Abstract
Autologous olfactory ensheathing cell (OEC) transplantation is a promising therapy for spinal cord injury; however, the efficacy varies between trials in both animals and humans. The main reason for this variability is that the purity and phenotype of the transplanted cells differs between studies. OECs are susceptible to modulation with neurotrophic factors, and thus, neurotrophins can be used to manipulate the transplanted cells into an optimal, consistent phenotype. OEC transplantation can be divided into 3 phases: (1) cell preparation, (2) cell administration, and (3) continuous support to the transplanted cells in situ. The ideal behaviour of OECs differs between these 3 phases; in the cell preparation phase, rapid cell expansion is desirable to decrease the time between damage and transplantation. In the cell administration phase, OEC survival and integration at the injury site, in particular migration into the glial scar, are the most critical factors, along with OEC-mediated phagocytosis of cellular debris. Finally, continuous support needs to be provided to the transplantation site to promote survival of both transplanted cells and endogenous cells within injury site and to promote long-term integration of the transplanted cells and angiogenesis. In this review, we define the 3 phases of OEC transplantation into the injured spinal cord and the optimal cell behaviors required for each phase. Optimising functional outcomes of OEC transplantation can be achieved by modulation of cell behaviours with neurotrophins. We identify the key growth factors that exhibit the strongest potential for optimizing the OEC phenotype required for each phase.
Keywords: autologous transplantation, glia, growth factors, cell proliferation, neuron
Introduction
Spinal cord injury (SCI) can lead to permanent damage for which there is currently no cure. SCI causes damage to neural tissue, initially due to the direct trauma, which then progresses due to a series of secondary cellular events causing further damage. After injury, local inflammation, ischemia, and oxidative stress result in expansive cell death and damage at the SCI site1. Subsequently, reactive astrocytes undergo hypertrophy, proliferate, and migrate to the injury site. They then create a glial scar that impedes growth and reinnervation of neurons in this area and which acts as a tertiary lesion1–4.
A promising therapy for SCI is the autologous transplantation of olfactory ensheathing cells (OECs), the glial cells of the primary olfactory nervous system. OECs are taken from the olfactory epithelium of the nasal cavity, cultured in vitro, and then transplanted into the damaged SCI site (Fig. 1)5. OECs are present in the primary olfactory nervous system, which comprises the olfactory nerve and the nerve fiber layer (NFL) of the olfactory bulb (OB). OECs naturally promote the continuous regeneration of the olfactory nerve that occurs throughout life and therefore exhibit unique growth-promoting properties. OECs are also capable of migrating long distances into and interacting with astrocytic glial scar tissue3, as well as with other cells that may be present in the injury site6, resulting in a 3-dimensional framework conducive to axonal extension. This developing treatment has been trialed in rats, dogs, and humans, where it has been shown to be safe and capable of promoting functional repair in the form of motor and sensory innervation and allowing for weight bearing movement to varying levels of success7–11. However, in order to create a therapeutic intervention capable of providing consistent results, autologous OEC transplant therapies must be improved.
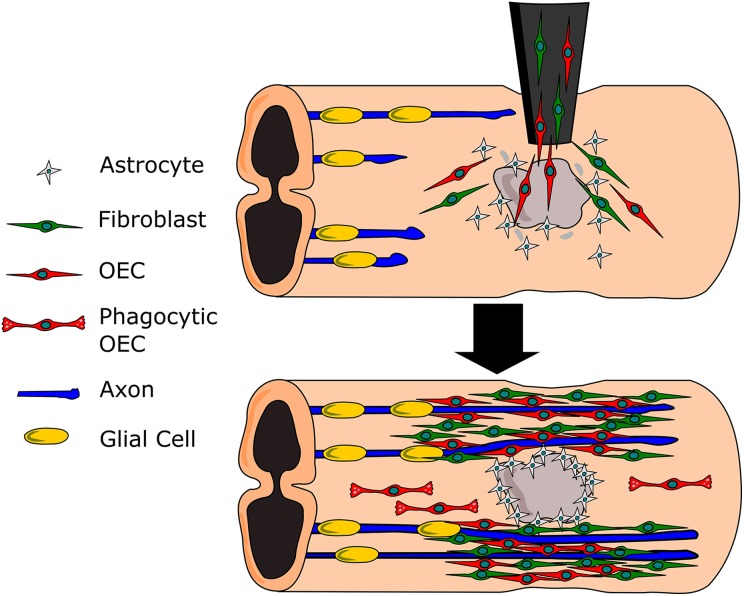
Fig. 1.
Olfactory ensheathing cells (OECs) and fibroblasts administered to a Schwann cell site (gray). The mixed cell culture supports and ensheathes the regenerating axons. OEC phagocytose scar and damaged tissues.
There are many reasons why outcomes of OEC intervention for spinal cord repair vary from trial to trial. There are several broadly different methods for inducing SCI in animal models including hemisection, transection, and contusion injuries, which all have different effects on the extent of injury. The injuries can all be performed at various cervical and thoracic levels which again lead to variations in outcomes of the OEC intervention. With respect to the use of OECs themselves, discrepancies between preclinical trial results can be broadly attributed to (1) exact anatomical source of the OECs (different subpopulations of OECs exist with distinct biological properties12), (2) OEC purity, and (3) OEC survival rates after transplantation.
As a first step toward improving outcomes and consistency in the use of OECs, the purification, survival, and behavior of OECs could be optimized for transplantation. One proposed method of achieving reproducibility is by using neurotrophins, for which OECs express a multitude of receptors, to promote OEC survival and to drive the cellular behaviors into those most favorable for transplantation and regeneration.
A number of studies have highlighted the potential of exploiting OEC characteristics for therapeutic use within the injured spinal cord. Within the olfactory system environment, OECs aid and support the extension of axons from olfactory sensory neurons which reside in the olfactory mucosa in the peripheral nervous system (PNS). The OECs guide the axons up to the OB within the central nervous system (CNS) where the OECs then contribute to the sorting of olfactory axons to their appropriate targets within the olfactory bulbar glomeruli as dictated by the olfactory receptor profile of the neurons13–15. Furthermore, OECs can infiltrate scar tissue and astrocytic environments. Due to their unique heparin profile3, OECs can interact and intermingle with astrocytes in ways that Schwann cells (SCs) and oligodendrocytes cannot. This feature may allow OECs to disperse throughout the glial scar which is formed following SCI, providing a scaffold for neuronal growth through an otherwise neurotoxic environment. Furthermore, OECs have been shown to possess phagocytic capabilities16,17. In the olfactory system, this capability allows for the removal of axonal debris during early development as well as a mechanism for bacterial removal18; however, within the injured spinal cord, this may allow for removal of cytotoxic debris that can inhibit the regeneration of neurons. Further studies into OEC properties have demonstrated facilitation of axon extension19, secretion of neuroprotective and stimulatory proteins which can enhance the properties of neurons and other glia20,21, and an ability to integrate and support other cell types22. Within the context of SCI, these cellular behaviors may prove ideal for supporting and guiding axonal regeneration to reform severed connections. However, what is now needed is to further optimize these features to enhance the therapeutic potential of OECs and to achieve consistency between treatments.
OECs express a variety of neurotrophin receptors including p75 neurotrophin receptor (P75NTR); tropomyosin receptor kinase A (TrkA), tropomyosin receptor kinase B (TrkB), and tropomyosin receptor kinase C (TrkC); 2 types of glial-derived neurotrophic factor (GDNF) family receptors: GDNF receptor alpha 1 (GFRα-1) and the tyrosine kinase receptor RET; and the fibroblast growth factor (FGF) receptor 1 (FGFR1; Table 1). Neurotrophins acting on these receptors have been shown to promote favorable OEC behaviors, in particular cell migration and cell–cell interactions19,37, as well as influence the survival and viability of neurons and their axonal extensions38,39. Additionally, many of these receptors are also present on neurons, oligodendrocytes, SCs, astrocytes, and other supporting cells found throughout the nervous system. Using neurotrophins as therapeutic agents may not only be utilized to target transplanted OECs but also to aid in the regeneration of neurons at the injury site—a process involving modulation of these other cell types.
Table 1.
Receptor Expression and Corresponding Cell Types.
| Neurotrophin Receptor | Cell Type | Neurotrophin | References |
|---|---|---|---|
| TrkA | Neurons | NGF and NT-3 | 23 |
| TrkB | Neurons and OECs | BDNF, NT-3, and NT-4 | 23 |
| TrkC | SCs, neurons, and OECs | NT-3 | 23,24 |
| P75NTR | SCs, OECs, and dorsal root ganglion neurons | BDNF and NGF | 24–26 |
| GFRα-1 | SCs, OECs, and neurons | GDNF | 27–30 |
| RET | OECs and neurons | GDNF | 29,30 |
| VEGFR2 | Blood vessel endothelial cells | VEGF | 31,32 |
| EGFR | Activated astrocytes and oligodendrocytes | EGF | 33,34 |
| FGFR1 | Astrocytes, OEC, SCs, and fibroblasts | FGF2 | 3,35 |
| PDGFRα | Fibroblasts, oligodendrocytes, and astrocytes | PDGF-AA and PDGF-BB | 36 |
Abbreviations: TrkA, tropomyosin receptor kinase A; TrkB, tropomyosin receptor kinase B; TrkC, tropomyosin receptor kinase C; P75NTR, p75 neurotrophin receptor; GFRα-1, GDNF receptor alpha 1; RET, receptor tyrosine kinase; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; OEC, olfactory ensheathing cell; SCs, Schwann cells; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; NT, neurotrophin; GDNF, glial-derived neurotrophic factor; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor 2; EGF, epidermal growth factor; FGF2, fibroblast growth factor 2; PDGF-AA, dual alpha-subunit of PDGF; PDGF-BB, dual β-subunit of PDGF.
Using Neurotrophins as Therapeutic Agents in SCI Repair
Using OECs to repair the injured spinal cord involves (1) harvesting and expansion of OECs, then (2) transplantation followed by (3) integration and survival of the cells at the site of injury40. All 3 phases must be successful to achieve a significant functional outcome. Through the use of neurotrophins, it may be possible to enhance the outcomes of all 3 of these phases, with a combination of neurotrophins being tailored to the requirements of each phase. Thus, neurotrophins can (1) be applied to OECs following biopsy to promote proliferation in preparation for transplantation, (2) be coadministered along with OECs to support the cells during transplantation, and (3) act in the longer term at the SCI site, leading to greater integration and survival of OECs following transplantation. Within the final phase, the neurotrophins may also exert important effects on other cell types, such as cell–cell interactions or remyelination, as well as on the extracellular environment, for example, altered growth factor profile of the extracellular matrix.
OEC Proliferation and Preparation Prior to Transplantation into the Injured Spinal Cord
Key factors accounting for the variable outcomes of OEC transplantation are the quality, purity, and source of the cells—all of which may influence the phenotype and behavior of the OECs to be transplanted. A high-quality population of OECs must be prepared prior to application and should consist of cells that are (1) highly proliferative, to achieve an appropriate number of cells for transplantation in a prompt time period, allowing the transplantation to occur sooner, and (2) resilient to surviving the transplantation process—thus increasing the number of viable cells transplanted. It may be possible to manipulate this preparatory period using neurotrophins to adjust OEC phenotype, induce purification, and improve viability of OEC populations.
OEC Source and Phenotype
OECs are present throughout the entire olfactory nervous system from inside the nasal cavity to the OB14. OECs exhibit distinct roles based on their location and thus different phenotypes. OECs within the lamina propria (LP-OECs) of the olfactory mucosa are located along the length of the olfactory nerve and guide axons from olfactory sensory neurons as they project toward the OB. The OECs create channels through which the axons extend, and the channels formed by OECs are in turn encompassed by fibroblasts that act as a perineurium-like structure41. In contrast to SCs (the main glia of the PNS), OECs do not myelinate individual axons but rather ensheathe small fascicles (bundles) of unmyelinated axons in a process similar to the early stages of nervous system development42. OEC channels guide the immature olfactory sensory neuron axons through the cribriform plate into the NFL of the OB in the CNS. OECs within the NFL of the OB-OECs have characteristics different from that of the LP-OECs as they aid the sorting and directing of axons toward their appropriate glomerulus according to their respective odorant receptor (Fig. 2), for which there are 400 functionally distinct genes in humans13,14.
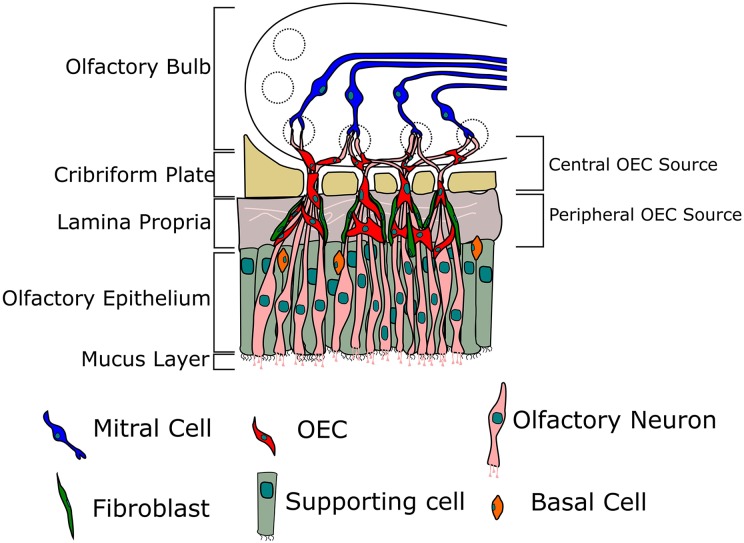
Fig. 2.
Location of olfactory ensheathing cells (OECs) within the olfactory system. Olfactory neurons extend axons from the nasal cavity through the cribriform plate and make connections within the glomeruli with mitral cells. OECs ensheathe the olfactory axons, aiding their growth and directing them to form their connections.
Both LP-OECs and OB-OECs have been used for SCI transplantation studies of animals and humans. A recent study showed that transplanting OB-OECs from the OB produced functional recovery in a 38-y-old SCI patient who had a spinal cord transection leaving an 8-mm gap in the spinal cord at thoracic vertebral level nine10. While the transplantation resulted in improved motor and sensory functional outcomes in this case, harvesting cells from the OB can cause severe side effects, such as hyposmia and depression43. For this reason, it is favorable to use a less invasive biopsy procedure to obtain LP-OECs from the olfactory mucosa5,44. It is, however, possible that OECs from distinct anatomical sources may exhibit differential ability to promote axonal regeneration. One review systematically compared multimetric outcomes (electrophysiological, behavioral, and magnetic resonance imaging results) between LP-OECs and OB-OECs and found that the 2 cell types induced comparable outcomes; however, the advantage of OB-OECs was that they do not need the same extent of purification as peripheral OECs45. Thus, both peripheral and bulb OECs exhibit advantages and disadvantages. If future studies determine that OB-OECs indeed do lead to better outcomes than LP-OECs, it is also possible that growth factor modulation can change the phenotype of LP-OECs toward a type with similar features to OB-OECs. Therefore, a thorough characterization of how growth factors modulate OECs is highly warranted in the field.
Cell Purity
Another variability between studies is the purity of the OECs. As the common biopsy region also contains fibroblasts and other contaminant cells, there have been studies focusing on purification methods for OECs46. Currently, the importance of OEC population purity for transplantation is debated, as other cell types from the olfactory epithelium may also exert important beneficial effects on neural regeneration7,10,47,48. In the previously described case showing functional improvement of a patient with thoracic level 9 SCI, OECs comprised only 16% of the cells transplanted with the majority of cells likely being fibroblasts10. Additionally, pure OECs have been suggested to be less resilient to the harsh environment of the SCI site10. However, regardless of whether a homogenous or heterogeneous OEC population is more beneficial, purifying these populations is essential so that the optimal ratio between OECs and other cell types, in particular fibroblasts, can be determined.
The aforementioned issues may be addressed using neurotrophins by favoring OEC purity through selective promotion of proliferation and by improving resilience of cells through neuroprotective effects. Neurotrophin-3 (NT-3) is currently used as a purification agent and has been shown to stimulate proliferation of OECs over other cells (Fig. 3). This method has been demonstrated to achieve a 90% to 95% pure population of OECs by passage 546. Furthermore, human OECs cultured in the presence of NT-3, demonstrated a 25% increase in cells that fit OEC receptor expression and appearance, and a 30% decrease in cells that did not46.
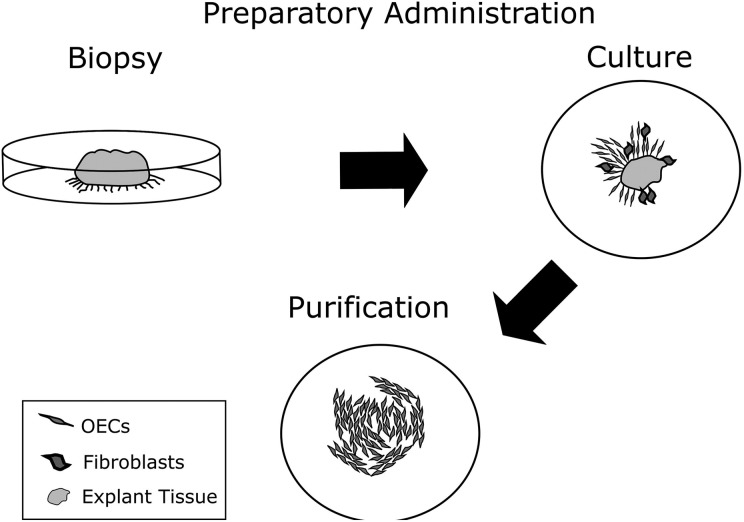
Fig. 3.
Primary olfactory ensheathing cells (OECs) are cultured from an explant tissue. Following the initial outgrowth, the explant is removed and the cells are passaged. Neurotrophin 3 may be used as a purification agent to facilitate expansion of OECs rather than fibroblasts8.
Cell Viability
An important point to consider is whether supporting the cells with neurotrophins prior to transplantation can improve the long-term quality of the OECs after transplantation. While OECs may thrive in vitro, following transplantation, around 99% of the transplanted cells die49. This issue may also be addressed by manipulating the cells with neurotrophins in culture to improve cellular resilience to pathological and noxious environments following transplantation. To date, relatively little is known regarding which neurotrophins enhance the viability of OECs. NT-3 is likely to aid in this process as it promotes proliferation and survival of OECs (discussed earlier). NT-3 also exerts beneficial effects on other neural cells; for example, it has been shown to combat apoptosis and prolong survival of chronically injured neurons when applied locally to an SCI site together with OECs50. Additionally, OECs transfected to express higher levels of NT-3 have been shown to induce a much greater recovery in an injured rat model compared to NT-3 or OECs alone51. While it is possible that NT-3 has a direct effect on OEC viability, this has not been proven experimentally to date.
In addition to NT-3 and GDNF, OECs secrete nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). LP-OECs and OB-OECs from the outer NFL express the NGF receptor P75, along with TrkA, TrkB, and TrkC which are receptors for NGF, BDNF, and NT-3, respectively52–54. Thus, while NGF may exert a regulatory role on OECs, the OEC features and behaviors regulated by NGF remain unknown. NGF is known to exert a neuroprotective role on other nervous system cells, for example, inhibiting apoptosis in a neural cell line (PC12 cells) via binding to the TrkA receptor55. Conversely, while NGF has been demonstrated to be neuroprotective, it has also been shown to play a role in the stimulation of apoptosis in other cell types. This variable effect is due to the differential binding affinities of the Trk receptors and P75NTR. For example, NGF, BDNF, and NT-3 bind to TrkA, TrkB, and TrkC, respectively, but also have low-affinity binding with P75NTR. P75NTR has been linked with effects of cell survival, apoptosis, proliferation, differentiation, regeneration, axonal extension, and neuronal outgrowth depending on the cells that express them56–59. The range of pathways and cobinding involving these receptors can lead to opposing effects depending on several factors including cell health, ceramide presence, neurotrophin concentration, and others4,59,60. To date, the effects of growth factors on OECs, in particular on different OEC populations, remain to be determined. In summary, NT-3, NGF, GDNF, and BDNF may all play important regulatory roles relating to OEC viability, but this remains to be systematically determined.
Coadministration of Neurotrophins during OEC Transplantation
Although cell quality may be improved by pretreatment with neurotrophins, neurotrophic factors can also be coadministered during transplantation. This can address the issue of cell death primarily in 2 ways: (1) by stimulating protective pathways preventing cell death and (2) by stimulating cell proliferation to promote cell replenishment. Furthermore, neurotrophin stimulation can promote cell–cell interactions, resulting in better cell survival, contact-mediated migration, and integration6. The successful use of neurotrophins during the transplantation process should aid in the increase of the cellular resilience, decrease in the cytotoxic environment, and promote the successful integration of the transplanted cells.
Neuroprotection during Transplantation
NGF would appear to be a possible candidate for coadministration with OECs. NGF exhibits protective effects against TrkA-positive cells such as neurons and may be able to further support neurons in addition to the effects of OECs55. However, NGF binding to P75NTR during stress stimulates a stress-related pathway. In this pathway, ceramide, (a lipid molecule found in the cell membrane), phosphorylates the stress-activated protein kinase (SAPK), the downstream effect of which is cell death. Therefore, NT-3 may be a more appropriate candidate as it has demonstrated clear effects on OECs that are likely to be beneficial in vivo46,55.
Phagocytosis during Transplantation
As mentioned previously, following cell transplantation, most cells will not survive49. In addition to being counterproductive to transplantation efforts, this cell death leaves a large swath of toxic cellular debris within the transplantation site, which can be detrimental to neuron growth. One aspect of glial cell biology that has only recently been characterized is glial-mediated phagocytosis. Phagocytosis of axonal debris is a crucial aspect of the continuous regeneration that occurs in the olfactory nerve, and it is a property of OECs that may be particularly important in spinal cord regeneration61. OECs are highly phagocytic61, and so by engulfing this cellular debris, OECs create an environment more favorable for axonal regeneration. Increasing the phagocytic capability of OECs may therefore be highly beneficial for functional outcomes following transplantation, and thus identifying neurotrophins that enhance this effect is highly warranted.
To date, the effects of neurotrophins on phagocytosis are largely unknown. Natural products have been utilized to increase phagocytic potential of OECs, which is highly correlated with increased activity of lamellipodial (membranous) extensions62. While the direct effect on phagocytosis by growth factors is not yet characterized, neurotrophins capable of inducing lamellipodia, such as GDNF6, may also enhance the phagocytic potential of OECs. Additionally, NGF and BDNF play a neuroprotective role and can cause behavioral changes in OECs via the Mitogen-activated protein kinase kinase / extracellular signal-related kinase (Mek/Erk) pathways63 which also regulate phagocytic activity62. While the possibility of these neurotrophins influencing the phagocytic activity of OECs has not been investigated, they present a possible starting point for research into using neurotrophins to manipulate OECs into a state that is conducive for consistent functional SCI repair.
Modulation of the Inflammatory Response within the SCI Site by Transplanted OECs
In addition to phagocytosing debris, which may aid in clearing the injury site, OECs may modulate the endogenous inflammatory response (mainly mediated by astrocytes and microglia) in a manner favoring neural regeneration. Microarray analyses have shown that OECs express a large number of cytokines involved in the recruitment of immune cells64–66, suggesting that OECs do indeed interact with immune cells and regulate inflammatory responses. After injury (bulbectomy), OECs upregulate expression of interleukin 6 and its receptor, which may play a key role both in mediated axonal regeneration and in modulation of inflammation67,68.
The presence of OECs does not induce stress responses in astrocytes69. In fact, the presence of OECs reduced the expression of glial fibrillary acidic protein (GFAP; a marker of reactive astrocytes) in an in vitro model of astrogliosis70. One study compared the expression of proinflammatory cytokines and markers of astrocyte reactivity between OEC-transplanted and mock-transplanted rats with SCI. OEC-transplanted rats exhibited an earlier peak in the immunoreactivity of inducible nitric oxide synthase, GFAP (a marker of reactive astrocytes), and tomato lectin (a marker of microglia) than mock-transplanted animals, suggesting that one of the mechanisms by which OECs are neuroprotective is by causing an earlier, shorter immune response by astrocytes and microglia71. OECs have also been shown to attenuate stimulated nuclear factor κβ translocation in astrocytes, a key response involved in astrocyte activation/inflammation, along with expression of the proinflammatory cytokine granulocyte macrophage colony-stimulating factor. It is thought that insulin-like growth factor-1 secreted by OECs contributes to this modulation of astrocyte activation72.
Thus far, the mechanisms by which OECs may modulate the inflammatory state and activation of astrocytes and microglia after transplantation into the injured cord remain a largely unexplored field. In particular, it remains unknown how neurotrophins regulate OEC-mediated modulation of inflammation. The astrocyte scar is detrimental for axonal regeneration, and thus a characterization of how OECs modulate inflammatory cells within the injury site may lead to significantly enhanced therapeutic potential of transplanted OECs.
Cell Transplant Integration
A successful therapeutic outcome following OEC transplantation into an SCI site is highly dependent on the ability of the transplanted cells to integrate into the transplantation site. OECs have shown to rely upon cell–cell contact for growth stimulation, and so a neurotrophin that encourages this action would be beneficial for integration of the transplanted cells within the SCI site. GDNF is an interesting candidate with already proven beneficial effects on OECs, such as membrane protrusions (so-called lamellipodial waves) that promote cell–cell contacts, resulting in contact-mediated migration6,8,39. These features may allow the transplanted OECs to penetrate into the SCI site and integrate following transplantation6,8. Additionally, the total membrane protrusion surface area seen in OECs has been shown to increase up to 13 times following GDNF administration, resulting in a contact-mediated migration and the formation of a cellular network6. Similarly, at lower concentrations (10 to 20 ng/mL), OECs have been shown to decrease intercellular distance and form tight chains of adherent cells which act as a growth substrate for neurons to follow during regeneration73. As OECs are capable of intermingling with reactive astrocytes found in the astrocytic scar, OEC chain formations may allow axonal extension through this otherwise impenetrable environment3,21. Furthermore, GDNF is strongly implicated in the survival of dopaminergic and autonomic neurons, where it dramatically increases neurite differentiation and outgrowth at a half-maximal effect concentration of 1.2 pM38. Thus, GDNF is unlikely to have detrimental effects on cells endogenous to the injury site.
BDNF is a neurotrophin secreted from OECs, dorsal root ganglion neurons, and myocytes and is a promising neurotrophic agent for SCI therapies when used alone or in conjunction with OEC transplants25,74,75. BDNF has been implicated in neuroprotective and morphological changes76. For example, BDNF has been shown to stimulate thicker cellular processes of retinal ganglion cells (RGCs) and has greatly increased RGC migration in scratch assays77. Another study showed that following transplantation of OECs into a transected optic nerve, the RGC life span was increased, correlating with the concentration of BDNF secreted from OECs. This suggests that the key to increased RGC survival was both the presence of OECs and OEC-secreted BDNF. In the PNS, BDNF secretion can promote migration and myelination by SCs. Signaling through GTPase Ras-related C3 botulinum toxin substrate 1 (Rac1) influence myelination by SCs25,78. Increasing levels of active Rac1 are caused by upregulated 3’,5’-cyclic adenosine monophosphate (cAMP) production due to BDNF stimulation. These high levels of Rac1 have been shown to produce radial lamellae in SCs—a phenotype that is highly conducive to myelination and sorting of axons79. By increasing cAMP production further through the addition of forskolin (which stimulates cAMP production), it may be possible to amplify the BDNF-induced myelination; however, further investigation into whether these same myelinating effects can be seen in OECs is still required78,80.
Effects of Neurotrophins at the SCI Site Following Transplantation
A continued regimen of neurotrophins, applied postsurgery, could be used to further support the SCI patient during their recovery. In addition to being used during OEC expansion in vitro and as a supportive compound during transplantation, neurotrophins could be used to support the greater injury site area. Neurons, astrocytes, oligodendrocytes, and surrounding cells all express a variety of neurotrophic receptors which may be used to manipulate the SCI site to promote neurogenesis, transplant integration, and angiogenesis.
Neurons within the SCI Site
As the primary goal of SCI therapies focusses on the regeneration of neurons, neurotrophins that aid the regeneration and migration of neurons are the factors most likely to be beneficial to repair67,81. One candidate neurotrophin beneficial to repair might be NT-3. It has been demonstrated that transplanted OECs genetically modified to produce high amounts of NT-3 cause significantly greater axonal regeneration and neuronal sprouting in a thoracic 9 contusion SCI site than control OECs51. Furthermore, in RGCs, BDNF has been shown to stimulate thicker cellular processes, greatly increase migration, and extend survival of RGCs following injury suggesting that the same effects may be seen in neurons found in an SCI site77. Fibroblast growth factor 2 (FGF2) has been shown to play a role in neuronal maturation which may also be applied to a SCI site for therapeutic benefit. Similarly, FGF2 (25 ng/mL) has been shown to stimulate the maturation of bipolar ganglionic cells from embryonic rat cochlear ganglion tissue82,83.
Astrocytes within the SCI Site
One of the features that makes OECs a valuable transplant candidate for further investigation is that they possess the unique ability to interact with astrocytes while other glial cells do not3. This phenotype is attributed to the interaction of FGF2 and heparin molecules on the target cells, which are cell-type specific82,84. In contrast, SCs do not naturally interact with astrocytes. However, inhibition of FGFR1, which specifically inhibits the binding of FGF2, leads to an increased mingling of SCs with astrocytes. Additionally, the use of heparinase (a heparin sulfate digestive enzyme) results in the removal of the boundary between the 2 cell types3,85,86. The degradation of the boundary between SCs and astrocytes is due to the interaction of FGF with the heparin sulfate proteoglycan profile of which SCs and OECs are different3. These results show that while neurotrophins play a beneficial role for 1 cell type, such as promoting survival or migration of OECs, in other circumstances they can be detrimental to the efforts of SCI treatment, for example, by preventing cell integration.
Oligodendrocytes within the SCI Site
Following SCI, a large number of oligodendrocytes die49. Stimulating the survival or proliferation of these cells to support recovering neurons through remyelination and continued survival could be an important step in SCI recovery. It has previously been shown that EGF (20 ng/mL) when applied to injured oligodendrocytes can stimulate the outgrowth of myelinating lamellipodial processes87. Interestingly, this feature is not seen in developing or mature oligodendrocytes, only in damaged cells. Conversely, EGF has been demonstrated to suppress the expression of myelin basic protein expression in developing oligodendrocytes and could cause issues with oligodendrocyte precursors found within the SCI site1,88.
Angiogenesis within the SCI Site
As previously discussed, GDNF would be a useful candidate for a coadministrative approach to transplantation. However, GDNF also possesses properties that would be beneficial after the cells have been transplanted, both for the transplanted cells and for the cells at the injury site. The role of angiogenesis in SCI repair is often overlooked; however, the reinnervation of blood supply to regenerating tissue is important for supporting the newly generating neurons and glia. While OECs have previously been shown to encourage angiogenesis89, GDNF additionally stimulates an increase in the number of blood vessels compared to a control19,39. Similarly, NGF and VEGF demonstrate an ability to stimulate angiogenesis. NGF demonstrates an ability to increase angiogenesis in ischemic hindlimbs90, and VEGF can stimulate vascular sprouting in endothelial cells when under hypoxic conditions91–93.
A variety of neurotrophins could be applied to the SCI therapy. As autologous OECs constitute a promising therapy option, supporting them following transplantation using NGF, BDNF, NT-3, and GDNF may improve the efficacy of this treatment. Additionally, by influencing cell comingling and angiogenesis using the FGF family of neurotrophins, VEGF, and GDNF, it may be possible to further support the SCI site by improving nutrient innervation within the damaged tissues. If effects of neurotrophins on different cell types in a multicellular environment can be defined, the outcomes may also pave the way for enhancing therapies using other transplanted cell types, such as SCs, oligodendrocytes, and neural stem cells, perhaps in combination with OECs. Neurons, SCs, and oligodendrocytes all express a different profile of neurotrophin receptors as shown in Table 1.
Future Avenues for Optimization of OEC Transplantation Using Neurotrophins
The neurotrophins discussed in this review and their potential applications in SCI therapies offer an avenue by which the therapeutic potential of transplanted OECs may be significantly enhanced. However, the process of defining a cocktail of neurotrophins to be used to complement OEC transplantation may be hindered by unforeseen interactions between these different proteins. For this reason, the application of neurotrophins in phases may provide the least complications with this treatment. Additionally, application outside of these proposed phases may further aid the patient, for example, as an emergency application following injury or used prior to biopsy so that the greatest yield of functional cells can be achieved.
Compounding Neurotrophic Effects
As discussed so far, there are several neurotrophins that are applicable in different stages of the transplantation process; however, examining the compounding effects of these different neurotrophins has not been thoroughly investigated. Understanding how neurotrophins interact with each other may provide insight into how to achieve the greatest effect. For example, when exposed to FGF2, platelet-derived growth factor receptor alpha (PDGFRα) expression has been shown to increase. When FGF2 administration was followed with PDGF administration, the resulting increase of mitogenic activity was 30% greater than PDGF alone94. Similarly, EGF has shown altered effects when combined with another growth factor. EGF (10 ng/mL) has been shown to promote cell proliferation and differentiation within the basal olfactory neuroepithelium. However, when transforming growth factor beta (TGFβ-2) is used in conjunction with EGF, neuron differentiation is stimulated whereas EGF alone encourages fibroblast differentiation33,95. By exploring the use of EGF with varying levels of TGFβ-2, it may be possible to cotransplant OECs and basal cells and then further differentiate neurons and fibroblasts gradually in the days following transplantation, replicating the regenerative abilities of the olfactory system at the level of the cytoenvironment.
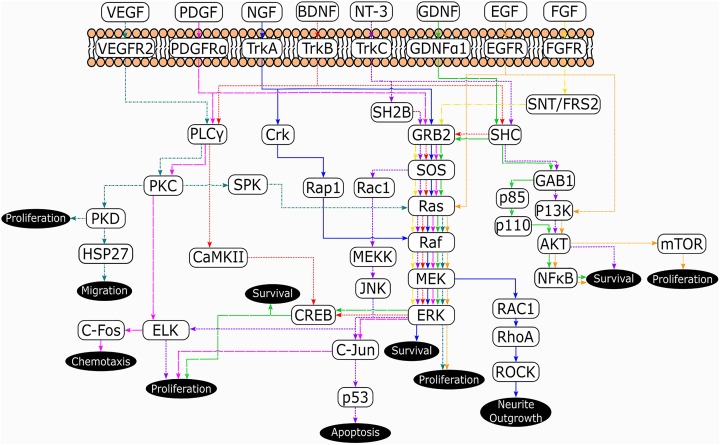
The modulation of neurotrophic factor receptors by other neurotrophins is an area that requires further research. As many of the target cells possess more than a single neurotrophin receptor, understanding the interplay between these receptors could prove important in the division of an effective treatment. Several of the signaling pathways for different neurotrophins share elements (Fig. 4). In particular, the GRB2-SOS-Ras-Raf-MEK-ERK pathway is shared by all neurotrophins. There are also other similarities such as PDGFRα, VEGFR2, and TrkB signaling via phospholipase C gamma-protein kinase C (PLCγ-PKC), and TrkA, and GFRα1/Ret signaling via RAC1-RhoA. While what is presented in Fig. 4 is a simplified signaling map, it demonstrates the crossover between several neurotrophin signaling cascades and so interaction between these proteins should be further characterized before being applied in future treatments32,52–54,96–98.
Fig. 4.
Simplified pathway map of the neurotrophic signaling cascades described in this review and their subsequent effects. Source: Adapted from references 32, 52–54, and 96–98.
Neurotrophins for Emergency Administration
As with many acute injuries, addressing an SCI quickly following injury yields the greatest functional outcome. However, beyond providing therapeutic hypothermia99, there is little that can be done to diminish the secondary damage that will occur following SCI. However, neurotrophins may be suited to decreasing this secondary damage when applied acutely. The dual β-subunit of PDGF (PDGF-BB) has been previously used to ameliorate ischemic stroke damage in a dose-dependent manner. Additionally, PDGF-BB has been shown to decrease neuronal death in forebrain ischemia, improving ischemic outcome. While this has not been investigated in the spinal cord, these anti-ischemic properties of PDGF may be translatable to SCI100. An interesting combinatorial effect of PDGF with VEGF has been observed for acute SCI, where the combination of the 2 growth factors resulted in a dramatic reduction of secondary degeneration compared to when either growth factor alone was applied to the injury site101,102. This combinatorial effect of growth factors highlights the importance of considering multiple outcomes on different cell types when using growth factors.
Conclusion
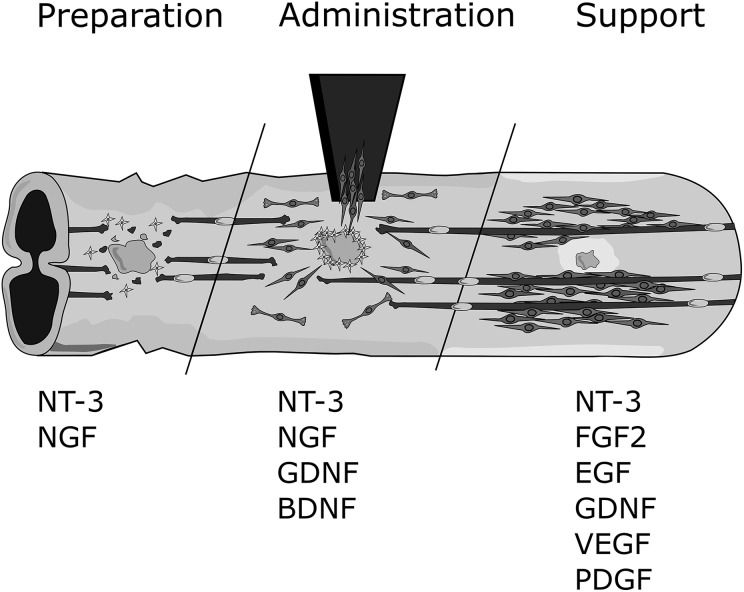
OEC transplantation is a very promising therapy for SCI; however, results are highly variable and the method needs improvement. Neurotrophins can stimulate favorable behaviors in OECs, such as proliferation, migration, phagocytosis, and interaction with other cells. Prior to transplantation, neurotrophins can be used to optimize the health, quality, and purity of OECs. Coadministration of neurotrophins with OECs at the time of transplantation may also be beneficial; here, the neuroprotective factors NGF and NT-3 are likely candidates. GDNF can also be used in this stage of treatment, by aiding the migration and integration of OECs into the SCI site. Finally, neurotrophins may be used as general support for the SCI environment in the longer term. NT-3 (neuroprotection), BDNF (neuronal survival), FGF2 (astrocyte–OEC integration), NGF, GDNF, VEGF, and PDGF (vascularization) are all promising candidates for this purpose (Fig. 5).
Fig. 5.
Summary of neurotrophins and their application in spinal cord injury (SCI). The potential roles for key neurotrophins during the 3 phases of olfactory ensheathing cell (OEC) transplantation therapy for SCI (cell preparation, cell administration, and continuous support). OEC preparation may be enhanced using neurotrophin 3 (NT-3) and nerve growth factor (NGF). Cell administration may be supported using NT-3, NGF, glial-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF). Ongoing support to the injury/transplantation site may be achieved through the use of NT-3, fibroblast growth factor 2 (FGF2), epidermal growth factor (EGF), GDNF, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF).
Using neurotrophin manipulation, it may be possible to adjust the phenotype and behaviors of OEC into those most beneficial for SCI repair. Determining how neurotrophins modulate OECs and cell types endogenous to the injury site is therefore highly warranted.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Perry Cross Spinal Research Foundation grant to JSJ and JE, an ARC Discovery grant (DP150104495) to JE and JSJ, a Motor Accident Insurance Commission of Queensland grant to JSJ and JE and a Clem Jones Foundation grant to JSJ. The granting bodies had no role in the experimental design, interpretation, and writing of the report or decision to submit the article.
References
- 1. Baldoino Leal-Filho M. Spinal cord injury: from inflammation to glial scar. Surg Neurol Int. 2011;2:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Santos-Silva A, Fairless R, Frame MC, Montague P, Smith GM, Toft A, Riddell JS, Barnett SC. FGF/heparin differentially regulates Schwann cell and olfactory ensheathing cell interactions with astrocytes: a role in astrocytosis. J Neurosci. 2007;27(27):7154–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Domeniconi M, Hempstead BL, Chao M V. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34(2):271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackay-Sim A. Olfactory ensheathing cells and spinal cord repair. Keio J Med. 2005;54(1):8–14. [DOI] [PubMed] [Google Scholar]
- 6. Windus LCE, Claxton C, Allen CL, Key B, St. John JA. Motile membrane protrusions regulate cell-cell adhesion and migration of olfactory ensheathing glia. Glia. 2007;55(14):1708–1719. [DOI] [PubMed] [Google Scholar]
- 7. Granger N, Blamires H, Franklin RJM, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135(11):3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128(12):2951–2960. [DOI] [PubMed] [Google Scholar]
- 9. Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J Neurosci. 2003;23(28):9428–9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tabakow P, Raisman G, Fortuna W, Czyz M, Huber J, Li D, Szewczyk P, Okurowski S, Miedzybrodzki R, Czapiga B, Salomon B, Halon A, Li Y, Lipiec J, Kulczyk A, Jarmundowicz W. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplant. 2014;23(12):1631–1655. [DOI] [PubMed] [Google Scholar]
- 11. Cordero MI, Santos-Benito FF. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25(2):425–435. [DOI] [PubMed] [Google Scholar]
- 12. Windus LCE, Lineburg KE, Scott SE, Claxton C, MacKay-Sim A, Key B, St John JA. Lamellipodia mediate the heterogeneity of central olfactory ensheathing cell interactions. Cell Mol Life Sci. 2010;67(10):1735–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilad Y, Lancet D. Population differences in the human functional olfactory repertoire. Mol Biol Evol. 2003;20(3):307–314. [DOI] [PubMed] [Google Scholar]
- 14. Ekberg JAK, Amaya D, MacKay-Sim A, St. John JA. The migration of olfactory ensheathing cells during development and regeneration. NeuroSignals. 2012;20(3):147–158. [DOI] [PubMed] [Google Scholar]
- 15. Ekberg JAK, St. John JA. Crucial roles for olfactory ensheathing cells and olfactory mucosal cells in the repair of damaged neural tracts. Anat Rec (Hoboken). 2014;297(1):121–128. [DOI] [PubMed] [Google Scholar]
- 16. Nazareth L, Lineburg KE, Chuah MI, Tello Velasquez J, Chehrehasa F, St John JA, Ekberg JAK. Olfactory ensheathing cells are the main phagocytic cells that remove axon debris during early development of the olfactory system. J Comp Neurol. 2015;523(3):479–494. [DOI] [PubMed] [Google Scholar]
- 17. Su Z, Chen J, Qiu Y, Yuan Y, Zhu F, Zhu Y, Liu X, Pu Y, He C. Olfactory ensheathing cells: the primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia. 2013;61(4):490–503. [DOI] [PubMed] [Google Scholar]
- 18. Panni P, Ferguson IA, Beacham I, Mackay-Sim A, Ekberg JAK, St. John JA. Phagocytosis of bacteria by olfactory ensheathing cells and Schwann cells. Neurosci Lett. 2013;539:65–70. [DOI] [PubMed] [Google Scholar]
- 19. Yang H, He BR, Hao DJ. Biological roles of olfactory ensheathing cells in facilitating neural regeneration: a systematic review. Mol Neurobiol. 2015;51(1):168–179. [DOI] [PubMed] [Google Scholar]
- 20. Au E, Richter MW, Vincent AJ, Tetzlaff W, Aebersold R, Sage EH, Roskams AJ. SPARC from olfactory ensheathing cells stimulates Schwann cells to promote neurite outgrowth and enhances spinal cord repair. J Neurosci. 2007;27(27):7208–7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li C, Yan-Ling Z, Zhida S, Baolai L, Zhihui H, Lifang M, Cheng H. Olfactory ensheathing cells promote migration of Schwann cells by secreted nerve growth factor. Glia. 2007;55(14):897–904. [DOI] [PubMed] [Google Scholar]
- 22. Chehrehasa F, Windus LCE, Ekberg JAK, Scott SE, Amaya D, Mackay-Sim A, St John JA. Olfactory glia enhance neonatal axon regeneration. Mol Cell Neurosci. 2010;45(3):277–288. [DOI] [PubMed] [Google Scholar]
- 23. Huang EJ, Wilkinson GA, Fariñas I, Backus C, Zang K, Wong SL, Reichardt LF. Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development. 1999;126(10):2191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298(5596):1245–1248. [DOI] [PubMed] [Google Scholar]
- 25. Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci U S A. 2004;101(23):8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vickland H, Westrum LE, Kott JN, Patterson SL, Bothwell MA. Nerve growth factor receptor expression in the young and adult rat olfactory system. Brain Res. 1991;565(2):269–279. [DOI] [PubMed] [Google Scholar]
- 27. Trupp M, Belluardo N, Funakoshi H, Ibáñez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17(10):3554–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274(30):20885–20894. [DOI] [PubMed] [Google Scholar]
- 29. Cao L, Su Z, Zhou Q, Lv B, Liu X, Jiao L, Li Z, Zhu Y, Huang Z, Huang A, He C. Glia cell line-derived neurotrophic factor promotes olfactory ensheathing cells migration. Glia. 2006;(54):536–544. [DOI] [PubMed] [Google Scholar]
- 30. Huang EJ, Zang K, Schmidt A, Saulys A, Xiang M, Reichardt LF. POU domain factor Brn-3a controls the differentiation and survival of trigeminal neurons by regulating Trk receptor expression. Development. 1999;126(13):2869–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widenfalk J, Lipson A, Jubran M, Hofstetter C, Ebendal T, Cao Y, Olson L. Vascular endothelial growth factor improves functional outcome and decreases secondary degeneration in experimental spinal cord contusion injury. Neuroscience. 2003;120(4):951–960. [DOI] [PubMed] [Google Scholar]
- 32. Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb Perspect Med. 2012;2(7):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–1034. [DOI] [PubMed] [Google Scholar]
- 34. Mazzoni IE, Kenigsberg RL. Localization and characterization of epidermal growth-factor receptors in the developing rat medial septal area in culture. Brain Res. 1994;656(1):115–126. [DOI] [PubMed] [Google Scholar]
- 35. Morikawa Y, Ishihara Y, Tohya K, Kakudo K, Seo MK, Matsuura N. Expression of the fibroblast growth factor receptor-1 in human normal tissues and tumors determined by a new monoclonal antibody. Arch Pathol Lab Med. 1996;120(5):490–496. [PubMed] [Google Scholar]
- 36. Hart IK, Richardson WD, Heldin CH, Westermark B, Raff MC. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 1989;105(3):595–603. [DOI] [PubMed] [Google Scholar]
- 37. Lipson AC, Widenfalk J, Lindqvist E, Ebendal T, Olson L. Neurotrophic properties of olfactory ensheathing glia. Exp Neurol. 2003;180(2):167–171. [DOI] [PubMed] [Google Scholar]
- 38. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. [DOI] [PubMed] [Google Scholar]
- 39. Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57(11):1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mackay-Sim A, St John JA. Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Exp Neurol. 2011;229(1):174–180. [DOI] [PubMed] [Google Scholar]
- 41. Li Y, Field PM, Raisman G. Olfactory ensheathing cells and olfactory nerve fibroblasts maintain continuous open channels for regrowth of olfactory nerve fibres. Glia. 2005;52(3):245–251. [DOI] [PubMed] [Google Scholar]
- 42. Higginson JR, Barnett SC. The culture of olfactory ensheathing cells (OECs)—a distinct glial cell type. Exp Neurol. 2011;229(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29(4–5):627–647. [DOI] [PubMed] [Google Scholar]
- 44. Choi D, Li D, Law S, Powell M, Victor F, Raisman G, Horsley V. A prospective observational study of the yield of olfactory ensheathing cells cultured from biopsies of septal nasal mucosa. Neurosurgery. 2008;62(5):1140–1145. [DOI] [PubMed] [Google Scholar]
- 45. Mayeur A, Duclos C, Honoré A, Gauberti M, Drouot L, do Rego JC, Bon-Mardion N, Jean L, Vérin E, Emery E, Lemarchant S, Vivien D, Boyer O, Marie JP, Guérout N. Potential of olfactory ensheathing cells from different sources for spinal cord repair. PLoS One. 2013;8(4).e62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bianco JI, Perry C, Harkin DG, Mackay-Sim A, Féron F. Neurotrophin 3 promotes purification and proliferation of olfactory ensheathing cells from human nose. Glia. 2004;45(2):111–123. [DOI] [PubMed] [Google Scholar]
- 47. Deumens R, Koopmans GC, Lemmens M, Möllers S, Honig WM, Steinbusch HW, Brook G, Joosten EA. Neurite outgrowth promoting effects of enriched and mixed OEC/ONF cultures. Neurosci Lett. 2006;397(1–2):20–24. [DOI] [PubMed] [Google Scholar]
- 48. Toft A, Tome M, Barnett SC, Riddell JS. A comparative study of glial and non-neural cell properties for transplant-mediated repair of the injured spinal cord. Glia. 2013;61(4):513–528. [DOI] [PubMed] [Google Scholar]
- 49. Li Y, Yu H, Chen L, Duan C, Zhang J, Li B. Survival and number of olfactory ensheathing cells transplanted in contused spinal cord of rats. Chin J Traumatol. 2010;13(6):356–361. [PubMed] [Google Scholar]
- 50. Houle JD, Ye JH. Survival of chronically-injured neurons can be prolonged by treatment with neurotrophic factors. Neuroscience. 1999;94(3):929–936. [DOI] [PubMed] [Google Scholar]
- 51. Ma Y-H, Zhang Y, Cao L, Su J-C, Wang Z-W, Xu A-B, Zhang S-C. Effect of neurotrophin-3 genetically modified olfactory ensheathing cells transplantation on spinal cord injury. Cell Transplant. 2010;19(2):167–177. [DOI] [PubMed] [Google Scholar]
- 52. Wang J, Gutala R, Sun D, Ma JZ, Sheela RCS, Ticku MK, Li MD. Regulation of platelet-derived growth factor signaling pathway by ethanol, nicotine, or both in mouse cortical neurons. Alcohol Clin Exp Res. 2007;31(3):357–375. [DOI] [PubMed] [Google Scholar]
- 53. Chong ZZ, Tor SB, Loh NH, Wong TN, Gañán-Calvo AM, Tan SH, Nguyen N-T. Acoustofluidic control of bubble size in microfluidic flow-focusing configuration. Lab Chip. 2014;15:996–999. [DOI] [PubMed] [Google Scholar]
- 54. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ivanisevic L, Banerjee K, Saragovi HU. Differential cross-regulation of TrkA and TrkC tyrosine kinase receptors with p75. Oncogene. 2003;22(36):5677–5685. [DOI] [PubMed] [Google Scholar]
- 56. Yoon SO, Casaccia-Bonnefil P, Carter B, Chao M V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998;18(9):3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raucci F, Tiong JD, Wray S. P75 nerve growth factor receptors modulate development of GnRH neurons and olfactory ensheathing cells. Front Neurosci. 2013;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rydén M, Hempstead B, Ibáñez CF. Differential modulation of neuron survival during development by nerve growth factor binding to the p75 neurotrophin receptor. J Biol Chem. 1997;272(26):16322–16328. [DOI] [PubMed] [Google Scholar]
- 59. Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265(5178):1596–1599. [DOI] [PubMed] [Google Scholar]
- 60. Testi R. Sphingomyelin breakdown and cell fate. Trends Biochem Sci. 1996;21(12):468–471. [DOI] [PubMed] [Google Scholar]
- 61. Nazareth L, Tello Velasquez J, Lineburg KE, Chehrehasa F, St John JA, Ekberg JAK. Differing phagocytic capacities of accessory and main olfactory ensheathing cells and the implication for olfactory glia transplantation therapies. Mol Cell Neurosci. 2015;65:92–101. [DOI] [PubMed] [Google Scholar]
- 62. Tello Velasquez J, Watts ME, Todorovic M, Nazareth L, Pastrana E, Diaz-Nido J, Lim F, Ekberg JAK, Quinn RJ, St John JA. Low-dose curcumin stimulates proliferation, migration and phagocytic activity of olfactory ensheathing cells. PLoS One. 2014;9(10):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res. 1999;253(1):131–142. [DOI] [PubMed] [Google Scholar]
- 64. Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23(21):7922–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184(3):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McKimmie CS, Graham GJ. Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun. 2010;394(4):1006–1011. [DOI] [PubMed] [Google Scholar]
- 67. Asan E, Langenhan T, Holtmann B, Bock H, Sendtner M, Carroll P. Ciliary neurotrophic factor in the olfactory bulb of rats and mice. Neuroscience. 2003;120(1):99–112. [DOI] [PubMed] [Google Scholar]
- 68. Wewetzer K, Grothe C, Claus P. In vitro expression and regulation of ciliary neurotrophic factor and its alpha receptor subunit in neonatal rat olfactory ensheathing cells. Neurosci Lett. 2001;306(3):165–168. [DOI] [PubMed] [Google Scholar]
- 69. Lakatos A, Franklin RJM, Barnett SC. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia. 2000;32(3):214–225. [DOI] [PubMed] [Google Scholar]
- 70. O’Toole DA, West a K, Chuah MI. Effect of olfactory ensheathing cells on reactive astrocytes in vitro. Cell Mol Life Sci. 2007;64(10):1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. López-Vales R, García-Alías G, Forés J, Vela JM, Navarro X, Verdú E. Transplanted olfactory ensheathing cells modulate the inflammatory response in the injured spinal cord. Neuron Glia Biol. 2004;1(3):201–209. [DOI] [PubMed] [Google Scholar]
- 72. Hale DM, Ray S, Leung JY, Holloway AF, Chung RS, West AK, Chuah MI. Olfactory ensheathing cells moderate nuclear factor kappaB translocation in astrocytes. Mol Cell Neurosci. 2011;46(1):213–221. [DOI] [PubMed] [Google Scholar]
- 73. Windus LCE, Chehrehasa F, Lineburg KE, Claxton C, MacKay-Sim A, Key B, St John JA. Stimulation of olfactory ensheathing cell motility enhances olfactory axon growth. Cell Mol Life Sci. 2011;68(19):3233–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boruch A V., Conners JJ, Pipitone M, Deadwyler G, Storer PD, Devries GH, Jones KJ. Neurotrophic and migratory properties of an olfactory ensheathing cell line. Glia. 2001;33(3):225–229. [PubMed] [Google Scholar]
- 75. Niederhauser O, Mangold M, Schubenel R, Kusznir EA, Schmidt D, Hertel C. NGF ligand alters NGF signaling via p75(NTR) and TrkA. J Neurosci Res. 2000;61(3):263–272. [DOI] [PubMed] [Google Scholar]
- 76. Lang B-C, Zhang Z, Lv L-Y, Liu J, Wang T-Y, Yang L-H, Liao D-Q, Zhang W-S, Wang T-H. OECs transplantation results in neuropathic pain associated with BDNF regulating ERK activity in rats following cord hemisection. BMC Neurosci. 2013;14(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang T, Cong R, Yang H, Wu MM, Luo N, Kuang F, You SW. Neutralization of BDNF attenuates the in vitro protective effects of olfactory ensheathing cell-conditioned medium on scratch-insulted retinal ganglion cells. Cell Mol Neurobiol. 2011;31(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. J Neurosci. 2012;32(48):17251–17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML. β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177(6):1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sulaiman OAR, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002;37(3):206–218. [DOI] [PubMed] [Google Scholar]
- 81. Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367(6459):170–173. [DOI] [PubMed] [Google Scholar]
- 82. Taylor V, Zgraggen C, Naef R, Suter U. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: Immunohistochemistry and antibody perturbation. J Neurosci Res. 2000;62(1):40–55. [DOI] [PubMed] [Google Scholar]
- 83. Stachowiak MK, Maher PA, Stachowiak EK. Integrative nuclear signaling in cell development—a role for FGF receptor-1. DNA Cell Biol. 2007;26(12):811–826. [DOI] [PubMed] [Google Scholar]
- 84. Hsu P. Basic fibroblast growth factor and fibroblast growth factor receptors in adult olfactory epithelium. Brain Res. 2001;896(1–2):188–197. [DOI] [PubMed] [Google Scholar]
- 85. Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV., Yeh BK, Yayon A, Linhardt RJ, Mohammadi M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000;6(3):743–750. [DOI] [PubMed] [Google Scholar]
- 86. Reiland J, Kempf D, Roy M, Denkins Y, Marchetti D. FGF2 binding, signaling, and angiogenesis are modulated by heparanase in metastatic melanoma cells. Neoplasia. 2006;8(7):596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Knapp PE, Adams MH. Epidermal growth factor promotes oligodendrocyte process formation and regrowth after injury. Exp Cell Res. 2004;296(2):135–144. [DOI] [PubMed] [Google Scholar]
- 88. Sheng HZ, Turnley A, Murphy M, Bernard CC, Bartlett PF. Epidermal growth factor inhibits the expression of myelin basic protein in oligodendrocytes. J Neurosci Res. 1989;23(4):425–432. [DOI] [PubMed] [Google Scholar]
- 89. Richter MW, Fletcher PA, Liu J, Tetzlaff W, Roskams AJ. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci. 2005;25(46):10700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Emanueli C, Salis MB, Pinna A, Graiani G, Manni L, Madeddu P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation. 2002;106(17):2257–2262. [DOI] [PubMed] [Google Scholar]
- 91. Kubota Y, Suda T. Feedback mechanism between blood vessels and astrocytes in retinal vascular development. Trends Cardiovasc Med. 2009;19(2):38–43. [DOI] [PubMed] [Google Scholar]
- 92. Liem RKH, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest. 2009;119(7):1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58(10):1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schollmanns C, Grugels R, Tatjej D, Hoppej J, Folkmanq J, Marme D, Ii HAW. Basic fibroblast growth factor modulates the mitogenic potency of the platelet-derived growth factor (PDGF) isoforms by specific upregulation of the PDGF alpha receptor in vascular smooth muscle cells. Biochemistry. 1992;267(25):18032–18039. [PubMed] [Google Scholar]
- 95. Calof AL, Lander AD. Relationship between neuronal migration and cell-substratum adhesion: laminin and merosin promote olfactory neuronal migration but are anti-adhesive. J Cell Biol. 1991;115(3 I):779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kaihola H, Olivier J, Poromaa IS, Åkerud H. The effect of antenatal depression and selective serotonin reuptake inhibitor treatment on nerve growth factor signaling in human placenta. PLoS One. 2015;10(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Reuss B, Von Bohlen Und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313(2):139–157. [DOI] [PubMed] [Google Scholar]
- 98. Postigo A, Calella AM, Fritzsch B, Knipper M, Katz D, Eilers A, Schimmang T, Lewin GR, Klein R, Minichiello L. Distinct requirements for TrkB and TrkC signaling in target innervation by sensory neurons. Genes Dev. 2002;16(5):633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dietrich WD, Levi AD, Wang M, Green BA. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics. 2011;8(2):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sakata M, Yanamoto H, Hashimoto N, Iihara K, Tsukahara T, Taniguchi T, Kikuchi H. Induction of infarct tolerance by platelet-derived growth factor against reversible focal ischemia. Brain Res. 1998;784(1–2):250–255. [DOI] [PubMed] [Google Scholar]
- 101. Chehrehasa F, Cobcroft M, Young YW, Mackay-Sim A, Goss B. An acute growth factor treatment that preserves function after spinal cord contusion injury. J Neurotrauma. 2014;31(21):1807–1813. [DOI] [PubMed] [Google Scholar]
- 102. Lutton C, Young YW, Williams R, Meedeniya ACB, Mackay-Sim A, Goss B. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J Neurotrauma. 2012;29(5):957–970. [DOI] [PubMed] [Google Scholar]