Abstract
Background and purpose
Embolization of the middle meningeal artery (MMA) has recently been proposed as an alternative to surgery for treatment of chronic subdural hematoma (SDH), and several case reports have been published supporting its efficacy. It has been suggested that the primary pathologic process in chronic SDH is repeated microhemorrhaging into the subdural collection from fragile neovasculature within the SDH membrane that arises from distal branches of the MMA. Embolization could thus provide a means of eliminating this chronic rebleeding.
Materials and methods
Images were selected from MMA embolization procedures performed at our institution in order to illustrate the technique and theory behind its efficacy for treatment of chronic SDH.
Results
Images from MMA angiograms demonstrate the variability of MMA anatomy and help illustrate the importance of avoiding potential ophthalmic collaterals and branches supplying cranial nerves. The findings of irregular wispiness of the distal MMA vasculature, contrast outlining of the SDH membrane on angiography, and homogenous increased density within the SDH on postembolization head computed tomography are described.
Conclusion
MMA embolization may provide a safe alternative for treatment of chronic SDH, but careful angiographic assessment of MMA anatomy should be performed to avoid potential complications. The findings illustrated here lend support to the theory that the pathologic process of chronic SDH is repeated leakage of blood products from an inflamed, abnormal arterial neovasculature within the SDH membrane that arises from the MMA, and thus selective embolization could provide an effective treatment.
Keywords: Angiography, embolization, middle meningeal artery, minimally invasive, subdural hematoma
Introduction
Chronic subdural hematoma (SDH) is a relatively common pathology in neurosurgery. Treatment, which consists of surgical evacuation with burr holes or craniotomy, can be difficult because of high recurrence rates (11%–33%) and the significant medical comorbidities that tend to afflict this patient population.1–4 Recently, embolization of the middle meningeal artery (MMA) has been described as an adjunct to surgical evacuation or as an alternative treatment for recurrent or primary chronic SDH.5–8
Early in the development of a chronic SDH, inflammatory cells and fibroblasts migrate from the dura to form the membrane that encapsulates the subdural collection.9 Within this membrane, neovasculature forms that contains poorly developed endothelial cell junctions.9,10 It is hypothesized that this fragile neovasculature is largely responsible for the chronic, repeated rebleeding that causes growth or recurrence of chronic SDH.10–12 Chronic exudate and hemorrhage from fragile neovasculature is a well-described pathologic mechanism and has been studied in the context of other conditions as well, most notably exudative macular degeneration. Histologic analysis has confirmed these findings, and has suggested that there is connection to distal branches of the MMA.5,11 Embolization of the MMA would thus provide a means of eliminating the blood supply to the neovasculature of the subdural membrane and thus prevent further rebleeding.
Here, we present various findings from cases of MMA embolization for chronic SDH that support this theory. We first describe our technique in detail and provide pearls and pitfalls to look out for in order to avoid complications. We then describe radiographic images that lend support to the idea that the primary pathologic process responsible for the formation and persistence of chronic SDH is the neovasculature of the SDH membrane and that this can be addressed with embolization of the MMA.
Methods
Embolization procedure
MMA embolization for chronic SDH is performed at our institution in the neurointerventional suite in an inpatient setting. If the patient is cooperative, the procedure is performed under light or no sedation and local anesthesia. If patient motion precludes this during superselective catheterization, general anesthesia is used. The patient is prepped and draped in the usual sterile fashion. Common femoral artery access is obtained using a 5 French micropuncture kit (Terumo, Tokyo, Japan). Usually, a 5 French diagnostic catheter (Merit Medical, Piscataway, NJ) is advanced over a standard angled glidewire into the common carotid artery (CCA) of the affected side. Angiography of the CCA and external carotid artery is performed. The diagnostic catheter is then connected to heparinized saline flush and used as a guide catheter for microcatheter access. In select cases of difficult vascular anatomy, larger or stiffer guide catheters and/or exchange techniques are required.
Using roadmap guidance, a microcatheter (typically an Echelon 10; Medtronic, Minneapolis, MN) is then advanced over a 0.014-inch standard Synchro microwire (Stryker, Kalamazoo, MI) selectively into the MMA. Angiography of the MMA is then performed to assess the anatomy and various branches in relation to the distribution of the SDH. Depending on the anatomy of the MMA, particular branches are selectively catheterized for embolization, or embolization is performed proximally before branch points depending on the size and location of the subdural hematoma. Embolization is performed by slowly infusing polyvinyl alcohol particles (150–250 microns in diameter) suspended in 70% Omnipaque contrast and 30% heparinized saline in a pulsatile fashion using negative fluoroscopic roadmap until there is stasis of anterograde flow and/or reflux around the catheter tip. MMA angiography is repeated to demonstrate elimination of flow into the distal branches of the MMA and the microcatheter is removed. CCA angiography is repeated to ensure patency of all intracranial vessels and the guide catheter is then removed.
Hemostasis is achieved using a closure device and manual compression, and the patient is brought to the recovery room. Patients are observed in a neurological step-down unit overnight and repeat head computed tomography (CT) is obtained the following day. The patient is then discharged to home or a rehabilitation facility, as appropriate. Follow-up head CT is obtained two and six weeks post-procedure.
Interpretation of MMA anatomy
The anatomy of the MMA can be variable but it is important to interpret the angiogram with attention toward potential anastomoses in order to avoid dangerous complications. The most common configuration is a common trunk that enters the skull base through the foramen spinosum and then divides into frontoparietal and squamosal/temporal branches (Figure 1(a)).13 In these cases, embolization is performed with the microcatheter tip proximal to the bifurcation of these branches in order to supply the largest portion of dura (Figure 1(b)).
Figure 1.
(a) Common configuration of the middle meningeal artery with a common trunk that divides into frontoparietal (1) and squamosal/temporal (2) branches. (b) With this configuration, embolization can be performed with the microcatheter tip proximal to the bifurcation of these branches to achieve embolization of the maximal amount of dura.
Avoidance of the petrosal branch
In some cases, a prominent petrosal branch is identified (Figure 2(a)). It is important to navigate the microcatheter past the take-off of this branch and avoid reflux into it, as this branch can supply the vasa nervorum of cranial nerve VII within the petrous bone.13 At our institution, we always take care to ensure that the microcatheter tip is well past the petrous temporal bone on the unsubtracted lateral x-ray, even if a petrosal branch is not visible on angiography (Figure 2(b)).
Figure 2.
(a) A prominent petrosal branch (asterisk) is identified coursing posteriorly within the petrous bone. The microcatheter tip (circled) is visible on both the subtracted (a) and unsubtracted (b) views past the take-off of this branch in order to avoid potential harm to cranial nerve VII.
Avoidance of ophthalmic collaterals
An important note in this technique, as in all procedures within the MMA, is the careful evaluation for MMA branches that collateralize with the ophthalmic artery. In some cases, an orbital/meningolacrimal branch is present that can potentially form dangerous anastomoses with the ophthalmic artery.13 The microcatheter must be navigated past this branch distally into the frontoparietal branch and great care must be taken to avoid reflux into it (Figure 3). If the vessel is too diminutive to navigate distally, the procedure is aborted.
Figure 3.
(a) An orbital branch is noted coursing anteriorly (asterisk). The microcatheter tip (circled) was navigated distally past this branch and great care was taken to avoid reflux of particles. (b) Postembolization angiogram shows reduced flow into the frontoparietal branch, but persistent supply of the dura over the posterior convexity. (c) The squamosal/temporal branch was selectively catheterized and again great care was taken to avoid reflux of particles. (d) Postembolization external carotid artery angiogram shows reduced flow into both frontoparietal and squamosal/temporal branches, but preserved, unchanged flow into the orbital branch.
Results
Abnormal angiographic findings
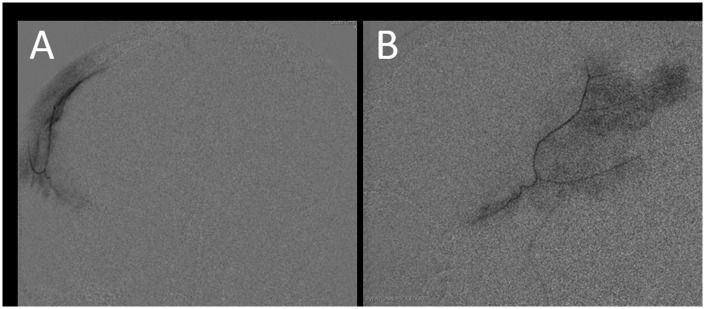
In a 2013 study in which five patients underwent MMA embolization for recurrent chronic SDH, Hashimoto et al.6 described a “cotton wool-like staining” appearance of the distal vasculature of the MMA on angiogram. This finding of abnormal distal vasculature is often seen on MMA angiogram in these cases, and examples from our cases are described in Figure 4. The “wispiness” of the distal MMA branches may represent the neovasculature within the subdural membrane around the hematoma.
Figure 4.
Lateral middle meningeal artery (MMA) angiograms ((a) and (b)) demonstrate a “cotton wool-like” appearance of the distal vasculature. (c) A zoomed-in example of the abnormal distal vasculature in another lateral MMA angiogram. Lateral projections of frontoparietal (d) and squamous temporal (e) MMA injections in another patient undergoing embolization for subdural hematoma. A “cotton wool-like” appearance is again demonstrated. Postembolization external carotid artery angiogram (f) shows a truncated MMA (circled) with minimal residual anterograde flow.
This abnormal distal MMA vasculature is further appreciated on continuous injection into the MMA on a blank fluoroscopic roadmap. Contrast pooling around the distal vasculature is observed, suggesting leakiness and immaturity of these neovessels that penetrate the subdural membrane (Figure 5).
Figure 5.
Posteroranterior (PA) (a) and lateral (b) projections of a prolonged middle meningeal artery (MMA) microcatheter injection under blank fluoroscopic roadmap. Contrast pooling is observed within the distal MMA vasculature.
Opacification of the SDH membrane
Another important observation in our cases is the appearance of posteroranterior projections during blank roadmap fluoroscopy. With the microcatheter situated within the MMA, slow, continuous contrast injection creates the appearance of contrast outlining the SDH (Figure 6). This appearance is consistent with the membrane that encapsulates the subdural collection, which can be appreciated by comparing it to coronal projections of the CT. This observation lends support to the theory that the vasculature within the SDH membrane is supplied by the MMA and there is a direct connection between the arterial feeders in what was traditionally thought to be a strictly “venous” pathology.
Figure 6.
Posteroranterior projections during contrast injection of the left middle meningeal artery (MMA), unsubtracted (a) and blank roadmap (b) views. Contrast appears to outline the subdural collection as the subdural hematoma membrane does, suggesting a vascular connection between this membrane and the MMA. A slice from the coronal computed tomography performed one day prior to this procedure is provided (c) to demonstrate the distribution of blood that is identical to the contrast outline in (b).
Contrast pooling within the SDH on postembolization CT
A final observation is the finding of a subtle but noticeable homogenous increase in density on the postembolization CT scan (Figure 7). Often, comparison of the pre- and post-procedural non-contrast head CT scans shows this distinct difference in density. The homogeneity of this change in density distinguishes contrast pooling from acute blood products, which tend to be heterogeneous and in a more loculated and sporadic pattern.
Figure 7.
(a) Pre- and (b) postembolization non-contrast head computed tomography scans on a patient who underwent right middle meningeal artery (MMA) embolization for a right-sided chronic subdural hematoma (SDH). There is a subtle but noticeable homogeneous increase in density within the subdural collection, suggesting the pooling of contrast from the procedure. This finding lends support to the theory of an arterial connection between the MMA and the membrane surrounding the SDH.
Discussion
In this paper, we describe our technique for treating chronic SDH with MMA embolization, including several technical pearls involving MMA anatomy in order to avoid complications, and provide radiographic findings that lend support to the theory that the development of chronic SDH is an arterial pathology that can be treated with embolization. Endovascular treatment for chronic SDH is particularly attractive as an alternative to surgical evacuation given the high recurrence rates (reported between 11% and 33% in the literature) and the medical comorbidities that tend to afflict the chronic SDH patient population.1–4 MMA embolization can often be performed under light or no sedation as opposed to general anesthesia, which is often required for surgical procedures. Furthermore, antiplatelet therapy or anticoagulation can be resumed sooner or not discontinued at all in select cases.
While there has not been a large-scale, controlled clinical trial as of yet, there have been several case series published supporting the efficacy of MMA embolization for chronic SDH. Hashimoto et al. in 2013 described five cases performed for multi-recurrent chronic SDH after several surgical evacuations and reported that all five had resolution of SDH without recurrence.6 Tempaku et al. in 2015 reported 21 total cases from seven series, including five of their own cases, and reported no recurrences.7 A recent retrospective case-control study from 2017 reported 20 cases compared to 23 controls (surgical evacuation) of recurrent chronic SDH after prior surgical evacuation.8 They found statistical significance in fewer recurrences and earlier brain re-expansion in the embolization group.8 Our group also recently reported the first series of patients treated up-front for symptomatic chronic SDH as an alternative to initial surgery that had failed medical management.5 Five patients underwent MMA embolization and in all five there was reduction in size of the SDH, resolution of symptoms, no recurrences, and no complications.5 All five patients were able to avoid subsequent surgery.5 Naturally, these studies are subject to the well-known forms of bias associated with retrospective case series, but they point collectively toward a treatment modality deserving more rigorous investigation.
This technique is supported by increasing evidence that growth and persistence of chronic SDH is an arterial pathologic process, as opposed to the venous process associated with formation of an acute SDH. The accepted theory of SDH formation is that in acute SDH, tearing of a bridging vein results in accumulation of blood within the subdural space.9 Based on the observations summarized here, we hypothesize that an inflammatory and angiogenic process then occurs resulting in the formation of a vascularized, complex membrane encapsulating the hematoma.9 The vascular supply to this membrane appears to arise from distal MMA branches within the overlying dura, and consists of immature neovessels with poorly developed endothelial junctions.9,10 It is the repeated seeping of blood products from these fragile neovessels into the subdural collection that is hypothesized to be responsible for growth and persistence of a chronic SDH.10–12 Histologic sections of this membrane from our own studies and others have demonstrated giant capillaries, inflammatory cells, and numerous small neovessels, which serves as supporting evidence for this hypothesis.5,11 In a sense, the chronic SDH behaves like benign tumors of the meninges, which frequently recruit blood supply from dural branches of the MMA, and have long been known to respond to devascularization by endovascular embolization.
The radiographic findings presented in this study provide further supporting evidence to this theory. The “cotton wool-like staining” appearance of the distal MMA branches on angiogram described by Hashimoto et al.6 and seen in Figure 4 likely represents the immature neovasculature of the subdural membrane. The observation that this vasculature arises from the MMA is further supported by Figure 6, which shows contrast outlining the entire hematoma. Since an SDH is by definition beneath the dura, the only plausible explanation for contrast lining the inner portion of the hematoma is that the membrane separating the hematoma from the brain parenchyma contains arterial vessels arising from the MMA. The contrast pooling in Figure 5 lends support to the idea that this neovasculature is leaky and potentially responsible for the recurrent bleeding and growth of chronic SDHs, and this is further supported by the homogeneous presence of contrast on the postembolization CT scan shown in Figure 7. Embolizing the MMA by occluding the distal branches with small particles could thus potentially eliminate this repeated, chronic leaking of blood products into the subdural collection and enable the brain to resorb the collection over time.
While the risk profile of endovascular procedures is generally low, care must be taken in order to avoid complications. In this study, we provided examples of variations of MMA anatomy to illustrate potential pitfalls. Careful evaluation of the MMA angiogram should be performed to identify dangerous ophthalmic anastomoses from the orbital/meningolacrimal branch or prominent petrosal branches that could lead to injury to cranial nerve VII. Selective catheterization of the frontoparietal or squamous temporal branches is encouraged in order to avoid injection of particles into these branches, as illustrated in Figures 2 and 3. Finally, care should be taken using blank fluoroscopic roadmapping while injecting particles to avoid reflux of particles into these branches or proximally into the internal carotid artery.
While these findings are compelling, care should be taken interpreting them. The conclusions drawn in this study are based on findings described in the literature and our own case-series evidence, but remain limited as the efficacy of this technique for treating SDH has not been evaluated in a large-scale study. A randomized, controlled clinical trial is necessary and warranted in order to demonstrate the continued efficacy and safety of this technique. Furthermore, it is important to note that this technique is reserved for patients with an acceptable amount of mass effect on the brain who can tolerate the slow, gradual nature with which the collection gets resorbed over time. Significant mass effect causing herniation or major neurologic deficit will always warrant urgent surgical evacuation.
Conclusions
Embolization of the MMA has been proposed as a potentially safe and minimally invasive treatment alternative to surgery for up-front or recurrent chronic SDH. In order to maximize efficacy and avoid complications, it is important to carefully evaluate the anatomy of the MMA on angiogram in order to provide complete embolization to all areas of the SDH, and to avoid dangerous anastomoses or cranial nerve damage via the orbital/ophthalmic and petrosal branches. The findings presented here of abnormal wispy vasculature of distal MMA branches, contrast outlining of the SDH membrane, and contrast pooling on angiography, as well as homogeneous increased density within the SDH on postembolization head CT, all lend support to the theory that the pathologic process of chronic SDH is repeated leakage of blood products from an inflamed arterial neovasculature within the SDH membrane that arises from distal branches of the MMA, and thus occlusion of these fragile vessels with embolization could provide an effective means of treatment. A randomized, controlled clinical trial comparing this method to the surgical standard of care is warranted prior to routine clinical use.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Liu W, Bakker N, Groen R. Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures. J Neurosurg 2014; 121: 665–673. [DOI] [PubMed] [Google Scholar]
- 2.Almenawer SA, Farrokhyar F, Hong C, et al. Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg 2014; 259: 449–457. [DOI] [PubMed] [Google Scholar]
- 3.Chon KH, Lee JM, Koh EJ, et al. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154: 1541–1548. [DOI] [PubMed] [Google Scholar]
- 4.Mehta V, Harward SC, Sankey EW, et al. Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci 2018; 50: 7–15. [DOI] [PubMed] [Google Scholar]
- 5.Link TW, Boddu S, Marcus J, et al. Middle meningeal artery embolization as treatment for chronic subdural hematoma: A case series. Oper Neurosurg (Hagerstown). Epub ahead of print 25 July 2017. DOI: 10.1093/ons/opx154. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto T, Ohashi T, Watanabe D, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int 2013; 4: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tempaku A, Yamauchi S, Ikeda H, et al. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: Five cases and a review of the literature. Interv Neuroradiol 2015; 21: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg 2017; 101: 520–527. [DOI] [PubMed] [Google Scholar]
- 9.Jafari N, Gesner L, Koziol JM, et al. The pathogenesis of chronic subdural hematomas: A study on the formation of chronic subdural hematomas and analysis of computed tomography findings. World Neurosurg 2017; 107: 376–381. [DOI] [PubMed] [Google Scholar]
- 10.Weigel R, Hohenstein A, Schilling L. Vascular endothelial growth factor concentration in chronic subdural hematoma fluid is related to computed tomography appearance and exudation rate. J Neurotrauma 2014; 31: 670–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T, Kaimori M. Histological study of vascular structure between the dura mater and the outer membrane in chronic subdural hematoma in an adult [article in Japanese]. No Shinkei Geka 1999; 27: 431–436. [PubMed] [Google Scholar]
- 12.Shono T, Inamura T, Morioka T, et al. Vascular endothelial growth factor in chronic subdural haematomas. J Clin Neurosci 2001; 8: 411–415. [DOI] [PubMed] [Google Scholar]
- 13.Morris P. Practical neuroangiography, 3rd ed Philadelphia: Lippincott Williams & Wilkins, 2007. [Google Scholar]