Abstract
High donor variation makes comparison studies between different dental sources dubious. Dental tissues offer a rare opportunity for comparing the biological characteristics of haploidentical mesenchymal stem cells (MSCs) isolated from the same donor. The objective was to identify the optimal dental source of MSCs through a biological and functional comparison of haploidentical MSCs from gingival (GMSCs) and dental pulp stem cells (DPSCs) focusing mainly on their angiogenic potential. The comparison study included (1) surface markers expression, (2) mesodermal differentiation capacity (chondrogenic, adipogenic, and osteogenic), (3) proliferation, (4) migration potential, (5) ability to form colony units, and (6) angiogenic potential in vitro and in vivo. Comparative analysis showed no difference in the immunophenotypic profile nor for the trilineage differentiation potential. Proliferation of GMSCs was higher than DPSCs at day 6 (2.6-fold higher, P < 0.05). GMSCs showed superior migratory capacity compared to DPSCs at 4, 8, and 12 h (2.1-, 1.5-, and 1.2-fold higher, respectively, P < 0.05). Furthermore, GMSCs formed a higher number of colony units for both cell concentrations (1.7- and 1.4-fold higher for 150 and 250 starting cells, respectively, P < 0.05). GMSCs showed an improved angiogenic capacity compared to DPSCs (total tube lengths 1.17-fold higher and 1.5-fold total loops, P < 0.05). This was correlated with an enhanced release of vascular growth factor under hypoxic conditions. Finally, in the plug transplantation assay evaluating the angiogenesis in vivo, the DPSC and GMSC hemoglobin content was 3.9- and 4-fold higher, respectively, when compared to the control (Matrigel alone). GMSCs were superior to their haploidentical DPSCs in proliferation, migration, and angiogenic potentials. This study positions GMSCs in the forefront of dental cell sources for applications in regenerative medicine.
Keywords: haploidentical, dental and gingival mesenchymal stem cells, migration, angiogenesis
Introduction
Mesenchymal stem cells (MSCs) hold great promise for revolutionizing the dental regeneration field1–3. They can be derived from the bone marrow (BM)4, the adipose tissue5, the dental pulp6, the gingival tissue7, or the periodontal ligament (PDL)2,8. MSCs are pluripotent cells that have the ability to differentiate into different type of cells (osteogenic, chondrogenic, and adipogenic)9–12. Despite the various sources of MSCs, the bone marrow mesenchymal stem cell (BMSC) has been considered the gold standard and used extensively in cell therapy applications. One of the major drawbacks is the high invasiveness and low yield of their harvesting, making access to a large volume of BM from healthy donors very difficult13–15. Currently, five different human dental stem cells have been isolated and characterized: dental pulp stem cells (DPSCs), stem cells from exfoliated deciduous teeth, PDL stem cells (PDLSCs), stem cells from apical papilla (SCAP), and dental follicle progenitor cells16. Gronthos et al. isolated and characterized DPSCs and compared them with BMSCs. While the comparative analysis led to a similar immunophenotype, their osteogenic differentiation potential resulted in sporadic calcified nodules in comparison with BMSCs. Additionally, DPSCs were unable to differentiate toward an adipogenic pheynotype6. Human gingiva plays an important role in the maintenance of oral health and shows a unique fetal-like scarless healing process after wounding. The gingival tissue is an accessible source from the oral cavity; it is often resected during general dental treatments, and is treated as a biomedical waste17. Gingival connective tissue is a reservoir of MSCs that could be used in regenerative procedures based on tissue engineering. Donor variability and the site of stem cell extraction play a crucial role in the quality of stem cells in order to ensure a successful therapy18,19. Therefore, taking into consideration all of the variables, a head-to-head comparative study addressing the pros and cons for the use of each of the dental cell sources is still missing. In fact, donor variation in MSC growth, differentiation, and in vivo ability is a bottleneck for standardization of therapeutic protocols20. Indeed, in a previously published work, we showed that donor-to-donor variation limits greatly comparison studies from any different MSC sources, as demographically matched donors presented different MSC performance that did not cluster according to age range21. Although the ideal situation would be to study haploidentical MSCs, this has been difficult to achieve in many cases owing to the difficulty in obtaining a matched pair of samples from the same human donor. However, dental tissue offers the rare opportunity for comparing the biological characteristics of haploidentical MSCs isolated from the same donors. Hence, in order to dismiss the donor-to-donor variation, we designed this comparative study of the biological characteristics using haploidentical MSCs from gingival and dental pulp tissue. All the isolated cells were fully characterized and compared for their biological activities including proliferation, the ability to form colony forming units (CFUs), mesodermal differentiation, surface marker expression, and most importantly for their angiogenic potential both in vitro and in vivo. A great number of functionally competent clinical-grade MSCs can be generated over a short period of time from human gingiva or dental pulp for cell therapy in the future. Results of this comparative study will assist health professionals in selecting the optimal MSC source for dental regeneration.
Materials and Methods
Isolation of Dental Pulp, GMSCs and In Vitro Expansion
Three healthy individuals (1 man and 2 women) aged between 18 and 25 without any evidence of dental caries were recruited. The third molars and the gingival tissue were collected in the dental school of Universidad de Los Andes, San Bernardo. After signing an informed consent form and following the ethical approval of the Universidad de los Andes, the surgery was performed after disinfecting the patient’s mouth with 0.2% chlorhexidine and then local anesthesia was injected in the area of interest. The third molars (wisdom teeth) often develop in abnormal positions, and most of the time they are unable to erupt properly. The third molar removal is necessary under different indications. In this study, the third molars were extracted by surgical and orthodontic indications for one of the following diagnoses: decubitus position of the third molar, periodontal commitment of the second molar, nonfunctional (unopposed and soon to supraerupt), or prophylaxis. The inclusion and exclusion criteria were added along the study design in the Supplementary Material, therefore avoiding the appearance of any future complication, such as oral or lingual ulcer, lesion to the second molar, and dental displacements22. The MSCs were considered haploidentical as they were isolated from the dental pulp of the third molars and from gingival tissues of the same patient during the same dental appointment. The third molars and the gingival tissue were removed and were transferred in a tube containing 2 mL of cell culture medium (α-minimum essential medium Eagle [α-MEM], Thermofisher, Massachusetts, USA with 10% and 1% Penicillin Streptomycin [Penn Strep], Thermofisher, Massachusetts, USA). The extracted teeth and mesenchymal gingival tissue were washed with serum (Invitrogen, California, USA) and transported to the laboratory for further processing. The DPSCs and the GMSCs were isolated with direct cell outgrowth from the tissue explants. All 3 explants from each tissue were processed separately and incubated for 20 d until the dish reached confluence and pulp cells with a fibroblast-like morphology were observed. Therefore, 3 different DPSC and GMSC populations were generated based on these biopsies. At confluency, cells were washed with 1× phosphate-buffered saline (PBS; Sigma, Missouri, USA), trypsinized (Invitrogen), and centrifuged at 1,500 rpm for 5 min and subcultured to a flask (T75-Nunc, Roskilde, Denmark, USA). The cultures were continued until reaching passage 3 to 4 where they were used for all the experiments. For all the biocompatibility experiments, the cells from each donation of DPSCs and GMSCs were analyzed separately, and the cells from different donors or sources were never pooled together.
Characterization of MSCs Derived from DPSCs and GMSCs
DPSCs and GMSCs were characterized by their plastic adherence capacity, fibroblast-like morphology, proliferation potential, immunophenotypic profile, colony–forming unit frequency (CFU-F), and the capacity to differentiate into adipocytes, chondrocytes, and osteoblasts.
Immunophenotypical Profile by Flow Cytometry
For the immunophenotypic characterization, cells were incubated with the specific labeled antibodies Immunoglobulin G1 light chain kappa Fluorescein isothiocyanate (IgG1k-FITC), IgG1k-R-phycoerythrin (PE), CD90-FITC, cluster of differentiation (CD) 105-PE, CD45-FITC, CD34-PE, CD44-FITC, CD73-PE, CD29-PE, IgG1k-AF488, IgG2bk-PE, CD11b-AF488, and Human Leukocyte Antigen – antigen D Related (HLADR)-PE (BD, Franklin Lakes, New Jersey, USA). The samples were incubated with the antibodies for 20 min at 4 °C in a dark area and then were washed with 4 mL of PBS 1× and centrifuged at 1,800 rpm for 6 min; the supernatant was removed. The cells were further washed with 1 mL of PBS 1× and centrifuged at 1,800 rpm for 6 min. Finally, the supernatant was removed, and the cells were resuspended in 500 μL of PBS 1×. In addition, LIVE/DEAD® Fixable Dead Cell Stain Kit (Invitrogen) was used to determine the viability of cells by flow cytometry according to the manufacturer’s protocol. Data (5,000 events) were collected using a FACS Canto II Flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo analysis software version, v10.4.2 (FlowJo LLC, Ashland, Oregon, USA).
Mesodermal Differentiation
The mesodermal differentiation was performed as previously described20. For adipogenic differentiation, 50,0000 cells were incubated in a 4-well plate (Nunc) and incubated for 24 h with proliferation medium α-MEM (Thermofisher, Massachusetts, USA) (10% fetal bovine serum [FBS], [Sigma, California, USA] and 1% Penn Strep, [Thermofisher, Massachusetts, USA]) at 37 °C, 5% CO2, and then switched to 500 μL adipogenic differentiation medium containing α-MEM, 10% FBS, Penn Strep (1%), dexamethasone (0.11 mM), insulin (10 mg/mL), and indometacin (0.02 mg/mL; Sigma, California, USA). The cells were incubated for 4 wks, and the medium was changed every other day. At day 30, the cells were washed with 1 mL of PBS 1× and stained with 1 m of Oil Red (Sigma, California, USA) in isopropanol 60% v/v for 1 h at room temperature. Then, the cells were washed 2 times with 1 mL of PBS 1×, and images were taken with an inverted microscope (Olympus CKX41, Tokyo, Japan). For osteogenic differentiation, 70,000 cells were plated in 4-well plates with proliferation medium and incubated for 24 h with proliferation medium α-MEM (10% FBS and 1% Penicillin-Streptomycin) at 37 °C, 5% CO2, and then switched to 500 μL differentiation medium α-MEM, 10% FBS, Penn Strep (1%), dexamethasone (0.1 mM), β-glycerophosphate (10 mM; Sigma, California, USA), and ascorbate-2-phosphate (50 mg/mL; Sigma). The cells were incubated for 4 wks, and the medium was changed every other day. At day 30, the cells were washed with 1 mL of PBS 1× and stained with 1 ml of Alizarin Red 40 mM in NaH2PO4 (0.1 M, pH 4.3; Sigma, California, USA). The cells were washed 2× with 500 μL of PBS 1× and fixed with 70% ethanol for 30 min at room temperature. Then, the cells were washed 2 times with 1 mL of PBS 1× and further stained with 500 μL of Alizarin Red 40 mM in NaH2PO4 (0.1 M, pH 4.3) for 10 min at room temperature. Finally, the cells were washed 2 times with 1 mL of PBS 1× and 5 times with 1 mL distilled water. Images were taken with an inverted microscope (Olympus CKX41). For chondrocyte differentiation, 60,000 cells were plated in a 10 μL drop in the middle of a 4-well plate for the creation of the micromass. The drop of cells was incubated for 1 h and then 500 μL of differentiation medium was used (α-MEM, 10% FBS, Penn Strep [1%], dexamethasone [0.1 mM], insulin [5 mg/mL], transforming growth factor-β1 [10 ng/mL], ascorbate-2-phosphate [50mg/mL]). The cells were cultured for 4 wks, and the medium was changed every other day. At day 30, the cells were washed with 1 mL of PBS 1× and stained with 1 ml Safranin O (0.1%). The cells were washed with 1 mL of PBS 1× and fixed with 250 μL ethanol (70%) for 10 min. Then, the cells were washed with 1 mL of PBS 1× and then further stained with 250 μL of Safranin for 5 min at room temperature. Finally, the cells were washed 5 times with 250 μL ethanol (100%) followed by a 5-time wash with distilled water. Images were taken with an inverted microscope (Olympus CKX41).
CFUs
The CFU assay was used to estimate the fibroblast colony forming ability of MSCs reflecting the quality of the different cell preparations. For this, 150 or 250 cells were seeded in a 6-well plate (Nunc) with 2.5 mL proliferation medium α-MEM and incubated at a 37 °C, 5% CO2 for 14 d. Cells were stained with Crystal Violet (Merck, Darmstadt, Germany) and counted using an inverted microscope (Olympus CKX41).
Water-soluble tetrazolium salts (WST)-1 Cell Proliferation Assay
In order to compare the proliferation capacity between DPSCs and GMSCs, 1,000 cells were plated in a 24-well plate (Nunc) for 24 h with proliferation medium α-MEM (10% FBS and 1% Penn Strep) at 37 °C, 5% CO2. The proliferation rate was measured at various time points (day 1, 3, 6, and 9) using the WST-1 methods following the manufacturer’s instruction (Roche Applied Science, Penzberg, Upper Bavaria, Germany, USA). The absorbance was measured using a plate reader (Tecan, Chapel Hill, North Carolina, USA) at 450 nm with a reference wavelength at 570 nm.
In Vitro Scratch Assay
The cell migration was evaluated with a scratch assay, where 350,000 cells were seeded in a 6-well plate with proliferation α-MEM (10% FBS and 1% Penn Strep) at 37 °C, 5% CO2. After 24 h of incubation, a scratch was made with a 10-μL pipet tip (Thermo Fisher Scientific, Massachusetts, USA). Images were taken at various time points (4, 8, 12, and 24 h) using an inverted microscope until the complete closure of the gap. The images were analyzed with the WimScratch Software (Wimasis, München, Germany).
In Vitro Tube Formation Assay and Measurement of Angiogenic Factors
The angiogenic potential of DPSCs or GMSCs was evaluated based on their capacity to form tube-like structures in vitro (total branching points, total tube length, and total loops). DPSCs or GMSCs (60,000 in total) were seeded on a precoated 24-well plate (Nunc) with standard Matrigel matrix (BD Biosciences) and incubated for 5 h at 37 °C, 5% CO2 with endothelial cell growth medium (EGM; Lonza, Cleveland, TN, USA). Additionally, to determine the angiogenic potential of MSC-conditioned media (CM), 500,000 cells (DPSC or GMSC) were incubated under hypoxic (1% O2) or normoxic conditions for 48 h. Subsequently, human umbilical vein endothelial cells (HUVECs) were plated with the MSC-CM, EGM-2 as positive control, or α-MEM (negative control) coated with Matrigel matrix. In both in vitro experiments described previously, images were taken after 5 h of incubation with an inverted microscope and analyzed with the WimTube software (Wimasis image analysis, München, Germany). Finally, the different MSC-CM was collected, and the secreted levels of vascular growth factor (VEGF) and hepatocyte growth factor (HGF) were measured using the DuoSet ELISA Development System (R&D Systems, Minneapolis, USA) following the manufacturer’s instruction.
Matrigel Plug Assay
To compare the angiogenic potential of DPSCs and GMSCs in vivo, the Matrigel plug assay was performed in an 8-wk-old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratories, Bar Harbor, ME, USA). All in vivo studies received approval by the Universidad de Los Andes ethical committee for animal experimentation. Additionally, the authors have completed and complied with animal research reporting of in vivo experiments guidelines/checklist for preclinical animal studies. Specifically, 3,000,000 cells were mixed with 250 μL of Matrigel high concentration growth factor reduced (HC GFR) (BD Biosciences) with 50 ng/mL VEGF (R&D Systems, USA) and injected subcutaneously using a 23-G syringe in both flanks of the mouse (2 Matrigel plugs per mouse—6,000,000 cells). The mice (24 mice in total) were divided into 4 different groups: (1) Matrigel alone, (2) GMSC + Matrigel, (3) DPSC + Matrigel, and (4) HUVECS + Matrigel (positive control). After 14 d postimplantation, the mice were euthanized, and the plugs were removed. Images were taken of the implanted plugs, and the quantity of new vessels formed around the implants was quantified with image processing and analysis in java. The Matrigel implants were homogenized, and hemoglobin content of the implant was determined by Drabkin’s reagent kit (Sigma). Thereafter, the Matrigel implants were removed and placed in 10% formalin (Sigma). Then, they were paraffin embedded, and longitudinal sections of 4 μm were stained for hematoxylin and eosin (H&E; Sigma). Some of the sections were used for immunohistochemical analysis.
Immunohistochemistry
Deparaffinized sections were dehydrated in a series of xylol and alcohol series, and then, the antigen recuperation was performed using citric buffer. The samples were immersed in 3% H2O2 for 15 min and then blocked with BSA for 30 min. The primary incubation was performed overnight at 4 °C using the following antibody: (1) human leukocyte antigen (anti-HLA-A; EP 1395Y, Abcam, (MA, USA). Isotype-matched control antibody was used under the same conditions as the primary antibody. For enzymatic immunohistochemical staining, VECTASTAIN® Universal ABC kit (Vector Laboratories, Burlingame, USA) was used according to the manufacturer’s protocol. All sections were counterstained with hematoxylin and mounted with a 10 µL drop of Entellan (Merck, Darmstadt, Germany). The amount of protein expression was calculated with ImageJ and was expressed as percentage of the area coverage.
Statistical Analysis
All experiments were performed in triplicate (n = 3) and data were expressed as the mean and standard error of the mean. The comparison between the groups was made with Kruskal–Wallis and Tukey’s tests. A probability value of 0.05 was considered statistically significant.
Results
GMSCs Display a Higher Proliferation Rate in Comparison with DPSCs
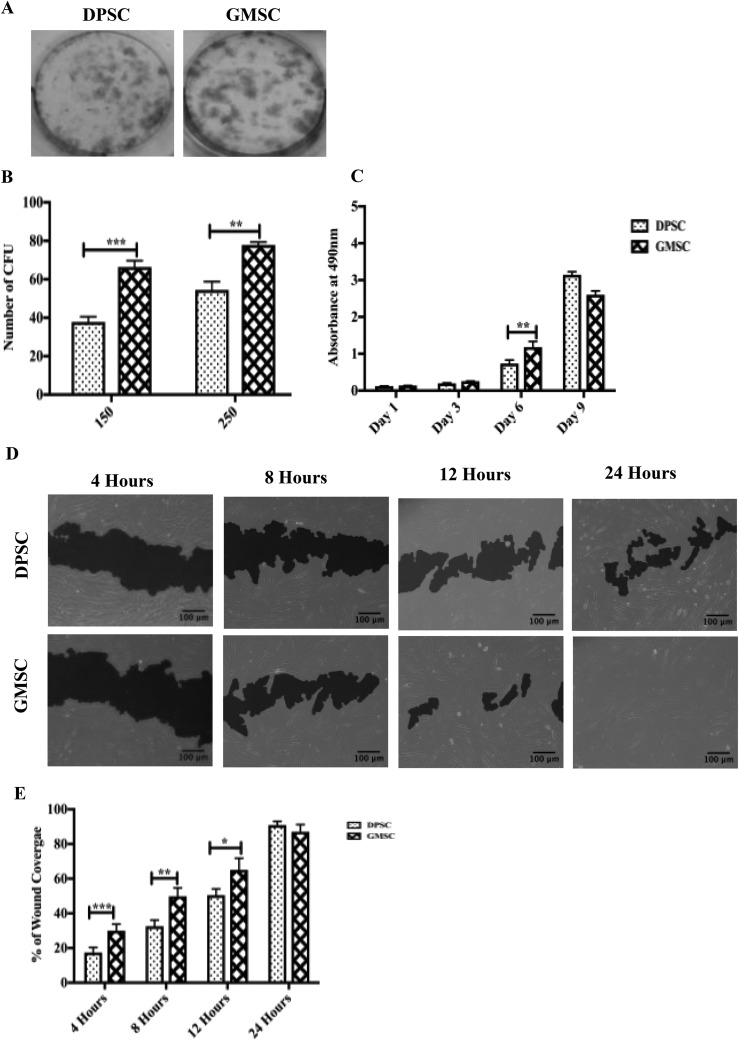
DPSCs adn GMSCs, similarly to previous observations, showed similar fibroblast-like characteristics as seen in previous reports17,23. To evaluate the fibroblastic-colony-forming ability of MSCs, the CFUs were calculated based on a series of cell dilutions. The results have shown that there was a significant increase in the formation of CFUs for both concentrations of GMSCs (1.7- and 1.4-fold higher, respectively, P < 0.05; Fig. 1A and B). Additionally, the proliferation between the DPSC and GMSC was investigated using a WST-1 cell proliferation assay. A significant increase in the proliferation of GMSCs at day 6 was observed (2.6-fold higher, P < 0.05; Fig. 1C).
Fig. 1.
Gingival mesenchymal stem cells (GMSCs) and dental pulp stem cells (DPSCs) showed different clonogenic and proliferation potentials. (A) Representative images of colony-forming units (CFUs) stained with crystal violet after 20 d in culture. (B) An increase in the formation of CFUs was observed for both concentrations (150 cells and 250 cells) for GMSCs compared to DPSCs with a P < 0.05. (C) Quantification of cell proliferation between DPSCs and GMSCs incubated at different time points (1, 3, 6, and 9 d). An increase in the proliferation of GMSCs compared to DPSCs was observed between day 6 compared to DPSCs with a P < 0.05. (D) In vitro migration comparison between DPSCs and GMSCs based on a 24-h scratch wound healing assay. (E) GMSCs display a better migratory capacity compared to DPSCs for 4, 8, and 12 h (P < 0.05). At 24 h, no significant change in the proliferation was observed. All data are represented as a mean with the associated standard error of the mean (n = 3) of a minimal 3 donors.
GMSCs Exhibit a Superior Migratory Capacity in a Wound Scratch Assay
To evaluate the migration potential of DPSCs and GMSCs, a wound scratch assay was performed. The migratory capacity was evaluated from each time point (4, 8, and 12 h) in correlation to 0 h (images not shown). There was a significant increase in the migration of GMSCs compared to DPSCs for 4, 8, and 12 h (2.1-, 1.5-, and 1.2-fold higher, respectively, P < 0.05). No significant difference was observed at 24 h, where full wound closure was reached by both cell sources. This experiment indicates that GMSCs possess a higher migration potential in comparison to DPSCs for all the different time points analyzed (Fig. 1D and E).
DPSCs and GMSCs Express Common MSC Markers with No Significant Difference
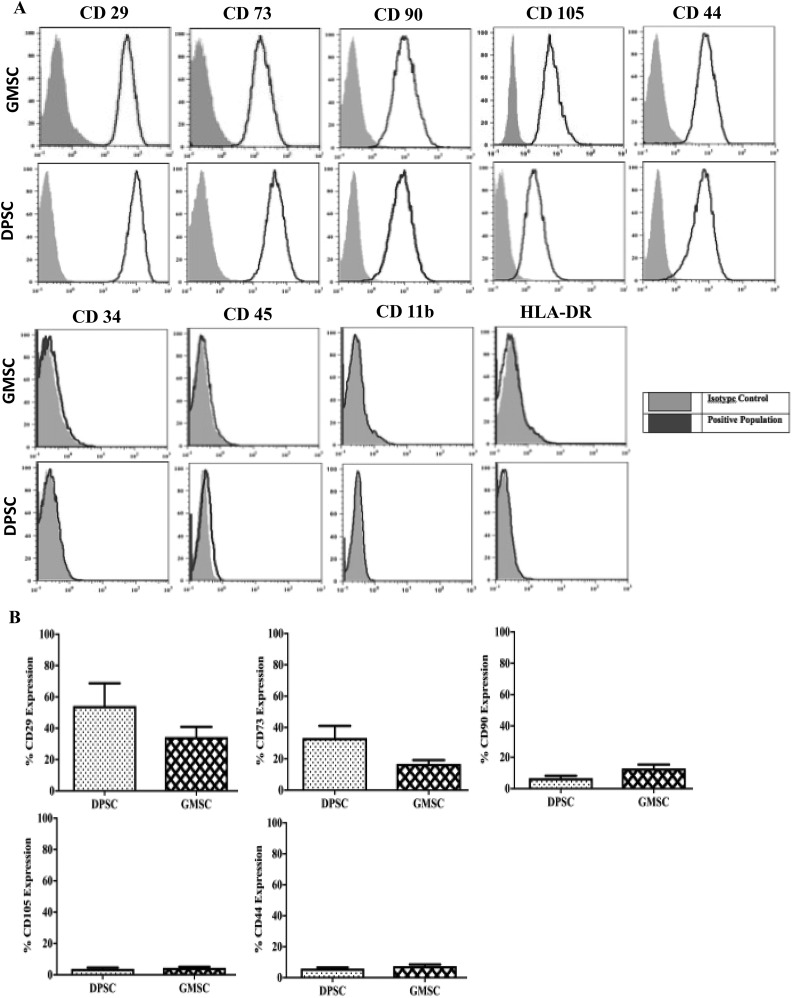
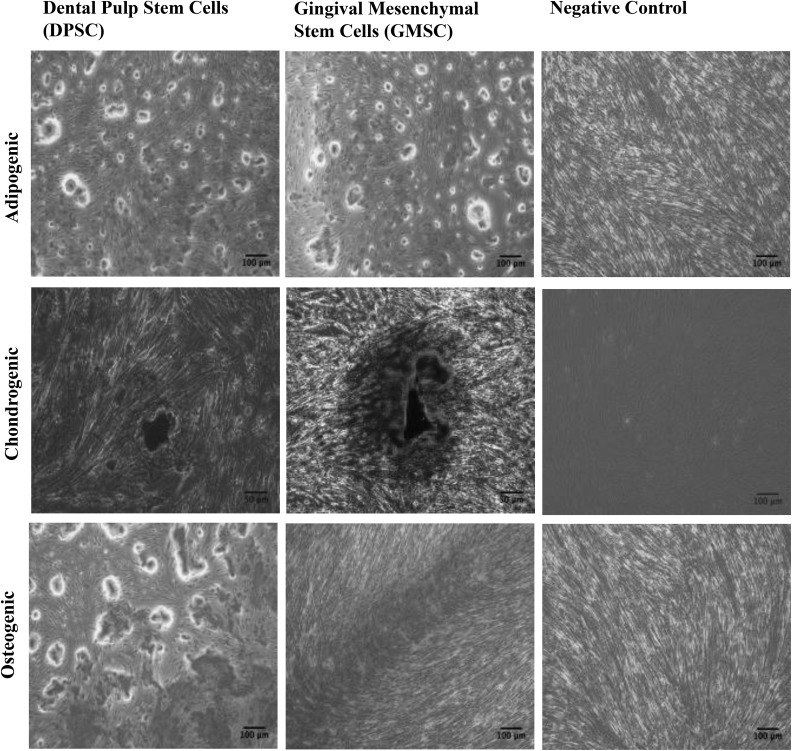
Both cell sources showed a positive expression of the common MSC markers such as CD29, CD73, CD90, CD105, and CD44 and a negative for CD34, CD45, CD11b, and HLA-DR for both DPSCs and GMSCs (Fig. 2A and B). GMSCs and DPSCs were induced to differentiate into mesodermal tissues (adipogenic, chondrogenic, and osteogenic) lineages. No immunophenotypical differences were observed between GMSCs and DPSCs (Fig. 3).
Fig. 2.
Gingival mesenchymal stem cells and dental pulp stem cells express common mesenchymal stem cell (MSC) markers. (A) MSCs were stained with labeled monoclonal antibodies against known MSC surface markers (blue) and their respective isotypes (gray), cells were analyzed by flow cytometry. All MSCs were positive for CD29, CD73, CD90, CD105, and CD44 and negative for CD34, CD11b, CD45, and human leukocyte antigen-DR. (B) No significant difference was observed for CD29, CD73, CD90, CD105, and CD44. All data are represented as a mean with the associated standard error of the mean (n = 3) of a minimal 3 donors.
Fig. 3.
Dental pulp stem cells and gingival mesenchymal stem cells display similar mesodermal differentiation potential. Images illustrating mesenchymal stem cell trilineage differentiation following incubation with differentiation medium for 30 d and stained with Oil Red O (adipocytes), Alizarin red (osteocytes), and Safranin O (chondrocytes).
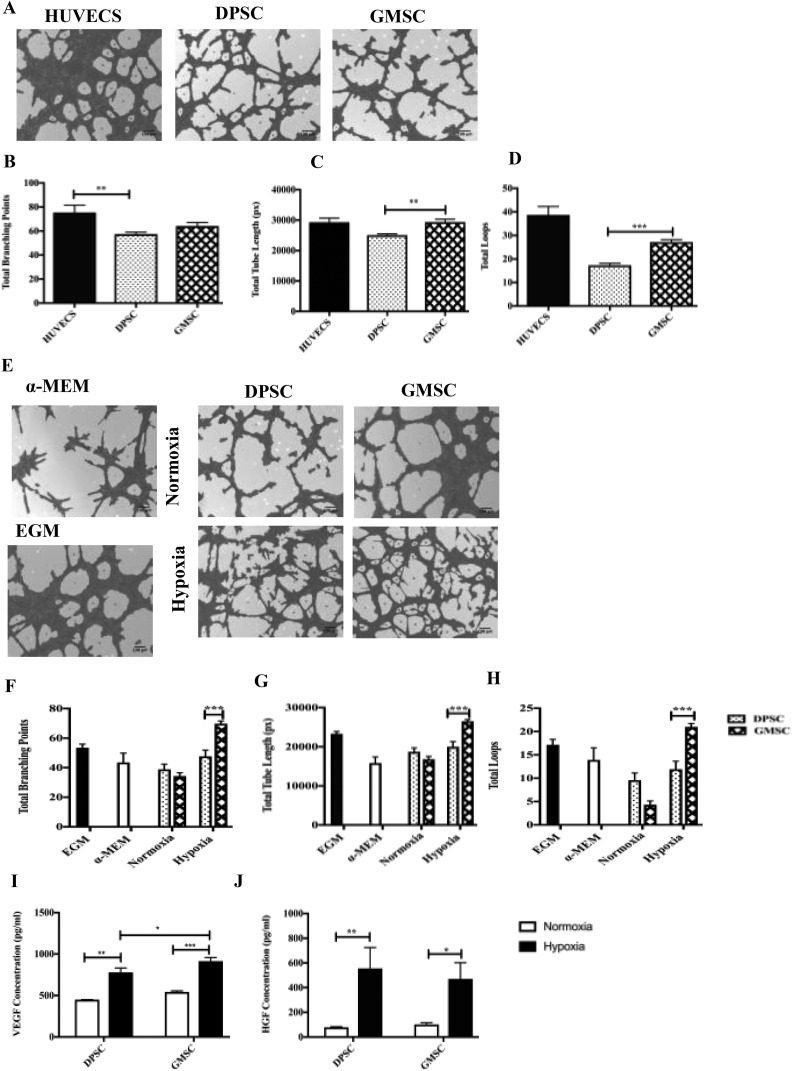
GMSCs Were Able to Form a Higher Number of Tube-like Structures Compared to DPSCs
The angiogenic ability designated by the ability of DPSCs and GMSCs to form tubular networks was investigated in vitro in a semisolid medium (Matrigel). The in vitro angiogenesis was evaluated with the following characteristics: (1) total branching points, (2) total tube length, and (3) total loops (Fig. 4A). Image analysis of the tube formation evaluated at 5 h postculture initiation showed a higher angiogenic capacity evidenced by a more extensive network of capillary-like structures for GMSCs as compared to DPSCs (1.17-fold higher for total tube lengths and 1.5-fold for total loops, P < 0.05; Fig. 4C and D). In order to evaluate their secreted paracrine factors, we measured in a separate experimental setting the angiogenic factors released in the CM harvested from DPSCs and GMSCs after 48 h incubation under hypoxic (1% O2) or normoxic conditions. HUVECS were resuspended with the CM and were seeded onto precoated plated with growth factor–reduced Matrigel. α-MEM (basal media) and EGM (angiogenic media) were used as the negative and positive controls, respectively. The tube formation was analyzed after 5 h of incubation. Images were taken, and the results have shown a higher tubular structure for the HUVECS incubated with the conditioned medium under hypoxic conditions versus normoxic (Fig. 4E). There was a significant difference in the formation of total tube lengths, total loops, and total branching points between hypoxia GMSCs and hypoxia DPSCs (1.3-fold higher for total tube lengths, 1.4 higher for total branching points, and 1.7 for fold total loops; P < 0.05; Fig. 4F–H). The quantification of angiogenic factors (Fig. 4I and J) revealed a significant increase in the VEGF release for GMSCs compared to DMPCs after 48 h of incubation under hypoxic conditions (P < 0.05). The release of HGF was higher after 48 h of incubation for both GMSCs and DPSCs between hypoxic and normoxic conditions.
Fig. 4.
In vitro angiogenesis comparison between dental pulp stem cells (DPSCs) and gingival mesenchymal stem cells (GMSCs) based on a 5-h culture in the Matrigel. (A) Images were analyzed using a Wimasis software. GMSCs were shown a higher potential to form (C) tube-like structure (P < 0.05) and (D) total loops (P < 0.05) in Matrigel-coating cultures compared to DPSC. (B) No statistical difference was observed in the formation of total branching points between DPSCs and GMSCs (b). Human umbilical vein endothelial cells were used as a positive control. All data are represented as a mean with the associated standard error of the mean (n = 3) of a minimal 3 donors. (E) In vitro angiogenesis comparison between DPSC and GMSC-conditioned media (CM) under hypoxic and normoxic conditions. The GMSC-CM under hypoxic conditions were shown a better potential to form (F) total branching points (P < 0.05), (G) total tube length (P < 0.05), and (H) total loops (P < 0.05) conditions compared to supernatant of DPSCs. Endothelial cell growth medium (EGM) and α-minimum essential medium Eagle were used as a control (E). An ELISA was performed to measure (I) protein levels of vascular growth factor (P < 0.05), (J) protein levels of hepatocyte growth factor (P < 0.05). All data are represented as a mean with the associated standard error of the mean (n = 3) of a minimal 3 donors.
Angiogenic Potential of GMSCs and DPSCs In Vivo
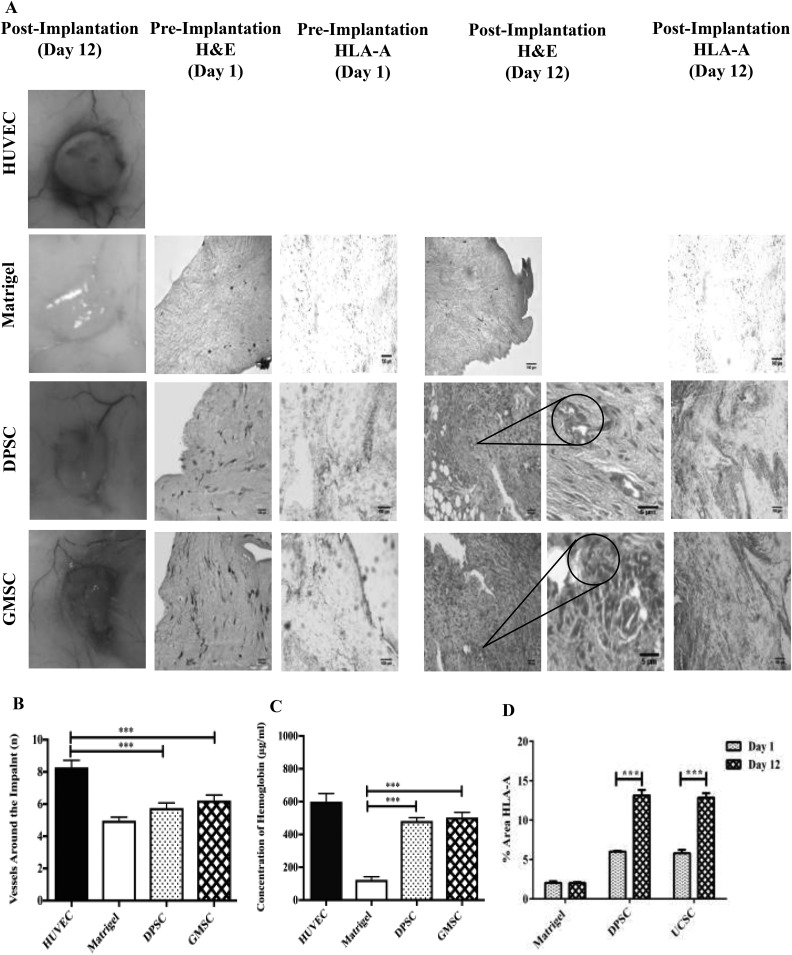
To comparatively evaluate the angiogenic potential of GMSCs and DPSCs, a Matrigel plug was implanted in a NSG mouse. After 15 d, the implants were collected and photographs were taken for image analysis. As shown in Fig. 5A, all plugs generated vessels around and inside the implant. After image analysis using ImageJ, the results have shown similar vessel formation for GMSCs versus DPSCs (Fig. 5B). Additionally, the implants were extracted and analyzed for their hemoglobin content. The quantification results show a significant difference and a higher hemoglobin content of GMSCs compared to Matrigel (negative control; P < 0.05); however, no significant difference was observed in the formation of new vessels around the implants between GMSCs and DPSCs (Fig. 5C). The H&E staining, 12 d after implantation, revealed several luminal structures containing red blood cells (Fig. 5 ). Also, the presence of cell invasion was revealed only in the plugs containing cells (MSCs). Additionally, specific HLA-A immunostaining revealed the presence of human MSCs within the Matrigel plug at days 1 and 12 (Fig. 5A). The quantification of HLA-A staining revealed the proliferation of the human cells in vivo. A comparable 2.2-fold increase (P < 0.05) was measured for both DPSCs and GMSCs at 12-d postimplantation (Fig. 5D).
Fig. 5.
Comparison of the angiogenic potential of dental pulp stem cells (DPSCs) and gingival mesenchymal stem cells (GMSCs) in a mouse plug assay model. In order to determine the angiogenic capacity between DPSCs and GMSCs, a Matrigel plug assay was performed in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice. The mice were divided into 4 different groups, namely, human umbilical vein endothelial cells (positive control), Matrigel (negative control), DPSCs, and GMSCs. The different cells (2 × 106) were mixed with a growth factor reduced Matrigel and implanted subcutaneously. At 15-d posttransplantation, the implants were harvested and (A) images were taken, and (B) quantification of the vessels around the implant was performed using the ImageJ software (P < 0.05). (C) Also a quantification of the hemoglobin content (μg/mL) was performed using Drabkin’s reagent at different concentrations (P < 0.05). Histological staining (A) Matrigel implants containing DPSCs or GMSCs were evaluated at 12-d postsubcutaneous implantation in mice. Macroscopic view of explanted Matrigel plugs. Hematoxylin and eosin (H&E)-staining of implants containing DPSCs, GMSCs, or Matrigel alone (control) preimplantation and 12-d post implantation (20× and 40× magnification). H&E-staining showing (40× magnification) high-power view of 1 microvessel containing hematopoietic cells. (A) Human leukocyte antigen (HLA-A) immunostaining revealed the presence of human mesenchymal stem cells within the Matrigel at days 1 and 12. (D) The amount of HLA-A expression was measured using Image J, showing an increase for both DPSCs and GMSCs at day 12 (postimplantation) in comparison to day 1 (preimplantation; P < 0.05).
Discussion
Stem cells from the oral cavity such as DPSCs and GMSCs offer a promising source for cell therapy due to their abundance and accessibility. In this study, we examined differentially expressed regulatory factors for MSCs involved in key biological function of haploidentical MSCs isolated from the gingival and DPSCs. The characterization included comparisons of their proliferation potential, their ability to form colonies, mesodermal differentiation, surface antigen expression, and finally for their angiogenic potential, both in vitro and in vivo through tubule and plug transplantation assays. Both DPSCs and GMSCs showed similar expression of the typical MSC surface markers and the trilineage differentiation potential; however, no differences were observed between DPSCs and GMSCs. Previous studies have demonstrated a trilineage differentiation for DPSCs24 and GMSCs23,25–28. In a comparison study between DPSCs, PDLSCs, and PAFSCs (periapical follicle stem cells), no chondrocyte differentiation was observed29. Another aspect including the proliferation, migration, and the ability to form colony units between GMSCs and DPSCs was investigated. The results have shown a significant increase in the proliferation of GMSCs compared to DPSCs at day 6 of culture. Similarly, GMSCs have exposed an accelerated migration profile at all the different time points (4, 8 and 12 h) based on a scratch wound assay. Additionally, the CFU-F assay confirmed that GMSCs and DPSCs were clonogenic, with a significant advantage of GMSCs over the other cell source. These results coincide with previous studies where GMSCs were compared to BMSCs, and the results have shown that GMSCs did not lose their MSC characteristics at higher passages and the proliferation rate of GMSCs was significantly higher compared to BMSCs7. Similarly, in a different study, the proliferation between GMSCs and PDLSCs pointed at a higher cell proliferation rate for GMSCs following 8 d of culture17. It is very challenging to draw conclusions or extrapolate these results as the extent of donor variability throughout the characterization process can lead to high inconsistencies. MSCs from the same source have shown significant differences that were associated with demographic or genetic variations18,19. However, the advantage of studying haploidentical cells, as done in this study, circumvents this limitation. The current regenerative approaches, based on the use of MSCs, consider their multiple biological properties including angiogenic potential. The angiogenic function is relevant in multiple conditions, including local ischemia, where the activation and proliferation of endothelial cells are required to form neovasculature or remodel existing collaterals. The angiogenic effect of GMSCs versus DPSCs was investigated in vitro through a tubule formation assay. The results point at an increase in the formation of total tube length and total loops for GMSCs compared to DPSCs. No significant difference in the formation of total branching points was observed between DPSCs and GMSCs. Additionally, we demonstrate that GMSCs and DPSCs incubated for 48 h under hypoxic conditions induce an angiogenic effect on the cells compared to normoxic conditions. Interestingly, GMSCs have demonstrated higher angiogenic potential under hypoxic conditions with a significant increase in total loops, total branching points, total tube length and also the increase of VEGF compared to DPSCs. Different studies have demonstrated that DPSCs have a better angiogenic potential compared to different oral stem cell populations. Hilkens et al. investigated the paracrine angiogenic properties of DPSCs, SCAPs, and FSCs. They showed an increase in the tubulogenesis of DPSC-conditioned medium compared to the negative control situation as was shown by an in vitro Matrigel assay30. The results of the in vivo plug assay showed a significant increase in the hemoglobin content of GMSCs compared to the control (Matrigel alone); no differences were observed between DPSCs and GMSCs in the formation of new vessels around the implant. The difference between the angiogenic results obtained in the tubule versus the plug transplantation assay could be related to both experimental timing and microenvironment conditions. In vivo hypoxic conditions such as limb ischemia models might be useful to consider in the future assessing the angiogenic properties. Previously, it has been demonstrated that DPSCs injected in rats to induce angiogenesis by secreting proangiogenic and antiapoptotic factors have shown after 4 wk that DPSC-treated animals have shown an improvement in cardiac function, in parallel with a reduction in infarct size31. Importantly, cell invasion and proliferation were only noted when the plug contained MSCs, independently of their origin. The human origin of the cells detected in the plugs at different time points demonstrates their survival and proliferation capacities. It is important to mention that cell rejection was not assessed in those experiments, as the engraftment assay was performed in immunodeficient NSG mice.
Conclusion
The present work describes the differences between haploidentical MSCs isolated simultaneously from 2 different sites of the oral cavity of 3 donors. GMSCs displayed a higher capacity to proliferate, migrate, and form angiogenic tubules compared with DPSCs in vitro and in vivo. Additionally, GMSCs are abundant, and their harvest is less invasive, therefore providing a good cell source for regenerative purposes. By using cells from the same donor, our comparative studies bypass the donor variation and hence, present robust comparison data between DPSCs and GMSCs. These results provide clinicians with strong arguments and considerations when it comes to single out the best cell origin among dental sources for different regenerative and tissue engineering applications24,25.
Supplemental Material
Supplementary_Material for Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential by Ioannis Angelopoulos, Claudia Brizuela, and Maroun Khoury in Cell Transplantation
Acknowledgments
The authors specially acknowledge the technical expertise and assistance of Dr. Macarena Ocaña with animal experimentation. We are thankful for Dr. Karina Pino Lagos for her help on the analysis of the flow cytometry data, Paz Gonzalez for the help in the tubule and plug assays, Dr. Nicole Saint-Jean for helping us with the mesodermal differentiation, and Dr. Luis Castrillon for his help analyzing the colony-forming units experiment; very grateful for the help and support of Carolina Inostrosa.
Footnotes
Author Contributions: Ioannis Angelopoulos and Maroun Khoury contributed to the experimental design of the study. Ioannis Angelopoulos was involved in collecting and analyzing the data. Claudia Brizuela obtained the samples of pulp and gingival tissue. The final manuscript was read and approved by all authors.
Ethical Approval: All in vivo studies received approval by the Universidad de Los Andes ethical committee for animal experimentation. Additionally the authors have completed and complied with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines/checklist for preclinical animal studies.
Statement of Human and Animal Rights: I undersign, and certify that the procedures and the experiments I’ve done respect the ethical standards in the Helsinki Declaration of 1975, as well as the national law.
Statement of Informed Consent: All individuals that participated in the study signed an informed consent form following ethical approval of the Universidad de los Andes for the donation of samples.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Maroun Khoury is the chief scientific officer of Cells for Cells and REGENERO. The other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the “Corporación de fomento de la producción” (CORFO L1 13IDL1-25418 and CORFO L2 14IDL2-30051).
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Chen FM, Sun HH, Lu H, Yu Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials. 2012;33(27):6320–6344. [DOI] [PubMed] [Google Scholar]
- 2. Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26(4):1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, Chen JH, Wang BB, Huang GT, Wang S, Shi S. Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis. 2010;16(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Y, Rossi FM, Putnins EE. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials. 2010;31(33):8574–8582. [DOI] [PubMed] [Google Scholar]
- 5. Tobita M, Uysal AC, Ogawa R, Hyakusoku H, Mizuno H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng Part A. 2008;14(6):945–953. [DOI] [PubMed] [Google Scholar]
- 6. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393(3):377–383. [DOI] [PubMed] [Google Scholar]
- 8. Tsumanuma Y, Iwata T, Washio K, Yoshida T, Yamada A, Takagi R, Ohno T, Lin K, Yamato M, Ishikawa I, Okano T, Izumi Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32(25):5819–5825. [DOI] [PubMed] [Google Scholar]
- 9. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. [DOI] [PubMed] [Google Scholar]
- 10. Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–1835. [DOI] [PubMed] [Google Scholar]
- 11. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 12. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. [DOI] [PubMed] [Google Scholar]
- 13. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68(11):4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3(5):e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34(29):7033–7047. [DOI] [PubMed] [Google Scholar]
- 18. Yang HJ, Kim KJ, Kim MK, Lee SJ, Ryu YH, Seo BF, Oh DY, Ahn ST, Lee HY, Rhie JW. The stem cell potential and multipotency of human adipose tissue-derived stem cells vary by cell donor and are different from those of other types of stem cells. Cells Tissues Organs. 2014;199(5–6):373–383. [DOI] [PubMed] [Google Scholar]
- 19. Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ, van Milligen FJ. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332(3):415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez PL, Carvajal C, Cuenca J, Alcayaga-Miranda F, Figueroa FE, Bartolucci J, Salazar-Aravena L, Khoury M. Chorion mesenchymal stem cells show superior differentiation, immunosuppressive, and angiogenic potentials in comparison with haploidentical maternal placental cells. Stem Cells Transl Med. 2015;4(10):1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6:199 doi: 10.1186/s13287-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steed MB. The indications for third-molar extractions. J Am Dent Assoc. 2014;145(6):570–573. [DOI] [PubMed] [Google Scholar]
- 23. Otabe K, Muneta T, Kawashima N, Suda H, Tsuji K, Sekiya I. Comparison of Gingiva, dental pulp, and periodontal ligament cells from the standpoint of mesenchymal stem cell properties. Cell Medicine. 2012;4(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waddington RJ, Youde SJ, Lee CP, Sloan AJ. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs. 2009;189(1–4):268–274. [DOI] [PubMed] [Google Scholar]
- 25. Tang L, Li N, Xie H, Jin Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J Cell Physiol. 2011;226(3):832–842. [DOI] [PubMed] [Google Scholar]
- 26. Ge S, Mrozik KM, Menicanin D, Gronthos S, Bartold PM. Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: potential use for clinical therapy. Regen Med. 2012;7(6):819–832. [DOI] [PubMed] [Google Scholar]
- 27. Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, Yang P, Pei X. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011;20(12):2093–2102. [DOI] [PubMed] [Google Scholar]
- 28. Mitrano TI, Grob MS, Carrion F, Nova-Lamperti E, Luz PA, Fierro FS, Quintero A, Chaparro A, Sanz A. Culture and characterization of mesenchymal stem cells from human gingival tissue. J Periodontol. 2010;81(6):917–925. [DOI] [PubMed] [Google Scholar]
- 29. Navabazam AR, Sadeghian Nodoshan F, Sheikhha MH, Miresmaeili SM, Soleimani M, Fesahat F. Characterization of mesenchymal stem cells from human dental pulp, preapical follicle and periodontal ligament. Iran J Reprod Med. 2013;11(3):235–242. [PMC free article] [PubMed] [Google Scholar]
- 30. Hilkens P, Fanton Y, Martens W, Gervois P, Struys T, Politis C, Lambrichts I, Bronckaers A. Pro-angiogenic impact of dental stem cells in vitro and in vivo. Stem Cell Res. 2014;12(3):778–790. [DOI] [PubMed] [Google Scholar]
- 31. Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008;26(3):638–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Material for Gingival Mesenchymal Stem Cells Outperform Haploidentical Dental Pulp-derived Mesenchymal Stem Cells in Proliferation Rate, Migration Ability, and Angiogenic Potential by Ioannis Angelopoulos, Claudia Brizuela, and Maroun Khoury in Cell Transplantation