Abstract
Monoclonal gammopathies are characterized by the overproduction of monoclonal Ig (MIg) detectable in the serum or urine resulting from a clonal proliferation of plasma cells or B lymphocytes. The underlying hematologic conditions range from malignant neoplasms of plasma cells or B lymphocytes, including multiple myeloma and B-cell lymphoproliferative disorders, to nonmalignant small clonal proliferations. The term MGUS implies presence of an MIg in the setting of a “benign” hematologic condition without renal or other end organ damage. The term MGRS was recently introduced to indicate monoclonal gammopathy with MIg-associated renal disease in the absence of hematologic malignancy. Most MIg-associated renal diseases result from the direct deposition of nephrotoxic MIg or its light- or heavy-chain fragments in various renal tissue compartments. Immunofluorescence microscopy is essential to identify the offending MIg and define its tissue distribution. Mass spectrometry is helpful in difficult cases. Conditions caused by direct tissue deposition of MIg include common disorders, such as cast nephropathy, amyloidosis, and MIg deposition diseases, as well as uncommon disorders, such as immunotactoid glomerulopathy, proliferative GN with MIg deposits, light-chain proximal tubulopathy, and the rare entities of crystal-storing histiocytosis and crystalglobulinemia. Indirect mechanisms of MIg-induced renal disease can cause C3 glomerulopathy or thrombotic microangiopathy without tissue MIg deposits. Treatment of MIg-associated renal disease is aimed at eliminating the clonal plasma cell or B-cell population as appropriate. Both the renal and the underlying hematologic disorders influence the management and prognosis of MIg-associated renal diseases.
Keywords: kidney disease, Renal pathology, multiple myeloma
The term monoclonal gammopathy refers to the overproduction of a monoclonal Ig (MIg) that is detectable in the serum or urine resulting from the clonal proliferation of Ig-producing plasma cells or B lymphocytes.1,2 Etiologies include hematologic malignancy, such as multiple myeloma (MM), lymphoplasmacytic lymphoma (including Waldenström macroglobulinemia [WM]), or a B-cell lymphoproliferative neoplasm,3 or a nonmalignant small clonal proliferation of plasma cells or B lymphocytes. All of these conditions can secrete intact MIg and/or its monoclonal light- or heavy-chain fragments, which are detectable in the serum or urine as monoclonal protein (M protein) on electrophoresis or measurement of free light chains (FLCs).
The development of renal disease in the setting of monoclonal gammopathies depends on the nephrotoxic potential of the secreted M protein, which is a function of its unique physicochemical properties. Although many patients with MIg never develop renal disease, others manifest diverse patterns of renal injury known as dysproteinemia-associated or MIg-associated renal diseases, which are the subject of this review.
Definitions of MM, Smoldering Myeloma, Monoclonal Gammopathy of Undetermined Significance, and Monoclonal Gammopathy of Renal Significance
MM is a plasma cell malignancy defined by clonal bone marrow plasma cells ≥10% and the presence of one or more of the following myeloma-defining events: hypercalcemia, renal insufficiency, anemia, bone lesions (CRAB) features; clonal plasma cells ≥60%; serum FLC ratio of ≥100; or more than one focal lesion on magnetic resonance imaging. Approximately 10% of patients with Ig light chain amyloidosis (AL) will also meet criteria for MM on the basis of the presence of CRAB features.4 In almost all patients with MM, an M protein can be detected in the serum and/or urine. When M protein is detected in the absence of plasma cell or lymphoid malignancy or end organ damage, the term monoclonal gammopathy of undetermined significance (MGUS) is applied and implies a “benign” condition. MGUS is defined by <10% bone marrow plasma cells, <3 g/dl of M protein, and no myeloma-defining events (Table 1). Smoldering multiple myeloma (SMM) is an intermediate clinical entity between MM and MGUS, and it is defined by clonal bone marrow plasma cells 10%–60%, ≥3 g/dl of M protein, or ≥500 mg/24 h of urinary M protein and no myeloma-defining events or amyloidosis.5
Table 1.
Criteria for monoclonal gammopathy of underdetermined significance, monoclonal gammopathy of renal significance, smoldering multiple myeloma, and multiple myeloma
| MGUS | MGRS | Smoldering Multiple Myeloma | Multiple Myeloma |
|---|---|---|---|
| <10% Clonal BMPCs and | <10% Clonal BMPCs and | 10%–60% Clonal BMPCs or | Clonal plasma cell disorder (≥10% clonal BMPCs or biopsy-proven plasmacytoma) and one or more of following MDEs: ≥60% BMPCs, ≥100 FLC ratio, more than one MRI focal lesion, or CRAB features (see below) |
| <3 g/dl M protein | <3 g/dl M proteina and | ≥3 g/dl Serum M protein or ≥500 mg/24 h urinary M protein | M protein in serum and/or urine present in all patients except true nonsecretory myeloma |
| No end organ damage | Monoclonal Ig–associated renal diseaseb | No end organ damageb | CRAB featuresb attributable to plasma cell disorder |
| No MDE | No MDE | No MDE | MDE present |
MGUS, monoclonal gammopathy of underdetermined significance; MGRS, monoclonal gammopathy of renal significance; BMPC, bone marrow plasma cell; MDE, myeloma-defining event; FLC, free light chain’ MRI, magnetic resonance imaging; CRAB, hypercalcemia, renal insufficiency, anemia, bone lesions; M protein, monoclonal protein.
In small percentage of MGRS, there is a monoclonal Ig–associated renal disease but no detectable M protein, and in a small percentage of MGRS, there may be no detectable M protein but an abnormal FLC ratio.
Any of the renal disorders included under the term MGRS can occur in patients with smoldering multiple myeloma or multiple myeloma.
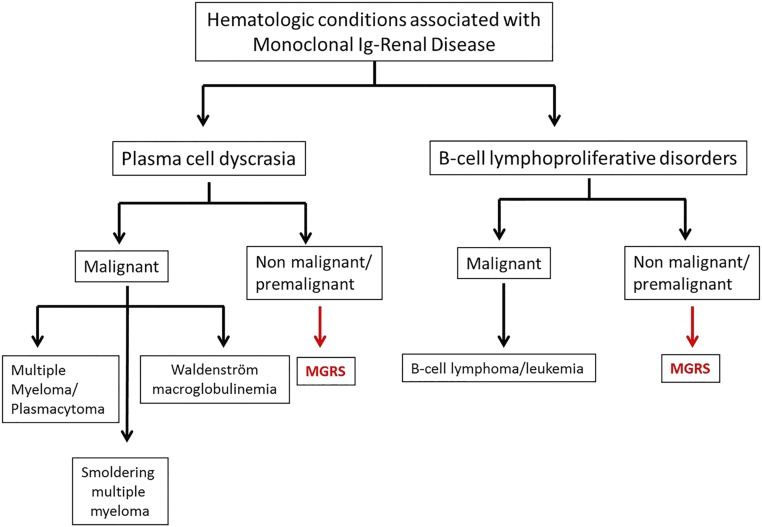
The term monoclonal gammopathy of renal significance (MGRS) was introduced to acknowledge a clonal plasma cell or B lymphocyte proliferation causing a renal lesion in the absence of hematologic malignancy or other myeloma-defining events; the renal lesion is nonetheless a consequence of the MIg, which carries major implications for management and prognosis, including the potential for progressive renal injury and ESRD.6–9 Its usage has facilitated the adoption of therapies directed to the clonal proliferation, which hematologists had formerly been reluctant to treat, because the underlying clonal disorder itself does not meet current hematologic criteria for immediate therapy. Conceptually, MGRS is neither a specific renal disease nor a specific hematologic disorder. MGRS due to a plasma cell clone would be defined as <10% bone marrow plasma cells, <3 g/dl of M protein, and the presence of renal lesions (but absence of any other myeloma-defining events) (Figure 1, Table 1).
Figure 1.
Hematologic conditions causing monoclonal Ig–associated renal disease. The hematologic conditions are divided into plasma cell derived and B-cell derived, which are further subdivided into malignant and nonmalignant/premalignant conditions. MGRS, monoclonal gammopathy of renal significance.
Although most cases of MIg-associated renal diseases in patients are caused by plasma cell disorders, others are related to B-cell lymphoproliferative disorders, including lymphoplasmacytic lymphoma, chronic lymphocytic leukemia (CLL)/small cell lymphocytic lymphoma, and marginal zone or mantle cell lymphomas. Analogous to low-mass plasma cell disorders associated with MGRS, the concept of dangerous B cell clones producing small yet nephrotoxic amounts of MIg in the absence of malignancy was also recently introduced.3
It should be noted that, in some settings of MGRS, in particular proliferative GN with monoclonal Ig deposits (PGNMID) or rare cases of amyloidosis, no M protein is detectable in serum or urine by electrophoresis, measurement of FLCs, or more sensitive immunoblotting techniques; however, kidney biopsy confirms the presence of MIg in the form of tissue deposits. In such patients, the small quantity of MIg synthesized and/or its high tissue avidity on the basis of charge, hydrophobicity, and particular matrix interactions may explain the absence of detectable M protein. Nonetheless, the ability of PGNMID to recur in the allograft lends support to the view that this condition represents a type of MGRS.10 Complicating matters further, the MIg detected in the serum or urine may not be identical to the MIg in the kidney deposits, and some patients may have more than one coexistent M protein in the serum and/or urine.
Laboratory Techniques for Clonal Identification in Body Fluids
A major inexpensive screening technique for detection of M protein is the serum protein electrophoresis (SPEP) and urine protein electrophoresis (UPEP), which separates proteins by electrophoresis on agarose gel or using light absorbance techniques by capillary zone followed by quantification of the M protein using densitometer tracing of the gel. The M protein typically produces a narrow peak on the densitometer tracing in the γ-region or less commonly, the β- or α2-globulin region. On agarose gel, M protein usually forms a discrete band. To determine the identity of the MIg, protein electrophoresis must be performed in conjunction with immunofixation, which applies antibodies to the specific Ig heavy- and light-chain components. Although UPEP is generally less sensitive than SPEP for MIg detection, it allows determination of the amount of proteinuria attributable to urinary albumin versus light chains. Whereas immunoblotting is even more sensitive than immunofixation, especially for detection of truncated heavy chains, its availability is limited to specialized laboratory settings.
Serum FLC assays provide a sensitive quantitation of total serum free (unbound) κ-light chain and λ-light chain, which is especially useful for clones synthesizing only a light chain fragment. The assay reports free κ-light chains and free λ-light chains in milligrams per liter and converts these numbers into a ratio. Normal range for free κ-light chains is 3.3–19.4 mg/L, and normal range for free λ-light chains is 5.7–26.3 mg/L. Thus, the normal free κ-to-λ ratio is 0.26–1.65.11 Importantly, in the setting of impaired renal function, the ratio rises to 0.34–3.1 owing to reduced renal clearance.12 The presence of a κ-clone or λ-clone is inferred from an abnormal ratio.
Tissue detection of the plasma cell or B lymphocyte clone requires a bone marrow biopsy and aspiration, lymph node biopsy, or extranodal biopsy, as appropriate, with adjunct flow cytometry and molecular immunophenotyping.
Clinical Presentation and Pathology of MIg-Associated Renal Diseases
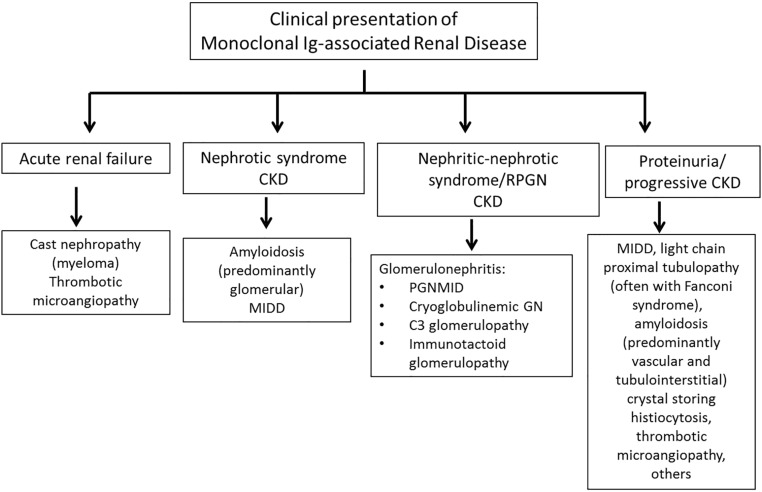
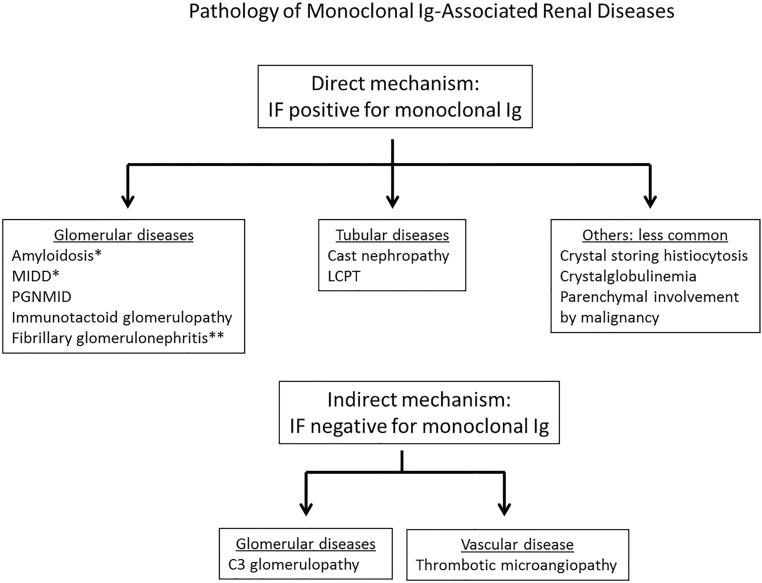
The clinical presentation often provides clues to the underlying renal disease (Figure 2). However, diagnosis of the specific renal disease requires kidney biopsy. Most cases in patients are due to renal deposition of the MIg (direct mechanism) as shown on immunofluorescence (IF) using antibodies to the Ig classes (i.e., heavy chains of IgG, IgM, and IgA), Ig light chains (κ and λ), and in specialized cases, the IgG subclasses (IgG1–IgG4). M proteins are restricted to a single Ig class, a single Ig subclass, and/or a single Ig light chain isotype. Thus, IF is essential to accurately diagnose MIg-associated renal diseases. In a smaller subset, the kidney disease can result from MIg-induced complement activation or endothelial injury without tissue deposition of MIg (indirect mechanism) (Figure 3).
Figure 2.
Clinical presentation of monoclonal Ig–associated renal disease. MIDD, monoclonal Ig deposition disease; PGNMID, proliferative GN with monoclonal Ig deposits; RPGN, rapidly progressive GN.
Figure 3.
Pathology of monoclonal Ig–associated renal disease. IF, immunofluorescence; LCPT, light-chain proximal tubulopathy; MIDD, monoclonal Ig deposition disease; PGNMID, proliferative GN with monoclonal Ig deposits. *Interstitium, tubules, and vessels may be involved in addition of glomeruli. **Usually polyclonal.
MIg-associated renal diseases are complex and heterogeneous with respect to clinical, pathogenetic, pathologic, and prognostic findings, requiring close collaboration between the pathologist, the nephrologist, and the hematologist/oncologist for optimal patient management. In general, the treatment of MIg-associated renal lesions is directed at eliminating the underlying clonal plasma cell or B-cell population to decrease or eradicate production of the offending MIg. This is done most effectively by using chemotherapy regimens that have been developed for the treatment of MM, AL, and B-cell lymphoproliferative disorders (Supplemental Figure 1).13
MIg-Associated Renal Disease Due to Deposition of MIg (Direct Mechanism)
This group of renal lesions is characterized by the finding of MIg deposited in the renal lesion. The physicochemical properties and size of the MIg are likely to be important pathogenetic determinants. Deposition of MIg can result in diseases involving the glomeruli, tubulointerstitium, and/or blood vessels. Larger molecular weight MIg molecules consisting of heavy and light chains are unlikely to pass the glomerular filtration barrier, resulting in glomerular deposition of the MIg with ensuing glomerular inflammation (proliferative GN and immunotactoid glomerulopathy). However, low molecular weight MIg consisting of light chains only is more likely to be filtered and reach the tubular lumen, resulting in such tubular diseases as cast nephropathy and light-chain proximal tubulopathy (LCPT). Finally, MIg that interacts with other proteins, such as apolipoproteins (e.g., amyloidosis)14 or matrix proteins (e.g., MIg deposition disease [MIDD]), can influence the type and location of the MIg deposits.
Glomerular Diseases
Ig Light Chain Amyloidosis/Ig Heavy Chain Amyloidosis
Pathology
AL is one of the common renal diseases associated with MIg.15 On light microscopy (LM), glomerular amyloid deposits cause acellular mesangial expansion and capillary wall thickening by periodic acid–Schiff (PAS) and silver methenamine–negative material (Supplemental Figure 2). Silver-positive spicules often transect the glomerular basement membranes, producing a “cock’s comb.” Amyloid deposits can also involve the vessel walls, tubular basement membranes, and interstitium. Amyloid deposits are Congo red positive (orangiophilic) and produce apple green birefringence or other anomalous colors under polarized light. IF most commonly shows light chain restriction consistent with AL, and the pathogenic light chain is more often λ than κ, with frequency of approximately 7:1 and predominance of λ-subgroup VI. Heavy chain restriction consistent with Ig heavy chain amyloidosis (AH) is rarer, and it is most commonly γ-type (IgG). Rarely, both light and heavy chain restriction occur (consistent with ALH). Electron microscopy (EM) confirms the presence of randomly oriented amyloid fibrils measuring 7–13 nm in thickness. IF studies are typically adequate for diagnosis of most cases of AL/AH. However, because amyloid deposits tend to nonspecifically trap circulating plasma proteins, confirmation of AL/AH by laser microdissection (LMD) and mass spectrometry (MS) has become a critical tool in diagnosis and identification of the amyloid precursor protein.14,16–18 Immuno-EM is another helpful technology to confirm the diagnosis of AL in difficult cases.19
Clinical Presentation
Typical presentation is albuminuria and nephrotic syndrome (NS); however, those lacking glomerular involvement may present with progressive renal insufficiency.20 Hypertension is uncommon; orthostatic hypotension may be present, especially in patients with autonomic neuropathy. Kidneys often appear large by renal ultrasound. Extrarenal organ involvement is common, and cardiac amyloidosis is a major determinant of patient survival. Nearly 100% of patients have evidence of an MIg disorder,5 and approximately 10% will also meet criteria for MM on the basis of the presence of CRAB features.4
MIDD
Pathology
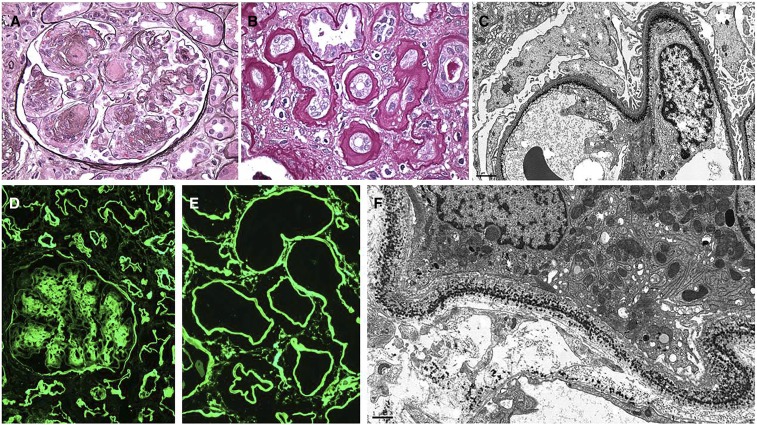
MIDD is characterized by deposition of MIg along the glomerular and tubular basement membranes (Figure 4). MIDD is classified into three types: light chain deposition disease (LCDD) when deposits are composed of light chains only, heavy chain deposition disease (HCDD) when deposits are composed of heavy chains only, and light heavy chain deposition disease when deposits are composed of both light and heavy chains. LCDD is the most common form; in approximately 80%–90% of cases, the deposits are composed of κ-light chains, in particular of the VκI or VκIV subgroup, which introduce hydrophobic side chains and glycosylation sites that promote light-chain precipitation and matrix interactions. However, the deposits of HCDD are typically composed of a truncated γ-chain and rarely, α- and μ-chains, and they lack the constant heavy 1 domain.21–27 Codeposits of C3 and C1q and resulting hypocomplementemia through activation of the classical complement pathway are not uncommon in HCDD, particularly the γ1- and γ3-subtypes. LM usually shows a nodular sclerosing glomerulopathy with membranoproliferative features, and crescents can occur, particularly in α-HCDD.25,28 PAS stain is helpful to highlight ribbon-like tubular basement membrane deposits. IF shows nodular mesangial and diffuse linear staining for the pathogenic light and/or heavy chain along the glomerular, Bowman’s capsular, tubular, and vascular basement membranes, where it has a tendency to ring the individual myocytes. Tubular basement membrane involvement is the most consistent IF finding. EM is characteristic and shows punctate powdery deposits in mesangium and along the glomerular, tubular, and arterial wall basement membranes. The ultrastructural demonstration of deposits may be subtle despite strong IF positivity.
Figure 4.
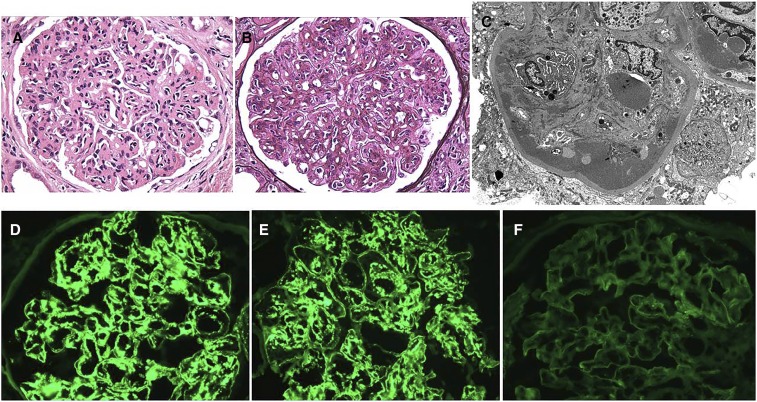
MIDD due to κ–LCDD. A 95-year-old man presents with AKI, nephrotic syndrome, and serum creatinine 2.7 mg/dl (increased from baseline 1.4 mg/dl 4 months prior) with lower extremity edema, urine protein 7 g/d, and serum protein electrophoresis with immunofixation showing elevated monoclonal κ-light chains. Renal biopsy shows the following. (A) Nodular sclerosing glomerulopathy with nodular mesangial expansion by partially silver-negative material associated with foci of circumferential mesangial interposition (Jones methenamine silver). Original magnification, ×400. (B) Thick ribbon-like periodic acid–Schiff (PAS)-positive deposits involving the tubular basement membranes (PAS). Original magnification, ×400. (C) Electron microscopic demonstration of finely granular punctate powdery deposits along the lamina rara interna of the glomerular capillary walls. Original magnification, ×8000. (D) Immunofluorescence staining for κ-light chain only distributed in the mesangial nodules, with weak linear staining of glomerular basement membranes and stronger linear staining of Bowman’s capsule and the tubular basement membranes. Original magnification, ×200. (E) Intense diffuse linear immunofluorescence staining for κ-light chain in the distribution of the tubular basement membranes associated with sparse interstitial positivity. Original magnification, ×400. (F) Punctate peppery deposits involving the tubular basement membrane and to a lesser extent, the adjacent interstitial capillary basement membrane (electron micrograph). Original magnification, ×10,000.
Clinical Presentation
Most patients present with proteinuria, NS, and renal insufficiency. The presence of hematuria and hypertension is variable. Monoclonal FLCs and albumin are the main proteins found in the urine, and the involvement of other target organs, such as heart, liver, and skin, can produce a myriad of clinical symptoms.29 In patients with HCDD, there is typically an abnormal FLC ratio,27 indicating cosecretion of monoclonal light chain by the abnormal clone producing the truncated heavy chain. A recent study of 64 patients with MIDD showed an abnormal FLC ratio in all patients, and 59% had MM, whereas one patient had a lymphoplasmacytic lymphoma.23 In another study of 34 patients of MIDD, 39% of the patients had MM, and 39% were diagnosed with MGUS (predating MGRS usage).24
PGNMID
Pathology
The kidney biopsy shows a proliferative GN with exclusively glomerular MIg deposits on IF studies and ordinary granular (nonorganized) electron dense deposits by EM (Figure 5).8,30 Membranoproliferative GN is the most common pattern. IF is essential for diagnosis by showing exclusively glomerular deposits of MIg with light chain restriction without involvement of Bowman’s capsule or tubular or vascular basement membranes. The deposits are usually in combined mesangial and glomerular capillary wall distribution. The MIg most often consists of IgG, less commonly consists of IgM, or rarely consists of IgA, with κ- or λ-light chain restriction. EM shows mesangial and subendothelial electron dense deposits and more variable subepithelial deposits. Glomerular capillary wall remodeling with double contour formation is often present. In patients with MIgG, subtyping for the IgG isotypes (1–4) is helpful to confirm the diagnosis by showing γ-subtype restriction. IgG3 is the most common subclass. Interestingly, this subclass is most likely to have undetectable M protein in serum and urine.31 By definition, the entity of PGNMID requires exclusion of type 1 cryoglobulinemia.
Figure 5.
PGNMID with monoclonal IgG3-κ deposits. A 56-year-old woman presents with edema and mixed acute nephritic and nephrotic syndrome, serum creatinine 2.75 mg/dl, urine protein 4.2 g/d, serum albumin 2.1 g/dl, urinalysis with 4+ protein and microhematuria with serum protein electrophoresis and urine protein electrophoresis with immunofixation negative for monoclonal protein, reduced serum C3, normal serum C4, and negative cryoglobulin titer. Renal biopsy shows the following. (A) Diffuse and global proliferative GN with membranoproliferative features and infiltrating mononuclear leukocytes (hematoxylin and eosin). Original magnification, ×400. (B) Well developed membranoproliferative pattern with mesangial interposition and duplication of glomerular basement membranes, causing luminal narrowing (Jones methenamine silver). Original magnification, ×400. (C) Electron micrograph showing subendothelial and mesangial electron dense deposits with capillary narrowing by infiltrating mononuclear leukocytes. The deposits have an ordinary granular texture, with no evidence of organized substructure. Original magnification, ×6000. (D) Immunofluorescence showing positive staining for IgG subtype 3 involving the mesangium and semilinear glomerular capillary walls, similar to the staining seen for IgG. There was no staining for IgG subtypes 1, 2, or 4 and no staining for IgM or IgA. Stains for complement components were positive with 3+ C3 and 2+ C1q in the same distribution as IgG (not shown). Original magnification, ×600. (E) The same glomerulus shows intense staining for κ-light chain in a mesangial and peripheral capillary wall distribution. There was no staining for IgG or κ-light chain involving the tubular basement membranes, Bowman’s capsule, or vessel walls. Original magnification, ×600. (F) The same glomerulus has negative staining for λ-light chain. Original magnification, ×600.
Clinical Presentation
Nearly all patients present with significant proteinuria and often present with NS, variable degrees of hematuria, and renal insufficiency. Hypertension is common and may be severe. Serum levels of C3 and C4 are low in one third of patients. Only 20%–30% of patients with PGNMID have a detectable M protein in serum or urine, mostly IgG1 or IgG2 subclass, but associated hematologic malignancy is extremely rare.
Cryoglobulinemic GN
Pathology
Cryoglobulinemic GN associated with MIg (either type 1 or type 2 cryoglobulins) shows a membranoproliferative pattern with abundant infiltrating macrophages and intraluminal PAS-positive deposits (pseudothrombi) on LM; intraluminal deposits of MIg, C3, and C1q on IF; and substructures (microtubules, fibrils, or curvilinear fingerprints) on EM. The MIg most often consists of IgM and less commonly consists of IgG. Deposits may be scant due to effective phagocytosis by macrophages.
Clinical Presentation
Cryoglobulins are circulating Igs that reversibly precipitate on cooling of the serum and plasma and redissolve on rewarming.32 Cryoglobulins are divided into three types. Type 1 is a single MIg (IgG, IgM, or less commonly, IgA; usually κ restricted). It is due to an underlying hematologic malignancy, such as MM, WM, or CLL, and it is often associated with a hyperviscosity syndrome. Type 2 is a mixed cryoglobulin composed of MIg (usually IgM-κ) complexed to polyclonal Ig (usually IgG), and it can be associated with underlying viral infections, such as hepatitis C or B, dysproteinemias, or autoimmune diseases.33 Type 3 is a mixed cryoglobulin composed of polyclonal Ig (usually IgM and IgG), and it is often secondary to autoimmune diseases or infections. Both types 2 and 3 have rheumatoid factor activity and can present with weakness, arthralgias, and purpura (Meltzer triad), and they can less commonly present with a peripheral neuropathy. However, features of thrombotic microangiopathy (TMA), such as Raynaud phenomenon, livedo reticularis, and acrocyanosis, are more common in type 1 cryoglobulinemia. Most patients with renal involvement present with proteinuria, hematuria, and renal insufficiency. Serum levels of C4 and less commonly, C3 are typically low, especially in type 2 cryoglobulinemia.
Immunotactoid Glomerulopathy
Pathology
Immunotactoid glomerulopathy is characterized by proliferative GN on LM, IgG deposits on IF, and capillary wall deposits with a microtubular substructure on EM.34,35 LM typically shows a membranoproliferative pattern of injury with variable membranous features. In most patients, the IgG deposits are monoclonal with either κ- or λ-light chain restriction and codeposits of C1q and C3. Monoclonal IgG1 is the most common subclass. Codeposits of IgM occur in some patients. The characteristic EM finding is the microtubular substructure of the deposits, which range from 10 to 60 nm in diameter with hollow cores and focal parallel alignment. Intracytoplasmic Ig with microtubular structures may also be detected in lymphocytes or plasma cells in the peripheral blood, renal interstitium, and bone marrow.36 Immunotactoid glomerulopathy should be differentiated from the patient with the rare case of monoclonal fibrillary GN, in which the IgG deposits consist of fibrils ranging from 16 to 24 nm in diameter and show light chain restriction by IF.35,37
Clinical Presentation
Renal involvement is typically manifested by proteinuria, often with NS, hematuria, hypertension, and variable levels of renal insufficiency. Patients are generally older and often have an underlying lymphoproliferative disorder. Although most series have excluded patients with systemic lupus or cryoglobulinemia, in some series, a transiently positive test for serum cryoglobulin has been reported.35,36,38 In a recent study of 16 patients, M spike was detected in 63% of the patients, and a hematologic malignancy was present in 38% of patients, which included CLL, lymphoplasmacytic lymphoma, and rarely, MM.39
Tubular Diseases
Cast Nephropathy
Pathology
Myeloma cast nephropathy is characterized by tubular injury and the presence of casts that are brightly eosinophilic and pale or negative with PAS stain (Supplemental Figure 3). The casts form in the distal tubules through reciprocal binding with Tamm–Horsfall protein and exhibit a “fractured” and “brittle” appearance, sharp edges, and formation of geometric shapes, with a surrounding inflammatory reaction by intratubular mononuclear cells, neutrophils, and giant cells.40,41 IF typically shows dominant or exclusive staining of the casts for the pathogenic monoclonal light chain (κ- or λ-light chains).42 In some patients with cast nephropathy, there is also linear staining of renal basement membranes for the monoclonal light chain without associated electron dense punctate deposits, which is referred to as “LCDD by IF only.”24
Clinical Presentation
Most patients present with AKI, whereas others recognized late in their course may have more slowly progressive CKD. Hypertension is uncommon. Monoclonal light chains are identifiable in the blood or urine in virtually all patients. Urine dipstick testing may be only weakly positive in the face of marked light chain proteinuria, because it identifies albumin, not light chains. Patients with suspected cast nephropathy need a kidney biopsy for confirmation of diagnosis. However, if the serum FLC levels are >500 mg/L in a patient in whom MM/cast nephropathy is suspected, renal biopsy can be deferred.5 Myeloma cast nephropathy is the most common renal lesion associated with MM, and >90% of the patients with cast nephropathy have MM. Cast nephropathy, also known as “myeloma kidney,” is considered a myeloma-defining event, and hence, it is incompatible with a diagnosis of MGRS.5 Rarely, cast nephropathy can occur in other hematologic disorders, such as WM or CLL, in which case, the term “light-chain cast nephropathy” is preferred.
LCPT
Pathology
LCPT is characterized by cytoplasmic inclusions of monoclonal light chains (more commonly κ than λ) within proximal tubular cells (Figure 6).43,44 Monoclonal light chain is internalized within proximal tubular cells by uptake via the megalin-cubilin receptor and delivery to endosomes and phagolysosomes, where it resists proteolysis, forming inclusions. The inclusions can be crystalline or noncrystalline; the crystalline form predominates. κ-Light chains of the Vκ1 subgroup are the most pathogenic, because they display hydrophobic side chains on the variable domain that inhibit proteolysis and facilitate crystallization.45 The entity of noncrystalline LCPT is still poorly defined and requires differentiation from physiologic tubular reabsorption and trafficking of the excess filtered light chains. A helpful diagnostic feature of both crystalline and noncrystalline LCPT is the presence of acute tubular injury, including tubular degenerative changes, loss of brush border, and shedding of cytoplasmic fragments and apoptotic cells into the lumen. Variable tubular atrophy and interstitial fibrosis develop in the chronic phase. The glomeruli are essentially normal. IF shows κ light chain restriction in almost all of the LCPT with crystals, whereas one third of the LCPT without crystals have λ light chain restriction.43 In many crystalline cases, diagnosis by IF on frozen tissue is insensitive and requires antigen retrieval using pronase or proteinase K digestion applied to paraffin sections.43,46 This is presumably due to sequestration and inaccessibility of antigenic sites within the tertiary structure of the light chain crystals. EM confirms the presence of proximal tubular intracytoplasmic crystals appearing as rhomboidal, rectangular, or needle shaped (Figure 6). Crystals can be membrane bound within phagolysosomes or free within the cytosol. The noncrystalline cytoplasmic inclusions appear as cytoplasmic droplets, granules, or vacuoles.
Figure 6.
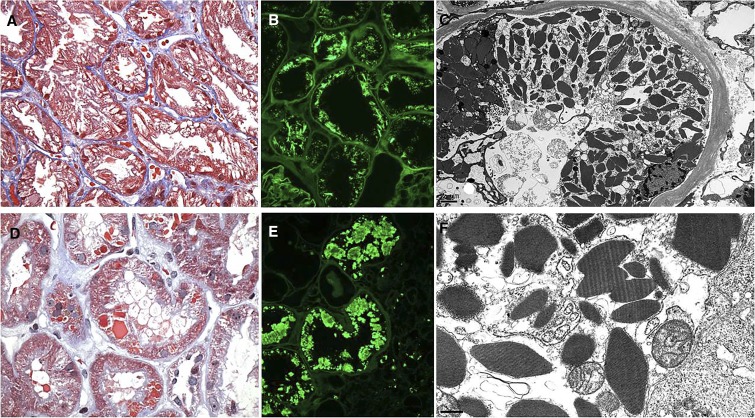
κ–LCPT, crystalline type. Several different examples of light-chain proximal tubulopathy are illustrated. (A) A case with abundant trichrome clear or weakly red intracellular crystals that distort the cytoplasm of proximal tubular epithelial cells throughout the biopsy (Masson trichrome). Original magnification, ×400. (B) Although the initial immunofluorescence stain for κ-light chain performed on frozen sections was negative (not shown), repeat immunofluorescence on pronase-digested paraffin sections is brightly positive for κ-light chain only in the distribution of the intracellular needle-shaped crystals. There was negativity for λ-light chain (not shown). Original magnification, ×400. (C) By electron microscopy, the proximal tubular cells are engorged with rhomboidal crystals throughout the full thickness of their cytoplasm. The proximal epithelial cells show acute injury with loss of brush border and shedding of cytoplasmic fragments and degenerating cellular debris into the lumen. Original magnification, ×5000. (D) An example with hexagonal trichrome red crystals irregularly distributed within the proximal tubular cell cytoplasm. The tubular epithelial cells exhibit acute injury but no intraluminal casts (Masson trichrome). Original magnification, ×400. (E) A case with large crystalline inclusions staining by immunofluorescence for κ-light chain only within the cytoplasm of proximal tubular cells was revealed following antigen retrieval by application of pronase digestion to paraffin sections. Original magnification, ×400. (F) High-power view of a representative proximal tubular cell from the same patient as in C above shows rhomboidal and other geometric-shaped crystals with lateral striations, indicating periodicity. The crystals are located within membrane-bound phagolyosomes or free within the cytosol of proximal tubular cells (electron micrograph). Original magnification, ×30,000.
Clinical Presentation
Proteinuria consisting of monoclonal light chains and albumin without NS is present in >95% of the patients, and most patients also exhibit renal insufficiency. Fanconi syndrome, characterized by normoglycemic glycosuria, aminoaciduria, and phosphaturia, is the classic presentation, particularly in the crystalline forms.47 Metabolic acidosis (proximal renal tubular acidosis), hypophosphatemia, and hypouricemia can also be present. In a recent series of 46 patients, LCPT was associated with an MGRS in 46% (of which 4% converted to MM), MM in 33%, SMM in 15%, non-Hodgkin lymphoma in 4%, and CLL in 2% of the patients.43 In another study of 49 patients with LCPT presenting with Fanconi syndrome, MGRS was identified in 27%, MM was identified in 14%, SMM was identified in 51%, and WM was identified in 8%.47
Other Rare Glomerular, Interstitial, and Vascular Diseases
Crystal-Storing Histiocytosis/Crystalglobulinemia
Crystal-storing histiocytosis (CSH) is a rare disorder in which numerous enlarged histiocytes containing MIg are noted in the glomeruli and/or interstitium (Figure 7).48 On LM, the glomerular capillary lumina are infiltrated with histiocytes containing eosinophilic, trichrome-red crystalline cytoplasmic inclusions, which are sometimes associated with membranoproliferative features and monoclonal subendothelial deposits. CD68 stain is helpful to confirm their histiocytic phenotype. Interstitial CSH can mimic an acute or chronic tubulointerstitial nephritis.49 The presence of pseudo-Gaucher cells in the interstitium and perirenal fat is suggestive of the disease.49 IF microscopy reveals the nature of the MIg, which typically has κ light chain restriction. EM identifies electron dense crystals within the histiocyte cytoplasm. Interstitial CSH can occur alone or in association with LCPT, presumably due to similar resistance of the monoclonal light chain to lysosomal proteolysis in both histiocytes and proximal tubular cells.43,49
Figure 7.
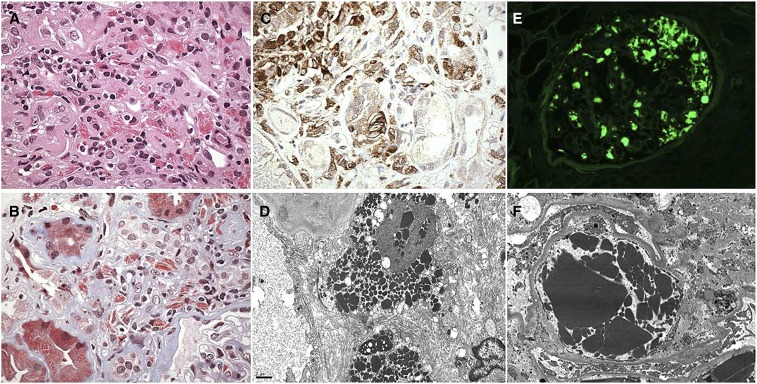
Crystal-storing histiocytosis (CSH) and crystalglobulinemia. (A) A case of CSH resembles acute interstitial nephritis with a mixture of interstitial lymphocytes and larger histiocytes containing eosinophilic needle-shaped inclusions (hematoxylin and eosin). Original magnification, ×600. (B) CSH: trichrome stain highlights the trichrome-red needle-shaped crystals within the interstitial histiocytes. There is tubular atrophy and interstitial fibrosis (Masson trichrome). By immunofluorescence, the crystals stained exclusively for κ-light chain (not shown). Original magnification, ×600. (C) CSH: immunostain for CD68 reveals the histiocytic phenotype of the interstitial cells, with cytoplasm that is distorted by crystalline inclusions. Original magnification, ×600. (D) CSH: electron micrograph showing interstitial histiocytes stuffed with electron dense intracytoplasmic crystals that invaginate the nucleus (electron micrograph). Original magnification, ×6000. (E) Crystalglobulinemia: a 47-year-old man presents with leukocytoclastic vasculitis of the skin, weakness, AKI, and creatinine 6.5 mg/dl. The glomerular capillaries are filled with pseudothrombi staining by immunofluorescence for κ and IgG (not shown), with negative staining for λ (not shown). Original magnification, ×400. (F) Crystalglobulinemia: a glomerular capillary lumen is occluded by a large plug of intraluminal electron dense crystals forming a type of crystalline “thrombus.” There are no deposits involving the glomerular basement membranes or the mesangial matrix (electron micrograph). After the kidney biopsy, a bone marrow biopsy was performed and showed 30% κ-restricted plasma cells, leading to a diagnosis of MM. Original magnification, ×4000.
Crystalglobulinemia, by contrast, results from the extracellular deposition of large MIg crystals within systemic vascular lumens, including renal arteries and glomerular capillaries (Figure 7).50 The crystals are visible by LM as eosinophilic, PAS positive, and trichrome red, and by EM, they appear electron dense and crystalline with sharp edges. By IF, the deposits usually consist of IgG-κ or IgG-λ, and less commonly, they are light chain only, which is usually λ-restricted. Crystalglobulinemia can occlude vascular lumina, mimicking a TMA, or incite arterial wall inflammation, producing a vasculitis.
Clinical Presentation
Glomerular involvement by CSH typically manifests as proteinuria with or without NS accompanied by renal insufficiency. Crystalglobulinemia often presents with AKI, and large vessel involvement can produce renal infarction; systemic manifestations, such as skin rash, polyarthralgias, and neuropathy, are common. Both CSH and crystalglobulinemia are most frequently seen in the setting of MM and lymphoproliferative disorders, and they are only rarely seen in MGRS.48,50–55
MIg-Associated Renal Disease with No MIg on IF Studies (Indirect Mechanism)
Monoclonal Gammopathy–Associated C3 Glomerulopathy
Pathology
C3 glomerulopathy is a rare disease resulting from dysregulation of the alternative pathway of complement and manifesting dominant or isolated glomerular deposition of C3. C3 glomerulopathy includes C3GN and dense deposit disease (DDD).56–59 Both C3GN and DDD are characterized by bright staining for C3 with absent or minimal staining for Ig.59,60
By EM, C3GN is characterized by mesangial, subendothelial, and occasionally, intramembranous and subepithelial deposits of moderate electron density, whereas DDD is characterized by highly electron-dense rounded mesangial and sausage-shaped intramembranous deposits.
Dysregulation of the alternative pathway of complement can be primary, resulting from mutations in complement regulatory proteins, or secondary (acquired) due to autoantibodies to complement regulatory proteins.61 A subset of patients with C3 glomerulopathy have an associated MIg.62–64 MIgs in the setting of C3 glomerulopathy likely act as autoantibodies to complement regulatory proteins, such as factor H or the C3 convertase (i.e., behaving as a C3 nephritic factor), thereby increasing the t1/2 of the C3 convertase,52–54 resulting in DDD, C3GN, or rarely, atypical hemolytic uremic syndrome.65 Indeed, in a recent large study, complement evaluation revealed C3 nephritic factor in 45.8% of patients with MIg-associated C3 glomerulopathy, whereas pathogenic variants in complement genes were rare.66
Clinical Presentation
In a recent study, 65.1% of patients with C3 glomerulopathy ≥50 years of age had an MIg.66 Other studies have similarly shown a high prevalence of MIg in older patients with C3 glomerulopathy.62–64,67,68 Hematuria, proteinuria, often NS, and some degree of renal impairment are present in most patients. In a study of 36 patients, C3 glomerulopathy was associated with MGRS in 77.8% (of which 4% converted to MM), MM in 13.9%, SMM in 5.6%, CLL/lymphoma in 5.6%, and type 1 cryoglobulinemia in 2.8% of the patients.66
TMA
Pathology
TMA comprises a diverse set of disorders mediated by endothelial injury. A subset of patients with TMA have an associated MIg.69 MIg can act as a potential trigger of TMA, producing atypical hemolytic uremic syndrome via dysregulation of the alternative pathway of complement, although precise mechanisms remain to be elucidated.70 Similar to MIg-associated C3 glomerulopathy, it was recently shown that, among patients ≥50 years of age, the prevalence of monoclonal gammopathy in TMA was much higher than in the general population.69 Kidney biopsy can show a constellation of findings, including thrombi in glomerular capillaries, mesangiolysis, and double contours of the glomerular basement membranes. Acute tubular injury is common. IF is negative for MIg, but staining for fibrin/fibrinogen and C3 is variably positive, involving the glomerular capillaries and vessel walls.
Clinical Presentation
Among patients with C3 glomerulopathy ≥60 years of age who underwent renal biopsy, the prevalence of monoclonal gammopathy was 24%, a rate five-fold higher than expected for this age group.71,72 Affected patients present with hematuria (in 64% patients), proteinuria, and AKI. In a comparative study, 50% of patients with MIg-associated TMA developed ESRD during follow-up compared with 33% of patients with MIg-negative TMA. Among those with MIg-associated TMA, 75% of patients had MGRS; 5% of patients had MM; 5% of patients had SMM; 10% of patients had polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes; and 5% of patients had had T-cell lymphocytic leukemia.
Proposed Terminology for MIg-Associated Renal Diseases
As described, the renal disorders caused by MIg are diverse and complex. Similarly, the underlying hematologic conditions vary from malignant to nonmalignant. Not all patients have detectable MIg in the kidney tissue, and some patients lack detectable M protein in the serum and urine. To standardize nomenclature and improve communication between the renal pathologist, the nephrologist, and the hematologist, we propose that the renal disease and associated hematologic disease should both be documented in the comment section of the kidney biopsy report, with the following caveats.
In some patients, the kidney biopsy is the first clue to the presence of MIg, and only subsequent evaluation reveals the nature of the hematologic disease. There is need to rapidly perform hematologic evaluation to identify the underlying etiology in such patients.
The hematologic disease may progress over time from benign to malignant.
In some patients, there may be no detectable hematologic disorder initially or after extensive workup.
Some examples are given in Supplemental Table 1.
MS in MIg-Associated Renal Diseases
LMD combined with MS-based proteomics is a new diagnostic tool that enables analysis of global protein expression patterns in specific tissue compartments, such as glomeruli, interstitium, and tubules. Using this technique, the protein profile of diseased glomeruli, tubulointerstitium, or vessels can be compared with tissue controls.73 LMD/MS is particularly useful in detecting MIg in renal diseases, because normal glomeruli only show small amounts of circulating polyclonal Ig. LMD/MS studies can detect not only the subtype of the MIg, such as IgG3, IgA1, etc., but also, the specific regions of the MIg, such as Ig-κ chain C region, Ig-λ V-I region, IgA1-C region, etc. Furthermore, MIgs comprising heavy chains Igε or Igδ not routinely tested for by IF performed on tissue sections or by protein electrophoresis with immunofixation on blood or urine can be detected by LMD/MS. Thus, LMD/MS is a valuable tool for diagnosis of many MIg-associated renal diseases as itemized below.
Confirmation of AL/AH. LMD/MS studies have shown that MIg amyloidosis has a specific protein signature that includes the MIg, apolipoproteins E and IV, and serum amyloid P component.14,17 Other forms of amyloidosis, such as amyloid A, leukocyte chemotactic factor 2, and fibrinogen α-chain amyloidosis, can have nonspecific tissue binding of circulating proteins in amyloid deposits by immunoperoxidase staining, leading to erroneous diagnoses.74 Because monoclonal gammopathy is common in the general population and 4.2% of the population ≥50 years old has a detectable serum MIg, tissue trapping of MIg may lead to false positive diagnosis of MIg amyloidosis. Conversely, the failure of some MIg amyloid to react with commercial antisera directed to light and heavy chain epitopes may lead to false negative diagnosis. In both scenarios, LMD/MS can identify amyloidosis subtype.
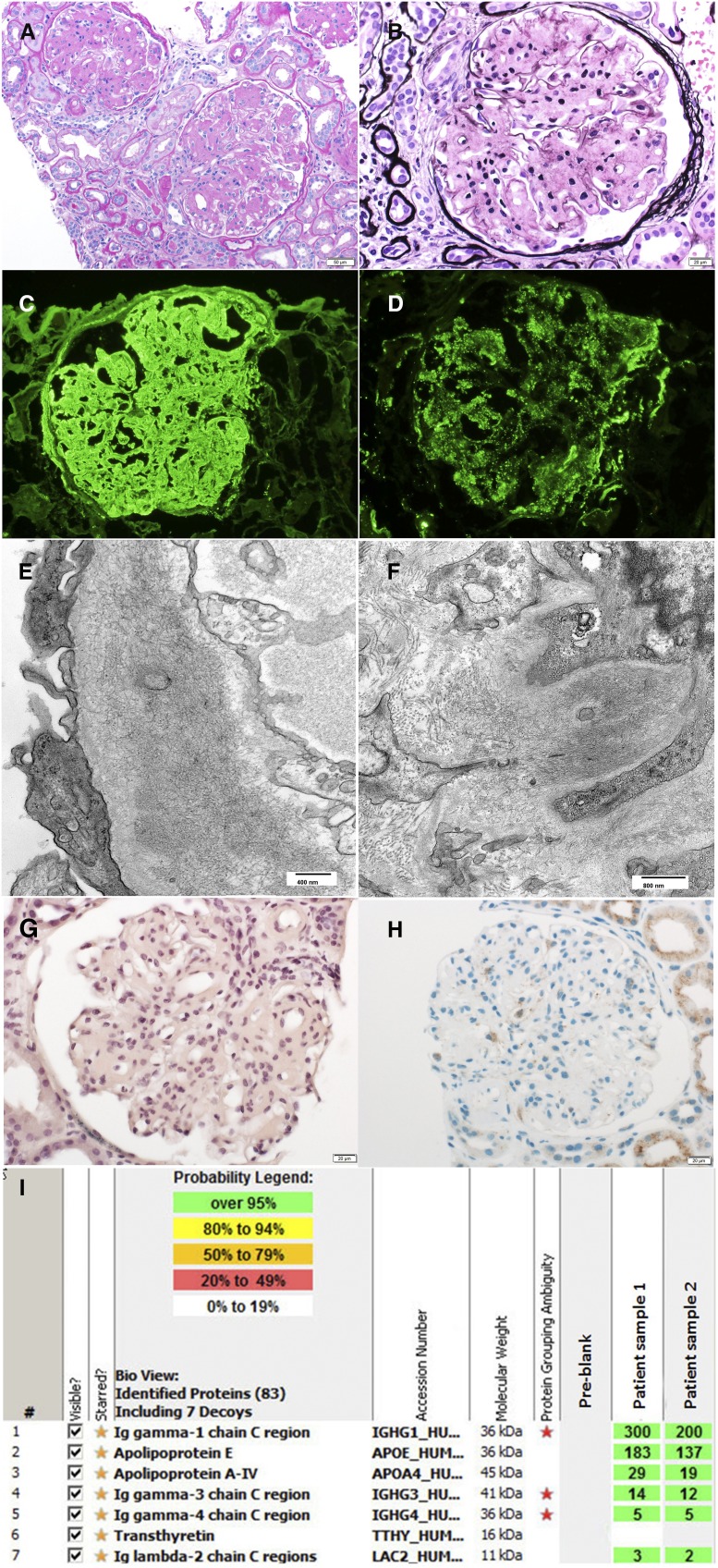
Differentiation of Ig amyloidosis from fibrillary GN. Recent studies on the basis of LMD/MS have shown that DNAJB9 is a marker of fibrillary GN that helps distinguish fibrillary GN from Ig amyloidosis.75,76 However, in some patients, it may be difficult to confirm the diagnosis of amyloidosis in the setting of equivocal Congo red and DNAJB9 staining. In such patients, LMD/MS can confirm the diagnosis of MIg amyloidosis as illustrated in a recent patient (Figure 8).
LMD/MS is useful in the setting of patients with unusual cases of MIDD where the MIg is truncated or is not tested for by routine IF studies. For example, HCDD comprising δ-heavy chains can be diagnosed using LMD/MS.77
In some patients with MPGN associated with MIg, in whom the lesions are chronic or the deposits are masked, the MIg may be undetectable by routine IF. In such instances, LMD/MS is again useful to identify MIg in the glomerular deposits.78 Some of these patients also benefit from repeat IF performed on protease-digested paraffin sections for antigen retrieval.79
Potential future applications of tissue LMD/MS include detection of complement pathways, cytokine and growth factor profiles, and MIg-matrix interactions in MIg-associated renal diseases. A promising future application of MS is the proteomic detection of MIg in the serum and urine of patients in whom both SPEP with immunofixation and FLC studies are negative.
Figure 8.
AH (IgG) amyloidosis diagnosed by mass spectrometry. A 62-year-old woman presented with progressive rise in serum creatinine and proteinuria over 1 year, leading to kidney biopsy. (A and B) Light microscopy showing (A) weakly periodic acid–Schiff-positive and (B) silver-negative mesangial nodules. (C and D) Immunofluorescence microscopy showed bright 3+ mesangial and capillary wall staining for (C) IgG and (D) C3, with negative staining for κ- and λ-light chains. (E and F) Electron microscopy showed fibrillary deposits in the mesangium and capillary walls, raising the possibility of AH (IgG) amyloidosis or fibrillary GN. However, both (G) Congo red and (H) DNAJB9 staining were negative. (I) Laser microdissection/mass spectrometry studies confirmed the AH (IgG) amyloidosis by detecting large signature amyloid spectra of IgG1, apo E, and A-IV. Subsequent serum electrophoresis studies revealed M spike consisting of monoclonal IgG heavy chains only. (Amyloid mass spectrometry data were courtesy Dr. Paul Kurtin.)
Summary
MIg results from both malignant and nonmalignant clonal proliferations of plasma cells or B lymphocytes; the secreted MIg can deposit in specific kidney compartments leading to diverse renal disorders. The term MGRS should be used to indicate monoclonal gammopathy caused by a nonmalignant yet clonal hematologic condition that results in a MIg-associated renal disease. Use of the term MGRS frees hematologists/oncologists to direct treatment to the hematologic disorder where appropriate. We propose that both the renal disease and the associated hematologic disease (as available at the time of reporting) should be included in the comment section of the renal biopsy report.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121319/-/DCSupplemental.
References
- 1.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al.: A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med 346: 564–569, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV: Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23: 3–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merlini G, Stone MJ: Dangerous small B-cell clones. Blood 108: 2520–2530, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kourelis TV, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR, et al.: Coexistent multiple myeloma or increased bone marrow plasma cells define equally high-risk populations in patients with immunoglobulin light chain amyloidosis. J Clin Oncol 31: 4319–4324, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al.: International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15: e538–e548, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Fermand J-P, Bridoux F, Kyle RA, Kastritis E, Weiss BM, Cook MA, et al.; International Kidney and Monoclonal Gammopathy Research Group : How I treat monoclonal gammopathy of renal significance (MGRS). Blood 122: 3583–3590, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand J-P, et al.; International Kidney and Monoclonal Gammopathy Research Group : Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 87: 698–711, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Zand L, Leung N, Smith RJH, Jevremonic D, Herrmann SS, et al.: Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol 5: 770–782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand J-P, et al.; International Kidney and Monoclonal Gammopathy Research Group : Monoclonal gammopathy of renal significance: When MGUS is no longer undetermined or insignificant. Blood 120: 4292–4295, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Nasr SH, Sethi S, Cornell LD, Fidler ME, Boelkins M, Fervenza FC, et al.: Proliferative glomerulonephritis with monoclonal IgG deposits recurs in the allograft. Clin J Am Soc Nephrol 6: 122–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al.: Serum reference intervals and diagnostic ranges for free κ and free λ immunoglobulin light chains: Relative sensitivity for detection of monoclonal light chains. Clin Chem 48: 1437–1444, 2002 [PubMed] [Google Scholar]
- 12.Hutchison CA, Harding S, Hewins P, Mead GP, Townsend J, Bradwell AR, et al.: Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 3: 1684–1690, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethi S, Rajkumar SV: Monoclonal gammopathy-associated proliferative glomerulonephritis. Mayo Clin Proc 88: 1284–1293, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Sethi S, Vrana JA, Theis JD, Leung N, Sethi A, Nasr SH, et al.: Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 82: 226–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said SM, Sethi S, Valeri AM, Leung N, Cornell LD, Fidler ME, et al.: Renal amyloidosis: Origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol 8: 1515–1523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung N, Nasr SH, Sethi S: How I treat amyloidosis: The importance of accurate diagnosis and amyloid typing. Blood 120: 3206–3213, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Sethi S, Theis JD, Leung N, Dispenzieri A, Nasr SH, Fidler ME, et al.: Mass spectrometry-based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol 5: 2180–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández de Larrea C, Verga L, Morbini P, Klersy C, Lavatelli F, Foli A, et al.: A practical approach to the diagnosis of systemic amyloidoses. Blood 125: 2239–2244, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Eirin A, Irazabal MV, Gertz MA, Dispenzieri A, Lacy MQ, Kumar S, et al.: Clinical features of patients with immunoglobulin light chain amyloidosis (AL) with vascular-limited deposition in the kidney. Nephrol Dial Transplant 27: 1097–1101, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Kambham N, Markowitz GS, Appel GB, Kleiner MJ, Aucouturier P, D’Agati VD: Heavy chain deposition disease: The disease spectrum. Am J Kidney Dis 33: 954–962, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Moulin B, Deret S, Mariette X, Kourilsky O, Imai H, Dupouet L, et al.: Nodular glomerulosclerosis with deposition of monoclonal immunoglobulin heavy chains lacking C(H)1. J Am Soc Nephrol 10: 519–528, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, D’Agati VD, et al.: Renal monoclonal immunoglobulin deposition disease: A report of 64 patients from a single institution. Clin J Am Soc Nephrol 7: 231–239, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, et al.: Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Alexander MP, Nasr SH, Watson DC, Méndez GP, Rennke HG: Renal crescentic alpha heavy chain deposition disease: A report of 3 cases and review of the literature. Am J Kidney Dis 58: 621–625, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Herrera GA: Renal lesions associated with plasma cell dyscrasias: Practical approach to diagnosis, new concepts, and challenges. Arch Pathol Lab Med 133: 249–267, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Bridoux F, Javaugue V, Bender S, Leroy F, Aucouturier P, Debiais-Delpech C, et al.: Unravelling the immunopathological mechanisms of heavy chain deposition disease with implications for clinical management. Kidney Int 91: 423–434, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Vignon M, Cohen C, Faguer S, Noel LH, Guilbeau C, Rabant M, et al.: The clinicopathologic characteristics of kidney diseases related to monotypic IgA deposits. Kidney Int 91: 720–728, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Pozzi C, D’Amico M, Fogazzi GB, Curioni S, Ferrario F, Pasquali S, et al.: Light chain deposition disease with renal involvement: Clinical characteristics and prognostic factors. Am J Kidney Dis 42: 1154–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, et al.: Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 20: 2055–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhutani G, Nasr SH, Said SM, Sethi S, Fervenza FC, Morice WG, et al.: Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc 90: 587–596, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Ramos-Casals M, Stone JH, Cid MC, Bosch X: The cryoglobulinaemias. Lancet 379: 348–360, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ferri C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, et al.: Mixed cryoglobulinemia: Demographic, clinical, and serologic features and survival in 231 patients. Semin Arthritis Rheum 33: 355–374, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Schwartz MM, Korbet SM, Lewis EJ: Immunotactoid glomerulopathy. J Am Soc Nephrol 13: 1390–1397, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D’Agati VD: Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63: 1450–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Bridoux F, Hugue V, Coldefy O, Goujon JM, Bauwens M, Sechet A, et al.: Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int 62: 1764–1775, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, Leung N, et al.: Fibrillary glomerulonephritis: A report of 66 cases from a single institution. Clin J Am Soc Nephrol 6: 775–784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogo A, Qureshi N, Horn RG: Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis 22: 367–377, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Nasr SH, Fidler ME, Cornell LD, Leung N, Cosio FG, Sheikh SS, et al.: Immunotactoid glomerulopathy: Clinicopathologic and proteomic study. Nephrol Dial Transplant 27: 4137–4146, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Cohen AH: The kidney in plasma cell dyscrasias: Bence-Jones cast nephropathy and light chain deposit disease. Am J Kidney Dis 32: 529–532, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Nasr SH, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, et al.: Clinicopathologic correlations in multiple myeloma: A case series of 190 patients with kidney biopsies. Am J Kidney Dis 59: 786–794, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Markowitz GS: Dysproteinemia and the kidney. Adv Anat Pathol 11: 49–63, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Stokes MB, Valeri AM, Herlitz L, Khan AM, Siegel DS, Markowitz GS, et al.: Light chain proximal tubulopathy: Clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol 27: 1555–1565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen CP, Bell JM, Harris AA, Messias NC, Wang YH, Walker PD: The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol 24: 1462–1469, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Messiaen T, Deret S, Mougenot B, Bridoux F, Dequiedt P, Dion JJ, et al.: Adult Fanconi syndrome secondary to light chain gammopathy. Clinicopathologic heterogeneity and unusual features in 11 patients. Medicine (Baltimore) 79: 135–154, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Nasr SH, Galgano SJ, Markowitz GS, Stokes MB, D’Agati VD: Immunofluorescence on pronase-digested paraffin sections: A valuable salvage technique for renal biopsies. Kidney Int 70: 2148–2151, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Vignon M, Javaugue V, Alexander MP, El-Karoui K, Karras A, Roos-Weil D, et al.: Current anti-myeloma therapies in renal manifestations of monoclonal light chain-associated Fanconi syndrome: A retrospective series of 49 patients. Leukemia 31: 123–129, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Sethi S, Cuiffo BP, Pinkus GS, Rennke HG: Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am J Kidney Dis 39: 183–188, 2002 [DOI] [PubMed] [Google Scholar]
- 49.El Hamel C, Thierry A, Trouillas P, Bridoux F, Carrion C, Quellard N, et al.: Crystal-storing histiocytosis with renal Fanconi syndrome: Pathological and molecular characteristics compared with classical myeloma-associated Fanconi syndrome. Nephrol Dial Transplant 25: 2982–2990, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Gupta V, El Ters M, Kashani K, Leung N, Nasr SH: Crystalglobulin-induced nephropathy. J Am Soc Nephrol 26: 525–529, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones D, Bhatia VK, Krausz T, Pinkus GS: Crystal-storing histiocytosis: A disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum Pathol 30: 1441–1448, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Hishida A, Honda N, Ito I, Shirasawa H, Nagase M: Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch Pathol Lab Med 115: 351–354, 1991 [PubMed] [Google Scholar]
- 53.Stokes MB, Aronoff B, Siegel D, D’Agati VD: Dysproteinemia-related nephropathy associated with crystal-storing histiocytosis. Kidney Int 70: 597–602, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Vankalakunti M, Bonu R, Shetty S, Siddini V, Babu K, Ballal SH: Crystalloid glomerulopathy in monoclonal gammopathy of renal significance (MGRS). Clin Kidney J 7: 296–298, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ball N, Wickert W, Marx L, Thaell J: Crystalglobulinemia syndrome. A manifestation of multiple myeloma. Cancer 71: 1231–1234, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, et al.: Primary glomerulonephritis with isolated C3 deposits: A new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet 44: 193–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, et al.: C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, et al.: Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol 6: 1009–1017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, et al.: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Stokes MB, et al.: Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 85: 450–456, 2014 [DOI] [PubMed] [Google Scholar]
- 61.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med 366: 1119–1131, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Sethi S, Sukov WR, Zhang Y, Fervenza FC, Lager DJ, Miller DV, et al.: Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis 56: 977–982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bridoux F, Desport E, Frémeaux-Bacchi V, Chong CF, Gombert JM, Lacombe C, et al.: Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: A fortuitous association? Clin J Am Soc Nephrol 6: 2165–2174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zand L, Kattah A, Fervenza FC, Smith RJH, Nasr SH, Zhang Y, et al.: C3 glomerulonephritis associated with monoclonal gammopathy: A case series. Am J Kidney Dis 62: 506–514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheungpasitporn W, Leung N, Sethi S, Gertz MA, Fervenza FC: Refractory atypical hemolytic uremic syndrome with monoclonal gammopathy responsive to bortezomib-based therapy. Clin Nephrol 83: 363–369, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Ravindran A, Fervenza FC, Smith RJ, Sethi S: C3 glomerulopathy associated with monoclonal Ig: A distinct subtype. Kidney Int doi: 10.1016/j.kint.2018.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, et al.: Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 4: 22–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chauvet S, Frémeaux-Bacchi V, Petitprez F, Karras A, Daniel L, Burtey S, et al.: Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy-associated C3 glomerulopathy. Blood 129: 1437–1447, 2017 [DOI] [PubMed] [Google Scholar]
- 69.Ravindran A, Go RS, Fervenza FC, Sethi S: Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int 91: 691–698, 2017 [DOI] [PubMed] [Google Scholar]
- 70.Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ 3rd, Colby CL, et al.: Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: A retrospective population-based cohort study. Lancet 375: 1721–1728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al.: Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354: 1362–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Sethi S, Vrana JA, Theis JD, Dogan A: Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens 22: 273–280, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Paueksakon P, Fogo AB, Sethi S: Leukocyte chemotactic factor 2 amyloidosis cannot be reliably diagnosed by immunohistochemical staining. Hum Pathol 45: 1445–1450, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Dasari S, Alexander MP, Vrana JA, Theis JD, Mills JR, Negron V, et al.: DnaJ heat shock protein family B member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol 29: 51–56, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andeen NK, Yang HY, Dai DF, MacCoss MJ, Smith KD: DnaJ homolog subfamily B member 9 is a putative autoantigen in fibrillary GN. J Am Soc Nephrol 29: 231–239, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Royal V, Quint P, Leblanc M, LeBlanc R, Duncanson GF, Perrizo RL, et al.: IgD heavy-chain deposition disease: Detection by laser microdissection and mass spectrometry. J Am Soc Nephrol 26: 784–790, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jain D, Green JA, Bastacky S, Theis JD, Sethi S: Membranoproliferative glomerulonephritis: The role for laser microdissection and mass spectrometry. Am J Kidney Dis 63: 324–328, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Larsen CP, Messias NC, Walker PD, Fidler ME, Cornell LD, Hernandez LH, et al.: Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int 88: 867–873, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.