusion, no IH | 2 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 19 | 4.0 × 14 | Yes | None | 1 | 2 | Near complete occlusion, no IH | 2 | 2 | N/A | N/A | N/A | N/A | N/A | N/A |
| 20 | 4.0 × 16 | No | None | 1 | 1 | Near complete occlusion, no IH | 2 | 1 | N/A | N/A | N/A | N/A | N/A | N/A |
| 21 | 4.0 × 14 | No | None | 1 | 0 | Complete occlusion, no IH | 2 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 22 | 4.0 × 18 | No | None | 1 | 0 | Partial occlusion, no IH | 2 | 0 | N/A | N/A | N/A | Partial occlusion, no IH | 2 | 0 |
| 23 | 3.25 × 14 | No | None | 1 | 1 | Complete occlusion, no IH | 3 | 1 | N/A | N/A | N/A | N/A | N/A | N/A |
| 24 | 4.0 × 16 | Yes | None | 2 | 1 | Partial occlusion, no IH | 2 | 1 | N/A | N/A | N/A | Complete occlusion, no IH | 2 | 1 |
| 25 | 4.0 × 14 | Yes | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 26 | 3.25 × 14 | Yes | None | 1 | 2 | Complete occlusion, no IH | 2 | 2 | N/A | N/A | N/A | N/A | N/A | N/A |
| 27 | 3.25 × 16 | No | None | 1 | 0 | – | – | – | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 2 | 0 |
| 28 | 3.5 × 12 | No | None | 1 | 1 | Complete occlusion, no IH | 3 | 1 | N/A | N/A | N/A | N/A | N/A | N/A |
| 29 | 4.5 × 20 | No | None | 1 | 2 | – | – | – | – | – | – | – | – | 1 |
| 30 | 3.75 × 14 | No | None | 1 | 0 | Near complete occlusion, no IH | 2 | 0 | Complete occlusion, no IH | 3 | 0 | a | a | a |
| 31 | 4.25 × 18 | No | None | 1 | 0 | – | – | – | Partial occlusion, no IH | 2 | 0 | Partial occlusion, no IH | 2 | 0 |
| 32 | 5 × 20 | No | None | 1 | 1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 33 | 4.0 × 16 | No | None | 1 | 0 | Partial occlusion, no IH | 1 | 0 | Partial occlusion, no IH | 1 | 0 | a | a | a |
| 34 | 3.25 × 14 | No | None | 3 | 1 | Near complete occlusion, no IH | 2 | 0 | a | a | a | a | a | a |
| 35 | 4.0 × 12 | No | None | 1 | 0 | Complete occlusion, no IH | 3 | 0 | – | – | – | Complete occlusion, no IH | 3 | 0 |
| 36 | 3.75 × 18 | No | Broken distal wire leading to aneurysm rupture, ICA sacrificed | 3 | 4 | – | – | – | – | – | – | – | – | 3 |
| 37 | 3.75 × 18 | No | None | 3 | 0 | Complete occlusion, no IH | 3 | 0 | – | – | – | Complete occlusion, no IH | 3 | 0 |
| 38 | 4.5 × 14 and 4.75 × 14 | Yes | None | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | – | – | – | Near complete occlusion, no IH | 1 | 0 |
| 39 | 3.4 × 14 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | – | – | – | Complete occlusion, no IH | 1 | 0 |
| 40 | 3.5 × 20 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | – | – | – | Complete occlusion, no IH | 1 | 0 |
| 41 | 3.75 × 14 | No | None | 1 | 0 | – | – | – | – | – | – | Complete occlusion, no IH | 1 | 0 |
| 42 | 3.25 × 20 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 43 | 4 × 18 | No | None | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 44 | 3.5 × 18 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 45 | 4 × 18 | No | None | 1 | 0 | Complete occlusion, no IH | 2 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 46 | 4 × 18 | No | None | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | Near complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 47 | 4 × 18 left3.75 × 12 right | Yes, left | None | 1 | 0 | Complete occlusion right and left, no IH | 1 | 0 | Complete occlusion right and left, no IH | 1 | 0 | N/A | N/A | N/A |
| 48 | 3 × 10 | No | None | 1 | 0 | Complete occlusion, no IH | 3 | 0 | a | a | a | a | a | a |
| 49 | 4 × 12 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 50 | 3.75 × 12 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 51 | 3.5 × 12 and 3.5 × 14 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 52 | 4 × 20 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | Complete occlusion, no IH | 1 | 0 |
| 53 | 3.5 × 14 | No | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| 54 | 3.5 × 14 | Yes | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
| 55 | 3.5 × 18 | Yes | None | 1 | 0 | – | – | – | – | – | – | Complete occlusion, no IH | 3 | 0 |
| 56 | 4 × 20 | Yes | None | 1 | 0 | Complete occlusion, no IH | 1 | 0 | Complete occlusion, no IH | 1 | 0 | N/A | N/A | N/A |
Patient did not reach follow-up time point.
Pt#: patient number; ICA: internal carotid artery; PED: Pipeline embolization device; mRS: modified Rankin Scale score; IHL: intimal hyperplasia; FU: follow-up; N/A: not available.
Unchanged PComA patency from discharge to 12- to 18-month follow-up was seen in 15 cases. Progression to Grade 2 and Grade 3 flow was seen in three and four patients, respectively. One patient showed improvement from a Grade 2 flow at discharge and six months to normal PComA flow (Grade 1). One patients showed stable diminished (Grade 2) PComA flow.
Long-term follow-up (24–36 months) was available for 20 patients (35.7%), who showed stable PComA patency to the last prior follow-up. Two aneurysms progressed to complete occlusion. The remaining aneurysms showed stable occlusion status from the last prior follow-up. No significant intimal hyperplasia was seen in these patients.
We did not observe an association between a larger PComA diameter and patency or a smaller vessel diameter and associated vessel occlusion on follow-up angiography.
Noninvasive imaging
A follow-up MRI was obtained in one patient who was found to have stroke symptoms after the procedure and revealed small distal middle cerebral artery (MCA) territory infarcts. No posterior circulation infarcts were seen. The patient recovered without residual neurological deficits.
Three patients underwent CT/CTA follow-up for change in mental status and stroke symptoms. One patient showed a small amount of subarachnoid hemorrhage on the treatment side in the posterior frontal lobe at the vertex but no infarcts. The other two patients showed small distal MCA territory infarcts on the treatment side. No posterior circulation territory infarcts were seen. All three patients recovered and were discharged without residual neurological deficits.
Discussion
The PED is placed during a single interventional procedure and alters flow dynamics in favor of aneurysm obliteration while securing parent artery blood flow. Curative reconstruction of the parent artery and exclusion of the aneurysm from the intracranial circulation, which ultimately leads to blood stasis and clot formation, develop over time. Thus, recanalization is less likely to be expected and need for re-treatment is markedly decreased if not unnecessary.
Three- to six-month follow-up angiography in our patient cohort confirmed near complete or complete aneurysm occlusion in the majority of cases (42/47, 89.4%). Twelve- to 18-month follow-up confirmed stable or progressive aneurysm occlusion. Our findings also demonstrate maintained therapeutic effect of the PED when used as a secondary treatment option in PComA aneurysms pre-treated with coil embolization or clipping.
Because of alteration of flow dynamics and opposed flow by distal collaterals through the ipsilateral P1 segment after flow-diverter placement, the gradient within the PComA artery may be neutralized, which can then lead to occlusion of the PComA. This phenomenon is well known and has shown not to be associated with neurological deficits since collaterals from the P1 segment can supply the PComA vascular territory.11 None of our patients who demonstrated either immediate or progressively diminished flow/occlusion post-procedure had any neurological symptoms at follow-up. These findings are in line with recent publications.10,12 Risk factors for branch vessel occlusions discussed in the current literature include ostium coverage with overlapping PEDs, neointimal hyperplasia across the covered branch vessel, suboptimal wall apposition of the PED or inadequate dual-antiplatelet response (clopidogrel resistance).12,13
Endovascular treatment has proven superior to surgical clipping with regards to invasiveness, procedural complications, and recovery time.14,15 In addition, surgical treatment of PComA aneurysms was found to have the second highest event rate of intra-procedural rupture among saccular aneurysms,16,17 further directing treatment of aneurysms in this specific location toward endovascular repair.
Recanalization rates for PComA aneurysms are as high as 37% in the current literature. Also, Campi et al.18 found a higher frequency of recurrences of coiled PComA aneurysms, which then often required reembolization. Despite the use of stents to assist the coiling procedure, recurrence rates still remain significant.19
Resolution of the oculomotor nerve paresis as seen in our two patients has previously been described in the literature after coil embolization20–26 and has been seen after two weeks to five months.26–28 Early endovascular intervention in the setting of third-nerve palsy is suggested to accomplish higher rates of complete resolution of oculomotor paresis.27–29
Limitations
Limitations of our study include its retrospective nature and short-term follow-up for the majority of our patients, especially because the definitive treatment results of flow-diversion technology rely on progressive reduction of aneurysm filling over time. Some of the aneurysms treated have a branch vessel incorporated into the aneurysm that may therefore take longer to achieve complete occlusion or that overall have a decreased occlusion rate. Also, the natural history of incompletely occluded aneurysms post-PED treatment is still unknown, and studies with long-term follow-up are needed for further evaluation. The majority of aneurysms treated in our study are less than 7 mm in size, which may limit applicability of our results to larger aneurysms. Also, follow-up cross-sectional imaging was obtained only in symptomatic patients and therefore evaluation of clinically silent infarctions potentially related to a PComA branch occlusion was not possible.
Conclusion
Our preliminary results show that flow-diversion technology is an effective and safe treatment option for incidental and pre-treated intracranial aneurysms involving a non-fetal PComA. Larger studies with long-term follow-up are needed to validate our promising results.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ALK: none declared. GD: consultant, speaker and proctor for Medtronic, Microvention, and Penumbra; shareholder of Medina, Stryker, and InNeuroCo Inc. PK: consultant for Stryker Neurovascular, Covidien, and MicroVention. AKW: consultant for Stryker Neurovascular; research grant: Philips Healthcare; speaker for Harvard postgraduate course, Miami Cardiovascular Institute; stocks in InNeuroCo Inc. ASP: consultant for Codman Neurovascular, Stryker Neurovascular, and Covidien; research grant from Stryker Neurovascular and Covidien; speaker for Miami Cardiovascular Institute; stocks in InNeuroCo Inc.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Beck J, Rohde S, Berkefeld J, et al. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol 2006; 65: 18–25. [DOI] [PubMed] [Google Scholar]
- 2.Birchall D, Khangure MS, McAuliffe W. Resolution of third nerve paresis after endovascular management of aneurysms of the posterior communicating artery. Am J Neuroradiol 1999; 20: 411–413. [PMC free article] [PubMed] [Google Scholar]
- 3.Kupersmith MJ, Heller G, Cox TA. Magnetic resonance angiography and clinical evaluation of third nerve palsies and posterior communicating artery aneurysms. J Neurosurg 2006; 105: 228–234. [DOI] [PubMed] [Google Scholar]
- 4.Paterson A. Direct surgery in the treatment of posterior communicating artery aneurysms. Lancet 1968; 2: 808–811. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton JG, Falconer MA. Immediate and late results of surgery in cases of saccular intracranial aneurysms. J Neurosurg 1959; 16: 514–541. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos VG, Fountas KN, Feltes CH, et al. Literature review regarding the methodology of assessing third nerve paresis associated with non-ruptured posterior communicating artery aneurysms. Neurosurg Rev 2005; 28: 256–260. [DOI] [PubMed] [Google Scholar]
- 7.Soni SR. Aneurysms of the posterior communicating artery and oculomotor paresis. J Neurol Neurosurg Psychiatry 1974; 37: 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassis SZ, Jouanneau E, Tahon FB, et al. Recovery of third nerve palsy after endovascular treatment of posterior communicating artery aneurysms. World Neurosurg 2010; 73: 11–16. [DOI] [PubMed] [Google Scholar]
- 9.Yanaka K, Matsumaru Y, Mashiko R, et al. Small unruptured cerebral aneurysms presenting with oculomotor nerve palsy. Neurosurgery 2003; 52: 553–557; discussion 556–557. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, Lanzino G, Cloft HJ, et al. Patency of the posterior communicating artery after flow diversion treatment of internal carotid artery aneurysms. Clin Neurol Neurosurg 2014; 120: 84–88. [DOI] [PubMed] [Google Scholar]
- 11.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–110. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Castilla L, Munich SA, Jaleel N, et al. Patency of anterior circulation branch vessels after Pipeline embolization: Longer-term results from 82 aneurysm cases. J Neurosurg 2017; 126: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 13.Mazur MD, Kilburg C, Wang V, et al. Pipeline embolization device for the treatment of vertebral artery aneurysms: The fate of covered branch vessels. J Neurointerv Surg 2016; 8: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 14.McDougall CG, Spetzler RF, Zabramski JM, et al. The Barrow Ruptured Aneurysm Trial. J Neurosurg 2012; 116: 135–144. [DOI] [PubMed] [Google Scholar]
- 15.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002; 360: 1267–1274. [DOI] [PubMed] [Google Scholar]
- 16.Leipzig TJ, Morgan J, Horner TG, et al. Analysis of intraoperative rupture in the surgical treatment of 1694 saccular aneurysms. Neurosurgery 2005; 56: 455–468. [DOI] [PubMed] [Google Scholar]
- 17.Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005; 366: 809–817. [DOI] [PubMed] [Google Scholar]
- 18.Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007; 38: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro M, Becske T, Sahlein D, et al. Stent-supported aneurysm coiling: A literature survey of treatment and follow-up. AJNR Am J Neuroradiol 2012; 33: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malisch TW, Guglielmi G, Viñuela F, et al. Unruptured aneurysms presenting with mass effect symptoms: Response to endosaccular treatment with Guglielmi detachable coils. Part I. Symptoms of cranial nerve dysfunction. J Neurosurg 1998; 89: 956–961. [DOI] [PubMed] [Google Scholar]
- 21.Inamasu J, Nakamura Y, Saito R, et al. Early resolution of third nerve palsy following endovascular treatment of a posterior communicating artery aneurysm. J Neuroophthalmol 2002; 22: 12–14. [DOI] [PubMed] [Google Scholar]
- 22.Stiebel-Kalish H, Maimon S, Amsalem J, et al. Evolution of oculomotor nerve paresis after endovascular coiling of posterior communicating artery aneurysms: A neuro-ophthalmological perspective. Neurosurgery 2003; 53: 1268–1273. [DOI] [PubMed] [Google Scholar]
- 23.Kim DJ, Kim DI, Lee SK, et al. Unruptured aneurysms with cranial nerve symptoms: Efficacy of endosaccular Guglielmi detachable coil treatment. Korean J Radiol 2003; 4: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn JY, Han IB, Yoon PH, et al. Clipping vs coiling of posterior communicating artery aneurysms with third nerve palsy. Neurology 2006; 66: 121–123. [DOI] [PubMed] [Google Scholar]
- 25.Hanse MC, Gerrits MC, van Rooji WJ, et al. Recovery of posterior communicating artery aneurysm-induced oculomotor palsy after coiling. Am J Neuroradiol 2008; 29: 988–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birchall D, Khangure MS, McAuliffe W. Resolution of third nerve paresis after endovascular management of aneurysms of the posterior communicating artery. AJNR Am J Neuroradiol 1999; 20: 411–413. [PMC free article] [PubMed] [Google Scholar]
- 27.Ko JH, Kim YJ. Oculomotor nerve palsy caused by posterior communicating artery aneurysms: Evaluation of symptoms after endovascular treatment. Interv Neuroradiol 2011; 17: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santillan A, Zink WE, Knopman J, et al. Early endovascular management of oculomotor nerve palsy associated with posterior communicating artery aneurysms. Interv Neuroradiol 2010; 16: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang SH, Pei W, Cai XS, et al. Endovascular management and recovery from oculomotor nerve palsy associated with aneurysms of the posterior communicating artery. World Neurosurg 2010; 74: 316–319. [DOI] [PubMed] [Google Scholar]