Abstract
Stem cells and biomaterials transplantation hold a promising treatment for functional recovery in spinal cord injury (SCI) animal models. However, the functional recovery of complete SCI patients was still a huge challenge in clinic. Additionally, there is no clinical standard procedure available to diagnose precisely an acute patient as complete SCI. Here, two acute SCI patients, with injury at thoracic 11 (T11) and cervical 4 (C4) level respectively, were judged as complete injury by a stricter method combined with American Spinal Injury Association (ASIA) Impairment Scale, magnetic resonance imaging (MRI) and nerve electrophysiology. Collagen scaffolds, named NeuroRegen scaffolds, with human umbilical cord mesenchymal stem cells (MSCs) were transplanted into the injury site. During 1 year follow up, no obvious adverse symptoms related to the functional scaffolds implantation were found after treatment. The recovery of the sensory and motor functions was observed in the two patients. The sensory level expanded below the injury level, and the patients regained the sense function in bowel and bladder. The thoracic SCI patient could walk voluntary with the hip under the help of brace. The cervical SCI patient could raise his lower legs against the gravity in the wheelchair and shake his toes under control. The injury status of the two patients was improved from ASIA A complete injury to ASIA C incomplete injury. Furthermore, the improvement of sensory and motor functions was accompanied with the recovery of the interrupted neural conduction. These results showed that the supraspinal control of movements below the injury was regained by functional scaffolds implantation in the two patients who were judged as the complete injury with combined criteria, it suggested that functional scaffolds transplantation could serve as an effective treatment for acute complete SCI patients.
Keywords: Acute complete spinal cord injury, collagen scaffold, mesenchymal stem cells, motor recovery
Introduction
Spinal cord injury (SCI) is a devastating injury. It is estimate that the annual incidence of SCI is approximately 54 cases per million populations, and as many as 500,000 people suffer from SCI every year in the world. SCI is often traumatic, mainly caused by trauma such as car accidents, falls, and sports injuries. Initial physical trauma directly disrupts blood vessels and cell membranes at the injury site. Subsequently, a secondary injury cascade is triggered involving vascular dysfunction, edema, ischemia, excitotoxicity, free radical production, and leads to the necrosis and apoptosis of neighboring neural cells. Eventually, an inhibitory environment is formed by local expression of inhibitory proteins, the formation of scarring and cystic cavitation, which hinders neural regeneration and results in the loss of sensory and motor function below the level of lesion1,2. Thus, the intervention in the acute phase can prevent the second damage in maximum and provide a chance to improve functional recovery in SCI patients.
Different strategies including biomaterial scaffolds, neurotrophin delivery or stem cells implantation have been used to rebuild a regenerative microenvironment for SCI repair. Biomaterial scaffolds are used to bridge the lesion gap and guide axonal growth across the injury site, they also act as an effective vehicle to deliver stem cells or functional biomolecules to reconstruct the microenvironment for SCI repair at the injury site3,4. Stem cell-based therapies for SCI aim to directly replace the damaged cells themselves or induce neural regeneration by secreting factors. Transplanting stem cells with biomaterial scaffolds can promote cell retention, integration or differentiation efficiently at the injury site. Combining biomaterials and stem cells may offer a promising treatment for the injured spinal cord5,6.
In our previous study, we developed a linear ordered collagen scaffold, NeuroRegen scaffold, which was consisted of ordered collagen fibers7. The collagen scaffold functionalized with multiple functional molecules such as neurotropic factors or stem cells was transplanted into SCI animal models. The results indicated that the functional collagen scaffolds could inhibit scar formation and induce newborn neuron production. More importantly, the locomotor recovery was also observed in animal models including complete transected rat and canine SCI models8–12.
Stem cell-based therapies have been reported to improve functional recovery in SCI animal models, including mesenchymal stem cells (MSCs), neural stem cells and embryonic stem cells. Among these cells, MSCs are self-renewing, multipotent progenitor cells. MSCs could exert positive immunomodulatory and neurotrophic influences on SCI repair, and it was considered as a promising source for cellular repair after SCI13,14. A number of preclinical studies have demonstrated that MSCs transplantation after SCI induced neural regeneration and improves functional recovery in animal models5. In our previous work, collagen scaffolds loaded with human MSCs were transplanted into acute and chronic complete canine SCI models, and the results showed that functional biomaterials promoted hindlimb locomotor recovery by reducing scar formation and inducing neural regeneration15,16. These data suggested that the combined treatment could serve as a beneficial method to treat complete SCI. Recently, the NeuroRegen scaffolds loaded with autologous bone marrow mononuclear cells (BMMCs) were transplanted to the gap in the chronic complete SCI patients following scar tissue resection. No obvious adverse effects related to scaffold transplantation were observed immediately after surgery or at the 12-month follow up and patients showed partially neural function recovery. This clinical study proved the safety and feasibility of the NeuroRegen scaffolds transplantation for SCI repair in clinic17.
Humans and experimental animals that suffer from incomplete SCI may show partially spontaneous functional recovery18–20. However, for the complete SCI, little progress has been made to improve the neural regeneration21–23. It often leads to the permanent loss of voluntary movements below the level of lesion in complete SCI patients. But there is no clinical standard procedure available to precisely diagnose a patient with acute complete SCI. The American Spinal Injury Association (ASIA) Impairment Scale was used to evaluate the severity of the SCI; ASIA A was considered as the complete injury in acute, sub-acute or chronic SCI patients24,25. But it was not suitable to judge acute SCI patients immediately after injury because of the existence of spinal shock. Spinal shock begins within a few minutes after a severe SCI. It often recovers within several days but may last over few weeks in less common cases. During the early phase of spinal shock, the nervous system is unable to transmit signals effectively26. Stricter standards should be used to judge the acute SCI patients as complete injury.
Here, a stricter standard was established to judge the acute SCI patient as complete injury combined with the ASIA Impairment Scale, magnetic resonance imaging (MRI) and nerve electrophysiology, NeuroRegen scaffolds loaded with human umbilical cord MSCs were transplanted into the injury site. During 1 year follow up, no obvious adverse symptoms related to NeuroRegen scaffolds and MSCs implantation were found after treatment, and the significant improvement of the sensory and motor function was observed in the two acute complete SCI patients.
Materials and Methods
Patient Enrolment and Diagnosis
Two patients with acute complete SCI, injured at thoracic and cervical level respectively, were enrolled at the Affiliated Hospital of Logistics University of CAPF. The clinical study was approved by the ethics committee of the Affiliated Hospital of Logistics University of CAPF and registered on the National Institute of Health database (ClinicalTrials.gov: NCT02510365). Ethical guideline provisions from the Helsinki Declaration were followed. Informed consent for participating in the study and publishing the results was obtained from the patients. The severity of the injury was judged with the ASIA Impairment Scale, MRI and nerve electrophysiology.
NeuroRegen Scaffolds and MSC Preparation
NeuroRegen scaffolds were prepared from bovine aponeurosis as described previously7,17. Briefly, fresh bovine aponeurosis were harvested from a local slaughter house (Wanlifa, Beijing, P.R. China) and rinsed with cold distilled water for several times, and then residual muscles, connective tissue, and fat were carefully removed. The samples were then repeatedly rinsed to completely remove the residual agents and freeze-dried. The standard of collagen scaffolds was established and the third-party inspection was complete by National institute of Food and Drug Control according to Chinese Criterion of Medical Device.
Umbilical cord MSCs were used in the trial, which obtained from the umbilical cord of human donor. Written consent for the use of the umbilical cord was obtained from the donor. The isolation, culture, and identification of the normal umbilical cord MSCs as described previously27,28. Briefly, the cords were dissected and the blood vessels were removed. The remaining tissues were cut into small pieces and digested with collagenase and trypsin. Then cells were seeded in the Dulbecco’s modified eagle’s culture medium containing serum replacements, 2 mM glutamine. Cells were passaged when they were confluent. MSCs had a fibroblast-like morphology and expressed the typical MSC markers CD105, CD73, and CD90, but not CD34, CD45, CD11b, CD19, or human leukocyte antigen-antigen D related (HLA-DR). When MSCs were cultured on the scaffolds, they adhered to the collagen scaffold, which would decrease diffusion of cells from the injury site after implantation as described in our previous work28. The pathogen- and virus-free status was also confirmed according the safety analysis such as sterility, mycoplasma, and endotoxin detection. The standard of MSCs was established and the third-party inspection was complete before transplantation.
Surgical Procedures and Intraoperative Nerve Electrophysiology
The patient was placed under general anesthesia. A midline skin incision was made, followed by paravertebral muscle dissection and laminectomy. Adhesion outside the injured dura was removed under an operating microscope. A midline durotomy was performed to expose the spinal cord. The intraoperative nerve electrophysiology was carried out as described previously with modification17. First, the stimulation electrodes of electromyography (XLTEK, Canada) were placed at the rostral side of the injured spinal cord, and the recording electrodes were placed on the anal sphincter. Then, the stimulation electrodes of electromyography were placed at the caudal side of the injured spinal cord, and the recording electrodes were placed on scalp. If there was no neural signaling was recorded across the injured spinal cord, the patient was judged as complete injury. The necrohemorrhagic tissues were carefully removed under an operating microscope. The collagen scaffolds were trimmed to fit the length of the gap, 4×107 umbilical blood MSCs were added to the collagen scaffolds, the functional scaffolds were grafted into the transected spinal cord gap to bridge the defect. Internal fixation was used to stabilize the subluxation in the T11 injury patient in this study.
Patient Follow Up
The patients underwent regular rehabilitation after surgery. The ASIA Impairment Scale was used to evaluate the recovery of the SCI patients every month. Nerve electrophysiology was used to evaluate the recovery of the SCI patients at 2, 4, 6, and 12 months after surgery. For nerve electrophysiology, somatosensory evoked potential (SSEP) and motor evoked potential (MEP) were used to determine the neural conduction. SSEP testing was carried out with tibial nerve stimulation for evaluation of the lower limbs, registration in the ankle, popliteal fossa, and scalp (Cz’ to Fpz) regions. A 100-ms square wave electrical pulse was delivered at intensities strong enough to cause a thumb twitch. We identified a P40 potential in response to target stimuli that could affect early cortical SSEP. For determining MEP of patients, the stimulation electrodes were positioned on scalp, and the recording electrodes were placed into target muscles, then single-pulse stimulation (160 V) was applied to produce neuroelectrical signals. The walking index for SCI (WISCI)29 was also used to evaluate the capacity to walk in the thoracic SCI patient at 1, 3, 6, and 12 months after surgery.
Results
Enrolment of the Patients
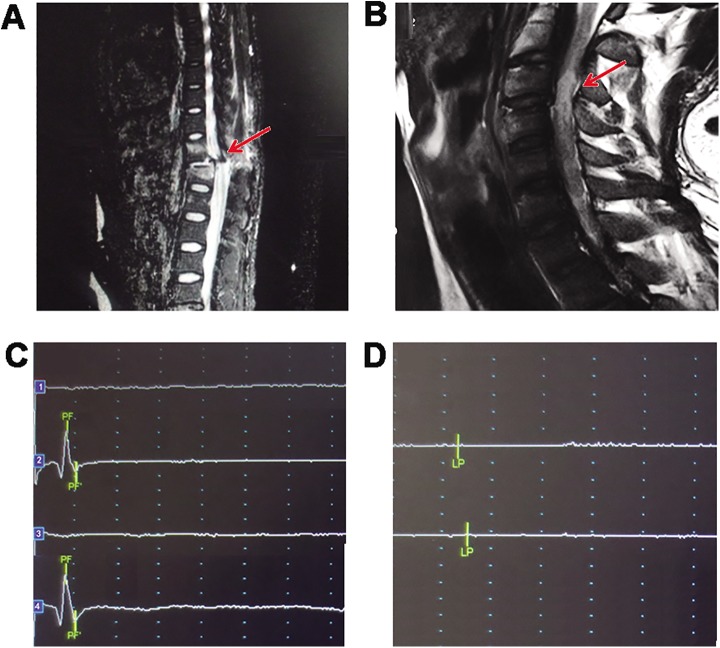
The first patient was a 28-year-old man who encountered a fall accident in April 2015. He completely lost the sensation and movements below the level of thoracic 11 (T11) with no voluntary anal contraction and sensation to deep anal pressure. Hence, the patient’s neurological deficit according to the ASIA Impairment Scale was grade A. MRI showed the spinal cord lost its continuity at T11 segment (Figure 1(a)). Another patient was a 30-year-old man who encountered a car accident in May 2016. He completely lost the sensation to deep anal pressure sensation and the voluntary movements of the muscles below the injury level. The patient’s neurological deficit according to the ASIA Impairment Scale was also grade A. MRI showed a total abnormal signal at cervical 4 (C4) level (Figure 1(b)).
Figure 1.
Patients were judged as complete injury with MRI and nerve electrophysiology. (a) MRI showed spinal cord lost its continuity at T11 segment in the thoracic SCI patient (red arrow). (b) MRI showed a total abnormal signal at the injury area in the cervical SCI patient (red arrow). (c) No SSEP of right and left tibial nerve was detected from the ankle to the cortex (1 and 3) after injury or even 2 months after surgery in the thoracic SCI patient, while the neural conduction from the ankle to the popliteal fossa was normal (2 and 4). (d) The MEP of the muscles below the injury was not detected in the thoracic SCI patient after injury. The figure showed the MEP of adductor magnus of the thoracic SCI patient. MEP: motor evoked potential; MRI: magnetic resonance imaging; SCI: spinal cord injury; SSEP: somatosensory evoked potential.
SSEP was carried out to detect the sensory nerve conduction of the right and left tibial nerve in the thoracic SCI patient. No neural conduction was detected from the ankle to the cortex immediately after the injury or even 2 months post-surgery, while the neural conduction from the ankle to the popliteal fossa was normal (Figure 1(c)). Motor MEP of the muscles below the injury level were also not detected at the same time (Figure 1(d)), which showed that the neural conduction was completely interrupted. The nerve electrophysiology of the cervical SCI patient was carried out at 8 days after injury when he was in a stable condition. The results of the SSEP and MEP of the lower extremities in the cervical SCI patient were similar as the first patient. Thus, the two patients were judged as a complete SCI injury by stricter combined method with ASIA Impairment Scale, MRI and nerve electrophysiology.
Transplantation of NeuroRegen Scaffolds with MSCs
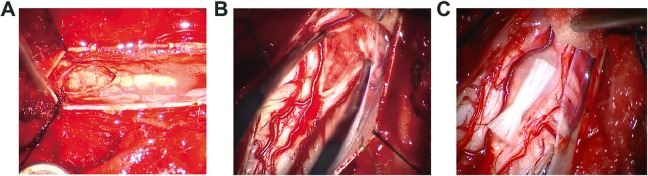
The surgery of the thoracic SCI patient was performed at approximately 24 h after injury. After open the dura, the injured spinal cord was exposed. The spinal cord tissue was destroyed at the injured area (Figure 2(a)). Following cleaning up the injured tissue, NeuroRegen scaffolds loaded with MSCs were implanted into the spinal cord gap with the length about 1.5 cm. The surgery of the cervical SCI patient was performed 8 days after injury. After open the dura, the injured spinal cord lost its continuity and was filled with the necrosis tissue (Figure 2(b)). Following cleaning up the necrosis tissue, NeuroRegen scaffolds loaded with MSCs were implanted into the spinal cord gap with the length about 1.1 cm. No obvious adverse symptoms such as infection, allergic reaction, high fever or perioperative complications related to the functional scaffolds implantation were observed post-surgery in the two patients. The intraoperative nerve electrophysiology showed that no neural signal was detected across the injured spinal cord in the patients.
Figure 2.
Intraoperative photographs of the SCI site under microscopic magnification. The images showed that the spinal cord lost its continuity and was filled with the necrosis tissue at the injured area in the thoracic SCI patient (a) and cervical SCI patient (b). (c) showed that collagen scaffolds were transplanted into the spinal cord gap of cervical SCI patient during surgery. SCI: spinal cord injury.
Functional Recovery in the Thoracic SCI Patient After Surgery
The baseline neurological examination of the patient showed a complete loss of sensory function below the injury level (T11). After surgery, the sensory function began to recover at 2 months, the sensation expanded from T11 to L2 assessed by the ASIA Impaired Scale. At 6-month follow up, the sensory score increased from 72 to 84 in the left and right side. At the patient’s 9-month follow up, the patient regained the sense function in bowel and bladder, and he was also able to empty his bladder in most times. The patient also had the sensation of deep anal pressure.
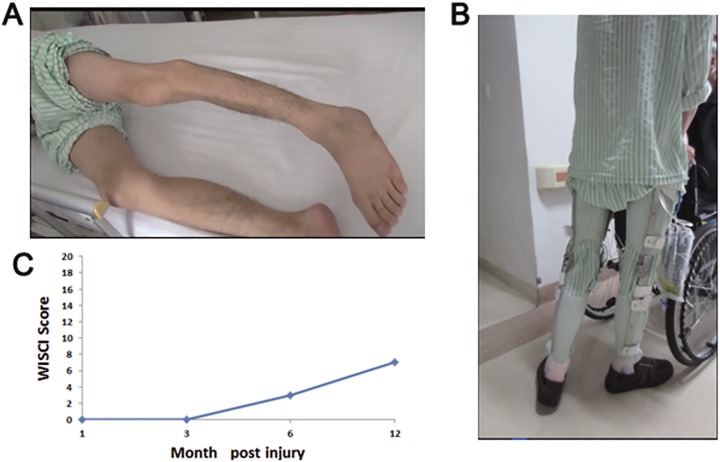
The movement of muscles below the T11 level was completely lost immediately after injury. The recovery of the muscle contraction of the adductor magnus was observed from 3 months post-surgery. The return of active movement of the hip flexors against gravity was observed from 6 months post-surgery, and the patient begun to walk under the support of brace (Figure 3(a) and (b)). The patient could walk voluntary with the hip under the help of brace at 12 months and keep this improvement at 24 months after surgery. The motor score increased from 25 to 27 in the left side and 25 to 28 in the right side. Accordingly, the WISCI score, which was used as a measure to evaluate the capacity to walk, was increased gradually from 0 point at 1 month to 7 points at 12 months after surgery (Figure 3(c)). According to this clinical improvement, the patient’s injury status was improved to ASIA C.
Figure 3.
The recovery of motor function in the thoracic SCI patient after NeuroRegen scaffolds with MSCs transplantation. The hip flexors against gravity was observed. (b) The patient begun to walk under the support of brace from 6 months post-surgery. (c) The WISCI score raised with time, it was 0, 0, 3, 7 points at 1, 3, 6, 12 months post-surgery respectively. MSC: mesenchymal stem cell; SCI: spinal cord injury; WISCI: walking index for SCI.
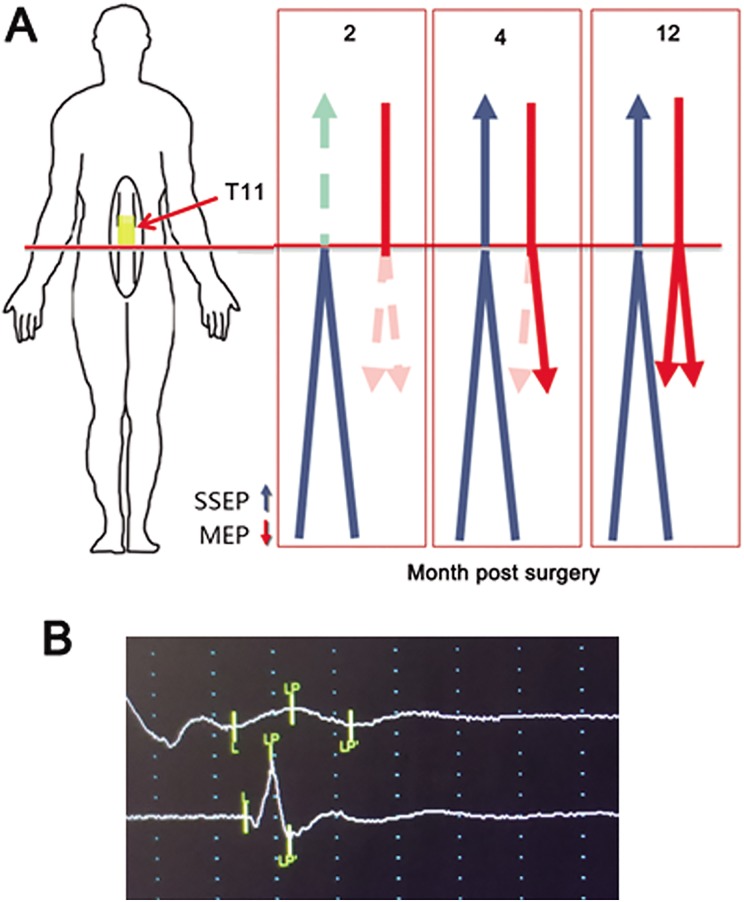
The SSEP of lower extremities could be detected from 4 months post-surgery from the ankle to the cortex, while no SSEP of lower extremities in patient was detected before surgery or 2 months post-surgery (Figure 4(a)). The MEP of the muscles in lower extremities was unable to be detected at two months post-surgery, it reappeared on the right side at 4 months post-surgery and there was significant recovery on both sides at 12 months post-surgery in adductor magnus (Figure 4(a) and (b)).
Figure 4.
The recovery of nerve electrophysiology in the thoracic SCI patient after NeuroRegen scaffolds with MSC transplantation. (a) Diagram of the recovery of SSEP and MEP. The SSEP and MEP of lower extremities could not be detected before surgery or 2 months post-surgery. The SSEP of lower extremities was detected at 4 months post-surgery. The MEP of the lower extremities reappeared on the right side at 4 months post-surgery and there was significant recovery on both sides at 12 months post-surgery. (b) The MEP of the left (upper) and right(lower) adductor magnus was significantly recovered at 12 months post-surgery.
MSC: mesenchymal stem cell; SCI: spinal cord injury; SSEP: somatosensory evoked potential.
Functional Recovery in the Cervical SCI Patient After Surgery
After surgery, the sensory function began to recover at 2 months. At 6 months post-surgery, the sensation level increased to S2 level at the left side and T10 at the right side assessed by ASIA Impaired Scale. At 9 months post-surgery, the sensation level increased to S5 level at the left side, and the sensory score increased from 46 to 110. The patient regained the accurate sense function in bowel and bladder at 12 months post-surgery, he had evidence of sacral sparing with the sensation of deep anal pressure.
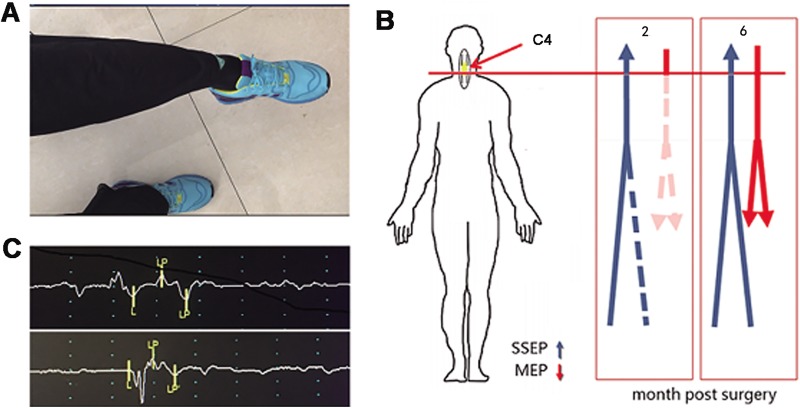
The muscles movement of lower extremities was completely lost after injury. The recovery of the movements in lower extremities began at 3 months post-surgery. At 6 months post-surgery, the patient could raise his lower legs against the gravity when sit on the wheelchair (Figure 5(a)), and he could move his toes under control, the motor score increased from 0 to 18 at the left side and 0 to 22 at the right side. It indicated that the supraspinal control of movements below the injury was partially regained. According to this clinical improvement, the patient’s injury status was improved to ASIA C at 12 months post-surgery.
Figure 5.
The recovery of the cervical SCI patient after NeuroRegen scaffolds with MSCs transplantation. (a) The patient could raise his lower leg against the gravity at 6 months post-surgery. (b) Diagram of the recovery of SSEP and MEP. The SSEP of lower extremities could be detected in left lower extremities at 2 months post-surgery, and it was detected on both sides at 6 months post-surgery accompanying with the recovery of the MEP. (c) The MEP of the left (upper) and right (lower) gastrocnemius muscles was detected at 6 months post-surgery.
MEP: motor evoked potential; MSC: mesenchymal stem cell; SCI: spinal cord injury; SSEP: somatosensory evoked potential.
The SSEP of left lower extremities began to recover at 2 months post-surgery, while no SSEP of right lower extremities was detected at the same time. The SSEP of right lower extremities reappeared at 6 months post-surgery (Figure 5(b)). The MEP of muscles in the lower extremities was unable to be detected at 2 months post-surgery, it also reappeared on both sides at 6 months post-surgery, especially in the gastrocnemius muscles (Figure 5(b) and (c)).
Discussion
ASIA A was considered as the complete injury in acute, sub-acute or chronic SCI patients. But it was not suitable to judge acute SCI patients immediately after injury because of the existence of spinal shock. In this study, a strict criterion including ASIA Impairment Scale, nerve electrophysiology and MRI was used to diagnose the patient as acute complete SCI. The two patients were firstly identified as ASIA Impairment Scale grade A. MRI also indicated that the spinal cord lost its continuity at the injury site, which was further confirmed by the observation during surgery. The intraoperative nerve electrophysiology further confirmed that there was no neural conduction was detected across the injured spinal cord. More importantly, when the patients were recovered from the spinal shock, they still showed the complete loss the movement below the injury accompanying with no detectable SSEPs and MEPs. These results all confirmed the patients as ‘complete SCI’.
Collagen has been proven a suitable biomaterial for clinical use due to its low antigenicity, excellent biocompatibility and biodegradability. MSCs could decrease cell apoptosis, promote angiogenesis and reduce lesion size in central nervous system injuries30. In addition, MSCs also could modulate the host immune microenvironment31,32. It was reported that allogeneic human MSCs transplantation showed low immunogenicity in vivo33 and a small number of patients treated with MSC transplantation showing no adverse effects34. In our previous work, we developed a collagen nerve guidance material from the bovine aponeurosis, NeuroRegen scaffolds7. When the NeuroRegen scaffolds loaded with neurotropic factors or stem cells were transplanted for treating SCI in animal models, it could reconstruct the microenvironment at the injury site to induce SCI repair in both rat and canine complete SCI models8,9,11. Then, we showed the safety and feasibility with the transplantation of collagen scaffolds with autologous BMMCs in chronic complete SCI patients17. In this study, we transplanted collagen scaffolds loaded with human MSCs into the acute complete SCI patients. During the patient’s follow up, no obvious early or late adverse events related to functional scaffolds transplantation such as infection, high fever, allergic reaction, or cancer were observed. It indicated that it is safe to treat the SCI patient with the functional collagen scaffolds.
Experimental animals and humans with incomplete spinal cord injuries often showed significant spontaneous functional recovery during the first months after injury. Such recovery may attribute to axons spared from the uninjured part or by propriospinal relay connection19,20. But it was a huge challenge to improve the functional recovery in complete SCI animals and patients. In our preclinical study, the significant locomotion improvement was observed about 3 months post-surgery in canine complete SCI models when collagen scaffolds with neurotrophic factors or stem cells were transplanted9,10,15,35. The NeuroRegen scaffolds loaded with neurotrophins or stem cells markedly induced neuronal differentiation of transplanted neural stem cells or endogenous neural stem cells in SCI rats. Moreover, the differentiated neurons could achieve neuronal relay formation throughout the lesion area. They may further rebuild the synaptic connections with each other or the host spinal neurons to transmit the neural signals and improve functional recovery in transected SCI animals10,35–37. In this study, the acute complete SCI patients also began to show striking motor function improvement after functional collagen transplantation at 3–4 months post-surgery accompanying with the recovery of SSEP and MEP of the lower extremities. The similar recovery pattern in animals and patients indicated that functional scaffolds might induce neuronal differentiation of endogenous neural stem cells to improve functional recovery in patients as in SCI animal models.
In complete SCI patients, the long-term absence of input from supraspinal often leads to degradation of neuronal function below the level of the lesion38, which results in the loss of the voluntary movement of the muscles below the injury. In our study, the thoracic SCI patient regained muscle contraction of the adductor magnus firstly. Then the return of active movement of the hip flexors against gravity was observed, and the patient could walk voluntary with the hip under the help of brace. The cervical SCI patient could raise his lower legs against the gravity and shake his toes under control. These results indicated that the voluntary movements of lower extremities in the complete SCI patient were partially regained with time, which was accompanied with the recovery of interrupted neural conduction. Furthermore, the recovery time of the voluntary movement in the two patients began at about 3–4 months post-surgery. It is different from the spontaneous recovery of the acute SCI patients in which the neurological improvement mostly happened within 3 months after injury39. The results suggested that the regained supraspinal control of movements in acute complete SCI patients was induced by functional biomaterials transplantation.
Conclusions
For the first time, NeuroRegen scaffolds combined with MSCs were transplanted to treat acute SCI patients who were judged as having ‘complete’ injury with a stricter method. The sensory and motor functions were improved significantly by transplantation of NeuroRegen scaffolds with MSCs. The injury status of the two patients was improved to ‘incomplete’ injury during 1 year of follow up. These data indicated that functional collagen scaffolds implantation could serve as an effective treatment for acute complete SCI patients.
Acknowledgments
Zhifeng Xiao, Fengwu Tang, and Yannan Zhao contributed equally to this work.
Footnotes
Ethical Approval: The clinical study was approved by ethics committee of the Affiliated Hospital of Logistics University of Chinese Armed Police Forces, Tianjin, China and registered on the National Institute of Health database (ClinicalTrials.gov: NCT02510365).
Statement of Human and Animal Rights: Guideline provisions from the Helsinki Declaration were followed. The patient was informed of the right to withdraw consent to participate at any time without reprisal.
Statement of Informed Consent: Written informed consent to participate the study and publish the results was signed. Patients were fully aware of the treatment process and possible adverse outcomes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from the Key Research Program of the Chinese Academy of Sciences (Grant No. ZDRW-ZS-2016-2) and Development Program of Ministry of Science and Technology (2016YFC1101500).
References
- 1. Fawcett JW, Schwab ME, Montani L, Brazda N, Muller HW. Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol. 2012;109:503–522. [DOI] [PubMed] [Google Scholar]
- 2. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25(5):E2. [DOI] [PubMed] [Google Scholar]
- 3. Sakiyama-Elbert S, Johnson PJ, Hodgetts SI, Plant GW, Harvey AR. Scaffolds to promote spinal cord regeneration. Handb Clin Neurol. 2012;109:575–594. [DOI] [PubMed] [Google Scholar]
- 4. Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res. 2015;10(5):726–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shrestha B, Coykendall K, Li Y, Moon A, Priyadarshani P, Yao L. Repair of injured spinal cord using biomaterial scaffolds and stem cells. Stem Cell Res Ther. 2014;5(4):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuhrmann T, Anandakumaran PN, Shoichet MS. Combinatorial therapies after spinal cord injury: how can biomaterials help? Adv Healthc Mater. 2017;6(10). Doi: 10.1002/adhm.201601130 [DOI] [PubMed] [Google Scholar]
- 7. Lin H, Chen B, Wang B, Zhao Y, Sun W, Dai J. Novel nerve guidance material prepared from bovine aponeurosis. J Biomed Mater Res A. 2006;79(3):591–598. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Han J, Zhao Y, Ding W, Wei J, Han S, Shang X, Wang B, Chen B, Xiao Z, Dai J. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl Mater Interfaces. 2015;7(25):13960–13971. [DOI] [PubMed] [Google Scholar]
- 9. Han S, Wang B, Jin W, Xiao Z, Li X, Ding W, Kapur M, Chen B, Yuan B, Zhu T, Wang H, Wang J, Dong Q, Liang W, Dai J. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials. 2015;41:89–96. [DOI] [PubMed] [Google Scholar]
- 10. Li X, Xiao Z, Han J, Chen L, Xiao H, Ma F, Hou X, Li X, Sun J, Ding W, Zhao Y, Chen B, Dai J. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials. 2013;34(21):5107–5116. [DOI] [PubMed] [Google Scholar]
- 11. Han Q, Jin W, Xiao Z, Ni H, Wang J, Kong J, Wu J, Liang W, Chen L, Zhao Y, Chen B, Dai J. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials. 2010;31(35):9212–9220. [DOI] [PubMed] [Google Scholar]
- 12. Fan J, Xiao Z, Zhang H, Chen B, Tang G, Hou X, Ding W, Wang B, Zhang P, Dai J, Xu R. Linear ordered collagen scaffolds loaded with collagen-binding neurotrophin-3 promote axonal regeneration and partial functional recovery after complete spinal cord transection. J Neurotrauma. 2010;27(9):1671–1683. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, Honjoh K, Johnson WE, Baba H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33(6):1902–1914. [DOI] [PubMed] [Google Scholar]
- 14. Vawda R, Fehlings MG. Mesenchymal cells in the treatment of spinal cord injury: current & future perspectives. Curr Stem Cell Res Ther. 2013;8(1):25–38. [DOI] [PubMed] [Google Scholar]
- 15. Han S, Xiao Z, Li X, Zhao H, Wang B, Qiu Z, Li Z, Mei X, Xu B, Fan C, Chen B, Han J, Gu Y, Yang H, Shi Q, Dai J. Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine. Sci China Life Sci. 2018;61(1):2–13. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Tan J, Xiao Z, Zhao Y, Han S, Liu D, Yin W, Li J, Li J, Wanggou S, Chen B, Ren C, Jiang X, Dai J. Transplantation of HUC-MSCS seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci Rep. 2017;7:43559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao Z, Tang F, Tang J, Yang H, Zhao Y, Chen B, Han S, Wang N, Li X, Cheng S, Han G, Zhao C, Yang X, Chen Y, Shi Q, Hou S, Zhang S, Dai J. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci China Life Sci. 2016;59(7): 647–655. [DOI] [PubMed] [Google Scholar]
- 18. Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2(4):263–273. [DOI] [PubMed] [Google Scholar]
- 19. Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, Nout YS, Roy RR, Miller DM, Beattie MS, Havton LA, Bresnahan JC, Edgerton VR, Tuszynski MH. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13(12):1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Illis LS. Central nervous system regeneration does not occur. Spinal Cord. 2012; 50(4):259–263. [DOI] [PubMed] [Google Scholar]
- 22. Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74(5):777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yokota K, Kobayakawa K, Kubota K, Miyawaki A, Okano H, Ohkawa Y, Iwamoto Y, Okada S. Engrafted neural stem/progenitor cells promote functional recovery through synapse reorganization with spared host neurons after spinal cord injury. Stem Cell Reports. 2015;5(2):264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theodore N, Hlubek R, Danielson J, Neff K, Vaickus L, Ulich TR, Ropper AE. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery. 2016;9(2):E305–E312. [DOI] [PubMed] [Google Scholar]
- 25. Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, Satti TM, Shahbaz N, Khan MA, Malik SA. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase i pilot study. Cytotherapy. 2016;18(4):518–522. [DOI] [PubMed] [Google Scholar]
- 26. Ditunno JF, Little JW, Tessler A, Burns AS. Spinal shock revisited: a four-phase model. Spinal Cord. 2004;42(7):383–395. [DOI] [PubMed] [Google Scholar]
- 27. Tu Y, Chen C, Sun HT, Cheng SX, Liu XZ, Qu Y, Li XH, Zhang S. Combination of temperature-sensitive stem cells and mild hypothermia: a new potential therapy for severe traumatic brain injury. J Neurotrauma. 2012;29(14):2393–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, Chen B, Jiang X, Yun C, Han W, Zhao C, Cheng S, Zhang S, Dai J. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017;26(5):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M, Ditunno JF. Walking index for spinal cord injury version ii in acute spinal cord injury: reliability and reproducibility. Spinal Cord. 2014;52(1):65–69. [DOI] [PubMed] [Google Scholar]
- 30. Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–619. [DOI] [PubMed] [Google Scholar]
- 31. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. [DOI] [PubMed] [Google Scholar]
- 32. Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012;33(3):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim JS, Jeon HB. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014;446(4):983–989. [DOI] [PubMed] [Google Scholar]
- 34. Mothe AJ, Tator CH. Advances in stem cell therapy for spinal cord injury. J Clin Invest. 2012;122(11):3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X, Zhao Y, Cheng S, Han S, Shu M, Chen B, Chen X, Tang F, Wang N, Tu Y, Wang B, Xiao Z, Zhang S, Dai J. Cetuximab modified collagen scaffold directs neurogenesis of injury-activated endogenous neural stem cells for acute spinal cord injury repair. Biomaterials. 2017;137:73–86. [DOI] [PubMed] [Google Scholar]
- 36. Li X, Han J, Zhao Y, Ding W, Wei J, Li J, Han S, Shang X, Wang B, Chen B, Xiao Z, Dai J. Functionalized collagen scaffold implantation and camp administration collectively facilitate spinal cord regeneration. Acta Biomaterialia. 2016;30:233–245. [DOI] [PubMed] [Google Scholar]
- 37. Xiao Z, Chen B, Dai J. Building the regenerative microenvironment with functional biomaterials cord injury repair. J Spine. 2016;S7:005. [Google Scholar]
- 38. Dietz V. Behavior of spinal neurons deprived of supraspinal input. Nat Rev Neurol. 2010;6(3):167–174. [DOI] [PubMed] [Google Scholar]
- 39. Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: Spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190–205. [DOI] [PubMed] [Google Scholar]