Abstract
Clinical islet transplantation effectively restores euglycemia and corrects glycosylated hemoglobin in labile type 1 diabetes mellitus (T1DM). Despite marked improvements in islet transplantation outcomes, acute islet cell death remains a substantial obstacle that compromises long-term engraftment outcomes. Multiple organ donors are routinely required to achieve insulin independence. Therapeutic agents that ameliorate cell death and/or control injury-related inflammatory cascades offer potential to improve islet transplant success. Apoptotic cell death has been identified as a major contributor to cellular demise and therapeutic strategies that subvert initiation and consequences of apoptotic cell death have shown promise in pre-clinical models. Indeed, in numerous pathologies and diseases apoptosis has been the most extensively described form of regulated cell death. However, recent identification of novel, alternative regulated cell death pathways in other disease states and solid organ transplantation suggest that these additional pathways may also have substantial relevance in islet transplantation. These regulated, non-apoptotic cell death pathways exhibit distinct biochemical characteristics but have yet to be fully characterized within islet transplantation. We review herein the various regulated cell death pathways and highlight their relative potential contributions to islet viability, engraftment failure and islet dysfunction.
Keywords: Islets, regulated cell death, apoptosis, ferroptosis, necroptosis, danger-associated molecular patterns
Introduction
Inroads in clinical islet transplantation have demonstrated repeatedly that this therapy protects against hypoglycemia, corrects hemoglobin A1C, improves overall glycemic control, and to a more variable degree can secure and maintain insulin independence for periods of time. Studies by the Clinical Islet Transplant consortium and a comprehensive Collaborative Islet Transplant Registry (CITR) strongly endorse these findings1,2. Several studies suggest that both pancreas and islet transplantation may impede evolution of several long-term secondary complications associated with type 1 diabetes mellitus (T1DM). However, despite marked improvements in outcome over the past two decades, a consistent finding is that except in highly selected series, multiple organ donors and a cumulative islet implant mass ≥10,000 islet equivalents per kilogram recipient weight are consistently required3. While both auto- and allo-immune-mediated mechanisms clearly contribute to long-term graft failure, mounting evidence strongly suggests that acute islet cell death in the immediate and peri-transplant period severely compromises engraftment outcomes. Islet transplantation is unique across organ transplantation as the complex enzymatic process required to mechanically separate islets away from their extracellular matrix, the purification and culture steps cumulatively result in injury. The subsequent transplantation to the hypoxic, intrahepatic portal site and many days to establishment of neovascularization render islets far more susceptible to injury than solid organ grafts.
Apoptosis is generally considered the primary form of regulated cell death, mediating biological functions such as homeostasis, development and pathogenesis4,5. Necrotic cell death was once considered as an all-encompassing, uncontrolled modality that occurred in response to unabated environmental triggers, hypoxia and physiological stress, resulting in the release of intracellular contents of dying cells6. Indeed, in instances of extreme stimuli, such as high temperatures, resultant necrotic cell death can occur in an accidental manner6. However, recent evidence has identified the existence of several alternative forms of regulated cell death, collectively termed ‘regulated necrosis’ (RN), elicited by pathophysiological conditions that occur in a genetically controlled fashion6. The recent identification of these cell death pathways has revealed non-apoptotic mechanisms, some of which are caspase-independent, characterized by morphologically and biochemically distinct events7 (Figure 1). In contrast to apoptosis, which is immunologically silent, at least in the initial phase, RN-pathways inevitably release damage-associated molecular patterns (DAMPs) when the plasma membrane ruptures, and thereby trigger an inflammatory cascade. Whereas all RN-pathways are to some extent immunogenic, active production of cytokines during the death process modulates the immunogenic response and may provide an evolutionary advantage to conserve different RN-subroutines6. These pathways include, but are not limited to, pyroptosis, ferroptosis, necroptosis and parthanatos (Table 1).
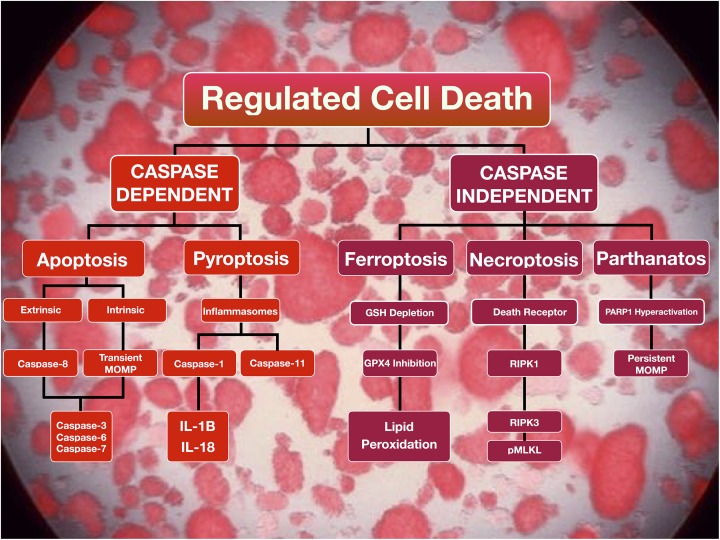
Figure 1.
Regulated cell death signaling pathways. Regulated cell death pathways may be differentiated by their dependence on caspase activity. Apoptosis, a caspase-dependent regulated cell death pathway, can be initiated by extrinsic or intrinsic cellular cues. DR binding from appropriate signals, including TNF-α, initiates the extrinsic pathway. Alternatively, internal signals, including hypoxia and ROS, activate the intrinsic pathway. Both pathways converge on caspase-3 activation and result in morphological changes, such as plasma membrane blebbing. Since membrane integrity is conserved during apoptosis, this cell death modality is a poor inducer of inflammation. Pyroptosis, a gasdermin-dependent form of regulated cell death requires the formation of inflammasomes which results in the activation of caspase-1 or caspase-11. Caspase-1 activates the pro-inflammatory cytokines IL-1β and IL-18. As such, pyroptosis is an immunogenic form of regulated cell death. Ferroptosis results in the accumulation of lipid peroxides as a result of glutathione depletion and GPx4 dysfunction. Necroptosis can be initiated by DR-ligand binding resulting in the activation of RIPK1 and RIPK3 with subsequent phosphorylation of MLKL. Parthanatos is triggered by diverse stimuli, including ROS production, resulting in the hyperactivation of PARP1 resulting in the prospective release of AIF.
AIF: apoptosis-inducing factor; DR: death receptor; GPx4: glutathione peroxidase 4; IL: interleukin; MLKL: mixed lineage kinase domain-like protein; MOMP: mitochondrial outer membrane permeabilization; PARP1: poly(ADP-ribose) polymerase 1; pMLKL: phosphorylated mixed lineage kinase domain-like protein; RIPK: receptor-interacting serine/threonine-protein kinase; ROS: reactive oxygen species; TNF: tumor necrosis factor.
Table 1.
Regulated cell death pathways influenced by key mediators, morphological characteristics associated with the cell death modality and their immunogenic potential.
| Regulated cell death pathway | Key mediator(s) | Morphological features | Immunogenicity/necroinflammation |
|---|---|---|---|
| Apoptosis | Initiator caspases (caspase-8 and -10) Executor caspases (caspase-3, -6 and -7) | Nuclear chromatin condensation Cellular shrinkage Membrane blebbing | Low |
| Pyroptosis | Caspases, gasdermins | Cellular necrosis – membrane rupture through a gasdermin D membrane pore – release of IL-1b and IL-18 | High |
| Ferroptosis | GPX4 | Cellular necrosis – membrane rupture by lipid peroxidation | Unknown |
| Necroptosis | RIPK1, RIPK3, phospho-MLKL | Cellular necrosis – membrane rupture – release of IL-33 and CXCL1 | Potent cross priming |
| Parthanatos | PARP1 | Cellular necrosis – membrane rupture | Unknown |
GPX4: glutathione peroxidase-4; IL: interleukin; MLKL: mixed lineage kinase domain-like protein; PARP1: poly(ADP-ribose) polymerase 1; RIPK: receptor-interacting protein kinase.
Within the context of islet isolation and transplantation, our group has examined several therapeutic interventions that truncate apoptotic cell death, either with pan-caspase inhibitors or agents that ameliorate onset of apoptosis3,8–12. The field of islet transplantation has identified apoptosis as the primary culprit of programmed cell death in experimental and clinical investigation13. Therapeutic strategies that dampen the inflammatory response in the acute transplant period have also been employed with considerable focus to subvert apoptotic cell death. With the emergence of recently defined, genetically and biochemically distinct pathways, re-examination of cell death modalities that contribute to islet loss is warranted. Indeed, some of the mechanisms attributed to the various regulated cell death pathways have been identified in β-cell death and islet transplantation, yet these modalities have not been exclusively defined. Herein, we discuss regulated cell death and their potential contributions to islet cell death in islet transplantation, and highlight prospective interventional strategies to ameliorate the consequences associated with islet loss.
Regulated Cell Death – Implications in Islet Transplantation
Caspase-Dependent Cell Death
Apoptosis
Once considered the only form of ‘programmed cell death’, apoptosis can be triggered by multiple stimuli. A distinct set of cysteine proteases termed caspases, are key mediators of apoptosis that become activated by pro-apoptotic stimuli14. Activation of initiator caspases (caspase-8 and -9) results in the downstream cleavage and further activation of executioner caspases (caspase-3, -6 and -7) with subsequent cellular morphological changes including DNA fragmentation and membrane blebbing15. Apoptosis is considered the least immunogenic form of programmed cell death, as it does not lead to plasma membrane rupture, however, it is not entirely immunologically silent6,16.
Apoptosis has largely been identified as the primary form of programmed cell death contributing to islet loss. Paraskevas et al. revealed that apoptosis was a key contributor to islet loss subsequent to islet isolation by evaluating a multitude of criteria13. Islets harvested from human cadaveric donors revealed morphological characteristics indicative of apoptosis within 3 to 5 days post-culture, including apoptotic bodies containing condensed chromatin. Immunohistochemical analysis for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was minimal in the immediate post-culture phase, and incrementally peaked 5 days post-culture. The authors also revealed that DNA fragmentation was also present within 48 hours of isolation13. Indeed, the findings from this study were paramount in directing therapeutic strategies during islet isolation and culture as a means to ameliorate the incidence of apoptosis prior to transplantation as a means to improve long-term engraftment outcomes.
Apoptosis can be triggered by intracellular (intrinsic) or extracellular (extrinsic) cues. The intrinsic apoptotic pathway is largely mediated by mitochondrial events and is regulated by the interplay between pro-apoptotic and anti-apoptotic members of the Bcl-2 family17. The initiation of the intrinsic pathway occurs through binding of the BH3-only proteins, including but not limited to Bim and Puma, to the Bcl-2-like anti-apoptotic proteins. These interactions result in the release of Bax and or Bak, promoting the loss of mitochondrial outer membrane potential and subsequent cytochrome c release. The release of cytochrome c results in the activation of the caspase cascade18–20. Within the context of islet cell death, the intrinsic pathway was revealed to occur in response to glucose toxicity by McKenzie and colleagues17. The authors demonstrated that islets exposed to high glucose concentrations resulted in increased DNA fragmentation relative to islets exposed to lower glucose concentrations. Moreover, islets over-expressing Bcl-2, as well as islets deficient in the BH3-only proteins, Bim or Puma, ameliorated the deleterious downstream events associated with glucose toxicity17. Notably, islets deficient in Fas or interleukin (IL)-1 receptors were not protected from glucose-induced DNA fragmentation. Other studies have also revealed that when exposed to high glucose concentrations, altered expression of Bcl-2 genes have been observed in human islets, further implicating the intrinsic apoptotic pathway in islet cell death21,22. Given that islets are susceptible to metabolic triggers that can initiate the intrinsic pathway, molecular mediators of this cell death modality may be attractive targets for therapeutic intervention. Binding of ligands to death receptors initiates the extrinsic pathway. Death receptors are members of the tumor necrosis factor (TNF) superfamily, which includes TNF receptor-1 (TNFR1) and CD95 (also called Fas and APO-1)15. Ligand-receptor binding (TNF-α to TNFR1) results in receptor clustering, adaptor molecule recruitment (i.e. Fas-associated death domain; FADD), and the formation of the death-inducing signaling complex (DISC). The initiator caspase, caspase-8, associates with the DISC complex, where it is activated. Caspase-8 initiates apoptosis by cleaving and activating executioner caspases15. It is now clear, however, that the most important function of caspase-8 is the prevention of necroptotic cell death23–25.
Intrinsic cues, such as reactive oxygen species (ROS) and hypoxia, as well as extrinsic cues, instant-blood mediated inflammatory reaction and inflammatory cytokine stimulation such as TNF-α and IL-1β, have demonstrated ability to initiate the apoptotic cascade. Methods of mitigating apoptosis activation in islets through inhibition of circulating inflammatory cytokines, IL-1β and TNF-α, have demonstrated improved engraftment outcomes in the experimental setting. In an immunocompromised murine model, human islet transplant recipients synergistically administered IL-1β receptor agonist (anakinra) and TNF-α receptor fusion protein (etanercept) exhibited improved islet engraftment outcomes26. Islet grafts harvested 24 hours post-transplant exhibited reduced apoptosis levels, as measured by TUNEL staining, suggesting these agents mitigated activation of the extrinsic apoptotic pathway.
Interventional strategies aimed to inhibit caspase activation directly, thus preventing the downstream apoptotic cascade, have been utilized in the pre-clinical setting to improve islet engraftment outcomes. Early generation pan-caspase-inhibitors, such as N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD-FMK) or N-benzyloxycarbonyl-Val-Asp-fluoromethyl ketone (zVD-FMK), have demonstrated the ability to augment islet graft survival and long-term outcomes when administered systemically in the acute transplant period3,9. When administered concomitantly with the co-stimulatory blockade agent, high affinity CTLA-4Ig (belatacept), zVD-FMK-enhanced islet engraftment outcomes in a murine allotransplantation model8. The development of novel, potent inhibitors of caspases, such as IDN-6556, have also demonstrated the ability to improve engraftment outcomes using marginal islet doses in a murine syngeneic model and porcine autograft model10,11. Most recently, our group demonstrated that the potent pan-caspase inhibitor, F573, could effectively reduce the incidence of apoptosis in murine and human islets in vitro. Furthermore, when administered to the recipient in the acute post-transplant period, F573 was able to deter apoptosis and improve long-term marginal islet engraftment outcomes in a murine transplant model. Most notably, the administration of F573 was able to augment engraftment in the clinically relevant portal site, as well as under the modified subcutaneous space12.
The utility of pan-caspase and other inhibitors in islet transplantation has identified apoptosis as a key player that influences islet viability and contributes to islet engraftment outcomes. Therapeutic strategies aimed at reducing apoptosis have clearly demonstrated efficacy pre-clinically and merits the utility of pan-caspase inhibitors as an adjuvant clinical therapy in the acute transplant period. While these studies highlighted the impact of apoptosis, most often overlooked other regulated cell death pathways and impact upon islet engraftment. With identification of newly defined, biochemically distinct, regulated cell death pathways, alternative interventions should now also be examined in islet transplantation to explore their potential as therapeutic targets for islet protection in the translational clinical setting.
Pyroptosis
Pyroptosis is a caspase-dependent form of regulated cell death that is biochemically and phenotypically distinct from apoptosis and other RN pathways. Dependent on caspase-1 and caspase-11, pyroptosis is unique from apoptosis in that it does not require the activation of caspase-3, -7, -8 or -927. Activation of caspase-1 or caspase-11 is reliant on the formation of multi-protein signaling complexes termed inflammasomes that assemble in response to various stimuli, including intracellular microbial ligands or cellular perturbations28. While inflammasomes are encompassed within three gene families, for the purpose of this review we will focus on the Nod-like receptors (NLRs), more specifically NLRs that contain a pyrin domain (PYD) that mediates the signaling event. The NLRs containing a PYD (NLRP) signal through the apoptosis-associated speck-like protein containing a caspase-associated recruitment domain which is responsible for the recruitment of caspase-1. Activation of caspase-1, or caspase-11, results in the maturation of the pyroptosis-specific cytokines, IL-1β and IL-18, and in the cleavage of the pyroptosis-mediating molecule gasdermin D (GSDMD) the translocation of which to the plasma membrane is required for subsequent cell death and IL-1β/IL-18 release23,29–31. Pyroptosis has largely been implicated in the host’s innate defense against intracellular pathogens32, however, it has also been identified in the promotion of chronic liver injury33,34. Within the context of islet transplantation, pyroptosis has not been fully elucidated, although emerging evidence suggests that mediators of pyroptosis may contribute to compromised β-cell function and viability, particularly within the context of type 2 diabetes mellitus (T2DM), which may have translational implications in islet transplantation.
Inflammation plays a critical role leading to β-cell dysfunction and death35,36. IL-1β, a key inflammatory cytokine has also been implicated in T2DM. Clinical studies inhibiting IL-1β either by IL-1 receptor antagonist (IL-1Ra) or IL-1β antibody, suggest improved glycemic control and β-cell function in T2DM37,38. Given that NLRP3 activation drives IL-1β secretion, it is at least plausible that this inflammasome contributes to islet dysfunction and death. Zhou and colleagues demonstrated that mice deficient in NLRP3 exhibited improved glucose tolerance and insulin sensitivity in contrast to their wild-type counterparts39. However, work by Wali et al. contested that NLRP3 inflammasome activation is not required for stress-induced cell death40. Using murine islets lacking functional NLRP3 or caspase-1, pharmacological induction of oxidative and endoplasmic reticulum stress sufficiently induced islet cell death similar to that of wild-type, control islets40. Indeed, these competing findings suggest that the role of NLRP3 activation in islet cell death remains controversial.
Islet amyloid polypeptide, a protein that forms amyloid deposits in islets of patients with T2DM, has demonstrated the capacity to trigger NLRP3 inflammasome activation and subsequent maturation and secretion of IL-1β41. Within the context of islet transplantation, amyloid deposition in human islets has been observed as early as 2 weeks in an immune-compromised murine transplant model42. Moreover, amyloid deposition was discovered in liver sections at the time of autopsy in clinical patients that exhibited marginal graft function at the time of death23,43,44. These findings suggest that amyloid deposition is not restricted to patients with T2DM and may trigger NLRP3 activation in islet transplant recipients, thus contributing to graft failure. Potter and colleagues revealed that human in vitro islet viability was preserved when treated with a potent inhibitor of amyloid formation45. Ongoing studies will determine if caspase-1-specific inhibitors or NLRP3-specific inhibitors can mitigate the deleterious effects associated with amyloid deposition in islets. In previous studies employing pan-caspase inhibitors, pyroptosis may have also been impaired in tandem with apoptosis, since these inhibitors indiscriminately inhibit multiple caspases, including caspase-1. Further studies evaluating the role of inflammasome activation and pyroptosis in islet dysfunction and death may play a critical role in identifying prospective therapeutic targets, thus preserving islet function and viability.
Caspase-Independent Regulated Cell Death – A Role in Islet Transplantation?
Ferroptosis
With the recent identification and expansion of cell death modalities in other disease states, it is likely that islets are indeed susceptible to non-caspase-dependent, regulated cell death mechanisms. Ferroptosis has recently emerged as a distinct form of regulated cell death that is morphologically, biochemically, and genetically distinct from apoptosis and alternative forms of RN46. Recently discovered using a pharmacological approach, ferroptosis is defined by the iron-dependent accumulation of lipid ROS46,47. The accumulation of toxic lipid ROS can be initiated by the inhibition of intracellular glutathione (GSH) synthesis or the GSH-dependent antioxidant enzyme, glutathione peroxidase 4 (GPX4)46–48. In mammals, ferroptosis has recently been implicated in numerous pathological conditions including stroke, traumatic brain injury, ischemia-reperfusion injury and kidney degeneration, as well as degenerative diseases, including Alzheimer’s and Huntington’s diseases49. Despite its observations in such pathologies, the discovery of ferroptosis occurred through a pharmacological approach47. The first ferroptosis-inducing compounds identified were erastin50 and RSL351. Erastin, a small potent molecule, demonstrated the ability to induce ferroptosis by selectively inhibiting the Xc -cystine/glutamate antiporter required for GSH biosynthesis47,48. The depletion of intracellular GSH results in the accumulation of lipid-based ROS molecules due to the impaired ability of the GSH-dependent, lipid repair enzyme glutathione peroxidase 4 (GPX4)46–48,52,53. RSL3 has the ability to induce ferroptosis by directly inhibiting the enzymatic activity of GPX4, with the biochemical hallmarks of ferroptosis ensuing, including elevated lipid peroxides. GPX4 has been implicated in models of ischemia-reperfusion-related diseases. Moreover, cancer cells in a high-mesenchymal therapy-resistant cell state are dependent GPX448. Loss of GPX4 function in these resistant cell types are susceptible to ferroptosis-induced cell death in vitro54.
The role of ferroptosis has yet to be clearly defined in context to islet loss, however, prior pre-clinical studies suggest that this regulated cell death pathway may have implications in islet transplantation. As a tri-peptide, GSH is synthesized from glutamate, cysteine and glycine, and has been implicated as a crucial antioxidant alleviating oxidative stress in islets55. In the presence of lipid peroxidation byproducts, islets exhibit impaired glucose-induced insulin secretion56. When elevated in β-cells, fatty acids impair insulin gene expression, glucose-stimulated insulin secretion, and increase cell death57. The administration of GSH precursors have demonstrated improved insulin secretion in response to glucose, as well as reduce lipid peroxidation levels27,58,59. Koulajian et al. demonstrated improved in vitro and in vivo β-cell function in islets over-expressing GPX4 in the presence of lipid peroxidation products60, further substantiating the necessity to deter lipid peroxidation for improved islet function. Most recently, our group has revealed that human islets exposed to the ferroptosis-inducing agents, erastin and RSL3, exhibit compromised islet function and viability. When islets were pre-treated with the ferroptosis-specific inhibitor, ferrostatin-1, the effects of erastin and RSL3 were abolished (unpublished data). These results suggest that islets are indeed susceptible to ferroptosis, at least in part, through pharmacological induction. The utility of inhibitors of ferroptosis capable of reducing intracellular lipid peroxidation may be attractive therapies to employ in islet isolation and transplantation61.
Given that ferroptosis requires abundant and accessible cellular iron, iron chelators, such desferrioxamine (DFO) have demonstrated the ability to protect from ferroptosis in other disease models52. Since DFO has led to improved islet function and engraftment outcomes, ferroptosis may play at least some role in islet injury after isolation and transplantation. In murine islet transplant models, DFO-treated islets and recipients exhibit improved engraftment outcomes due to preserved islet mass62,63. Furthermore, Vaithilingam et al. demonstrated that encapsulated human islets cultured in the presence of DFO exhibited enhanced insulin secretion relative to non-treated control islets64. DFO-treated islets restored euglycemia in immunocompromised NOD/SCID recipients at marginal doses relative to control islet recipients64. While ferroptosis was not identified as the cell death modality contributing to reduced islet function or engraftment, this regulated cell death pathway had yet to be defined. Stokes and colleagues also demonstrated that iron chelation with DFO potentiated islet survival subsequent to transplantation in pre-clinical models utilizing murine and human islets65. The authors revealed that islets pre-treated with DFO improved islet survival and engraftment through up-regulation of hypoxia-inducible factor-1α (HIF-1α). Consequently, the incidence of apoptosis was mitigated, as revealed by decreased caspase-3 activation. The role of HIF-1α has yet to be implicated in ferroptosis, and whether DFO can ameliorate multiple islet cell death pathways remains to be determined.
Within the context of islet isolation and transplantation, the relative contribution of ferroptosis has yet to be fully elucidated, however, prior pre-clinical observations has revealed that key contributors to ferroptosis, such as increased lipid peroxidation, iron accumulation and compromised GPX4 function, contribute to cellular demise. Interventional strategies, such as ferrostatins or newer potent longer-acting inhibitors of ferroptosis may potentially mitigate islet lipid peroxidation, improve islet function and thereby preserve islet mass. This newly identified regulated cell death pathway has garnered much interest in other disease pathologies and organ systems, and its role in islet transplantation is being actively investigated.
Necroptosis
Necroptosis is a newly identified form of RN induced by ligand binding to death receptors, TNFR1 and Fas, Toll-like receptors or intracellular receptors such as DNA-dependent activator of interferon (IFN)-regulatory factors (DAI). Emerging evidence suggests that TNF binding to TNFR1 in concomitant inhibition of caspase-8 through pan-caspase inhibition (such as, zVAD-FMK) induces necroptosis66. The upstream signaling elements of apoptosis and necroptosis are shared, and hence are tightly regulated. While TNFR1-induced apoptosis requires the activation of caspase-8, necroptosis requires caspase-8 function to be inhibited or disrupted61. The necroptosis signaling cascade requires the involvement of receptor interaction protein kinase 1 and 3 (RIPK1 and RIPK3, respectively). Subsequent RIPK3-mediated phosphorylation of mixed lineage kinase domain-like protein (MLKL) results in plasma membrane rupture, though the mechanism of MLKL-plasma membrane rupture remains unknown6. However, it is now clear that the ESCRT-3 complex is downstream of pMLKL and facilitates for plasma membrane rupture23. Early work identifying the embryonic lethality of caspase-8-null genes in mice led researchers to believe that apoptosis was a process required for vertebrate viability67. Further investigation reversing the lethal phenotype of caspase-8-deficient mice on a RIPK3-deficient background revealed other functions of caspase-8, not just as a mediator of the apoptotic pathway, but also as a key controller of RIPK325,67–69. These observations revealed that the primary function of caspase-8 was not solely for the execution of extrinsic apoptosis, but also in mediating the prevention of RIPK3-dependent necroptosis67.
In various whole organ murine transplant models, the role of RIPK3 has been identified as an important mediator of necroptosis. For example, Pavlosky et al. demonstrated delayed graft rejection when hearts from RIPK3-deficient mice were transplanted in rapamycin-immunosuppressed recipients when compared with mice receiving wild-type hearts70. In a kidney transplant model void of immunosuppression, kidneys from RIPK3-deficient C57Bl/6 mice transplanted into Balb/c recipients exhibited improved organ function and overall survival, in comparison with wild-type kidney recipients71,72. Given the importance of RIPK3 as a therapeutic target in whole organ transplantation, further investigation certainly permits the determination of necroptosis in islet transplantation.
Given that TNF-α has demonstrated to be toxic to β-cells, it is plausible that necroptosis contributes to islet cell death in islet transplantation. An initial study by Farney et al. demonstrated benefit of TNF-α blockade in a murine syngeneic islet transplant model73. Subsequently, in a single-donor clinical transplant protocol utilizing etanercept, a TNF-α fusion protein, Hering et al. achieved insulin independence in all eight patients transplanted74. Bellin et al. also revealed that islet transplant recipients receiving an induction therapy T-cell depleting antibodies with TNF-α-inhibition (TNF-α-i) exhibited significantly improved insulin independence rates up to 5 years post-transplant than recipients who did not receive TNF-α-i, regardless of maintenance immunosuppression75. Though these findings do not specifically address whether necroptosis or apoptosis account for TNF-α-induced β-cell death, this clearly merits comprehensive further delineation in experimental islet transplantation.
As previously noted, our laboratory examined the concerted administration of anti-inflammatory agents etanercept and the IL-1 receptor agonist (IL-1Ra) anakinra in a murine syngeneic islet transplant model, as well as in a human islet immunocompromised murine model26. The results from this study revealed that when administered alone, these agents could not augment engraftment outcomes in either model. However, when administered together, a significant proportion of islet transplant recipients became euglycemic as compared with non-treated control recipients. In the clinical setting, Matsumoto and colleagues achieved single-donor success in three islet transplant patients receiving etanercept and anakinra in a sirolimus-free immunosuppression regimen76.
While pre-clinical and clinical studies utilizing these anti-inflammatory agents suggest the role of reducing apoptosis to confer engraftment efficacy, it seems likely that necroptosis was also ameliorated in this setting. During necroptosis, IL-1α is actively produced77. Given that IL-1α and IL-1β bind to the same cell-surface receptor, and that anakinra demonstrates the ability to prevent IL-1α and IL-1β activity78, it is plausible that the administration of anakinra may have ameliorated the consequences of necroptosis.
The release of intracellular DAMPs from dying cells into the extracellular milieu has been identified as a downstream event associated with necroptosis, and other RN pathways79,80. In normal conditions, high-mobility group box 1 (HMGB1) has been associated with DNA winding and promotes protein assembly81. However, HMGB1 has also been implicated as a DAMP82. A novel classification of DAMPs has recently been introduced83,84. In a study by Itoh and colleagues, greater HMGB1 release from human and mouse islets correlated with poorer islet engraftment outcomes85. Matsumoto et al. further corroborated these experimental findings in a clinical autotransplantation model86. Paredes-Juarez et al. further established that when cultured in low oxygen conditions, human islets exhibit robust HMGB1 release into the extracellular milieu in vitro82 . In parallel, treatment with necrostatin-1 (Nec-1), a once perceived inhibitor of necroptosis, revealed the ability to significantly reduce HMGB1 release in islets82. When islets were challenged with nitric oxide, Tamura and colleagues revealed the release of HMGB1, as well as compromised islet viability, which could be completely abrogated in the presence of Nec-187. A caveat to these studies in pinpointing necroptosis as a defined cell death modality in islets is that Nec-1 has demonstrated the ability to potently inhibit necroptosis and ferroptosis67. Therefore, these results, and others employing Nec-1 to confer cytoprotection in islets, permits further evaluation to effectively delineate the contribution of necroptosis and/or ferroptosis in islet cell death. This can be accomplished through utilizing necroptosis-specific inhibitors, like Nec-1 stable (Nec-1 s), which may truly elucidate the role of necroptosis in solid organ and prospectively, islet transplantation.
Parthanatos
The over-activation of poly(ADP-ribose) polymerase (PARP)1 triggers parthanatos, a RN pathway that has been implicated in neurodegenerative disorders, such as Parkinson’s disease88. PARP1 has been shown to be involved in DNA repair, chromosome stability and the inflammatory response89. Moreover, while other isoforms of PARP have been identified, namely PARP2 and PARP3, specific inhibition of PARP1 solely prevents parthanatos. PARP1 activity has been demonstrated in response to stimuli, such as DNA damage and ROS production90. Under oxidative stress, activated PARP1 consumes nicotinamide adenine dinucleotide (NAD+), depleting cellular adenosine triphosphate (ATP), leading to eventual cellular energy collapse. PARP1 hyperactivation results in the translocation of apoptosis-inducing factor (AIF) from mitochondria to the nucleus, fragmenting DNA91. Given that islet viability is susceptible to both stimuli, it is conceivable that parthanatos may play a role in β-cell loss.
Murine studies have revealed that mice deficient in PARP1 exhibit resistance to single-bolus treatment of streptozotocin (STZ)92,93, a known β-cell toxin that induces DNA damage through alkylation94,95. Further work has also revealed that inhibition of PARP1 protects islets against free radical- and cytokine-mediated islet damage96–98. Islets deficient in PARP1 have also been associated with reduced cytokine and endotoxin signaling, as evidenced by reduced nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) activation and its inflammatory gene targets, such as inducible nitric oxide (NO) synthase (iNOS)99. Andreone et al. revealed that islets isolated from PARP1-deficient mice prevented islet cell death when exposed to inflammatory cytokines, IL-1β and IFN-γ, suggesting a role of parthanatos in inflammatory injury to islets99. In a study by Heller et al., islets pre-treated with the PARP1 inhibitor, 3-aminobenzamide, were partially protected when subsequently challenged with NO or ROS, further supporting a role of PARP1 in islet cell death100. As a contributor to islet cell death, PARP1 and other molecular targets in this pathway may serve as important opportunities for intervention.
Cross-Talk between Regulated Cell Death Pathways
As described above, there are numerous RN pathways that can be triggered by several molecular pathways. As such, there is considerable cross-talk between components in different forms of these pathways. For example, RIPK3 has been implicated in the processing of pro-IL-1β as a result of promoting the NLRP3 inflammasome, independent of necroptotic cell death101. Regulated cell death mechanisms have also been implicated in chronic kidney injury, as inflammasome activation and pyroptosis has been demonstrated to occur33,101. Moreover, Nec-1 has also demonstrated the capacity to inhibit ferroptosis, prospectively suggesting implications in off-target, to be determined mechanisms7. Within the context of islet transplantation, cross-talk of the various regulated cell death pathways has yet to be fully elucidated. It is conceivable that multiple regulated pathways can contribute to islet dysfunction and cell death, given that islets are susceptible to numerous stimuli that act as key contributors to the various regulated cell death mechanisms. Elucidating key molecules contributing to islet demise will prove crucial for the development of therapeutic treatments.
Conclusion
Despite substantial advances in clinical islet transplantation over the past two decades, islet loss in the acute and peri-transplant period remains a substantial obstacle to long-term success. As such, single-donor transplant success rates still remain elusive for the majority of islet recipients. Therapeutic strategies to ameliorate islet cell death in the acute and peri-transplant period provide an attractive approach to preserve early islet mass, potentially improving long-term engraftment outcomes. Apoptosis has been identified in numerous pathological conditions and transplant settings, including islet transplantation. Numerous pre-clinical and clinical strategies have been employed to ameliorate the deleterious events associated with apoptosis. However, recent research endeavors have identified other notable, regulated cell death modalities that are genetically and biochemically distinct from apoptosis. The identification of such RN pathways, including but not limited to, ferroptosis and necroptosis, exhibit distinct biochemical hallmarks with defined molecular machinery contributing to cellular demise.
Our detailed review of published literature reveals that hallmarks of various regulated cell death pathways contribute to islet β-cell death, but these pathways have not been fully characterized to date. Studies aimed to identify the key contributors of ferroptosis, necroptosis, and other regulated necrotic pathways may be new and exciting arenas to explore in islet transplantation. The key molecules identified in these regulated cell death modalities may be ideal targets for therapeutic intervention in the early isolation and acute transplant period. With the potential cross-talk of these cell death modalities, employing a single therapy to abate early islet death either post-isolation or in the acute transplant period may be of limited benefit. A multi-therapeutic approach is likely required, as targeted inhibition of some molecules may drive the incidence of other cell death pathways. Insights in pre-clinical and clinical investigations have revealed that a multi-therapeutic strategy to combat various biochemical pathways may be imperative to improve single-donor engraftment outcomes. The administration of these drugs to multi-organ donors and subsequently to transplant recipients may also have considerable implications in supporting islet viability and deterring the onset of islet cell death. The window at which these drugs are administered will likely be attributed to the time at which these regulated cell death processes occur, as well as the homeostatic importance of these pathways in the host. The efficacy of any interventions aimed at controlling regulated cell death will depend heavily on their half-life and durability of action. It will also be important to establish if these new target therapeutics have direct toxicity to islet beta cells, or to the engraftment and neovascularization process. Emerging evidence of alternative, regulated cell death pathways in other pathological conditions will continue to garner relevance in islet transplantation. However, elucidating the mechanisms that contribute to islet death in islet transplantation will be of much benefit to ameliorate graft attrition thus improving long-term engraftment outcomes.
Acknowledgments
Antonio Beruni, Stefan Bornstein, Andreas Linkermann, and A. M. James Shapiro participated in the design, writing and review of the manuscript.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A. M. James Shapiro was supported through a Senior Clinical Scholarship from Alberta Innovates Health Solutions (AIHS), and holds a Canada Research Chair in Transplantation Surgery and Regenerative Medicine funded through the Government of Canada. A. M. James Shapiro was also supported by AIHS CRIO Team Award #201201154, the Diabetes Research Institute Foundation Canada (DRIFCan), and the Canadian National Transplant Research Program. Antonio Beruni was supported by a grant from Stem Cell Network (NCESCN CTRA FY17/CT5). Andreas Linkermann was granted a Heisenberg-Professorship by the German Research Foundation.
References
- 1. Hering BJ, Clarke WR, Bridges ND, Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, et al. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2016;39(7):1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The CITR Coordinating Center and Investigators. The Collaborative Islet Transplant Registry (CITR) 2016 Ninth Annual Report. Bethesda, MD, USA: US Department of Health and Human Services; 2016. [cited 2017 Jun 3]. Available from https://citregistry.org/system/files/9AR_Report.pdf. [Google Scholar]
- 3. Emamaullee JA, Davis J, Pawlick R. The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes. 2008;57(6):1556–1566. [DOI] [PubMed] [Google Scholar]
- 4. Suzanne M, Steller H. Shaping organisms with apoptosis. Cell Death Differ. 2013;20(5):669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9(3):231–241. [DOI] [PubMed] [Google Scholar]
- 6. Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370(5):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emamaullee JA, Davis J, Pawlick R, Christian Toso, Shaheed Meran, Sui-Xiong Cai, Ben Tseng, Shapiro A.M. James. et al. Caspase inhibitor therapy synergizes with costimulation blockade to promote indefinite islet allograft survival. Diabetes. 2010;59(6):1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emamaullee JA, Stanton L, Schur C, Shapiro AM. Caspase inhibitor therapy enhances marginal mass islet graft survival and preserves long-term function in islet transplantation. Diabetes. 2007;56(5):1289–1298. [DOI] [PubMed] [Google Scholar]
- 10. McCall M, Toso C, Emamaullee J, Pawlick R, Edgar R, Davis J, Maciver A, Kin T, Arch R, Shapiro AM. The caspase inhibitor IDN-6556 (PF3491390) improves marginal mass engraftment after islet transplantation in mice. Surgery. 2011;150(1):48–55. [DOI] [PubMed] [Google Scholar]
- 11. McCall MD, Maciver AM, Kin T. Caspase inhibitor IDN6556 facilitates marginal mass islet engraftment in a porcine islet autotransplant model. Transplantation. 2012;94(1):30–35. [DOI] [PubMed] [Google Scholar]
- 12. Pepper AR, Bruni A, Pawlick R, Wink J, Rafiei Y, Gala-Lopez B, Bral M, Abualhassan N, Kin T, Shapiro AMJ. Engraftment site and effectiveness of the pan-caspase inhibitor F573 to improve engraftment in mouse and human islet transplantation in mice. Transplantation. 2017;101(10):2321–2329. [DOI] [PubMed] [Google Scholar]
- 13. Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20(3):270–276. [DOI] [PubMed] [Google Scholar]
- 14. Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. [DOI] [PubMed] [Google Scholar]
- 15. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4): a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15(2):135–147. [DOI] [PubMed] [Google Scholar]
- 17. McKenzie MD, Jamieson E, Jansen ES, Scott CL, Huang DCS, Bouillet P, Allison J, Kay TWH, Strasser A, Thomas HE. Glucose induces pancreatic islet cell apoptosis that requires the BH3-only proteins Bim and Puma and multi-BH domain protein Bax. Diabetes. 2010;59(3):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. [DOI] [PubMed] [Google Scholar]
- 19. Soria B, Gauthier BR. Dual Trade of Bcl-2 and Bcl-xL in islet physiology: balancing life and death with metabolism secretion coupling. Diabetes. 2013;62(1):18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi D, Schroer SA, Lu SY, Cai EP, Hao Z, Woo M. Redundant role of the cytochrome c-mediated intrinsic apoptotic pathway in pancreatic beta-cells. J Endocrinol. 2011;210(3):285–292. [DOI] [PubMed] [Google Scholar]
- 21. Costes S, Vandewalle B, Tourrel-Cuzin C, Broca C, Linck N, Bertrand G, Kerr-Conte J, Portha B, Pattou F, Bockaert J, Dalle S. Degradation of cAMP-responsive element-binding protein by the ubiquitin-proteasome pathway contributes to glucotoxicity in beta-cells and human pancreatic islets. Diabetes. 2009;58(5):1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Federici M, Hribal M, Perego L, Ranalli M, Caradonna Z, Perego C, Usellini L, Nano R, Bonini P, Bertuzzi F, Marlier LN, Davalli AM, Carandente O, Pontiroli AE, Melino G, Marchetti P, Lauro R, Sesti G, Folli F. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50(6):1290–1301. [DOI] [PubMed] [Google Scholar]
- 23. The effect of intensive treatment of diabetes on the development and progression of long–term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 24. Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471(7338):368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471(7338):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12(2):322–329. [DOI] [PubMed] [Google Scholar]
- 27. Avila J, Barbaro B, Gangemi A. Intra-ductal glutamine administration reduces oxidative injury during human pancreatic islet isolation. Am J Transplant. 2005;5(12):2830–2837. [DOI] [PubMed] [Google Scholar]
- 28. Jorgensen I, Zhang Y, Krantz BA, Miao EA. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. [DOI] [PubMed] [Google Scholar]
- 31. Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, Dueber EC. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci USA. 2016;113(28):7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sangiuliano B, Perez NM, Moreira DF, Belizario JE. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators Inflamm. 2014;2014:821043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21(10):1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D’Cruz AA, Hall C, Kaur Spall S, Anderton H, Masters SL, Rashidi M, Wicks IP, Alexander WS, Mitsuuchi Y, Benetatos CA, Condon SM, Wong WW, Silke J, Vaux DL, Vince JE. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. [DOI] [PubMed] [Google Scholar]
- 37. Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A, Berg JK, Landschulz WH. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36(8):2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. [Interleukin-1 receptor antagonist-treatment of patients with type 2 diabetes]. Ugeskr Laeger. 2007;169(45):3868–3871. [PubMed] [Google Scholar]
- 39. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. [DOI] [PubMed] [Google Scholar]
- 40. Wali JA, Gurzov EN, Fynch S, Elkerbout L, Kay TW, Masters SL, Thomas HE. Activation of the NLRP3 inflammasome complex is not required for stress-induced death of pancreatic islets. PLoS One. 2014;9(11): e113128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Westermark GT, Westermark P, Nordin A, Tornelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108(3):193–203. [DOI] [PubMed] [Google Scholar]
- 43. Westermark GT, Westermark P, Berne C, Korsgren O, Nordic Network for Clinical Islet T. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359(9):977–979. [DOI] [PubMed] [Google Scholar]
- 44. Westermark GT, Davalli AM, Secchi A, Folli F, Kin T, Toso C, Shapiro AM, Korsgren O, Tufveson G, Andersson A, Westermark P. Further evidence for amyloid deposition in clinical pancreatic islet grafts. Transplantation. 2012;93(2):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Potter KJ, Scrocchi LA, Warnock GL, Ao Z, Younker MA, Rosenberg L, Lipsett M, Verchere CB, Fraser PE. Amyloid inhibitors enhance survival of cultured human islets. Biochim Biophys Acta. 2009;1790(6):566–574. [DOI] [PubMed] [Google Scholar]
- 46. Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. [DOI] [PubMed] [Google Scholar]
- 47. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Bräsen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111(47):16836–16841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stockwell BR, Friedmann Angeli JP, Bayir H, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. [DOI] [PubMed] [Google Scholar]
- 51. Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reed JC, Pellecchia M. Ironing out cell death mechanisms. Cell. 2012;149(5):963–965. [DOI] [PubMed] [Google Scholar]
- 54. Hangauer MJ, Viswanathan VS, Ryan MJ, Ryan MJ4, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, McCormick F, McManus MT. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. do Amaral AS, Pawlick RL, Rodrigues E, Rodrigues E, Costal F, Pepper A, Galvão FH, Correa-Giannella ML, Shapiro AM. Glutathione ethyl ester supplementation during pancreatic islet isolation improves viability and transplant outcomes in a murine marginal islet mass model. PLoS One. 2013;8(2):e55288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miwa I, Ichimura N, Sugiura M, Hamada Y, Taniguchi S. Inhibition of glucose-induced insulin secretion by 4-hydroxy-2-nonenal and other lipid peroxidation products. Endocrinology. 2000;141(8):2767–2772. [DOI] [PubMed] [Google Scholar]
- 57. Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl. 1):S119–S124. [DOI] [PubMed] [Google Scholar]
- 58. Carobbio S, Ishihara H, Fernandez-Pascual S, Bartley C, Martin-Del-Rio R, Maechler P. Insulin secretion profiles are modified by overexpression of glutamate dehydrogenase in pancreatic islets. Diabetologia. 2004;47(2):266–276. [DOI] [PubMed] [Google Scholar]
- 59. Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM. A signaling role of glutamine in insulin secretion. J Biol Chem. 2004;279(14):13393–13401. [DOI] [PubMed] [Google Scholar]
- 60. Koulajian K, Ivovic A, Ye K, Desai T, Shah A, Fantus IG, Ran Q, Giacca A. Overexpression of glutathione peroxidase 4 prevents beta-cell dysfunction induced by prolonged elevation of lipids in vivo. Am J Physiol Endocrinol Metab. 2013;305(2): E254–E262. [DOI] [PubMed] [Google Scholar]
- 61. Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551–4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bradley B, Prowse SJ, Bauling P, Lafferty KJ. Desferrioxamine treatment prevents chronic islet allograft damage. Diabetes. 1986;35(5):550–555. [DOI] [PubMed] [Google Scholar]
- 63. Nomikos IN, Prowse SJ, Carotenuto P, Lafferty KJ. Combined treatment with nicotinamide and desferrioxamine prevents islet allograft destruction in NOD mice. Diabetes. 1986;35(11):1302–1304. [DOI] [PubMed] [Google Scholar]
- 64. Vaithilingam V, Oberholzer J, Guillemin GJ, Tuch BE. Beneficial effects of desferrioxamine on encapsulated human islets–in vitro and in vivo study. Am J Transplant. 2010;10(9):1961–1969. [DOI] [PubMed] [Google Scholar]
- 65. Stokes RA, Cheng K, Deters N, Lau SM, Hawthorne WJ, O’Connell PJ, Stolp J, Grey S, Loudovaris T, Kay TW, Thomas HE, Gonzalez FJ, Gunton JE. Hypoxia-inducible factor-1alpha (HIF-1alpha) potentiates beta-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 2013;22(2):253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187(9):1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Linkermann A. Nonapoptotic cell death in acute kidney injury and transplantation. Kidney Int. 2016;89(1):46–57. [DOI] [PubMed] [Google Scholar]
- 68. Oberst A, Green DR. It cuts both ways: reconciling the dual roles of caspase 8 in cell death and survival. Nat Rev Mol Cell Biol. 2011;12(11):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38(1):27–40. [DOI] [PubMed] [Google Scholar]
- 70. Pavlosky A, Lau A, Su Y, Lian D, Huang X, Yin Z, Haig A, Jevnikar AM, Zhang ZX. RIPK3-mediated necroptosis regulates cardiac allograft rejection. Am J Transplant. 2014;14(8):1778–1790. [DOI] [PubMed] [Google Scholar]
- 71. Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant. 2013;13(11):2797–2804. [DOI] [PubMed] [Google Scholar]
- 72. Lau A, Wang S, Jiang J, Haig A, Pavlosky A, Linkermann A, Zhang ZX, Jevnikar AM. RIPK3-mediated necroptosis promotes donor kidney inflammatory injury and reduces allograft survival. Am J Transplant. 2013;13(11):2805–2818. [DOI] [PubMed] [Google Scholar]
- 73. Farney AC, Xenos E, Sutherland DE, Widmer M, Stephanian E, Field MJ, Kaufman DB, Stevens RB, Blazar B, Platt J, et al. Inhibition of pancreatic islet beta cell function by tumor necrosis factor is blocked by a soluble tumor necrosis factor receptor. Transplant Proc. 1993;25(1 Pt 2):865–866. [PubMed] [Google Scholar]
- 74. Hering BJ. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79(10):1296–1297. [DOI] [PubMed] [Google Scholar]
- 75. Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N, Naziruddin B, Levy MF. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1beta and TNF-alpha. Cell Transplant. 2011;20(10):1641–1647. [DOI] [PubMed] [Google Scholar]
- 77. England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. J Biol Chem. 2014;289(23):15942–15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8(4):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–223. [DOI] [PubMed] [Google Scholar]
- 81. Daly KA, Liu S, Agrawal V, Brown BN, Johnson SA, Medberry CJ, Badylak SF. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials. 2012;33(1):91–101. [DOI] [PubMed] [Google Scholar]
- 82. Paredes-Juarez GA, Sahasrabudhe NM, Tjoelker RS, de Haan BJ, Engelse MA, de Koning EJ, Faas MM, de Vos P. DAMP production by human islets under low oxygen and nutrients in the presence or absence of an immunoisolating-capsule and necrostatin-1. Sci Rep. 2015;5:14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. DAMP-induced allograft and tumor rejection: the circle is closing. Am J Transplant. 2016;16(12):3322–3337. [DOI] [PubMed] [Google Scholar]
- 84. Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. Transplantation and damage-associated molecular patterns (DAMPs). Am J Transplant. 2016;16(12):3338–3361. [DOI] [PubMed] [Google Scholar]
- 85. Itoh T, Takita M, SoRelle JA, Shimoda M, Sugimoto K, Chujo D, Qin H, Naziruddin B, Levy MF, Matsumoto S. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant. 2012;21(7):1371–1381. [DOI] [PubMed] [Google Scholar]
- 86. Itoh T, Iwahashi S, Kanak MA, Masayuki Shimoda, Morihito Takita, Daisuke Chujo, Yoshiko Tamura, Rahman Ana M., Chung Wen Y., Onaca Nicholas, Coates P. Toby H., Dennison Ashley R., Naziruddin Bashoo, Levy Marlon F., Matsumoto Shinichi, et al. Elevation of high-mobility group box 1 after clinical autologous islet transplantation and its inverse correlation with outcomes. Cell Transplant. 2014;23(2):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tamura Y, Chiba Y, Tanioka T, Nobuyuki Shimizu, Shohei Shinozaki, Marina Yamada, Kentaro Kaneki, Seijiro Mori, Atsushi Araki, Hideki I, Masao Kanekia. NO donor induces Nec-1-inhibitable, but RIP1-independent, necrotic cell death in pancreatic beta-cells. FEBS Lett. 2011;585(19):3058–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15(5):348–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol Aspects Med. 2013;34(6):1217–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Virag L, Robaszkiewicz A, Rodriguez-Vargas JM, Oliver FJ. Poly(ADP-ribose) signaling in cell death. Mol Aspects Med. 2013;34(6):1153–1167. [DOI] [PubMed] [Google Scholar]
- 91. Jang KH, Do YJ, Son D, Son E, Choi JS, Kim E. AIF-independent parthanatos in the pathogenesis of dry age-related macular degeneration. Cell Death Dis. 2017;8(1):e2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yamamoto H, Uchigata Y, Okamoto H. DNA strand breaks in pancreatic islets by in vivo administration of alloxan or streptozotocin. Biochem Biophys Res Commun. 1981;103(3):1014–1020. [DOI] [PubMed] [Google Scholar]
- 93. Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294(5838):284–286. [DOI] [PubMed] [Google Scholar]
- 94. Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. [DOI] [PubMed] [Google Scholar]
- 95. Wilson GL, Hartig PC, Patton NJ, LeDoux SP. Mechanisms of nitrosourea-induced beta-cell damage. Activation of poly (ADP-ribose) synthetase and cellular distribution. Diabetes. 1988;37(2):213–216. [DOI] [PubMed] [Google Scholar]
- 96. Andersen HU, Jorgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J. Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes. 1994;43(6):770–777. [DOI] [PubMed] [Google Scholar]
- 97. Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Lakey JR, Rajotte RV. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab. 1994;79(4):1058–1062. [DOI] [PubMed] [Google Scholar]
- 98. Sandler S, Bendtzen K, Borg LA, Eizirik DL, Strandell E, Welsh N. Studies on the mechanisms causing inhibition of insulin secretion in rat pancreatic islets exposed to human interleukin-1 beta indicate a perturbation in the mitochondrial function. Endocrinology. 1989;124(3):1492–1501. [DOI] [PubMed] [Google Scholar]
- 99. Andreone T, Meares GP, Hughes KJ, Hansen PA, Corbett JA. Cytokine-mediated beta-cell damage in PARP-1-deficient islets. Am J Physiol Endocrinol Metab. 2012;303(2): E172–E179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Heller B, Wang ZQ, Wagner EF. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995;270(19):11176–11180. [DOI] [PubMed] [Google Scholar]
- 101. Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22(6):1007–1018. [DOI] [PubMed] [Google Scholar]