Abstract
The lateral foramen magnum region is defined as the bilateral occipital area that runs laterally up to the jugular foramen. The critical vasculatures of this region are not completely understood. Dural arteriovenous fistulas that occur in this region are rare and difficult to treat. Therefore, we searched PubMed to identify all relevant previously published English language articles about lateral foramen magnum dural arteriovenous fistulas, and we performed a review of this literature to increase understanding about these fistulas. Four types of dural arteriovenous fistulas occur in the lateral foramen magnum region. These include anterior condylar confluence and anterior condylar vein dural arteriovenous fistulas, posterior condylar canal dural arteriovenous fistulas, marginal sinus dural arteriovenous fistulas, and jugular foramen dural arteriovenous fistulas. These dural arteriovenous fistulas share similar angioarchitectures and clinical characteristics. The clinical presentations of lateral foramen magnum dural arteriovenous fistulas include pulsatile tinnitus, intracranial hemorrhage, myelopathy, orbital symptoms, and cranial nerve palsy. Currently, head computed tomography, computed tomography angiography, magnetic resonance imaging, magnetic resonance angiography and digital subtraction angiography (DSA) are useful for diagnosing dural arteriovenous fistulas, and of these, DSA remains the “gold standard.” Most lateral foramen magnum dural arteriovenous fistulas need to be treated due to their aggressive symptoms, and transvenous embolization presents the best options. During treatment, it is critical to accurately place the microcatheter into the fistula point, and intraoperative integrated computed tomography and DSA data are very helpful. Other treatments, such as transarterial embolization, microsurgery or conservative treatment, can also be chosen. After appropriate treatment, most patients with lateral foramen magnum dural arteriovenous fistulas achieve satisfactory outcomes.
Keywords: Dural arteriovenous fistula, lateral foramen magnum region, review
Introduction
The lateral part of the foramen magnum (FM) is its most complex and important area and contains many critical nerves and vasculatures.1–3 As a result, lesions in the lateral FM region often carry the potential to afflict devastating morbidity and mortality.4 Although dural arteriovenous fistulas (DAVFs) can occur in any structure that is covered by the dura mater, DAVFs that occur in the lateral FM region are uncommonly rare and difficult to treat, and they therefore pose substantial challenges during diagnosis and management.3,5,6
In order to completely understand lateral FM DAVFs, it is necessary to better define the lateral FM region and its normal vessel anatomy, to classify the angioarchitecture and clinical manifestations of its DAVFs, to improve image examinations, and to devise better treatment and prognostic techniques. Therefore, we searched PubMed for all previously published relevant English language articles about lateral FM DAVFs, and we performed a systematic review of the literature to increase understanding about lateral FM DAVFs.
Definition of the lateral FM region
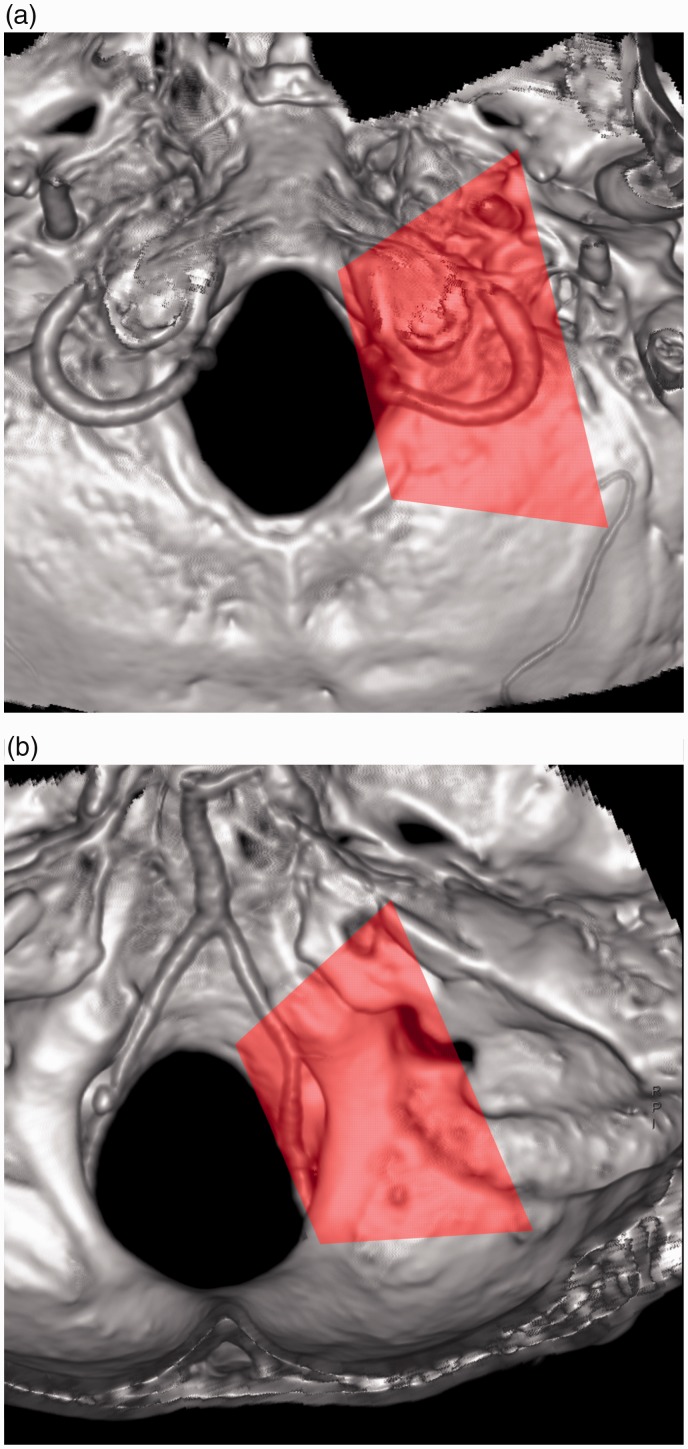
The FM region is an anatomically complex zone of the skull base.7–9 In the FM region, the lateral part is the most complex and important and contains many critical nerves and vasculatures. The lateral FM region should be defined as the bilateral occipital area that runs laterally up to the jugular foramen (JF) and includes both the inside and the outside of the occipital bone.10 DAVFs in this region form abnormal communications between dural branches of the external carotid artery (ECA) and vertebral artery (VA) in addition to the complex vein system of the craniovertebral junction. They are difficult to diagnose and treat.7,11 The lateral FM region is shown in Figure 1.
Figure 1.
Range of the lateral foramen magnum (FM). The region is indicated by a translucent pink rectangle shown on computed tomography angiography (CTA) skull base images: (a) outside the occipital bone, and (b) inside the occipital bone.
Normal vessel anatomy
In the lateral FM region, there are venous confluences, communicating veins, venous drainages, and important arteries.12
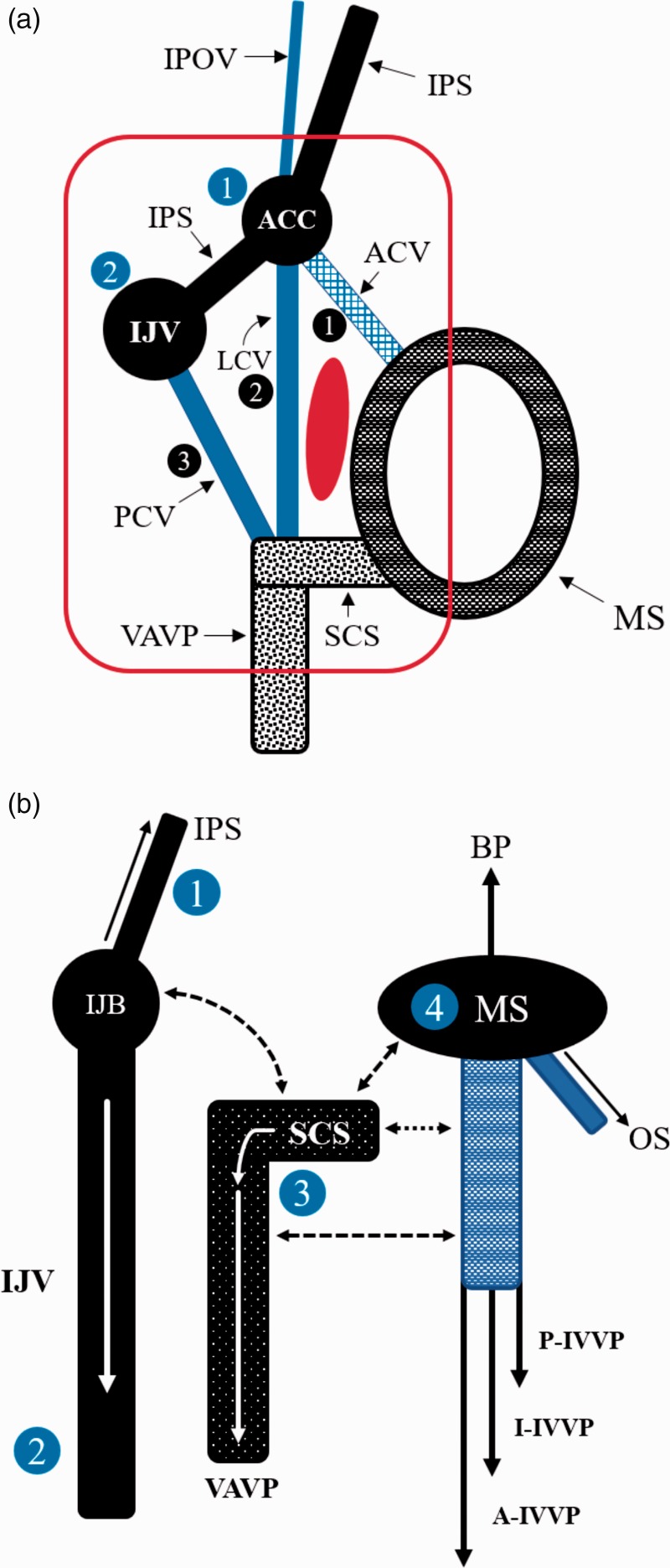
Venous confluences
In the lateral FM region, there are two venous confluences. These include the jugular bulb (JB) and anterior condylar confluence (ACC). The JB and adjacent veins connect the sigmoid and inferior petrosal sinus (IPS), posterior condylar vein (PCV), and occasional lateral condylar vein (LCV) and drain into the internal jugular vein (IJV).12–15 The ACC is located around the external orifice of the hypoglossal canal.14 It has multiple connections with the JB, IPS, inferior petro-occipital vein (IPOV), anterior condylar vein (ACV) and LCV.12,16,17
Communicating veins
In the lateral FM region, there are three communicating veins. These include the ACV, LCV, and PCV.12 The ACV communicates between the ACC and the marginal sinus (MS).3 Occasionally, the ACV communicates with the PCV through a bony channel.12 The LCV originates mainly in the ACC near the JB or occasionally from the jugular vein12 and drains into the vertebral artery venous plexus (VAVP).18 The PCV arises from the VAVP, runs into the posterior condylar canal (PCC) and connects the VAVP with the sigmoid sinus just proximal to the JB.12,19–22
Venous drainage
The lateral FM region has four main venous drainages, including the IJV, the VAVP and suboccipital cavernous sinus (SCS), the IPS and IPOV, and the MS.23 The VAVP surrounds the third segment of the VA, and its horizontal portion surrounding the VA is called the SCS.3 The VAVP and SCS drain the LCV and PCV.23 The IPS and IPOV connect the cavernous sinus to the JB and ACC and the ACC drains into the cavernous sinus.17,24 The MS receives the ACV of the lateral FM and drains into the anterior, lateral and posterior internal vertebral venous plexus (IVVP).25
Apart from the above vessels, in some cases, the brainstem or spinal vein connects the DAVF and acts as a draining vein.26 The above main venous structures are shown in Figure 2.
Figure 2.
Critical main veins in the lateral foramen magnum (FM). (a) A drawing showing the relationship among two venous confluences (blue: one and two) and three veins (black: one, two and three); (b) a drawing showed the four main drainages, which include the internal jugular vein (IJV), the vertebral artery venous plexus (VAVP) and suboccipital cavernous sinus (SCS), the inferior petrous sinus (IPS) and the inferior petro-occipital vein (IPOV), and marginal sinus (MS). The full line arrows indicate the direction of vein blood drainage, and the dotted line arrows indicate anastomosis between the venous structures.
A: anterior; ACC: anterior condylar confluence; ACV: anterior condylar vein; BP: basilar plexus; I: inferior; JB: jugular bulb; IVVP: internal vertebral venous plexus; LCV: lateral condylar vein; OS: occipital sinus; P: posterior; PCV: posterior condylar vein.
Brainstem veins (BVs)
The BVs are closely related to DAVFs of the lateral FM. BVs can be divided into longitudinal and transverse groups depending on their relationship with the brainstem. The longitudinal group comprises those that affect the midline axis of veins and a lateral group of veins.27,28 On the cerebral peduncles, the BVs drain into the basal vein of Rosenthal and the tentorial sinuses. Inferiorly, the BVs drain into the spinal veins and IVVP. Laterally, the BVs drain into the petrosal vein, which then joins the superior petrosal sinus.29,30
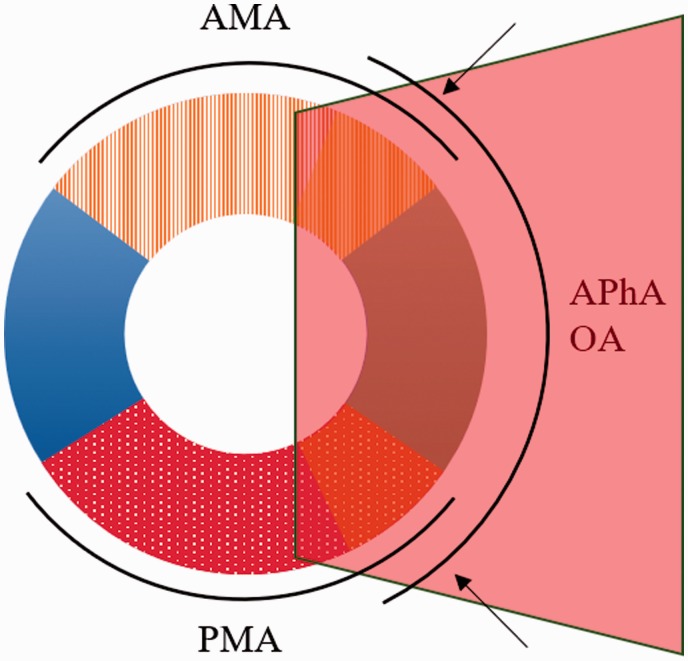
Feeding arteries
Four important arteries of the external carotid artery (ECA) and VA are involved in DAVFs of the lateral FM region. These include the hypoglossal and jugular branches of the ascending pharyngeal artery (APhA),31–34 the anterior meningeal artery (AMA) of the VA,32,35,36 the posterior meningeal artery (PMA)32,37 and the mastoid and hypoglossal branches of the occipital artery (OA).32,38 In these arteries, wide anastomoses often occur through the odontoid arch and via the cavernous internal carotid artery (ICA) through the meningeal branch.32 The above main feeding arteries are shown in Figure 3.
Figure 3.
Critical main arteries of the lateral foramen magnum (FM). The arteries of the lateral FM region are indicated by a pink transparent rectangle, which shows the region of the FM. The arrow indicates the anastomosis of adjacent arteries. AMA: anterior meningeal artery of the vertebral artery; APhA: ascending pharyngeal artery; OA: occipital artery; PMA: posterior meningeal artery.
Classification of DAVFs
Four types of DAVFs occur in the lateral FM region. These occur in a very limited area and include ACC and ACV DAVFs, PCC DAVFs, MS DAVFs, and JF DAVFs.
ACC and ACV DAVFs
The ACC is located at the extracranial aperture of the hypoglossal canal (HC) and has a rich venous network.1,39 ACV is an emissary venous plexus that is located in the HC, and ACV DAVFs are therefore also called HC DAVFs.40–43 HC DAVFs are rare and accounted for an incidence of only 3.6% of all DAVFs in a 2008 study by Manabe et al.,44 and only 4.2% in a 2013 study by Choi et al.41 ACC DAVFs are even rarer than HC DAVFs.44,45
PCC DAVFs
The PCC contains the PCV and meningeal branches of the OA. The PCV originates from the ACC and drains through the PCC into the SCS or VAVP.19,46 DAVFs located in the PCC are very rare.5,26,47,48
MS DAVFs
The MS surrounds the FM, and MS DAVFs are rare, comprising only 14 of 290 fistulas (4.8%) in a report published by McDougall et al.49
JF DAVFs
DAVFs located in the vicinity of the JF are rare, and JB DAVFs account for only 5.9% of all intracranial DAVFs.50
Angioarchitecture of the DAVF
In lateral FM DAVFs, fistulous points can be single or multiple, and their feeding arteries and drainage veins vary according to type of DAVFs.51
ACC and ACV DAVFs
ACC DAVFs and ACV DAVFs share a similar angioarchitecture, and they are therefore discussed together.51,52
Feeding artery
In most cases of ACC and ACV DAVFs, the APhA is the main feeding artery. In addition, the AMA, PMA, and OA can also feed ACC and ACV DAVFs to varying degrees.39 Rarely, the internal maxillary artery, the transosseous branch of the posterior auricular artery and the petrous branch of the middle meningeal artery of the ECA can be involved in ACC and ACV DAVFs.6,39,40,53
Draining vein
In ACC and ACV DAVFs, the drainages can be divided into the following three types: Type 1, including those with dominant anterograde venous drainage to the IJV and/or the VAVP via the LCV and PCV; Type 2, including those with dominant retrograde drainage to the cavernous sinus (CS) and/or orbital veins via the IPS and IPOV; and Type 3, including those with dominant or exclusive venous drainage into the intracranial pial or perimedullary veins via the ACV and MS.54
In addition, in ACV DAVFs, retrograde drainage via the bridging vein into perimedullary veins can occur.41,55 In these veins and sinuses, stenosis and obstruction are not uncommon.41 The ACC and ACV can be dilated, resulting in the formation of a venous pouch.39,41
PCC DAVFs
Feeding artery
In most cases of PCC DAVFs, the APhA is the main feeding artery. In addition, the AMA, PMA, and OA can also feed PCC DAVFs.5,26,47,48 Rarely, the dorsal meningeal artery of the ICA and, for example, the dural branches of the cervical VA can be involved in PCC DAVFs.5,26,47
Draining vein
In PCC DAVFs, the PCV acts as the main vein draining vein into the IJV or SCS. Rarely, PCC DAVFs can also directly drain into the MS and then via the MS upward into the occipital sinus (OS) or inferiorly into the IVVP system.26,47 PCC DAVFs may also rarely form a long, tortuous bridging vein into the brainstem venous system.26,48 This abnormal vein may represent an embryological developmental relic.56,57
MS DAVFs
Most MS DAVFs are located in the lateral MS. Hence, in this paper, MS DAVF means lateral MS DAVF.49
Feeding artery
The APhA and OA are the most common feeders involved in lateral MS DAVFs. Rarely, the transosseous branch of the posterior auricular artery and the meningeal branch of the ICA can be involved in a MS DAVF.58
Draining vein
The draining veins of lateral MS DAVFs can be divided into three grades, as follows. In Grade 1, the venous drainage is unrestricted and typically runs through the adjacent ipsilateral JB. Grade 2 MS DAVFs have restricted or obstructed venous drainage via the JB, and venous outflow is therefore retrogradely shunted through the IPS and into the CS. Grade 3 MS DAVFs have retrograde cortical venous drainage.49,58
JF DAVFs
Feeding artery
While the APhA is the most common feeder in JF DAVFs, the PMA, OA, and middle meningeal artery (MMA) can be involved in these DAVFs.50,59 Rarely, the internal maxillary artery is involved.60
Draining vein
JF DAVFs can directly drain into the IJV.59 Rarely, these veins drain into the right lateral medullary veins of the brainstem venous system and then reflux into the superior petrosal sinus.59
Clinical manifestations
The clinical presentations of lateral FM DAVFs are highly variable and reflect the pattern of venous drainage/reflux. Symptoms can include pulsatile tinnitus, intracranial hemorrhage, myelopathy, orbital symptoms, and cranial nerve palsy.47,54
General characteristics
The general clinical characteristics of FM DAVFs are similar to those of DAVFs that occur at the craniovertebral junction, as reviewed by Zhao et al. who evaluated data related to 56 patients with a mean age of 55.6 years old and a male to female ratio of 3:1.61
Pulsatile tinnitus
Since this type of DAVF is located very close to petrous bone, pulsatile tinnitus may be its main clinical presentation.50,58,62,63 For instance, in a 2015 study published by Spittau et al., pulsatile tinnitus was found in 92% and 75% of Type 1 and 2 HC DAVFs, respectively.54 In addition, in a report published by Liu et al. in 2008, 88% of ACC and HC DAVF patients presented with pulse-synchronous bruit or tinnitus.53
Intracranial hemorrhage
Intracranial hemorrhage is not rare in lateral FM DAVFs, and this type of hemorrhage is associated with brainstem venous system drainage.26,59,61 For instance, in PCC DAVFs with vein drainage that bridges to the brainstem venous system, a long and tortuous draining vein may often be present with multiple venous pouches and stenosis, making it prone to rupture.26,48 In a study by McDougall et al., three of 14 MS DAVF cases had subarachnoid and intracerebellar hemorrhage.49
Myelopathy
In lateral FM DAVFs, if venous reflux is entering inferiorly into the IVVP system, symptoms of spinal venous congestion, such as quadriparesis, are likely to occur.26,47,55,64 For instance, in a study by Spittau et al., 64.7% of Type 3 lateral FM DAVFs presented with myelopathy.54
Orbital symptoms
In lateral FM DAVFs, 38% of ACC and HC DAVFs may present with ocular symptoms, including chemosis, proptosis, secondary glaucoma, and diplopia.53,65 MS DAVFs can also result in ocular symptoms.49,58 This is because of retrograde venous flow into the IPS, CS, and superior ophthalmic vein.40,42,45,65,66 In a study by Spittau et al., 100% of Type 2 HC DAVF patients presented with orbital symptoms.54
Cranial nerve palsy
Cranial nerve palsy is rare and mainly presents as hypoglossal nerve palsy.6,39,53 For instance, in a study by Spittau et al., the rate of hypoglossal nerve palsy was 18.7% in Type 1 HC DAVFs.54 Occasionally, ACC DAVF patients may present with vagal nerve palsy.67 In these cases, there are two possible causes of cranial nerve palsy. The first is ischemic neuropathy of the vasa nervorum of the cranial nerve, and the second is neuropathy secondary to venous congestion.67
Other symptoms
Other symptoms that can be involved in lateral FM DAVFs include severe headache, otalgia, and seizures.41,67
Image examination
Diagnosing FM DAVFs remains a challenge due to the complex vascular anatomy of this region, which contains tiny and tortuous supplying vessels.54 Currently, head computed tomography (CT), CT angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) can be useful for achieving a diagnosis of FM DAVF.
Non-contrast CT
Preoperatively, CT can be used to identify acute intracranial hemorrhage in the medullary cistern or intraventricular hemorrhage.26,68 In addition, it can also detect the osseous destructive changes that can be caused by DAVFs.44 Postoperatively, CT can show whether there is intraoperative hemorrhage in cases treated with a glue cast or whether coils are appropriately located in a fistula point.5,26
CTA
CTA can help identify a fistula and determine its anatomy. Performing CTA in the venous phase can reveal increased vascularization around the lateral FM region.26,47,48 In addition, an oblique reconstruction of a CTA can show the bone structure and enhance the clinician’s understanding of the DAVF, which can be very helpful.41 Selective intra-arterial injection CTA is more useful for confirming the precise anatomy of the DAVF and developing a treatment strategy.69
MRI and MRA
MRI and MRA can reveal abnormal serpiginous flow-related enhancement vessels at the skull base.6,41 Sometimes, an MRA image of an HC DAVF can act as a “magic wand” by revealing the affected HC and the IJV.44 In addition, MRA source images can demonstrate the intraosseous extent of a DAVF, which can be helpful.41,70 Additionally, in patients with myelopathy, spinal cord swelling and extensive abnormally dilated veins can be observed.48,61 Moreover, MRI and MRA can be used to demonstrate the location of thrombosis in the draining vein or a large venous pouch on diffusion-weighted imaging and fluid-attenuated inversion imaging recovery.26
DSA
DSA remains the “gold standard” for evaluating DAVFs.61 Superselective arteriography and a venogram are very necessary, and selective dynamic 3D-DSA can be used to precisely identify the site of a fistula point and its drainage routes in addition to revealing venous reflux into the brainstem vein and IVVP systems.39,47,48
Treatment
Most lateral FM DAVFs need to be treated because of their aggressive symptoms, which can include orbital symptoms. Surgical resection is difficult because of their deep location, and endovascular treatments, including transvenous embolization (TVE), transarterial embolization (TAE) and combined embolization, are currently the best options.70–72
TVE
In lateral FM DAVFs, TVE is the best approach because it is easy to access the fistula point via the draining vein, especially because in most cases, the draining veins do not drain the normal cerebral parenchyma.3,26 When performing TVE, it is very important and necessary to obtain a comprehensive understanding of the imaging anatomy of the DAVF. Superselective arteriography and a venogram should be performed in such cases.6,65
During TVE, it is critical to accurately place the microcatheter into the fistula point. Because it is often difficult to navigate a microcatheter to the exact fistula site under 2D roadmap guidance, an intraoperative integrated CT of the DSA data may be very helpful.41,47 When necessary, a double-catheter technique can be used in TVE.54
During TVE, coils or Onyx and n-butyl-2-cyanoacrylate (NBCA) glues can be used. Coils are considered safer than Onyx and NBCA, and coil embolization should therefore be the first choice of treatment to avoid potentially compromising the anastomosis and spinal cord blood supply.6,39,41,43,45,47,49,70,72–74 Sometimes, combining Onyx and coils can be advantageous, especially in cases of high-flow DAVF, in which coils should be deployed to reduce the fistula flow, secure anchoring to the Onyx cast, and prevent the Onyx from inappropriately migrating.6,41,65
However, TVE also has disadvantages. For example, it can be difficult to access when sinus stenosis and tortuosity are present and is associated with a risk of wire perforation, bleeding, and venous flow redistribution to normal veins.43,70 In addition, it should be kept in mind that coil overpacking, Onyx or NBCA migrant embolization, or DMSO neurotoxicity can cause postoperative cranial nerve palsy. For instance, in a study of 24 cases of ACC DAVFs, three patients presented with hypoglossal nerve palsy after coil TVE.6
To avoid these complications, it is necessary to obtain a complete understanding of the local anatomy of the lateral FM region. Careful manipulations are also needed. Distal catheterization of the DAVF should be achieved to minimize reflux and prevent glue migrant embolization.6,65 To avoid postoperative cranial nerve palsy, the drainage veins in the lateral FM region should not be densely packed with coils.41,65
TAE
It is difficult to achieve a cure of lateral FM DAVFs using only TAE because using glue in TAE has a high risk of cranial nerve palsy, migration to the vertebrobasilar circulation can occur via dangerous anastomosis, and performing only parent artery embolization will not completely occlude the DAVF.59.70,73 For instance, in a 2017 study published by Maus et al. on PCC DAVF, a previous partial transarterial coil occlusion of feeding arteries was unsuccessful.47 In a 2013 study published by Choi et al., recurrence occurred in a patient who underwent transarterial particle embolization.41
However, it may be appropriate to treat some patients with lateral FM DAVFs with only TAE, such as those in which a DAVF has a single arterial feeder or an easily accessible draining vein.26,41,44,48–50,59,64 Since it is often difficult to identify the fistula point, selective angiography of the main feeding artery will provide more useful information. In addition, 3D-DSA is essential for determining the angioarchitecture of the DAVF and surrounding region.65
In TAE for lateral FM DAVFs, Onyx or NBCA can be used, and the microcatheter tip should be positioned at or immediately adjacent to the fistula point to avoid improper reflux; additionally, proper reflux may promote the continuous penetration of the glues into the fistula.48,73 However, if improper reflux results in the embolization of the vasa nervorum of the cranial nerve during the TAE, improper reflux is inevitable, and balloon protection may be useful.26
In JF DAVFs, TVE is sometimes not feasible because it is associated with a risk of major venous occlusion. In such cases, TAE can be attempted and may achieve success, but it will likely be difficult to perform TAE because of the large number of arterial feeders that traverse the occipital condyle and jugular process to enter the JB.50,59,64
Currently, TAE is more often used as an assistant method. For example, the superselective catheterization of arterial feeders can be used to control the inflow in concurrent TVE.44,67,74 In a 2008 report by Miyachi et al., of eight cases of ACC DAVFs, three were treated with TVE plus TAE.65 In 2007, Turner et al. described a case of MS DAVF in which the fistula was first partially embolized with coils via the TVE, and a transarterial Onyx injection was then used to completely occlude the fistula.58
The therapeutic options involving endovascular treatment of lateral FM DAVFs are shown according to type in Table 1.
Table 1.
Therapeutic options involving endovascular treatment of lateral foramen magnum (FM) dural arteriovenous fistulas (DAVFs) according to type.
| DAVF type | Venous drainage | Therapeutic option |
|---|---|---|
| ACC and ACV (HC) DAVFs | These DAVFs can be divided into three types according to drainage: Type 1 and Type 2 (85.8% of cases) have adjacent sinus drainage; and Type 3 (14.2% of cases) has only venous drainage into the intracranial pial or perimedullary veins.54 | For Type 1 and 2 DAVFs, TVE has demonstrated high safety and efficacy. Type 3 DAVFs must be treated with TAE.54 |
| PCC and MS DAVFs | In PCC and MS DAVFs, the main draining vein enters the adjacent sinus wall or the brainstem venous system.26,47,48,74 | When these DAVFs are adjacent to the sinus wall, TVE is feasible.5,58,72,77 Otherwise, TAE has to be performed.26,47,58,74 |
| JF DAVFs | The main draining vein enters the IJV, and rarely enters brainstem venous system.59 | In JF DAVFs, the IJV is very important and normally cannot be occluded. Hence, TVE is not applied, and TAE must be performed.50,59,64 |
ACC: anterior condylar confluence; ACV: anterior condylar vein; HC: hypoglossal canal; IJV: internal jugular vein; JF: jugular foramen; MS: marginal sinus: PCC: posterior condylar canal; TAE: transarterial embolization; TVE: transvenous embolization.
Other treatments
Conservative treatment
In some patients, conservative treatment may be effective. For instance, Manabe et al. published a report in 2008 in which four patients with HC DAVFs were treated conservatively, and the shunt disappeared during follow-up.44 However, the mechanisms that leads to spontaneous occlusion remains poorly understood.75 A conservative strategy could be appropriate in Type 1 HC DAVFs in which spontaneous regression (5.8%) is observed.54
Microsurgery
It is critical to surgically resect a lateral FM DAVF because of the difficult access and a significant risk of blood loss, nerve damage, and craniovertebral instability.49,61 However, microsurgery can be used in some selective cases of lateral FM DAVF. For instance, in a 2008 report published by Liu et al., a patient with ACC DAVF underwent preoperative TAE of the three main arterial feeders to decrease the blood supply, and the DAVF was then excised via a far lateral transcondylar approach.53
Since a transcondylar approach has been established and there are disadvantages to using TAE and TVE, an individually tailored transcondylar approach has been shown to be effective as a treatment for JF DAVFs.76 For instance, in 2007, Tirakotai et al. described four patients with JF DAVF who were treated after the main parent arteries were coiled. Complete obliteration of the DAVF was achieved in three of the patients, and significant flow reduction was achieved in one individual.60
Prognosis
After treatment, most lateral FM DAVFs are considered to be satisfactorily treated.44,72 For instance, in 2015, Spittau et al. showed that TVE demonstrated high safety and efficacy (2.9% morbidity, 92.7% total occlusion) in Type 1 and 2 HC DAVFs, but in Type 3 HC DAVFs or lesions with poor venous access, TAE or surgical disconnection were associated with high morbidity (13.3%-6.7%).54
Incomplete occlusion represents a major limitation.61 Sometimes, incomplete occlusion is necessary to avoid overpacking, which can result in cranial nerve palsy, and incomplete coil embolization can be associated with satisfactory outcomes. For instance, in 2013, Choi et al. described two of 10 cases in which near-complete occlusion was achieved with no recurrence during the follow-up period.41
In summary
The lateral FM region should be defined as the bilateral occipital region that runs laterally up to the JF and includes the inside and the outside of the occipital bone. There are critical vasculatures in this region. The DAVFs that occur in this region are rare and difficult to treat. The following four types of DAVFs can occur in the lateral FM region: ACC and ACV DAVFs, PCC DAVFs, MS DAVFs and JF DAVFs. These types of DAVF share similar angioarchitectures and clinical characteristics. Their clinical presentations included pulsatile tinnitus, intracranial hemorrhage, myelopathy, orbital symptoms, and cranial nerve palsy. Currently, DSA remains the “gold standard” for evaluating DAVFs. However, head CT, CTA, MRI, and MRA are also useful. Most lateral FM DAVFs need to be treated because of their aggressive symptoms, and there are several therapeutic options, of which TVE is currently viewed as the best. After appropriate treatment, most lateral FM DAVFs have a satisfactory prognosis.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.San Millan Ruiz D, Gailloud P, Rufenacht DA, et al. The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002; 23: 1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 2.Tanoue S, Kiyosue H, Sagara Y, et al. Venous structures at the craniocervical junction: Anatomical variations evaluated by multidetector row CT. Br J Radiol 2010; 83: 831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi S, Sakuma I, Omachi K, et al. Craniocervical junction venous anatomy around the suboccipital cavernous sinus: Evaluation by MR imaging. Eur Radiol 2005; 15: 1694–1700. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AJ, Scheer JK, Leibl KE, et al. Anatomy and biomechanics of the craniovertebral junction. Neurosurg Focus 2015; 38: E2. [DOI] [PubMed] [Google Scholar]
- 5.Kiyosue H, Okahara M, Sagara Y, et al. Dural arteriovenous fistula involving the posterior condylar canal. AJNR Am J Neuroradiol 2007; 28: 1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takemoto K, Tateshima S, Rastogi S, et al. Onyx embolization of anterior condylar confluence dural arteriovenous fistula. J Neurointerv Surg 2014; 6: e13. [DOI] [PubMed] [Google Scholar]
- 7.Morota N. Pediatric craniovertebral junction surgery. Neurol Med Chir (Tokyo) 2017; 57: 435–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel A, Jankharia B, Shah A, et al. Three-dimensional models: An emerging investigational revolution for craniovertebral junction surgery. J Neurosurg Spine 2016; 25: 740–744. [DOI] [PubMed] [Google Scholar]
- 9.Raybaud C. Anatomy and development of the craniovertebral junction. Neurol Sci 2011; 32: S267–S270. [DOI] [PubMed] [Google Scholar]
- 10.Kshettry VR, Thorp BD, Shriver MF, et al. Endoscopic approaches to the craniovertebral junction. Otolaryngol Clin North Am 2016; 49: 213–226. [DOI] [PubMed] [Google Scholar]
- 11.Offiah CE, Day E. The craniocervical junction: Embryology, anatomy, biomechanics and imaging in blunt trauma. Insights Imaging 2017; 8: 29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsushima K, Funaki T, Komune N, et al. Microsurgical anatomy of the lateral condylar vein and its clinical significance. Neurosurgery 2015; 11(Suppl 2): 135–145. discussion 145–146. [DOI] [PubMed] [Google Scholar]
- 13.Singla A, Gupta T, Sahni D, et al. High jugular bulb: Different osseous landmarks and their clinical implications. Surg Radiol Anat 2016; 38: 903–909. [DOI] [PubMed] [Google Scholar]
- 14.Katsuta T, Rhoton AL, Jr, Matsushima T. The jugular foramen: Microsurgical anatomy and operative approaches. Neurosurgery 1997; 41: 149–201. discussion 201–202. [DOI] [PubMed] [Google Scholar]
- 15.Rhoton AL, Jr, Buza R. Microsurgical anatomy of the jugular foramen. J Neurosurg 1975; 42: 541–550. [DOI] [PubMed] [Google Scholar]
- 16.Ozveren MF, Uchida K, Aiso S, et al. Meningovenous structures of the petroclival region: Clinical importance for surgery and intravascular surgery. Neurosurgery 2002; 50: 829–836. discussion 836–837. [DOI] [PubMed] [Google Scholar]
- 17.Tubbs RS, Watanabe K, Loukas M, et al. Anatomy of the inferior petro-occipital vein and its relation to the base of the skull: Application to surgical and endovascular procedures of the skull base. Clin Anat 2014; 27: 698–701. [DOI] [PubMed] [Google Scholar]
- 18.Wu A, Zabramski JM, Jittapiromsak P, et al. Quantitative analysis of variants of the far-lateral approach: Condylar fossa and transcondylar exposures. Neurosurgery 2010; 66(6 Suppl Operative): 191–198. discussion 198. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima K, Kawashima M, Matsushima T, et al. Posterior condylar canals and posterior condylar emissary veins–a microsurgical and CT anatomical study. Neurosurg Rev 2014; 37: 115–126. [DOI] [PubMed] [Google Scholar]
- 20.Rhoton AL., Jr The foramen magnum. Neurosurgery 2000; 47: S155–S193. [DOI] [PubMed] [Google Scholar]
- 21.Verma R, Kumar S, Rai AM, et al. The anatomical perspective of human occipital condyle in relation to the hypoglossal canal, condylar canal, and jugular foramen and its surgical significance. J Craniovertebr Junction Spine 2016; 7: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushima T, Natori Y, Katsuta T, et al. Microsurgical anatomy for lateral approaches to the foramen magnum with special reference to transcondylar fossa (supracondylar transjugular tubercle) approach. Skull Base Surg 1998; 8: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnano C, Belov P, Krawiecki J, et al. Internal jugular vein cross-sectional area enlargement is associated with aging in healthy individuals. PLoS One 2016; 11: e0149532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhoton AL., Jr The posterior fossa veins. Neurosurgery 2000; 47: S69–S92. [DOI] [PubMed] [Google Scholar]
- 25.Tubbs RS, Demerdash A, Loukas M, et al. Intracranial connections of the vertebral venous plexus: Anatomical study with application to neurosurgical and endovascular procedures at the craniocervical junction. Oper Neurosurg (Hagerstown) 2018; 14: 51–57. [DOI] [PubMed] [Google Scholar]
- 26.Mondel PK, Saraf R, Limaye US. Acute subarachnoid hemorrhage in posterior condylar canal dural arteriovenous fistula: Imaging features with endovascular management. J Neurointerv Surg 2015; 7: e26. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima K, Yagmurlu K, Kohno M, et al. Anatomy and approaches along the cerebellar-brainstem fissures. J Neurosurg 2016; 124: 248–263. [DOI] [PubMed] [Google Scholar]
- 28.Rhoton AL., Jr Microsurgical anatomy of the brainstem surface facing an acoustic neuroma. Surg Neurol 1986; 25: 326–339. [DOI] [PubMed] [Google Scholar]
- 29.Matsushima T, Kawashima M, Inoue K, et al. Anatomy of the superior petrosal veins and their exposure and management during petrous apex meningioma surgery using the lateral suboccipital retrosigmoid approach. Neurosurg Rev 2014; 37: 535–546. [DOI] [PubMed] [Google Scholar]
- 30.Matsushima K, Matsushima T, Kuga Y, et al. Classification of the superior petrosal veins and sinus based on drainage pattern. Neurosurgery 2014; 10(Suppl 2): 357–367. discussion: 367. [DOI] [PubMed] [Google Scholar]
- 31.Hacein-Bey L, Daniels DL, Ulmer JL, et al. The ascending pharyngeal artery: Branches, anastomoses, and clinical significance. AJNR Am J Neuroradiol 2002; 23: 1246–1256. [PMC free article] [PubMed] [Google Scholar]
- 32.Martins C, Yasuda A, Campero A, et al. Microsurgical anatomy of the dural arteries. Neurosurgery 2005; 56(Suppl 2): 211–251. discussion: 211–251. [DOI] [PubMed] [Google Scholar]
- 33.Quisling RG, Seeger JF. Ascending pharyngeal artery collateral circulation simulating internal carotid artery hypoplasia. Neuroradiology 1979; 18: 277–280. [DOI] [PubMed] [Google Scholar]
- 34.Uchino A. Collateral circulation via the ascending pharyngeal artery arising from the internal carotid artery. AJNR Am J Neuroradiol 2006; 27: 246. [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu S, Garcia AS, Tanriover N, et al. The so-called anterior meningeal artery: An anatomic study for treatment modalities. Interv Neuroradiol 2004; 10: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasjaunias P, Moret J, Theron J. The so-called anterior meningeal artery of the cervical vertebral artery. Normal radioanatomy and anastomoses. Neuroradiology 1978; 17: 51–55. [DOI] [PubMed] [Google Scholar]
- 37.Tanohata K, Maehara T, Noda M, et al. Anomalous origin of the posterior meningeal artery from the lateral medullary segment of the posterior inferior cerebellar artery. Neuroradiology 1987; 29: 89–92. [DOI] [PubMed] [Google Scholar]
- 38.Sloniewski P, Dzierzanowski J, Och W. Aneurysm of the meningeal branch of the occipital artery connecting with the distal portion of the posteroinferior cerebellar artery by the dural fistula. Folia Morphol (Warsz) 2008; 67: 292–295. [PubMed] [Google Scholar]
- 39.Okamura A, Nakaoka M, Ohbayashi N, et al. Intraoperative cone-beam computed tomography contributes to avoiding hypoglossal nerve palsy during transvenous embolization for dural arteriovenous fistula of the anterior condylar confluence. Interv Neuroradiol 2016; 22: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiyosue H, Tanoue S, Okahara M, et al. Ocular symptoms associated with a dural arteriovenous fistula involving the hypoglossal canal: Selective transvenous coil embolization. Case report. J Neurosurg 2001; 94: 630–632. [DOI] [PubMed] [Google Scholar]
- 41.Choi JW, Kim BM, Kim DJ, et al. Hypoglossal canal dural arteriovenous fistula: Incidence and the relationship between symptoms and drainage pattern. J Neurosurg 2013; 119: 955–960. [DOI] [PubMed] [Google Scholar]
- 42.Lee CY, Koh JS, Kwon GY, et al. Transvenous embolization of an intraosseous dural arteriovenous fistula of the anterior condylar vein with anomalous venous drainage causing ocular manifestations. J Clin Neurosci 2011; 18: 413–415. [DOI] [PubMed] [Google Scholar]
- 43.Arai Y, Ishii H, Handa Y, et al. Dural arteriovenous fistula within the hypoglossal canal successfully treated by transvenous embolization. Case report. Interv Neuroradiol 2004; 10: 59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manabe S, Satoh K, Matsubara S, et al. Characteristics, diagnosis and treatment of hypoglossal canal dural arteriovenous fistula: Report of nine cases. Neuroradiology 2008; 50: 715–721. [DOI] [PubMed] [Google Scholar]
- 45.Hatabe N, Chikama T, Sonod KH, et al. [A case of anterior condylar confluence dural arteriovenous fistula with initial onset of ocular symptoms]. Nippon Ganka Gakkai Zasshi 2011; 115: 905–909. [PubMed] [Google Scholar]
- 46.Weissman JL. Condylar canal vein: Unfamiliar normal structure as seen at CT and MR imaging. Radiology 1994; 190: 81–84. [DOI] [PubMed] [Google Scholar]
- 47.Maus V, Soderman M, Rodesch G, et al. Endovascular treatment of posterior condylar canal dural arteriovenous fistula. J Neurointerv Surg 2017; 9: e7. [DOI] [PubMed] [Google Scholar]
- 48.Shambanduram SS, Devarajan Sebastian LJ, Jain N, et al. Management of a rare case of posterior condylar canal dural arteriovenous fistula presenting with subarachnoid haemorrhage: A case report and review of literature. Interv Neuroradiol 2018; 24: 206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McDougall CG, Halbach VV, Dowd CF, et al. Dural arteriovenous fistulas of the marginal sinus. AJNR Am J Neuroradiol 1997; 18: 1565–1572. [PMC free article] [PubMed] [Google Scholar]
- 50.Liang G, Li Z, Gao X, et al. Endovascular treatment for dural arteriovenous fistulas at the jugular foramen. Neurol India 2011; 59: 420–423. [DOI] [PubMed] [Google Scholar]
- 51.Cyril C, Ofelia M, Herve D. Dural arteriovenous fistula involving the anterior condylar canal. J Neuroimaging 2013; 23: 425–428. [DOI] [PubMed] [Google Scholar]
- 52.Abiko M, Ikawa F, Ohbayashi N, et al. Endovascular treatment for dural arteriovenous fistula of the anterior condylar confluence involving the anterior condylar vein. A report of two cases. Interv Neuroradiol 2008; 14: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu JK, Mahaney K, Barnwell SL, et al. Dural arteriovenous fistula of the anterior condylar confluence and hypoglossal canal mimicking a jugular foramen tumor. J Neurosurg 2008; 109: 335–340. [DOI] [PubMed] [Google Scholar]
- 54.Spittau B, Millan DS, El-Sherifi S, et al. Dural arteriovenous fistulas of the hypoglossal canal: Systematic review on imaging anatomy, clinical findings, and endovascular management. J Neurosurg 2015; 122: 883–903. [DOI] [PubMed] [Google Scholar]
- 55.Tanoue S, Goto K, Oota S. Endovascular treatment for dural arteriovenous fistula of the anterior condylar vein with unusual venous drainage: Report of two cases. AJNR Am J Neuroradiol 2005; 26: 1955–1959. [PMC free article] [PubMed] [Google Scholar]
- 56.Mitsuhashi Y, Aurboonyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: Possible homologs of spinal dural arteriovenous fistulas. Clinical article. J Neurosurg 2009; 111: 889–899. [DOI] [PubMed] [Google Scholar]
- 57.Kiyosue H, Tanoue S, Sagara Y, et al. The anterior medullary-anterior pontomesencephalic venous system and its bridging veins communicating to the dural sinuses: Normal anatomy and drainage routes from dural arteriovenous fistulas. Neuroradiology 2008; 50: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 58.Turner RD, Gonugunta V, Kelly ME, et al. Marginal sinus arteriovenous fistulas mimicking carotid cavernous fistulas: Diagnostic and therapeutic considerations. AJNR Am J Neuroradiol 2007; 28: 1915–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Byun JS, Hwang SN, Park SW, et al. Dural arteriovenous fistula of jugular foramen with subarachnoid hemorrhage: Selective transarterial embolization. J Korean Neurosurg Soc 2009; 45: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tirakotai W, Benes L, Kappus C, et al. Surgical management of dural arteriovenous fistulas with transosseous arterial feeders involving the jugular bulb. Neurosurg Rev 2007; 30: 40–48. discussion: 48–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Xu F, Ren J, et al. Dural arteriovenous fistulas at the craniocervical junction: A systematic review. J Neurointerv Surg 2016; 8: 648–653. [DOI] [PubMed] [Google Scholar]
- 62.Yu J, Lv X, Li Y, et al. Therapeutic progress in pediatric intracranial dural arteriovenous shunts: A review. Interv Neuroradiol 2016; 22: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo Y, Yu J, Zhao Y, et al. Progress in research on intracranial multiple dural arteriovenous fistulas. Biomed Rep 2018; 8: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin H, Lv X, Li Y. Transarterial Onyx embolization of jugular foramen dural arteriovenous fistula with spinal venous drainage manifesting as myelopathy–a case report and review of the literature. Interv Neuroradiol 2016; 22: 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyachi S, Ohshima T, Izumi T, et al. Dural arteriovenous fistula at the anterior condylar confluence. Interv Neuroradiol 2008; 14: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miropolsky V, da Costa LB, Marotta TR, et al. Endovascular therapy of hypoglossal canal AVFs presenting with orbital symptoms. Can J Neurol Sci 2009; 36: 745–750. [DOI] [PubMed] [Google Scholar]
- 67.Chia GS, Lim WE. Anterior condylar confluence dural arteriovenous fistula: A rare cause of hoarseness. BMJ Case Rep 2017; pii: bcr2016218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batista UC, Joaquim AF, Fernandes YB, et al. Computed tomography evaluation of the normal craniocervical junction craniometry in 100 asymptomatic patients. Neurosurg Focus 2015; 38: E5. [DOI] [PubMed] [Google Scholar]
- 69.Kanemaru K, Yoshioka H, Yagi T, et al. Hypoglossal canal dural arteriovenous fistula embolized under precise anatomical evaluation by selective intra-arterial injection computed tomography angiography. Interv Neuroradiol 2015; 21: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ernst R, Bulas R, Tomsick T, et al. Three cases of dural arteriovenous fistula of the anterior condylar vein within the hypoglossal canal. AJNR Am J Neuroradiol 1999; 20: 2016–2020. [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai LK, Liu HM, Jeng JS. Diagnosis and management of intracranial dural arteriovenous fistulas. Expert Rev Neurother 2016; 16: 307–318. [DOI] [PubMed] [Google Scholar]
- 72.Choi HS, Kim DI, Kim BM, et al. Endovascular treatment of dural arteriovenous fistula involving marginal sinus with emphasis on the routes of transvenous embolization. Neuroradiology 2012; 54: 163–169. [DOI] [PubMed] [Google Scholar]
- 73.Pei W, Huai-Zhang S, Shan-Cai X, et al. Isolated hypoglossal nerve palsy due to endovascular treatment of a dural arteriovenous fistula with Onyx-18. Interv Neuroradiol 2010; 16: 286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tekle WG, Grigoryan M, Tummala RP. Marginal sinus fistula supplied exclusively by vertebral artery feeders. J Vasc Interv Neurol 2013; 6: 30–33. [PMC free article] [PubMed] [Google Scholar]
- 75.Clarencon F, Biondi A, Sourour NA, et al. Spontaneous closure of intracranial dural arteriovenous fistulas: A report of 3 cases. Clin Neurol Neurosurg 2013; 115: 971–975. [DOI] [PubMed] [Google Scholar]
- 76.Sweiss F, Jean WC. Transcondylar approach for resection of lateral medullary cavernous malformation. Acta Neurochir (Wien) 2018; 160: 291–294. [DOI] [PubMed] [Google Scholar]
- 77.Shambanduram SS, Devarajan Sebastian LJ, Jain N, et al. Management of a rare case of posterior condylar canal dural arteriovenous fistula presenting with subarachnoid haemorrhage: A case report and review of literature. Interv Neuroradiol 2018; 24: 206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]