Abstract
Ten mongrel dogs were used in this study. Diabetes was chemically induced in 7 dogs, and 3 dogs served as normal controls. For each diabetic dog, 5 million human bone marrow–derived mesenchymal stem cells/kg were differentiated to form insulin-producing cells using a trichostatin-based protocol. Cells were then loaded in 2 TheraCyte capsules which were transplanted under the rectus sheath. One dog died 4 d postoperatively from pneumonia. Six dogs were followed up with for 6 to 18 mo. Euglycemia was achieved in 4 dogs. Their glucose tolerance curves exhibited a normal pattern demonstrating that the encapsulated cells were glucose sensitive and insulin responsive. In the remaining 2 dogs, the fasting blood sugar levels were reduced but did not reach normal values. The sera of all transplanted dogs contained human insulin and C-peptide with a negligible amount of canine insulin. Removal of the transplanted capsules was followed by prompt return of diabetes. Intracytoplasmic insulin granules were seen by immunofluorescence in cells from the harvested capsules. Furthermore, all pancreatic endocrine genes were expressed. This study demonstrated that the TheraCyte capsule or a similar device can provide adequate immunoisolation, an important issue when stem cells are considered for the treatment of type 1 diabetes mellitus.
Keywords: human mesenchymal stem cells, insulin, diabetes, dogs, immunoisolation
Introduction
Recent progress in the field of regenerative medicine provided a new strategy to reconstitute pancreatic endocrine function. To this end, various sources of stem cells have been utilized. D’Amour et al. refined a protocol for the differentiation of human embryonic stem cells to form insulin-producing cells (IPCs)1. Successful generation of IPCs from human embryonic stem cells was also repeated by Pagliuca et al2. and Rezania et al3. The time required for the correction of hyperglycemia in mice after the transplantation of their differentiated cells was comparable among the 3 studies and ranged between 50 and 75 d4. The use of embryonic stem cells suffers from 2 drawbacks: immunogenicity and teratogenicity. These problems could be contained if these cells are transplanted within an immunoisolation device. The pluripotency of induced pluripotent stem (iPS) cells provides an opportunity for their differentiation to IPCs. Their use can resolve some of the problems that pertain to embryonic stem cells. Patient-derived iPS cells are autologous and do not evoke a process of immunorejection. Similar to embryonic stem cells, iPS cells have a high proliferation activity and can form teratomas. In a recent study, human iPS cells derived from both fetal and adult tissues were differentiated in vitro to form IPCs. Approximately 5% of cells became insulin-positive. When transplanted into immunodeficient mice, the transplanted cells lost their insulin secretion capacity in response to glucose stimulation5.
In our laboratory, we have provided evidence that a subpopulation of human bone marrow–derived mesenchymal stem cells (HBM-MSCs) can be differentiated to form IPCs, albeit in a modest proportion. Transplantation of these cells into diabetic nude mice resulted in control of their diabetes6. In a subsequent study, we compared the efficiency of 3 protocols utilized to induce differentiation of HBM-MSCs into IPCs. The yield of functional IPCs was similar among the 3 studied methods7. Given its simplicity and the short period required for its completion, the trichostatin-based protocol is currently our basic method. In a more recent study, we have demonstrated that the transplanted cells under the kidney capsule of nude mice undergo further differentiation in vivo. The proportion of IPCs from the harvested kidneys reached a peak of ≃18%, 4 wk after transplantation without a substantial change thereafter8. Encouraged by these outcomes, it was logical to apply such an approach in a large animal.
Materials and Methods
The required approvals for this study were obtained from the ethical committee of the University of Mansoura.
Retrieval, Isolation, and Expansion of HBM-MSCs
Bone marrow aspirates (BMAs) were obtained from 3 healthy subjects undergoing elective orthopedic surgical procedures for correction of closed fractures at the Mansoura University Hospital. These donors were 3 males aged 22, 27, and 36 y. The BMAs were collected in heparin, diluted 1:1 in low-glucose Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) layered atop a density gradient (Ficoll-Paque, 1.077 g/mL; Pharmacia, Uppsala, Sweden) and centrifuged for 20 min at 600 g. The cells were collected from the DMEM/Ficoll interface, washed twice in phosphate-buffered saline (PBS), and resuspended in 10 mL of low-glucose complete DMEM supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA), 100 U/mL penicillin, and 100 U/mL streptomycin (Sigma-Aldrich). One milliliter of BMA yielded ∼1.5 × 106 nucleated cells. The collected cells were cultured in complete DMEM at a density of 5 × 105 cells/mL (10 mL in 25-cm2 tissue culture flasks) and incubated at 37 °C in a 5% CO2 incubator. Aliquots were preserved in liquid nitrogen for subsequent expansion and examination. After 3 d, the nonadherent cells were discarded. The adherent MSCs were cultured to 80% confluence before passaging using trypsin. Cells were resuspended in complete DMEM and reseeded at a ratio of 1:2 and then cultured to reach 80% confluence. This step was repeated for a second passage. At this point, the cells were spindle-shaped and displayed a fibroblast-like appearance. Samples from each donor were examined in duplicate for their in vitro characterization.
Characterization of the Isolated HBM-MSCs
Phenotyping
At passage 3, HBM-MSCs were trypsinized, centrifuged at 300 g for 8 min, and resuspended in PBS at a concentration of 1 × 106 cells/mL. Next, 100 µL were incubated for 30 min in 20 µL of antibodies against CD14, CD45 (fluorescein isothiocyanate [FITC]) or CD73, CD34 phycoerythrin (PE) or in 5 µL of CD105 (PE), or CD90 (FITC; Becton-Dickinson, Franklin Lakes, NJ, USA); washed with 1 mL of stain buffer (BD-Pharmingen, San Diego, CA, USA); and resuspended in 500 µL of stain buffer. The labeled cells were analyzed using an argon ion laser with a wavelength of 488 nm (FACSCalibur, Becton-Dickinson). A total of 10,000 events was obtained and analyzed with the Cell Quest software program, Version 5.2.1 (Becton-Dickinson). Control staining with the appropriate isotype-matched monoclonal antibodies was included.
Multilineage differentiation potential
HBM-MSCs were induced to differentiate into adipocytes, chondrocytes, and osteocytes using appropriate differentiation media. Oil Red O was used to stain adipocytes, alcian blue was used to stain chondrocytes, and Alizarin-Red was used to stain osteocytes.
Differentiation of HBM-MSCs into Endocrine Cells
The expanded and characterized cells from the 3 donors were pooled prior to differentiation. Differentiation was performed according to a protocol previously reported by Tayaramma et al9. Initially, the cells were cultured for 3 d in serum-free DMEM supplemented with trichostatin-A (TSA; Sigma-Aldrich) at a concentration of 55 nanomoles. Then, the cells were cultured for an additional 7 d in high-glucose (25 millimoles) medium consisting of a 1:1 ratio of DMEM:DMEM/F12 (Sigma-Aldrich). This mixture was supplemented with 10% fetal bovine serum and 10 nanomoles glucagon (GCG)-like peptide 1 (Sigma-Aldrich).
In Vivo Transplantation Studies in Dogs
Experimental animals
Ten male mongrel dogs were obtained from the animal farm belonging to the faculty of veterinary medicine, Mansoura University. Their age ranged between 6 and 12 mo and they weighed between 15 and 20 kg. As approved and supervised by an institution veterinarian, the animals were kept in individual well-ventilated cages at a room temperature (RT) of 24 °C and humidity of 50% and were provided with a standardized diet of bread, milk, cooked chicken, and water ad libitum.
Induction of diabetes
Diabetes was chemically induced by a single intravenous injection of a mixture of 40 mg/kg alloxan (Sigma-Aldrich) and 35mg/kg streptozotocin (Sigma-Aldrich) in citric buffer (pH 4.5) as recommended by Anderson et al10. Fasting blood sugar was determined 3 d thereafter. Diabetes was confirmed when blood sugar readings exceeded 250 mg/dL for 2 consecutive readings.
Device loading
Bioisolator devices were obtained from TheraCyte Inc. (Irvine, CA, USA). For each dog, 2 capsules were utilized. Using a Hamilton syringe, 5 million differentiated cells/kg were equally loaded into 2 capsules via the access port which was then sealed with medical grade silicone gel (NuSil Technology, Carpinteria, CA, USA).
Device implantation
The dogs were anaesthetized by thiopental sodium (10 to 25 mg/kg) with endotracheal intubations and mechanical ventilation. The abdomen was sterilized and draped. An antibiotic was given intravenously. A midline incision was made in the skin and fascia. The skin was then retracted to one side and a transverse incision was made in the anterior rectus sheath. By blunt dissection, a pocket was created under the rectus sheath into which one of the devices was embedded. The procedure was repeated on the other side. The abdomen was then closed in layers. Antibiotics were given for 2 more days. Azathioprine (Excella, Nurnberger, Germany) was given orally at a dose of 1 mg/kg throughout the observation period.
Follow-up studies
One dog died 4 d postoperatively from pneumonia (dog #3). For the remaining 6 dogs, urinary sugar was tested daily using Keto-Diabur Test 5000 (Roche Diagnostics, Mannheim, Germany). Body weight and blood glucose levels (measured by glucometer strips) were determined weekly. Blood samples were withdrawn and tested for human and canine insulin and C-peptide. An intravenous glucose tolerance test (1 g/kg) was carried out for all the animals every 6 mo. Blood samples were collected before and 30, 60, 90, and 120 min after glucose administration. Samples were tested at each time point for glucose, human insulin, and canine insulin levels. The K-values representing the decline rate of blood sugar in percentage per minute were determined for the cured, partially controlled, and normal dogs using a formula suggested by Lundbaek11. The mathematical basis used to construct these curves is given in Online Supplementary Table 1. Glycated hemoglobin (HbA1c) levels were also determined using the A1CD2 kit (Roche Diagnostics).
Estimation of Serum Insulin and C-peptide
Serum human insulin (µIU/mL) and C-peptide (ng/mL) were measured using ELISA kit (DRG Diagnostics, Marburg, Germany) according to the manufacturer’s instructions. Serum canine insulin (µIU/mL) and C-peptide (ng/mL) levels were measured using ELISA kit (Nova, Bioneovan Co., Ltd, Beijing, China) according to the manufacturer’s instructions.
Retrieval of the TheraCyte capsules
The implanted devices were removed from the dogs at the following time points: 6 mo in (dog #1), 8 mo in (dogs #2 and #4), 12 mo in (dog #5), and 18 mo in (dogs #6 and #7). From each dog, one capsule was utilized for histological studies and the second was utilized for relative gene expression. In addition, a sample from the tail of the right lobe of the pancreas was excised. Thereafter, each dog’s euglycemia was maintained by regular insulin therapy.
Gene Expression by Real-time Polymerase Chain Reaction
Total RNA was extracted from the undifferentiated cells, at the end of in vitro differentiation and from the cells retrieved from the TheraCyte capsule, using an RNeasy Plus Mini Kit (Qiagen GmbH, Hilden, Germany). Three micrograms of total RNA were converted to cDNA using an RT2 First Strand Kit (Qiagen Sciences, Germantown, MD, USA). Custom gene arrays were designed and supplied in 96-well plates (Qiagen Sciences). The genes tested in this study were selected based on their important role in the development of α and β cells6. Expression was determined for the following genes: pancreatic endocrine hormones: insulin, GCG, and somatostatin; relevant transcription factors: pancreatic and duodenal homeobox-1, neurogenin3, paired box4 (Pax4), regulatory factor X6, neurogenic differentiation 1, V-maf musculoaponeurotic fibrosarcoma oncogene homologue A and B, and POU (Pituitary-specific Pit-1 the Octamer transcription factor proteins Oct-1 and Oct-2 [octamer sequence is ATGCAAAT] the neural Unc-86 transcription factor from Caenorhabditis elegans) class 5 homeobox 1 (OCT4); an endocrine precursor marker: nestin; a glucose transporter: solute carrier family 2 member 2; and a pancreatic enzyme: glucokinase (GCK). Glyceraldehyde-3-phosphate dehydrogenase was included in the custom gene array as an internal control and for normalization. Amplifications were performed in each well using a 25 µL reaction volume consisting of 12.5 µL of 2×SYBR Green Master Mix (Qiagen Sciences,), 1 µL of cDNA template, and 11.5 µL of nuclease-free water. The plate was inserted into a real-time thermal cycler (CFX96 Real-Time System, Bio-Rad, Hercules, CA, USA) that was programmed according to the manufacturer’s instructions. The procedure was performed in duplicate for each sample. A mathematical model introduced by Pfaffl was used for the relative quantification of target genes12. In this study, gene expression results were relative to those obtained for human islets.
Immunolabeling
Antibodies
The primary antibodies employed were insulin in a dilution of 1:200 (mouse monoclonal, Novus Biologicals, Littleton, CO, USA), GCG in a dilution of 1:400 (rabbit monoclonal anti-GCG; Cell Signaling Technology), polyclonal rabbit anti-human SST in a dilution of 1:300 (Novus Biologicals), human C-peptide in a dilution of 1:100 (rabbit polyclonal; Cell Signaling Technology), CD3 and CD20 (Genemed Biotechnologies, San Francisco, CA, USA).
The secondary antibodies employed were anti-mouse immunoglobulin G (IgG; H + L) Alexa Fluor 488 conjugate in a dilution of 1:200 (Cell Signaling Technology) and anti-rabbit IgG (H + L) Alexa Fluor 555 conjugate in a dilution of 1:100 (Cell Signaling Technology). Universal Kit Power-Stain Version 1.0 Poly Horseradish peroxidase (HRP) 3,3-diaminobenzidine (DAB) for mouse and rabbit (Genemed Biotechnologies) was employed as a secondary antibody for CD3 and CD20.
Immunocytochemistry
Cell preparations were cultured on chamber slides (Nunc, Thermo Scientific, Rochester, NY, USA). The cells were fixed in 4% paraformaldehyde, permeabilized using chilled 100% methanol for 10 min, blocked with 5% normal goat serum for 60 min at RT, and incubated overnight in the primary antibodies at 4 °C. Thereafter, the cells were washed with PBS and incubated with the secondary antibodies for 3 h at RT. Negative controls were performed by omitting treatment with the primary antibody.
Immunofluorescent and histological studies
The harvested capsules were processed according to the manufacturer’s instructions (10% formalin, alcohol gradients, xylene gradients, and finally paraffin embedding). Sections were taken at a thickness of 3 µm and mounted on positive-charged coated slides (Citoglas, Citotest Labware Manufacturing Co., Haimen, China). The slides were then deparaffinized using xylene and a decreasing ethanol gradient. Antigens were unmasked by boiling the slides in 10 millimoles of sodium citrate buffer (pH 6.0) for 30 min and a subboiling temperature was maintained for 10 min. The sections were blocked with 5% normal goat serum and then incubated overnight with the primary antibody at 4 °C. Thereafter, the slides were washed 3 times in PBS and incubated with the secondary antibody for 3 h at RT. Nuclei were counterstained using 4’,6-diamidino-2-phenylindole. Image J software 1.51h (developed by NIH) was used to determine the proportion of insulin-producing cells within the TheraCyte capsule. To this end, 10 fields were randomly selected for cell counting which was carried out by 2 independent histopathologists. The results from all fields were calculated and expressed as the mean proportion of insulin-positive cells among total transplanted cells. In all the above studies, confocal images were captured using a Leica TCS SP8 microscope (Leica Microsystems, Mannheim, Germany). For immunolabeling of the native pancreas, the primary antibody was mouse monoclonal anti-insulin (Novus Biologicals), and the secondary antibody was the Power-Stain Version 1.0 Poly HRP DAB Kit for mouse (Genemed Biotechnologies). The retrieved capsules and pericapsular tissues were stained with silver, Congo red, and phosphotungstic acid hematoxylin. The pericapsular cellular infiltrate was also studied for CD3 and CD20 expression.
Statistical Methods
To measure central tendencies, median values were chosen because they are not affected by extreme observations when small amounts of data are available; otherwise mean values were utilized. T-tests were used to compare continuous data, and a P value <0.05 was considered significant.
Results
Characterization of the Cultured HBM-MSCs
Cultured cells became spindle-shaped, fibroblast-like cells that were arranged in monolayers. Flow cytometry revealed that these cells expressed high levels of CD73, CD90, and CD105 but negligible levels of CD14, CD34, and CD45 (Online Supplementary Fig. 1). These cells could be differentiated to form adipocytes, chondrocytes, and osteocytes when the appropriate growth factors were added (Online Supplementary Fig. 2). Taken together, these findings confirmed that these cells were indeed stromal MSCs and met the minimal criteria proposed by the International Society of Cellular Therapy.
Functional Evaluation of Differentiated HBM-MSCs
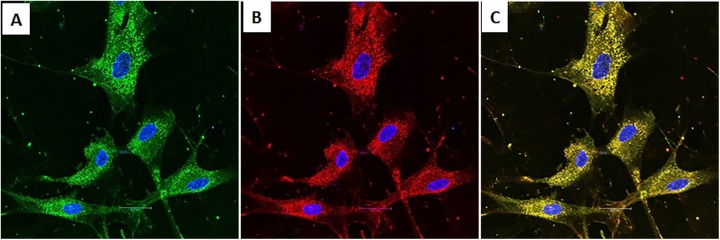
The presence of insulin granules within the cytoplasm of the IPCs was detected by immunocytochemistry (Fig. 1). The proportion of insulin-positive cells was ≃3%. Immunostaining for C-peptide was also positive. Coexpression of insulin and C-peptide was observed within the same cells by electronic merging.
Fig. 1.
Immunocytochemistry of human bone marrow–derived mesenchymal stem cells at the end of in vitro differentiation. (A) Cells with insulin-positive granules (green). (B) Cells with C-peptide-positive granules (red). (C) Coexpression of insulin and C-peptide within the same cells (yellow). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (blue).
Outcomes of the Transplantation Experiments
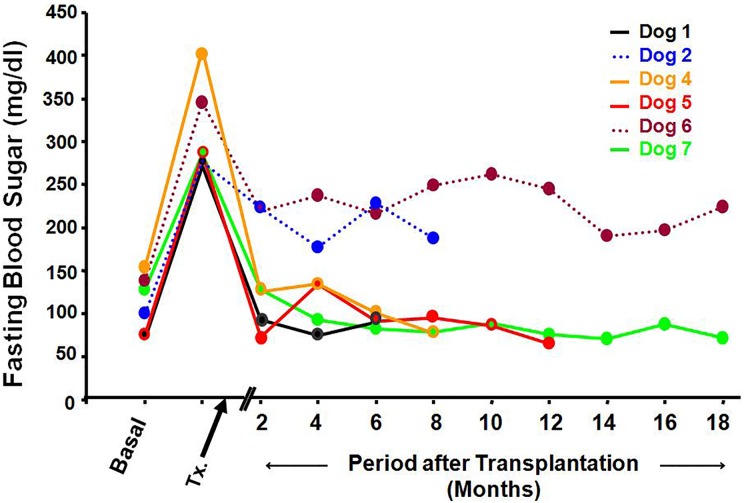
Fasting blood sugar (Fig. 2)
Fig. 2.
Fasting blood sugar levels in the 6 treated dogs. Basal values ranged between 76 and 154 mg/dL. After chemical induction, the values increased substantially (range between 276 and 407 mg/dL). Four weeks after transplantation, blood sugar values became normal in 4 dogs (solid lines). Two dogs were partially controlled (dotted lines).
After chemical induction of diabetes, the fasting blood sugar was increased in all animals reaching values ranging between 276 and 407 mg/dL. Fasting blood sugar became normal 8 wk after transplantation in 4 animals. These dogs remained euglycemic throughout the observation period. Partial control was achieved in the remaining 2 dogs. Their fasting blood sugar was reduced but did not reach normal values (Online Supplementary Table 2). The HbA1c levels for normal dogs ranged from 3.1% to 3.5%. Values for the cured experimental animals ranged from 3.6 to 3.8%. Higher values (4.7% - 6.7%) were recorded for dogs with incompletely controlled diabetes. Following removal of the capsules, fasting blood sugar levels returned promptly to pretransplantation readings. This necessitated their treatment with exogenous human insulin to maintain their survival (10 IU of a mixture of 70% isophane insulin and 30% regular insulin were injected subcutaneously, once daily).
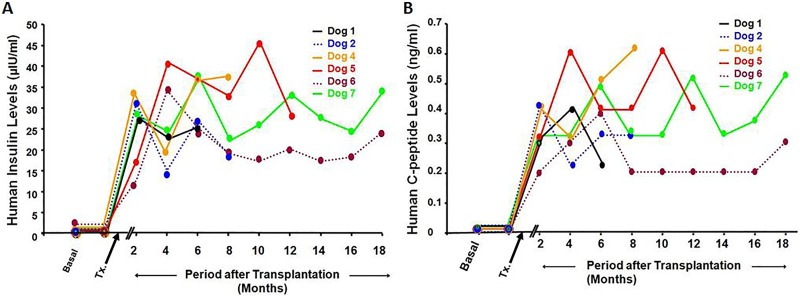
Serum human insulin and C-peptide (Fig. 3A and B)
Fig. 3.
(A) Human insulin levels. Under basal conditions and following the induction of diabetes, human insulin was not detected. Four weeks after transplantation, human insulin became measurable in all 6 dogs with a range of 10.5 to 30 µIU/mL. Thereafter, human insulin levels were sustained among the 4 cured dogs with a range of 25 to 33 µIU/mL. (B) After transplantation, human C-peptide levels became measurable in all treated dogs.
After transplantation, the serum human insulin levels increased to reach a peak at 8 wk. These values remained measurable and stable throughout the follow-up period. Human serum C-peptide followed a similar pattern (Online Supplementary Tables 3 and 4).
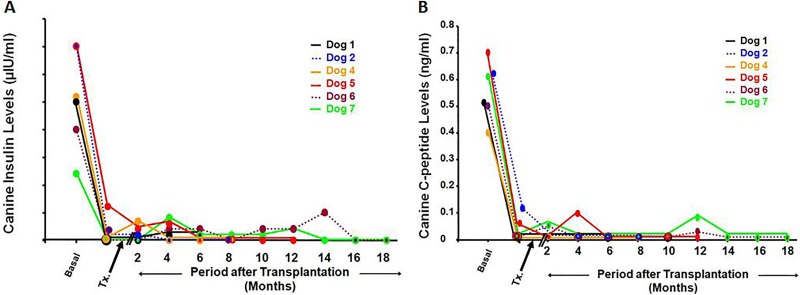
Serum canine insulin and C-peptide (Fig. 4A and B)
Fig. 4.
(A) Canine insulin levels. Following the induction of diabetes, the canine insulin levels were sharply reduced. Thereafter, canine insulin levels became negligible throughout the observation period for all dogs. (B) Canine C-peptide. Following the induction of diabetes, these levels were sharply reduced. Thereafter, they became negligible throughout the observation period for all dogs.
After the chemical induction of diabetes, serum canine insulin and C-peptide values were immediately reduced and continued to be negligible throughout the observation period in all dogs (Online Supplementary Tables 5 and 6).
Weights of the experimental animals (Online Supplementary Fig. 3)
After the induction of diabetes, the weights of the animals were reduced for a period of 2 to 3 mo. Thereafter, there was a gradual increase even in the 2 dogs whose diabetes was partially controlled (Online Supplementary Table 7).
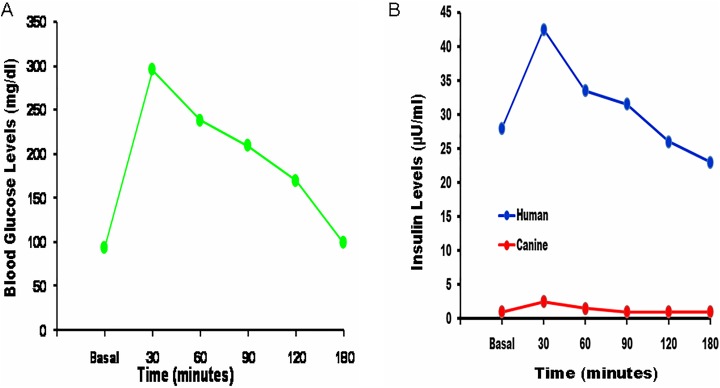
Glucose tolerance curve (Fig. 5A and B)
Fig. 5.
Glucose tolerance test for the cured dogs. (A) After glucose administration, the blood glucose level increased sharply from a mean basal value of 93 mg/dL to a high of 296 mg/dL after 30 min. Thereafter, there was a gradual decline to reach normal values after 120 to 180 min. (B) Human and canine serum insulin levels. These were measured at the same time points as those used for the glucose tolerance test. Human insulin levels exhibited a profile similar to that of blood glucose levels. On the other hand, there were negligible changes in the canine insulin levels.
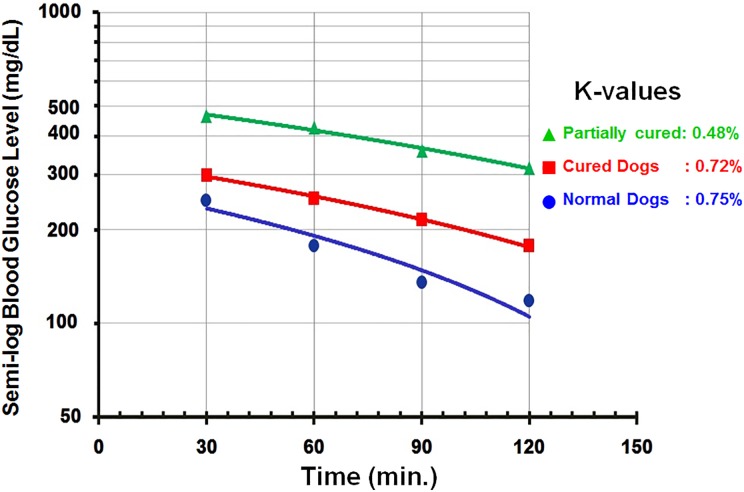
After the intravenous injection of glucose, blood sugar levels increased sharply to reach a maximum after 30 min. Thereafter, there was a gradual decline to reach normal levels after 120 to 180 min. There was a parallel change in serum human insulin levels while serum canine insulin levels remained unchanged (Online Supplementary Tables 8 to 10). Changes in the blood glucose levels in the control animals demonstrated a similar profile, with an initial increase to reach a maximum after 60 min which was followed by a gradual decline to reach basal values after 120 min (Online Supplementary Table 11). The K-values for euglycemic dogs were higher than those for diabetic ones but slightly lower than those for normal dogs (Fig. 6).
Fig. 6.
The results of the intravenous glucose tolerance test are expressed as K-values, which are the decline rates in blood glucose in percentage per minute. When drawn on semilogarithmic paper, the blood sugar curve forms a straight line. The rate of decline of blood sugar levels among the cured dogs was much higher than that for the partially controlled dogs and close to that of normal dogs.
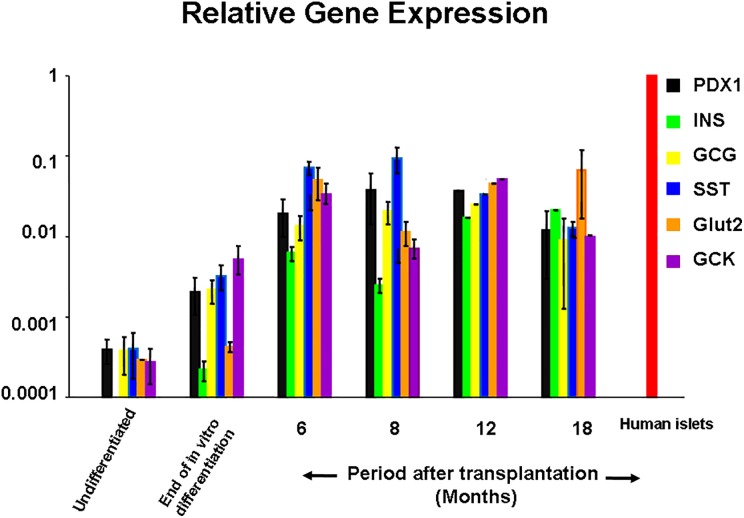
Relative gene expression (Fig. 7)
Fig. 7.
A representative histogram of the relative gene expression of specific pancreatic endocrine genes determined by real-time polymerase chain reaction. At 6 mo posttransplantation, relative gene expression was increased by 20 folds compared with relative gene expression at the end of in vitro differentiation. At 12 and 18 mo posttransplantation, there was a further increase reaching ≃100 folds. Vertical bars represent the standard errors.
At the end of in vitro differentiation, genes for pancreatic hormones, pancreatic enzymes, and endocrine precursors as well as transcription factors were expressed. Among cells retrieved from the isolation device, there was a marked increase in the relative expression of all relevant pancreatic endocrine genes. Insulin gene expression levels were increased by ≃30 folds at 6 mo and by ≃100 folds at 12 and 18 mo compared to values at the end of in vitro differentiation. These differences were statistically significant (P < 0.05; Online Supplementary Table 12).
Immunofluorescence and histological studies
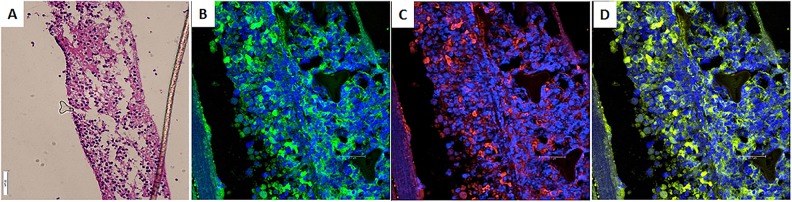
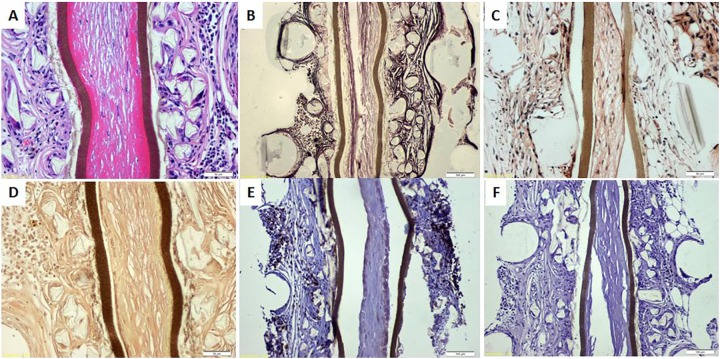
The TheraCyte capsules were removed at different time intervals to detect changes in the functional longevity of the transplanted cells. At 6 mo, approximately 22% of cells were insulin positive (Fig. 8). There was a very slight reduction in this proportion with time. Figure 9 shows representative histological and histochemical findings in a capsule removed 18 mo after transplantation. Hematoxylin and eosin staining revealed extracapsular cellular infiltrate. Silver staining was positive for fibrous tissue deposition outside the capsule. Congo red staining was negative for amyloid deposits. Similarly, phosphotungstic acid hematoxylin staining did not reveal fibrin, glial tissue, collagen, or elastic fiber deposits inside the capsule. The pericapsular cellular infiltrate was CD-3 positive but CD20 negative, indicating the predominance of the cell-mediated immune response. Immunolabeling of the native pancreata of the treated dogs was negative for insulin-secreting cells (Online Supplementary Fig. 4).
Fig. 8.
Removal of TheraCyte capsule 6 mo after transplantation (dog #1). (A) Hematoxylin and eosin. The wall of the capsule and enclosed cells is seen. (B) Immunofluorescent study was positive for intracytoplasmic insulin (green). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (blue). (C) Immunofluorescent study was also positive for intracytoplasmic C-peptide (red). (D) Electronic merging confirmed the coexpression of insulin and c-peptide within the same cells (yellow).
Fig. 9.
Removal of TheraCyte capsule 18 mo posttransplantation (from partially cured dog). (A) Hematoxylin and eosin staining revealed pericapsular cellular infiltrate. (B) Silver staining revealed pericapsular fibrous tissue. (C) Congo red was negative for amyloid deposit inside the capsule. (D) Phosphotungstic acid hematoxylin did not show fibrin, glial tissue, collagen, or elastic fibers inside the capsule. (E) Pericapsular cellular infiltrate was strongly CD3-positive indicating a cell-mediated immune response. (F) CD20 expression was negative, indicating the lack of an antibody-mediated response.
Discussion
The use of large animals as a diabetic model can provide an opportunity to estimate the number of required IPCs/kg body weight to induce euglycemia. This model also permits the evaluation of the functional longevity of these cells. Reports using dogs as a diabetic model are uncommon. Sullivan and associates13 implanted canine islet allografts within a selectively permeable membrane in 10 pancreatectomized dogs. These implants resulted in good control of diabetes in 6 animals for a period of up to 18 mo. Wang et al14. induced diabetes in 9 dogs by pancreatectomy. The authors designed a special capsule to protect their allotransplanted islets. Exogenous insulin independence was achieved in 6 dogs over a period ranging between 64 and 214 d. Using a different approach, adeno-associated viral vectors encoded with GCK and insulin genes were given to diabetic dogs by a single intramuscular injection15. This method resulted in the normalization of the fasting blood sugar for more than 4 y.
In our study, the IPCs employed were of human origin. Their transplantation in dogs within an immunoisolation device was imperative. Some investigators reported the successful xenotransplantation of encapsulated islets without immunosuppression16–18. Mckenzie et al. observed that the combination of immunoisolation and a single dose of anti-CD4 was necessary to protect xenografts19. Tredget and associates reported that monotherapy using a Leucocyte-function-associated antigen-1 (LFA-1) monocolonal antibody promoted the long-term survival of rat islet xenografts without immunoisolation20. We opted to provide additional protection of the encapsulated xenograft by conventional immunosuppression with azathioprine. We used a macroencapsulation device because they are easily removed and it is possible to examine their contents at predetermined periods. To this end, we used the TheraCyte capsule for immunoprotection. Experimental studies using this device in diabetes research are abundant21–25. In summary, all studies provided evidence that these devices protect transplanted pancreatic islets from allograft rejection. In addition, they allowed further maturation of human β-cell precursors after their transplantation into immunodeficient mice26–28. Motte and associates transplanted human embryonic cell–derived pancreatic endoderm into non obese diabetic (NOD)/severe combined immunodeficiency mice29 and compared 3 methods for implantation: a macroencapsulation device, microencapsulation, or free grafts in the cutis or under the renal capsule. Plasma human C-peptide was detected earlier in mice receiving the macroencapsulated graft. In the recipients of microencapsulation, human C-peptide was only marginally detected. Determination of the proinsulin:C-peptide ratio was used to mark the functional state of β cells. Animals receiving the macroencapsulated graft had the lowest ratio, reflecting a higher reserve of converted hormone.
In our experiments, fasting blood sugar levels surged following chemical induction of diabetes up. After transplantation of the loaded devices, it took 6 to 8 wk for the blood glucose to reach basal values in 4 dogs. In the remaining 2 animals, fasting blood sugar was also reduced but did not reach euglycemic levels. We have provided evidence that these changes were due to human insulin released from the encapsulated IPCs. Two months after transplantation, serum human insulin and C-peptide became measurable in all 6 dogs. Thereafter, their detection was sustained throughout the different observation periods. Meanwhile, serum canine insulin and C-peptide were not detected throughout the duration of the experiment. Furthermore, there was no histological evidence for regeneration in the examined samples from the native pancreata. The results of the glucose tolerance curves confirmed that the transplanted cells were glucose sensitive and insulin responsive. Thirty minutes following the intravenous infusion of glucose, blood levels reached a peak of ≃350 mg/dL. This was followed by a gradual decline to reach basal values after 120 to 180 min. The rate of decline of blood glucose levels/minute (K-value) among the cured dogs was much higher than that for the partially controlled animals and very close to that of the normal controls. Human insulin levels were measured at the same time points. Changes in their values followed a pattern parallel to that of the blood glucose levels. Meanwhile, serum canine insulin levels were negligible and did not have detectable changes during the testing period. At the end of in vitro differentiation, the proportion of the resulting IPCs was ≃3%. Six months after their encapsulated transplantation, this proportion increased to ≃22%. Evidence of further in vivo differentiation of the encapsulated cells was also reported in other studies26–28. Thereafter, this proportion exhibited a slight reduction with time. This may be a result of pericapsular fibrosis interfering with an adequate blood supply. Nevertheless, the human insulin levels and the relative gene expression of relevant pancreatic hormones were sustained throughout the duration of this experiment. Notably, there was no histochemical evidence of degenerative changes inside the capsule. Additionally, a cellular immune response against the xenograft was observed, while a humoral response was not observed. Presumably, the humoral response was abrogated because of adjuvant treatment with azathioprine. In addition, it was previously reported that MSCs themselves possess certain immunomodulatory capabilities30. Direct evidence for the presence or absence of an antibody-mediated response was lacking because anticanine IgG and immunoglobulin M antibodies were not available. However, this issue is of no concern in the clinical setting when definitive treatment of type 1 diabetes mellitus (DM) is considered. In this situation, immunoprotection of the encapsulated auto or allografts would be assured without a need for adjuvant immunosuppression.
Despite these promising results, there is always a concern regarding the efficient vascularization of the macroencapsulated devices. Insufficient supply of nutrients and oxygen leads to the necrosis of cells in the center of the graft. Sorenby et al. suggested a 2-step procedure31. Initially, empty capsules were implanted under the skin to induce vascularization. Three months later, islets were injected into the preimplanted device. Adjunctive application of vascular endothelial growth factor (VEGF) was also suggested32,33. Phelps and associates reported that proteolytically degradable hydrogels incorporating VEGF ensured its controlled release34. The addition of exendin-4 to the culture medium resulted in less graft necrosis of the encapsulated islets and promoted more blood vessels around the capsule35. Alternatively, oxygen may be delivered by suitable chemical compounds such as per fluorocarbon,36 calcium oxide,37 and calcium peroxide38.
Experiments with large animals lay the foundation for translational applications in the clinical setting. In this study, evidence was provided that IPCs derived from human MSCs are able to treat chemically induced diabetes in dogs. It was also confirmed that this benefit was the result of human insulin released from the encapsulated cells. An approximate estimate for the number of cells required to provide euglycemia was also determined (≃5 × 106 cells/kg body weight). Although the encapsulated cells were xenogeneic, their functional longevity was maintained for a reasonable period of 18 mo. The TheraCyte capsule in combination with azathioprine, a mild immunosuppressive agent, protected the xenogeneic IPCs from host immune responses. This issue will be of trivial importance when stem cell therapy is considered for the definitive treatment of type 1 DM.
IPCs resulting from the directed differentiation of HBM-MSCs did cure chemically induced diabetes in small animals. Of note, these outcomes were reproduced in a large animal. The next logical step in these research efforts is clinical application for the treatment of type 1 DM. However, initial problems such as a poor blood supply at the transplantation site must first be solved. Several experimental studies to ensure an early adequate oxygen and nutrient supply until a vascular bed is well established are currently underway in our laboratory.
Supplementary Material
Acknowledgment
The authors would like to thank Mrs. Fathia Gado for her immunolabeling work.
Footnotes
Author Contributions: Mahmoud M. Gabr: Cell culture. Mahmoud M. Zakaria: Gene expression. Ayman F. Refaie: Planning of the study protocol and revision of the manuscript. Amani M. Ismail: Flow cytometry. Sherry M. Khater: Immunofluorescence and histologic studies. Sylvia A. Ashamallah: Immunofluorescence studies. Maha M. Azzam: Care of animals and collection of samples. Mohamed A. Ghoneim: Study protocol, supervision of the experiments, and writing of the manuscript.
Ethical Approval: The required approvals for this study were obtained from the ethical committee of the University of Mansoura.
Statement of Human and Animal Rights: As approved and supervised by an institution veterinarian, the animals were kept in individual well-ventilated cages at a room temperature of 24 C and humidity of 50% and were provided with a standardized diet of bread, milk, cocked chicken, and water ad libitum.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article. This work was financially supported by Misr El-Kheir, a nonprofit charity organization, and the National Bank of Egypt and Misr Bank.
Supplemental Material: Supplementary material for this article is available online.
References
- 1. D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Beratge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. [DOI] [PubMed] [Google Scholar]
- 2. Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β-cells in vitro. Cell. 2014;159(2):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61 (8):2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shultz IC. Concise review: manufacturing of pancreatic endoderm cells for clinical trials in type 1 diabetes. Stem Cells Transl Med. 2015;4(8):927–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pellegrini S, Ungaro F, Mercalli A, Melzi R, Sebastiani G, Dotta F, Broccoli V, Piemonti L, Sordi V. Human pluripotent stem cells differentiate into insulin-producing cells able to engraft in vivo. Acta Diabetol. 2015;52 (6):1025–1035. [DOI] [PubMed] [Google Scholar]
- 6. Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Abou-El-Mahasen MA, Ashamallah SA, Khater SM, El-Halawani SM, Ibrahim RY, Uin GS, Kloc M, Calne RY, Ghoneim MA. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell transplant. 2013;22(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gabr MM, Zakaria MM, Refaie AF, Khater SM, Ashamallah SA, Ismail AM, El-Badri N, Ghoneim MA. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gabr MM, Zakaria MM, Refaie AF, Khater SM, Ashamallah SA, Ismail AM, El-Halawani SM, Ghoneim MA. Differentiation of human bone marrow-derived mesenchymal stem cells into insulin-producing cells: evidence for further maturation in vivo. Biomed Res Int. 2015;2015:575837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tayaramma T, Ma B, Rohde M, Mayer H. Chromatin remodeling factors allow differentiation of bone marrow cells into insulin-producing cells. Stem Cells. 2006;24(12):2858–2867. [DOI] [PubMed] [Google Scholar]
- 10. Anderson HR, Stitt AW, Gardiner TA, Lioyd SJ, Archer DB. Induction of alloxan, streptozotocin diabetes in dogs: a revised experimental technique. Lab Anim. 1993;27(3):281–285. [DOI] [PubMed] [Google Scholar]
- 11. Lundbaek K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br Med J. 1962;1(5291):1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ptaffl MW. A new matheuratical model for relative quantification in real-time RT-PCR. Nucleic Acid Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sullivan SJ, Maki T, Borland KM, Mahoney MD, Solomon BA, Muller TE, Monaco AP, Chick WL. Biohybrid pancreas: long-term implantation studies in diabetic, pancreatectomized dogs. Science. 1991;252(5006):718–721. [DOI] [PubMed] [Google Scholar]
- 14. Wang T, Adcock J, Kühtreiber W, Qiang D, Salleng KJ, Trenary I, Williams P. Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without immunosuppression. Transplantation. 2008;85(3):331–337. [DOI] [PubMed] [Google Scholar]
- 15. Callejas D, Mann CJ, Ayuso E, Lage R, Grifoll I, Roca C, Andaluz A, Ruiz-de Gopegui R, Montané J, Muñoz S, Ferre T, Haurigot V, Zhou S, Ruberte J, Mingozzi F, High KA, Garcia F, Bosch F. Treatment of diabetes and long-term survival after insulin and glucokinase gene therapy. Diabetes. 2013;62(5):1718–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanza RP, Butler DH, Borland KM, Staruk JE, Faustman DL, Solomon BA, Muller TE, Rupp RG, Maki T, Monaco AP. Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppression. Proc Natl Acad Sci. 1991;88(24):11100–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syn Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98(6):1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z, Chen M, Fialkow LB, Ellet JD, Wu R Nadler JL. Survival of pancreatic islet xenografts in NOD mice with the theracyte device. Transplant Proc. 2002;34(8):3349–3350. [DOI] [PubMed] [Google Scholar]
- 19. Mckenzie AW, Georgio HM, Zhan Y, Brady JL, Lew AM. Protection of xenografts by a combination of immunoisolation and a single dose of anti-CD4. Cell Transplantation. 2001;10(2):183–193. [PubMed] [Google Scholar]
- 20. Tredget FB, Arfanian H, Gill RG, Rajott RV, Rayat GR. Monotherapy with anti-LFA-1 monocolonal antibody promotes long-term survival of rat islet xenografts. Cell Transplantation. 2008;17(6):599–608. [DOI] [PubMed] [Google Scholar]
- 21. Yong Z, Chen M, Fialrow LB, Ellett JD, Wu R, Nadler JL. Survival of pancreatic islet xeografts in NOD mice with the theracyte device. Transplant Proc. 2002;34(8):3349–3350. [DOI] [PubMed] [Google Scholar]
- 22. Sörenby AK, Wu GS, Zhu S, Wernerson AM, Sumitran-Holgersson S, Tibell AB. Macroencapsulation protects against sensitization after allogenic transplantation in rats. 2006;82(3):393–397. [DOI] [PubMed] [Google Scholar]
- 23. Sweet IR, Yanay O, Waldron L, Gilbert M, Fuller JM, Tupling T, Lernmark A, Osborne WR. Treatment of diabetic rats with encapsulated islets. J Cell Mol Med. 2008;12(68):2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A. The TheraCyte™ device protects against islet allograft rejection in immunized hosts. Cell Transplant. 2013;22 (7):1137–1146. [DOI] [PubMed] [Google Scholar]
- 25. Boettler T, Schneider D, Cheng Y, Kadoya K, Brandon EP, Martinson L, von Herrath M. Pancreatic tissue transplanted in TheraCyte encapsulation devices is protected and prevents hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transplant. 2016;25(3):609–614. [DOI] [PubMed] [Google Scholar]
- 26. Lee SH, Hao E, Savinov A, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapy. Transplantation. 2009;87(7):983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruin JE, Rezania A, Xu J, Narayan K, Fox JK, O’Neil JJ, Kieffer TJ. Maturation and function of human embryonic stem cells-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56(9):1987–1998. [DOI] [PubMed] [Google Scholar]
- 28. Kirk K, Hao E, Lahmy R, Itkin-Ansari P. Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12(3):807–814. [DOI] [PubMed] [Google Scholar]
- 29. Motté E, Szepessy E, Suenens K, Stangé G, Bomans M, Jacobs-Tulleneers-Thevissen D, Ling Z, Kroon E, Pipeleers D, Beta Cell Therapy Consortium EU-FP7. Composition and function of macroencapsulated human emboryonic stem-cell derived implants: comparison with clinical human islets cells graft. Am J Physiol Endocrinol Metab. 2014;307(9):E838–E846. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–1016. [DOI] [PubMed] [Google Scholar]
- 31. Sorenby AK, Kumangi-Braesch M, Sbarma M, Hultenby KR, Wernerson AM, Tibell AB. Preimplantation of an immunoprective device can lower the curative dose of islets to that of free islet transplantation. Studies in a rodent model. Transplantation. 2008;86(2):364–366. [DOI] [PubMed] [Google Scholar]
- 32. Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell transplant. 2000;9(1):115–124. [DOI] [PubMed] [Google Scholar]
- 33. Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braum S, Legeay G, Bellocq JP, Pinget M, Kessler L. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12(6):627–635. [DOI] [PubMed] [Google Scholar]
- 34. Phelps EA, Headen DM, Taylor WR, Thule PH, Garcia AJ. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34(19):4602–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia X, Sharma A, Kumagai-Braesch M, Wernerson AM, Sörenby AK, Yamamoto S, Wang F, Tibell AB. Exendin-4 increased the expression of hypoxia-inducible factor-1α in rat islets and preserves the endocrine cell volume of both free and macroencapsulated islet graft. Cell Transplant. 2002;21(6): 1269–1283. [DOI] [PubMed] [Google Scholar]
- 36. Fraker CA, Cechin S, Álvarez-Cubela S, Echeverri F, Bernal A, Poo R, Ricordi C, Inverardi L, Domínguez-Bendala J. A physiological pattern of oxygenation using per fluorocarbon-based culture devices maximizes pancreatic islet viability and enhances β-cell function. Cell Transplant. 2013;22(9):1723–1733. [DOI] [PubMed] [Google Scholar]
- 37. Coronel MM, Geusz R, Stanler CL. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials. 2017;129:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced death in beta cells and islets via hydrolytically activated oxygen-generating biomaterials. Proc Natl Acad Sci USA. 2012;109(11):4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.