The cardiorenal syndromes (CRSs) are defined as disorders of the heart and kidneys, whereby acute or chronic dysfunction of one organ induces acute or chronic dysfunction of the other.1 Generally, CRSs are classified into five subtypes, which are conceptual and largely on the basis of expert opinion. Since the recognition of CRS as an entity, multiple studies have described its magnitude and pathophysiology. However, the majority of the patients in these trials have had left heart failure (LHF) with reduced ejection fraction.2 There is a dearth of information regarding pulmonary hypertension (PH) and isolated right heart failure (RHF) as a cause of CRS. Here, we discuss the potential pathophysiology of renal disease in patients with PH and isolated RHF with the purpose to stimulate further investigation in this understudied topic. To understand renal dysfunction in PH and RHF, it is useful to review the pathophysiology of CRS in LHF, because mechanisms of renal dysfunctions are better studied in LHF, and some of these are shared by RHF.
Pathogenesis of CRS in LHF
The main mechanisms behind renal dysfunction in LHF involve (1) reduced renal blood flow (RBF) as a result of neurohormonal activation and (2) increased central and renal venous pressure. Concisely, in heart failure with reduced ejection fraction, as a result of decreases in stroke volume and cardiac output, arterial integrity (or effective arterial blood volume) is compromised, leading to activation of baroreceptors residing in the high-pressure system, which results in activation of neurohormones, including the sympathetic nervous system, renin-angiotensin-aldosterone system (RAAS), and vasopressin.3 These neurohormones cause systemic and renal vasoconstriction and stimulation of sodium retention throughout the nephron to compensate for arterial underfilling and reduced BP but at the expense of increased systemic vascular resistance and higher plasma volume. Initially, the GFR is maintained with increased filtration fraction; however, after the autoregulatory capacity is exhausted, decreases in GFR ensue. On the basis of associations between increases in central venous pressure (CVP) and reduced GFR in patients with heart failure, venous congestion has been recognized as an additional important determinant of renal dysfunction.4,5 Venous congestion, in addition to causing hemodynamics perturbances on transglomerular pressure gradient, also causes release of inflammatory mediators, neurohormones, and activation of endothelial cells, resulting in decompensation of heart and kidney function.4,6
Etiology and Pathogenesis of CRS in RHF
PH is a syndrome characterized by the presence of mean pulmonary artery pressure >25 mm Hg at rest measured by right-sided cardiac catheterization. The World Health Organization has classified PH into five groups on the basis of etiology and mechanisms7 (Table 1). RHF encompasses right heart dysfunction due to any of above etiologies of PH. Among all, PH due to LHF (group 2 PH) remains the most common cause of RHF, and pathophysiology of CRS in this group is shared with the patients with LHF. However, there is a paucity of data regarding the prevalence of renal disease in patients with PH and isolated RHF. In retrospective analyses of hospitalized patients and PH registries, acute kidney diseases and CKDs exist in 4%–50% of patients and significantly associate with worse outcomes.8,9 However, it is not clear whether PH and RHF are the primary causes of kidney disease. To answer this question, it is necessary to show that PH/RHF can contribute to renal dysfunction in the absence of LHF. In sleep apnea, severity of nocturnal hypoxemia, and in chronic obstructive pulmonary disease (COPD), degree of hypercapnia closely associate with decreased RBF and GFR, suggesting a possible causal association between renal dysfunction and COPD/sleep apnea (group 3 PH). In mechanistic studies of COPD, hypercapnia is associated with decreases in systemic vascular resistance and thereby, threatening arterial pressure, resulting in unloading of arterial baroreceptors and increases in NE and RAAS. The net effect is reduced RBF as well as sodium and water retention just as in low left ventricle (LV) cardiac output state.10
Table 1.
Classification of pulmonary hypertension/right heart failure and potential pathophysiologic mechanisms of cardiorenal syndrome in the respective groups
| PH/RHF | Etiology | Potential Pathophysiologic Mechanism |
| Group 1 | Pulmonary arterial hypertension | PH → ventricular dyssynchrony and leftward ventricular septal bowing → impaired LV filling → ↓ LV cardiac output → neurohormonal activation |
| Central venous congestion | ||
| Group 2 | Left heart failure | ↓ LV cardiac output → neurohormonal activation |
| Central venous congestion | ||
| Group 3 | Lung disease and/or hypoxemia | Hypercapnia → ↓ in SVR → neurohormonal activation |
| Hypoxia → ↓ RBF and renal oxidative stress | ||
| PH → ventricular dyssynchrony and leftward ventricular septal bowing | ||
| Group 4 | Chronic thromboembolic disease | PH → ventricular dyssynchrony and leftward ventricular septal bowing → ↓ LV cardiac output |
| Central venous congestion | ||
| Group 5 | Miscellaneous | PH → ventricular dyssynchrony and leftward ventricular septal bowing → ↓ LV cardiac output |
| Central venous congestion |
PH, pulmonary hypertension; RHF, right heart failure; LV, left ventricle; SVR, systemic vascular resistance; RBF, renal blood flow.
The cause of kidney disease in patients with pulmonary arterial hypertension (PAH; group 1 PH) or chronic thromboembolic disease (group 4 PH) is more complex, because neither hypercapnia nor decreased ejection fraction are present in these patients. Peripheral edema and ascites are common, and resistance to diuretics often occurs as the disease progresses in advanced PAH. Do these patients have neurohormonal activation, impaired natriuresis, or reduced RBF to explain salt and water retention? If so, what is the stimulus? One study of patients with PAH showed increases in plasma endothelin, atrial natriuretic peptide, and NE concentrations but normal plasma renin levels.11 In an experimental model of pulmonary stenosis in dogs, as the pulmonary artery pressure was increased, LV cardiac output and mean arterial pressure (MAP) decreased, and the RAAS was activated. Salt and water retention occurred over time. Subsequently, cardiac output, MAP, and renin/aldosterone returned to baseline levels.12 An additional canine model of RHF induced by graded pulmonary valvular damage showed a similar decrease in RBF as well as intense sodium and water retention.13 Because pulmonary baroreceptors were not activated, the cause for neurohormonal activation and renal dysfunction was unclear. Recent work has shown that LV cardiac output can be reduced in patients with PH as a consequence of two mechanisms. First, increased right ventricle (RV) afterload leads to decreases in RV output and thus, decreases in left atrial and LV filling. Second, as a result of direct ventricular interaction, (1) because both ventricles are enclosed within a relatively nondistensible pericardium, increase in RV volume may occur at the expense of reduced LV volume; also, (2) RV pressure overload may lead to prolonged contraction of RV free wall, causing a right to left trans-septal pressure gradient at the early diastolic phase of LV, and thus, results in dyssynchrony and leftward ventricular septal bowing. The consequence is not only ineffective RV end systolic contraction but also, impaired LV early diastolic filling, causing decreased left stroke volume and reduced cardiac output, despite normal LV ejection fraction.14,15 In 46 patients with PAH, the LV stroke volume index and the LV end diastolic volume index were 25% lower compared with in control healthy patients.14
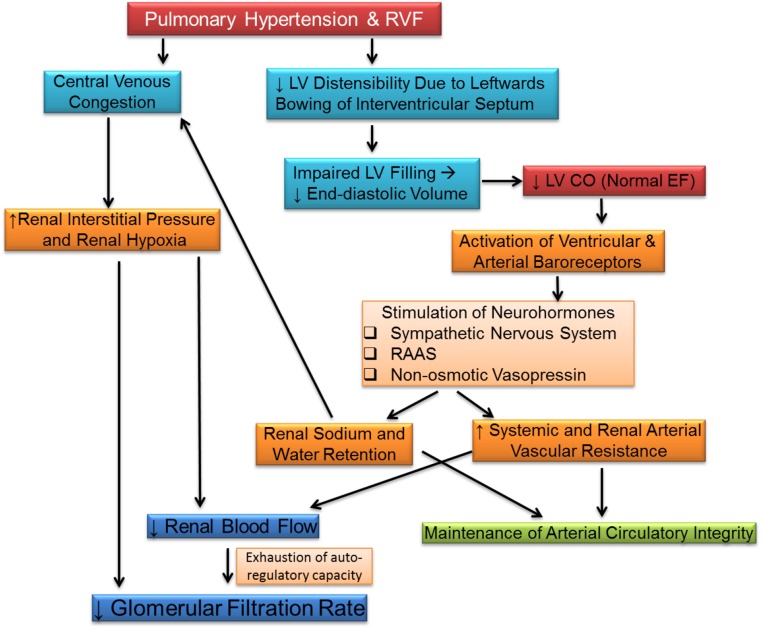
Similar to LHF, venous congestion may be an important contributor to the renal dysfunction in patients with isolated RHF. In patients with RHF due to PH and normal LV function, increase in right atrial pressure rather than cardiac index independently predicted GFR.16,17 It is possible that, compared with LHF, patients with RHF are more prone to renal consequence of venous congestion, because central venous congestion may develop earlier in the course without significant prior sodium and water retention from high RV afterload and reduced RV output. These changes in right-sided hemodynamics result in increased renal venous pressure and decreased GFR. Further studies are clearly required to prove this hypothesis. The potential pathogenesis of renal dysfunction in PH/RHF is illustrated in Figure 1.
Figure 1.
Potential mechanisms of renal dysfunction in patients with right heart failure. These include decreased left ventricle cardiac output as a result of leftwards bowing of interventricular septum and central venous congestion. CO, cardiac output; EF, ejection fraction; LV, left ventricle; RAAS, renin-angiotensin-aldosterone system; RHF, right heart failure.
Clinical Presentation and Diagnosis
The clinical manifestations are nonspecific and usually reflect the underlying cause of PH/RHF (i.e., COPD, PAH, or signs and symptoms related to volume overload and congestion). The diagnosis of CRS should be considered in patients with PH/RHF presenting with worsening renal function either at the time of presentation or during hospitalization. Multiple biomarkers reflecting neurohormonal disarray, myocardial stress and injury, inflammation and oxidative stress, and renal clearance and injury have been considered for their diagnostic and prognostic value in heart failure. Natriuretic peptides are endorsed by current guidelines. However, their role in the clinical care of patients with RHF is not defined.18
Treatment
Treatment of CRS from RHF requires treatment of underlying reversible processes, which may involve thrombolysis/thrombectomy, embolectomy, percutaneous intervention, continuous positive airway pressure, bronchodilators, steroid therapy, or lung protective ventilation. Vasopressors and/or inotropes are indicated in acute RHF with hemodynamic instability; however, data supporting recommendations are limited, and effects on renal function are not reported in these studies. Volume optimization is complex in RHF. Classically, RHF is considered a preload-dependent condition. Animal studies show that the RV responds more favorably to increases in preload than the LV by increasing both the stroke work of the RV and systemic cardiac output.19 However, clinical studies in failing human hearts suggest a more tempered approach to volume resuscitation. These studies support volume loading only in patients with low CVP and satisfactory MAPs.20 Volume loading can be beneficial in the presence of a collapsed RV, but after the RV is adequately filled, further volume expansion can become detrimental. Furthermore, in patients with low MAP, particular caution should be taken to avoid increasing RV filling pressures relative to systemic pressures. The physiology supporting these recommendations include ventricular interdependence (lessened LV filling with increasing RV volume), worsened tricuspid regurgitation with increased RV volume, and hypoperfusion of the RV, particularly in patients with systemic hypotension or RV ischemia. In sum, these observations call for fluid loading to a goal CVP of 10–12 mm Hg, taking care to avoid excessive volume loading (>2 L of fluid), especially in patients with MAP<60 mm Hg. Nonetheless, the data on the effectiveness of these strategies on renal function are limited.
In patients with congestive symptoms, intravenous diuretics are recommended. It is important to monitor the rate of diuresis, because overaggressive rates of fluid removal from the vascular space may exceed movement of fluid from the interstitial to the vascular space and result in hypotension or worsening of renal function. Clearly, decongestion requires careful clinical and hemodynamic assessment. However, monitoring of MAP or urine output alone can be misleading, because MAP is maintained at the expense of renal vasoconstriction and volume retention. In this regards, studies have shown decreased incidence of worsening of renal function when treatment is guided by invasively measured CVP and pulmonary capillary wedge pressure compared with clinical assessment alone.21 Moreover, special consideration should be given in these patients to avoid or minimize the use of nephrotoxins, because they may have apparently normal creatinine but poor renal reserve due to decreased underlying RBF; thus, they may not tolerate further vasoconstriction as induced with administration of contrast agents or nonsteroidal anti-inflammatory drugs.
Conclusions
CRS due to isolated RHF remains an under-recognized entity. Renal dysfunction is an important independent predictor of death and hospitalization in RHF; however, the pathophysiology is not well known. Moreover, there is a dearth of evidence regarding biomarkers, diagnosis, or interventions to improve renal outcomes in this population. Increasing awareness of interaction between right heart disease and kidneys is important, because it is bidirectional with causation involved. This perspective calls for further investigation in this important area.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Ronco C, House AA, Haapio M: Cardiorenal and renocardiac syndromes: The need for a comprehensive classification and consensus. Nat Clin Pract Nephrol 4: 310–311, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, et al. : Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43: 61–67, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Schrier RW: Decreased effective blood volume in edematous disorders: What does this mean? J Am Soc Nephrol 18: 2028–2031, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Ross EA: Congestive renal failure: The pathophysiology and treatment of renal venous hypertension. J Card Fail 18: 930–938, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. : Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 53: 589–596, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo PC, Doran AC, Onat D, Wong KY, Ahmad M, Sabbah HN, et al. : Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: Cause or effect? Curr Heart Fail Rep 12: 215–222, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. : Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54[Suppl]: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Haddad F, Fuh E, Peterson T, Skhiri M, Kudelko KT, De Jesus Perez V, et al. : Incidence, correlates, and consequences of acute kidney injury in patients with pulmonary arterial hypertension hospitalized with acute right-side heart failure. J Card Fail 17: 533–539, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa T, Okada K, Furuichi S, Ishiguro T, Yoshizawa A, Akahoshi T, et al. : Prevalence of chronic kidney diseases in patients with chronic obstructive pulmonary disease: Assessment based on glomerular filtration rate estimated from creatinine and cystatin C levels. Int J Chron Obstruct Pulmon Dis 10: 1283–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, et al. : Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation 86: 12–21, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, et al. : Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: Relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol 26: 1581–1585, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Watkins L Jr, Burton JA, Haber E, Cant JR, Smith FW, Barger AC: The renin-angiotensin-aldosterone system in congestive failure in conscious dogs. J Clin Invest 57: 1606–1617, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barger AC, Yates FE, Rudolph AM: Renal hemodynamics and sodium excretion in dogs with graded valvular damage, and in congestive failure. Am J Physiol 200: 601–608, 1961 [DOI] [PubMed] [Google Scholar]
- 14.Gan C, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JG, et al. : Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 290: H1528–H1533, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, Postmus PE, Heethaar RM, Van Rossum AC, et al. : Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: Noninvasive monitoring using MRI. Chest 119: 1761–1765, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, van Veldhuisen DJ, et al. : Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9: 872–878, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Hanberg JS, Sury K, Wilson FP, Brisco MA, Ahmad T, Ter Maaten JM, et al. : Reduced cardiac index is not the dominant driver of renal dysfunction in heart failure. J Am Coll Cardiol 67: 2199–2208, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines: 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62: e147–e239, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Cohn JN, Guiha NH, Broder MI, Limas CJ: Right ventricular infarction. Clinical and hemodynamic features. Am J Cardiol 33: 209–214, 1974 [DOI] [PubMed] [Google Scholar]
- 20.Piazza G, Goldhaber SZ: The acutely decompensated right ventricle: Pathways for diagnosis and management. Chest 128: 1836–1852, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. ; ESCAPE Investigators and ESCAPE Study Coordinators: Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 294: 1625–1633, 2005 [DOI] [PubMed] [Google Scholar]