Abstract
Background Everolimus permits reduced calcineurin inhibitor (CNI) exposure, but the efficacy and safety outcomes of this treatment after kidney transplant require confirmation.
Methods In a multicenter noninferiority trial, we randomized 2037 de novo kidney transplant recipients to receive, in combination with induction therapy and corticosteroids, everolimus with reduced-exposure CNI (everolimus arm) or mycophenolic acid (MPA) with standard-exposure CNI (MPA arm). The primary end point was treated biopsy-proven acute rejection or eGFR<50 ml/min per 1.73 m2 at post-transplant month 12 using a 10% noninferiority margin.
Results In the intent-to-treat population (everolimus n=1022, MPA n=1015), the primary end point incidence was 48.2% (493) with everolimus and 45.1% (457) with MPA (difference 3.2%; 95% confidence interval, −1.3% to 7.6%). Similar between-treatment differences in incidence were observed in the subgroups of patients who received tacrolimus or cyclosporine. Treated biopsy-proven acute rejection, graft loss, or death at post-transplant month 12 occurred in 14.9% and 12.5% of patients treated with everolimus and MPA, respectively (difference 2.3%; 95% confidence interval, −1.7% to 6.4%). De novo donor-specific antibody incidence at 12 months and antibody-mediated rejection rate did not differ between arms. Cytomegalovirus (3.6% versus 13.3%) and BK virus infections (4.3% versus 8.0%) were less frequent in the everolimus arm than in the MPA arm. Overall, 23.0% and 11.9% of patients treated with everolimus and MPA, respectively, discontinued the study drug because of adverse events.
Conclusions In kidney transplant recipients at mild-to-moderate immunologic risk, everolimus was noninferior to MPA for a binary composite end point assessing immunosuppressive efficacy and preservation of graft function.
Keywords: everolimus, calcineurin inhibitor, kidney transplantation, efficacy graft, function, randomized
Despite improvements in early kidney graft survival, long-term results remain unsatisfactory with one in four patients returning to dialysis within 5 years post-transplant.1 Several studies have implicated chronic antibody-mediated rejection as the most common cause of late graft failure, particularly in patients who are nonadherent, including the ongoing Long-Term Deterioration of Kidney Allograft Function (DeKAF) study.2,3 However, in a recently published study by Stegall et al. on the basis of a large cohort of patients who underwent prospective kidney biopsies over 10 years, the most frequently noted pathologies were arterial hyalinosis and glomerulosclerosis4—injuries associated with exposure to calcineurin inhibitors (CNIs) as well as with antibody-mediated rejection.5 Transplant glomerulopathy was present in only 12% of patients. Thus, even in the absence of rejection, the graft is vulnerable to chronic insults which can lead to deteriorating kidney function. The most notable of such insults is the dose-dependent nephrotoxicity associated with long-term CNI therapy.6 Kidney function during the first year post-transplant is a well established predictor of long-term graft survival7–10 and the importance of preserving good graft function, in addition to avoiding rejection, is now recognized.11,12
The use of mammalian target of rapamycin (mTOR) inhibitors with low tacrolimus levels may mitigate the chronic injury reported by Stegall et al.4 Additional potential long-term benefits of mTOR inhibitors described in experimental models and reported in clinical trials include suppression of cytomegalovirus (CMV) replication,13 protection from intimal hyperplasia14 and atherosclerosis,15,16 and antiangiogenic17,18 and antiproliferative19 actions that may induce an antioncogenic action although clinical evidence for an effect on post-transplant malignancy remains mixed.20–23 The initial enthusiasm for mTOR inhibitors after sirolimus was approved for de novo use in kidney transplantation, however, has waned and use of mTOR inhibitors has become less common. In part, this was because of complications associated with early high-exposure mTOR inhibitor regimens given de novo24,25 or in maintenance patients with deteriorating graft function,26 and reduced efficacy of CNI-free regimens on the basis of mTOR inhibition.27,28
The protocol of the Advancing renal TRANSplant eFficacy and safety Outcomes with an eveRoliMus-based regimen (TRANSFORM) trial incorporates lessons learned after almost two decades of mTOR inhibition in kidney transplantation. TRANSFORM was designed to test the hypothesis that everolimus with reduced-exposure CNI is noninferior to current standard of care, mycophenolic acid (MPA) and standard-exposure CNI, both with induction therapy and maintenance corticosteroids, in preventing acute rejection and preserving graft function. Results at 1 year, including the novel primary composite end point of treated biopsy-proven acute rejection (tBPAR) or suboptimal kidney function (eGFR<50 ml/min per 1.73m2), are reported here.
Methods
Study Design and Oversight
TRANSFORM was a randomized, open-label, two-arm study. Patients were recruited at 186 centers in 42 countries worldwide.
The study protocol was approved by the Institutional Review Board or Independent Ethics Committee at participating centers and was conducted according to the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent.
Patients
De novo kidney transplant patients aged ≥18 years who had received a graft from a living or deceased heart-beating donor were eligible. Patients who had undergone a previous kidney transplant could be enrolled if the first graft had not been lost due to immunologic reasons. Exclusion criteria included multiorgan transplantation, HLA-identical living-related donation, cold ischemia time >30 hours, high risk of rejection (on the basis of local practice for assessment of anti-donor reactivity, e.g., high panel reactive antibodies or presence of preexisting donor-specific antibodies [DSA]), recipient or donor positive for hepatitis C virus, and body mass index >35 kg/m2. Full inclusion and exclusion criteria are shown in Supplemental Table 1. Recruitment was planned to ensure that the population included ≥50% living-donor recipients and ≤20% patients receiving cyclosporine (CsA).
Randomization and Study Treatment
Patients were randomized in a 1:1 ratio within 24 hours after transplantation. A randomization sequence, stratified within treatment groups by donor type (living, deceased standard criteria, or deceased expanded criteria) and by the type of CNI (CsA or tacrolimus), was generated by a computer program and implemented by telephone-based interactive response technology.
All patients received induction therapy with basiliximab (20 mg×2 doses, on days 0 and 4) or rabbit anti-thymocyte globulin (rATG, 1.5 mg/kg per day, total dose ≤6 mg/kg).
In the everolimus group, the starting dose of everolimus was 1.5 mg twice a day or 0.75 mg twice a day, respectively, in patients receiving concomitant tacrolimus or CsA. The dose was subsequently adjusted to target an everolimus trough concentration (C0) of 3–8 ng/ml throughout the study. The tacrolimus dose was adjusted to target C0 concentrations of 4–7 ng/ml during months 0–2, 2–5 ng/ml during months 3–6, and 2–4 ng/ml thereafter; corresponding target ranges for CsA were 100–150, 50–100, and 25–50 ng/ml, respectively.
In the MPA group, MPA was given as enteric-coated mycophenolate sodium (1.44 g/d) or mycophenolate mofetil (2.0 g/d), which could be reduced after week 2 to enteric-coated mycophenolate sodium 1.08 g/d or mycophenolate mofetil 1.5 g/d in patients receiving tacrolimus but not those given CsA. The tacrolimus dose was adjusted to target C0 concentrations of 8–12 ng/ml during months 0–2, 6–10 ng/ml during months 3–6, and 5–8 ng/ml thereafter; corresponding target ranges for CsA were 200–300, 150–200, and 100–200 ng/ml, respectively.
Blood concentrations of everolimus, tacrolimus, and CsA were determined locally.
Corticosteroid therapy was mandatory for all patients, administered according to local practice but with a minimum dose of prednisolone 5 mg/d or equivalent.
CMV prophylaxis (given for at least 3 months post-transplant) was recommended for all donor positive/recipient negative (D+/R−) patients. Pneumocystis jirovecii (Pneumocystis carinii) pneumonia prophylaxis was given for at least 6 months to all patients.
Study End Points
The primary end point was a binary composite of tBPAR or eGFR<50 ml/min per 1.73 m2 (Modification of Diet in Renal Disease [MDRD]29) at 12 months post-transplant. eGFR was calculated at each study visit (baseline; day 1; weeks 1, 2, 4; months 2, 4, 6, 9, and 12) on the basis of assays at a central laboratory. tBPAR was defined as receipt of antirejection treatment and local histologic diagnosis of acute rejection according to Banff 2009 criteria.30 A threshold of 50 ml/min per 1.73 m2 was selected because it represents a moderate level of renal dysfunction31 and is associated with a significantly increased risk for subsequent death-censored graft loss.9,10 The key secondary end point was a composite efficacy failure end point of tBPAR, graft loss, or death at 12 months post-transplant.12 A full list of other secondary and exploratory end points is shown in Supplemental Table 2.
Statistical Analyses
On the basis of results from the A2309 study,32 it was assumed that the event rate in each arm would be 50%. A sample size of 1020 patients in each treatment arm (2040 in total) with a one-sided α error of 0.025 was estimated to provide at least 95% power to demonstrate noninferiority for the primary end point at month 12 in the overall study population, and in the tacrolimus-treated subpopulation with an assumption that approximately 80% of patients would be randomized to tacrolimus. A noninferiority margin of 10% was used in this study because it was used previously (A230932) and accepted by health authorities.
Event rates were compared between groups using a hierarchic testing strategy: (1) noninferiority of everolimus versus MPA for the primary end point using a 10% noninferiority margin; (2) noninferiority of everolimus versus MPA for the key secondary end point with a 10% noninferiority margin; and (3) superiority of everolimus versus MPA on the basis of the primary end point. The fixed hypothesis testing procedure would not inflate overall type I error rate. Therefore, each hypothesis was tested at the one-sided 0.025 significance level and no multiplicity adjustment was needed. Supportive analyses for the primary end point included an analysis on the basis of the per protocol population.
Details of other statistical methods, and the populations for analysis, are shown in Supplemental Table 3. Analyses were performed using SAS statistical software, Version 9.4 (or higher) for Unix.
Results
Patients
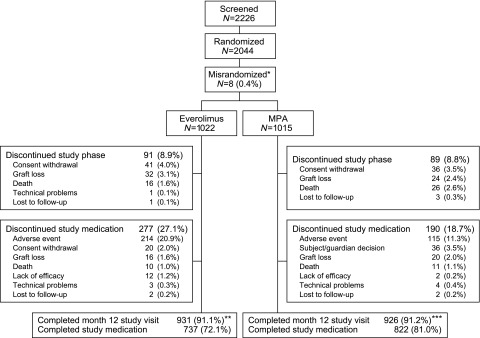
Between December of 2013 and January of 2016, 2226 patients were screened, of whom 2037 were transplanted and randomized and comprised the intent-to-treat population (Figure 1). The month 12 study visit was completed by 90.5% (925 of 1022) and 90.8% (922 of 1015) of patients in the everolimus and MPA groups, respectively, with 72.1% (737 of 1022) and 81.0% (822 of 1015) of patients still on study medication. The per protocol population (i.e., patients with no major protocol violations) included 1925 patients (everolimus 946, MPA 979).
Figure 1.
In total, 2037 patient were transplanted and randomized and comprised the intention-to-treat (ITT) population. The month 12 study visit was completed by more than 90% of patients in both treatment groups. *One misrandomized patient received study medication and was included in the ITT population. **Six of these patients completed their month 12 visit after day 450. ***Four of these patients completed their month 12 visit after day 450.
Recipient and donor characteristics were comparable between treatment groups (Table 1).
Table 1.
Characteristics at baseline (intention-to-treat population)
| Characteristic | Everolimus (n=1022) | MPA (n=1015) |
|---|---|---|
| Mean age (SD), yr | 49.3 (14.1) | 49.3 (14.5) |
| Male, n (%) | 710 (69.5) | 707 (69.7) |
| Race, n (%) | ||
| White | 743 (72.7) | 735 (72.4) |
| Asian | 136 (13.3) | 157 (15.5) |
| Black | 43 (4.2) | 35 (3.4) |
| Other | 100 (9.8) | 88 (8.7) |
| Mean BMI (SD), kg/m2 | 25.6 (4.2) | 25.6 (4.3)a |
| ESRD leading to transplant, n (%) | ||
| Glomerular disease | 157 (15.4) | 176 (17.3) |
| Polycystic disease | 147 (14.4) | 149 (14.7) |
| Diabetes mellitus | 128 (12.5) | 131 (12.9) |
| Hypertension/nephrosclerosis | 124 (12.1) | 125 (12.3) |
| IgA nephropathy | 88 (8.6) | 103 (10.1) |
| Other | 377 (36.9) | 331 (32.6) |
| Missing | 1 (0.1) | 0 (0.0) |
| Diabetes at baseline, n (%) | 279 (27.3) | 269 (26.5) |
| Hemodialysis, n (%) | 674 (65.9) | 679 (66.9) |
| Mean PRA (SD) (most recent evaluation) | 2.7 (10.7) | 2.7 (9.5) |
| HLA mismatching, n (%) | ||
| Loci A | ||
| 0 | 178 (17.4) | 172 (16.9) |
| 1 | 550 (53.8) | 545 (53.7) |
| 2 | 286 (28.0) | 295 (29.1) |
| Missing | 8 (0.8) | 3 (0.3) |
| Loci B | ||
| 0 | 114 (11.2) | 124 (12.2) |
| 1 | 518 (50.7) | 494 (48.7) |
| 2 | 382 (37.4) | 394 (38.8) |
| Missing | 8 (0.8) | 3 (0.3) |
| Loci DR | ||
| 0 | 240 (23.5) | 193 (19.0) |
| 1 | 495 (48.4) | 557 (54.9) |
| 2 | 279 (27.3) | 262 (25.8) |
| Missing | 8 (0.8) | 3 (0.3) |
| Mean cold ischemia time (SD), h | 8.4 (7.8) | 8.4 (7.8) |
| Delayed graft function, n (%) | 79 (7.7) | 72 (7.1) |
| Induction, n (%) | ||
| Basiliximab | 849 (83.1) | 843 (83.1) |
| rATG | 171 (16.7) | 171 (16.8) |
| Missing | 2 (0.2) | 1 (0.1) |
| Donor | ||
| Mean age (SD), yr | 48.4 (15.18) | 48.2 (15.48) |
| Male, n (%) | 493 (48.2) | 508 (50.0) |
| Race, n (%) | ||
| White | 620 (60.7) | 599 (59.0) |
| Asian | 120 (11.7) | 134 (13.2) |
| Other | 282 (27.6) | 279 (27.5) |
| Donor category, n (%) | ||
| Living related | 302 (29.5) | 315 (31.0) |
| Living unrelated | 209 (20.5) | 192 (18.9) |
| Deceased heart-beating | 506 (49.5) | 505 (49.8) |
| Deceased non-heart-beating | 5 (0.5) | 3 (0.3) |
| Donor criteria, n (%)a | ||
| Standard criteria deceased donor | 354 (70.0) | 345 (68.3) |
| Expanded criteria deceased donor | 152 (30.0) | 160 (31.7) |
| CMV serology, n/N (%) | ||
| D+/R− | 153 of 978 (15.6) | 139 of 976 (14.2) |
| D+/R+ | 514 of 978 (52.6) | 522 of 976 (53.5) |
| D−/R+ | 141 of 978 (14.4) | 147 of 976 (15.1) |
| D−/R− | 170 of 978 (17.4) | 168 of 976 (17.2) |
BMI, body mass index; IgA, immunoglobulin A; PRA, panel reactive antibodies; HLA, human leukocyte antigen; D, donor; R, recipient.
Denominator is the number of deceased heart-beating donors.
Study Medication
The majority of patients (83.1%, 1692 of 2037) received basiliximab induction (Table 1).
By weeks 1 and 2, respectively, 70.4% and 83.9% of patients randomized to everolimus had an everolimus trough concentration ≥3 ng/ml. Mean everolimus trough concentration was within target range in the everolimus group throughout the 12-month study phase (tacrolimus-treated patients 4.4−5.9 ng/ml; CsA-treated patients 5.0−6.1 ng/ml). At study entry, 913 patients (90.0%) and 100 patients (9.9%) in the everolimus group were receiving tacrolimus and CsA, respectively, compared with 916 patients (90.6%) and 95 (9.4%) in the MPA group. Mean tacrolimus trough concentration remained near or above the upper limit of the target range in the everolimus arm with 25%−44% of patients above the upper limit at any study visit, but it was within range in the MPA arm (Supplemental Figure 1). The mean CsA trough concentration was near or above the target range in both groups from month 2 onwards, with 17%−61% and 7%−32% of patients in the everolimus and MPA groups above the upper limit at any study visit, respectively (Supplemental Figure 1).
The mean (SD) MPA dose in the MPA group at month 12 was 1110 (398) mg/d. The median (range) corticosteroid dose at month 12 was 0.09 (0.0−16.0) mg/kg per day and 0.09 (0.0−25.4) mg/kg per day in the everolimus and MPA groups, respectively. Doses of MPA and steroids during the study are presented in Supplemental Table 4.
Efficacy
Binary Composite End Point
The everolimus group was noninferior to the MPA group for the primary end point (tBPAR or eGFR<50 ml/min per 1.73 m2 [MDRD4] at month 12 post-transplant). The incidence was 48.2% (493 of 1022) with everolimus versus 45.1% (457 of 1015) with MPA, a difference of 3.2% with 95% confidence interval (95% CI) −1.3% to 7.6%, where the upper limit of the 95% CI was below the noninferiority margin of 10%. Similar results were shown for the per protocol population, in which the incidence of the primary end point was 47.3% (448 of 946) with everolimus versus 44.0% (431 of 979) with MPA (difference 3.3%; 95% CI, –1.2% to 7.8%).
When analyzed in the subgroup of patients treated with tacrolimus, the primary end point occurred in 47.8% and 44.7% of patients in the everolimus and MPA groups, respectively (Table 2). For the smaller subgroup treated with CsA, the incidence was 51.5% and 47.3%, respectively (Table 2). Rates of the primary end point were somewhat higher in patients treated with basiliximab induction (everolimus 49.2%, MPA 46.9%) than those given rATG induction (everolimus 42.7%, MPA 36.2%) (Table 2).
Table 2.
Efficacy end points at month 12 (intention-to-treat population)
| End Point | Everolimus n/N (%) | MPA n/N (%) | Difference (Everolimus − CNI) | 95% CI |
|---|---|---|---|---|
| tBPAR or eGFR<50 ml/min per 1.73 m2 | ||||
| All patients | 493 of 1022 (48.2) | 457 of 1015 (45.1) | 3.2 | −1.3 to 7.6 |
| Tacrolimus-treated patients | 437 of 914 (47.8) | 410 of 917 (44.7) | 3.1 | −1.6 to 7.7 |
| CsA-treated patients | 51 of 100 (51.1) | 45 of 95 (47.3) | 3.9 | −10.3 to 18.0 |
| Basiliximab-treated patients | 418 of 849 (49.2) | 395 of 843 (46.9) | 2.3 | −2.5 to 7.2 |
| rATG-treated patients | 73 of 171 (42.7) | 62 of 171 (36.2) | 6.5 | −4.2 to 17.1 |
| tBPAR, graft loss, or deatha | ||||
| All patients | 137 of 1022 (14.9) | 122 of 1015 (12.5) | 2.3 | −1.7 to 6.4 |
| Tacrolimus-treated patients | 120 of 914 (13.3) | 104 of 917 (11.9) | 1.4 | −1.7 to 4.6 |
| CsA-treated patients | 16 of 100 (29.2) | 15 of 95 (15.9) | 13.3 | −13.7 to 40.3 |
| Basiliximab-treated patients | 115 of 849 (15.4) | 107 of 843 (13.3) | 2.1 | −2.7 to 6.8 |
| rATG-treated patients | 21 of 171 (12.6) | 15 of 171 (9.0) | 3.6 | −3.0 to 10.2 |
| tBPARa | ||||
| All patients | 100 of 1022 (11.5) | 83 of 1015 (8.8) | 2.7 | −1.2 to 6.5 |
| Tacrolimus-treated patients | 88 of 914 (10.0) | 70 of 917 (8.3) | 1.7 | −1.1 to 4.5 |
| CsA-treated patients | 12 of 100 (26.1) | 13 of 95 (14.0) | 12.1 | −15.8 to 40.0 |
| Basiliximab-treated patients | 89 of 849 (12.5) | 76 of 843 (9.8) | 2.7 | −1.9 to 7.3 |
| rATG-treated patients | 11 of 171 (6.8) | 7 of 171 (4.2) | 2.6 | −2.3 to 7.6 |
Kaplan–Meier estimate of incidence rate was used.
Other Efficacy End Points
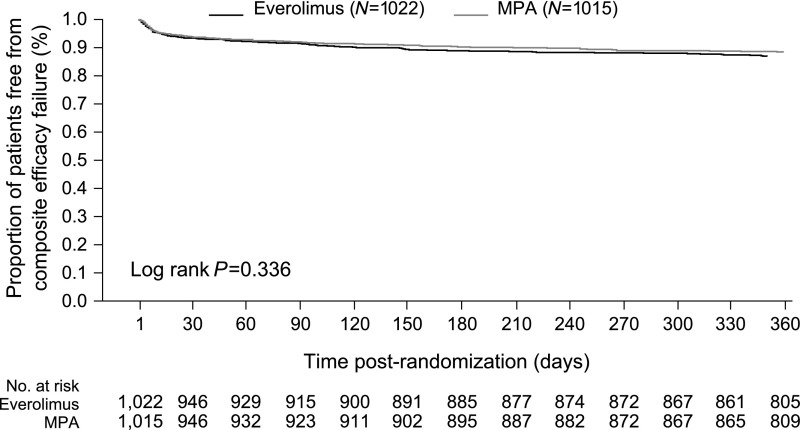
The Kaplan–Meier estimate of the key secondary end point of tBPAR, graft loss, or death at month 12 post-transplant, was 14.9% versus 12.5% in the everolimus versus MPA groups, respectively; difference 2.3%, with 95% CI, –1.7% to 6.4%, supporting the noninferiority of everolimus to MPA (Figure 2). The Kaplan–Meier estimate of tBPAR was 11.5% in everolimus patients (100 of 1022) and 8.8% in MPA patients (83 of 1015) (difference 2.7%; 95% CI, –1.2% to 6.5%).
Figure 2.
The Kaplan-Meier estimate of the key secondary endpoint of tBPAR, graft loss or death at month 12 post-transplant was 14.9% versus 12.5% in the everolimus versus MPA groups, respectively; difference 2.3% with 95% CI [–1.7% to 6.4%], supporting the non-inferiority of everolimus to MPA (ITT population). ITT, intention-to-treat.
Within the subgroup of patients given tacrolimus, the incidence of tBPAR was 10.0% and 8.3% in the everolimus and MPA groups, respectively; for the CsA-treated subgroup the incidences were 26.1% and 14.0% (Table 2). Low rates of tBPAR were observed in the subgroup given rATG induction (everolimus 6.8%, MPA 4.2%) (Table 2).
Graft loss occurred in 32 patients in the everolimus group and in 25 patients in the MPA group (Kaplan–Meier estimates 3.2% versus 2.6%). The most common cause of graft loss in both groups was infarction/thrombosis with no identified technical cause (ten everolimus, nine MPA). Rejection led to graft loss in four everolimus patients (one hyperacute, one acute T cell–mediated, two acute antibody-mediated) and five MPA patients (one hyperacute, three acute T cell, one chronic antibody-mediated). There were 16 and 27 deaths, respectively (Kaplan–Meier estimates 1.6% and 2.8%, P=0.08), most frequently due to cardiac arrest (everolimus n=2, MPA n=3), myocardial infarction (n=2 in each group), and sepsis (n=3 and n=2). No other cause of death occurred in more than one patient.
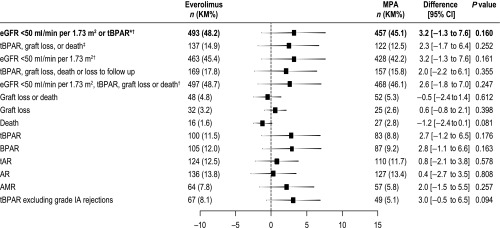
All other efficacy end points at month 12 occurred at a similar rate between groups (Figure 3).
Figure 3.
All efficacy endpoints at month 12 occurred at a similar rate in the everolimus and MPA groups (ITT population). Difference was defined as everolimus − MPA. 95% CI and P values test for no difference. The primary end point is shown in bold text. *P=0.001 for noninferiority; ‡P<0.001 for noninferiority; †compared using raw incidence rates (other end points are compared using Kaplan–Meier incidence rates). AMR, antibody-mediated rejection; AR, acute rejection; ITT, intention-to-treat; KM, Kaplan-Meier; tAR, treated acute rejection.
Renal Function
Using multiple imputation for missing values, mean (SD) eGFR at 12 months was 53.0 (0.7) and 54.4 (0.7) ml/min per 1.73 m2 in the everolimus and MPA groups, respectively. Renal function was stable from week 4 through to month 12: mean (SD) change was 0.0 (0.5) ml/min per 1.73 m2 versus 2.3 (0.5) ml/min per 1.73 m2 for the everolimus and MPA groups, respectively (Supplemental Figure 2). The predicted slope (SEM) of change in eGFR (ml/min per 1.73 m2) over time, calculated using a mixed effect model, was 0.0007 (0.0015) in the everolimus group versus 0.0074 (0.0015) in the MPA group. This was significantly different between groups (P=0.001) but is unlikely to be clinically relevant.
DSA and Antibody-Mediated Rejection
The presence of DSA was assessed using local assays and according to locally determined thresholds in a subset of subjects at participating centers. In total, 476 and 477 patients participated in this substudy from the everolimus and MPA groups, respectively. Rates of DSA at baseline and month 12 were similar between groups, as were the incidence and type of de novo DSA at month 12 (Table 3).
Table 3.
DSA
| Variable | Everolimus (n=476) | MPA (n=477) |
|---|---|---|
| DSA at baseline | ||
| Yes | 29 of 263 (11.0) | 37 of 292 (12.7) |
| Anti-class I | 11 of 263 (4.2) | 11 of 292 (3.8) |
| Anti-class II | 9 of 263 (3.4) | 12 of 292 (4.1) |
| Both anti-class I and anti-class II | 10 of 263 (3.8) | 14 of 292 (4.8) |
| No | 234 of 263 (89.0) | 255 of 292 (87.3) |
| Missing | 213 | 185 |
| DSA at month 12 | ||
| Yes | 51 of 374 (13.6) | 47 of 339 (13.9) |
| Anti-class I | 12 of 374 (3.2) | 16 of 339 (4.7) |
| Anti-class II | 24 of 374 (6.4) | 24 of 339 (7.1) |
| Both anti-class I and anti-class II | 15 of 374 (4.0) | 7 of 339 (2.1) |
| No | 323 of 374 (86.4) | 292 of 339 (86.1) |
| Missing | 102 | 138 |
| de novo DSA at month 12a | ||
| Yes | 19 of 187 (10.2) | 25 of 184 (13.6) |
| Anti-class I | 5 of 187 (2.7) | 8 of 184 (4.3) |
| Anti-class II | 10 of 187 (5.3) | 14 of 184 (7.6) |
| Both anti-class I and anti-class II | 4 of 187 (2.1) | 3 of 184 (1.6) |
| No | 168 of 187 (89.8) | 159 of 184 (86.4) |
In patients without DSA at baseline in whom DSA were assessed at month 12.
Acute antibody-mediated rejection occurred at a similar rate in both groups (7.8% versus 5.8%; difference 2.0%; 95% CI, –1.5% to 5.5%) (Figure 3).
Safety
Adverse Events
The incidences of adverse events and serious adverse events were similar between treatment groups (Table 4). Rates of new-onset diabetes mellitus, major cardiovascular events, and malignancies were comparable. Hyperlipidemia, interstitial lung disease, peripheral edema, proteinuria, stomatitis/mouth ulceration, thrombocytopenia, thrombotic/thromboembolic events, wound healing events/complications, hypokalemia, and proteinuria were more frequent in the everolimus group than the MPA group (Table 4). Diarrhea, nausea, vomiting, tremor, leukopenia, and insomnia were more frequent in the MPA group (Table 4).
Table 4.
Adverse events (safety population)
| Adverse Event | Everolimus (n=1014) | MPA (n=1012) | RR (Everolimus versus MPA) (95% CI) |
|---|---|---|---|
| Any adverse event, n (%) | 993 (97.9) | 984 (97.2) | 1.01 (0.99 to 1.02) |
| Any serious adverse event, n (%) | 557 (54.9) | 568 (56.1) | 0.98 (0.91 to 1.06) |
| Any adverse event leading to study drug discontinuation, n (%) | 233 (23.0) | 120 (11.9) | 1.94 (1.58 to 2.37) |
| Adverse events occurring more frequently in the everolimus group, n (%)a | |||
| Hyperlipidemia | 340 (33.5) | 188 (18.6) | 1.86 (1.59 to 2.17) |
| Interstitial lung disease | 11 (1.1) | 3 (0.3) | 3.66 (1.02 to 13.08) |
| Peripheral edema | 373 (36.8) | 262 (25.9) | 1.42 (1.25 to 1.62) |
| Stomatitis and mouth ulceration | 78 (7.7) | 21 (2.1) | 3.71 (2.31 to 5.95) |
| Thrombocytopenia | 82 (8.1) | 40 (4.0) | 2.05 (1.42 to 2.96) |
| Thrombotic and thromboembolic events | 119 (11.7) | 84 (8.3) | 1.41 (1.08 to 1.84) |
| Wound healing events/complications | 201 (19.8) | 164 (16.2) | 1.22 (1.01 to 1.47) |
| Hypokalemia | 144 (14.2) | 82 (8.1) | 1.75 (1.36 to 2.27) |
| Proteinuria | 128 (12.6) | 57 (5.6) | 2.24 (1.66 to 30.2) |
| Adverse events occurring more frequently in the MPA group, n (%)a | |||
| Diarrhea | 219 (21.6) | 316 (31.2) | 0.69 (0.60 to 0.80) |
| Nausea | 177 (17.5) | 214 (21.1) | 0.83 (0.69 to 0.99) |
| Vomiting | 110 (10.8) | 141 (13.9) | 0.78 (0.62 to 0.98) |
| Tremor | 98 (9.7) | 137 (13.5) | 0.71 (0.56 to 0.91) |
| Leukopenia | 94 (9.3) | 192 (19.0) | 0.49 (0.39 to 0.62) |
| Insomnia | 91 (9.0) | 130 (12.8) | 0.70 (0.54 to 0.90) |
| Infections, n (%) | |||
| Any infection | 527 (52.0) | 605 (59.8) | 0.87 (0.80 to 0.94) |
| Any serious infection | 272 (26.8) | 305 (30.1) | 0.89 (0.78 to 1.02) |
| Any viral infection | 174 (17.2) | 296 (29.2) | 0.59 (0.50 to 0.69) |
| CMV infection | 36 (3.6) | 135 (13.3) | 0.27 (0.19 to 0.38) |
| BK virus infection | 44 (4.3) | 81 (8.0) | 0.54 (0.38 to 0.77) |
| Any bacterial infection | 353 (34.8) | 381 (37.6) | 0.92 (0.82 to 1.04) |
| Any fungal infection | 69 (6.8) | 46 (4.5) | 1.50 (1.04 to 2.15) |
Events captured by standard adverse event reporting, or prespecified events of interest, which occurred in ≥10% of patients in either group and for which the 95% CI of the RR did not include 1.0 (excluding prespecified events of interest).
Discontinuation of study drug due to adverse events was more frequent in the everolimus group (23.0% versus 11.9% in the MPA group; risk ratio [RR], 1.94; 95% CI, 1.58 to 2.37). Graft rejection, proteinuria, and impaired healing were more frequent causes of discontinuation in the everolimus group, whereas polyomavirus-associated nephropathy and BK virus infection were more frequent causes in the MPA group (Supplemental Table 5). Conversely, adverse events leading to dose adjustment or temporary interruption were less frequent with everolimus versus MPA (25.4% versus 48.2%; RR, 0.53; 95% CI, 0.47 to 0.60).
Infections
Infections occurred less frequently in the everolimus group (52.0% versus 59.8% in the MPA group; RR, 0.87; 95% CI, 0.80 to 0.94), a difference accounted for by fewer viral infections (17.2% versus 29.2%; RR, 0.59; 95% CI, 0.50 to 0.69) (Table 4). CMV infection was less frequent in the everolimus group (3.6% versus 13.3% in the MPA group; RR, 0.27; 95% CI, 0.19 to 0.38), with clinical signs of CMV infection, CMV syndrome, and histologic signs of CMV in 3.9% (40 of 1014), 1.5% (15 of 1014), and 0.1% (1 of 1014) of everolimus patients, respectively, and in 11.2% (113 of 1012), 4.9% (50 of 1012), and 0.6% (6 of 1012) of patients in the MPA group.
BK virus infection (viruria or viremia) occurred in 4.3% and 8.0% of everolimus and MPA patients, respectively (RR, 0.54; 95% CI, 0.38 to 0.77), with histologic evidence for organ involvement in 1.2% (12 of 1014) and 2.1% (21 of 1012).
Infections reported as serious adverse events occurred in 26.8% (272 of 1014) and 30.1% (305 of 1012) of patients in the everolimus and MPA groups, respectively (RR, 0.89; 95% CI, 0.78 to 1.02).
Laboratory Results
The mean urine protein-to-creatinine ratio was higher in the everolimus group up to week 4, but similar from month 2 onwards (Supplemental Figure 3). Proteinuria in the nephrotic range (≥3000 mg/g) was present in 3.1% (30 of 953) of patients and 1.4% (13 of 940) of patients in the everolimus and MPA groups, respectively, at month 12 or end of treatment visit (Supplemental Table 6).
Total cholesterol, LDL-cholesterol, and triglycerides were higher in the everolimus group versus the MPA group; the LDL/HDL-cholesterol level was comparable (Supplemental Table 5). Lipid-lowering medication was used by 59.3% (601 of 1014) and 45.9% (465 of 1012) of patients, respectively. One or more clinically abnormal liver function values, among patients with normal liver function tests at baseline, were detected in 19.5% of everolimus patients (84 of 435) and 22.0% (117 of 484) of MPA patients.
Discussion
The primary end point of this study, uniquely, combined the efficacy end point of graft rejection (tBPAR) with an assessment of graft function (eGFR<50 ml/min per 1.73 m2). This novel approach was considered more indicative of potential long-term graft and patient survival than a conventional end point comprising only efficacy parameters such as biopsy-proven acute rejection (BPAR), graft loss, or death, or only eGFR. In this large international population of de novo kidney transplant patients, everolimus was noninferior to MPA for this composite primary end point. Similar between-treatment differences in incidences were observed in the subgroups of patients who received either tacrolimus or CsA. Both regimens achieved good and comparable immunosuppressive efficacy with a low rate of tBPAR. The two treatment groups were also associated with similarly well preserved graft function during the first post-transplant year. Rates of CMV and BK virus infection were lower with everolimus.
Combination therapy with tacrolimus and MPA has become the standard of care after kidney transplantation, partly on the basis of findings from the landmark SYMPHONY trial.33 SYMPHONY showed higher eGFR and a lower incidence of BPAR with MPA+tacrolimus versus MPA+CsA or versus a CNI-free regimen combining MPA and the mTOR inhibitor sirolimus.33 Since then, an increased rate of acute rejection using sirolimus in a CNI-free regimen from time of transplant has been confirmed elsewhere.34 In contrast, everolimus with reduced CsA from time of transplant does not increase the risk for BPAR.32 In the CsA-treated subgroup of this trial, the Kaplan–Meier estimate of tBPAR, graft loss, or death was high for everolimus-treated patients (29.2%), due to the last occurrence of a tBPAR event, which influenced the Kaplan-Meier estimate. The raw incidence rates of tBPAR, graft loss, or death were similar with everolimus/CsA (16.0%) or MPA/CsA (15.9%). Similar results were observed in the incidence of tBPAR (Table 2). Inclusion of reduced-exposure tacrolimus in the investigational arm of this study ensured equivalent efficacy, on the basis of tBPAR, to the MPA group. Previously, the US92 study also randomized de novo kidney transplant patients to everolimus with reduced tacrolimus or to MPA with standard-exposure tacrolimus, but in that trial the starting dose of everolimus was only 0.75 mg twice a day compared with 1.5 mg twice a day for tacrolimus-treated patients in this trial, giving rise to inadequate early everolimus exposure and a higher rate of tBPAR versus standard therapy.35 At weeks 1 and 2, the proportion of patients with everolimus trough concentration above the minimum target level (3 ng/ml) was only 64.2% and 66.5% in the US92 study compared with 70.4% and 83.9% in this trial. Here, the comparable rates of de novo DSA in the two treatment arms also point to equivalent immunosuppressive potency with the everolimus plus reduced CNI regimen versus MPA plus standard CNI regimen. Although between-study comparisons must always be approached cautiously, the 1-year incidence of tBPAR in the subgroup treated with everolimus and tacrolimus (10.0%) in our trial is comparable with the reported rate of BPAR excluding borderline values in the tacrolimus arm of the SYMPHONY study (12.3%).33
The validity of the DSA data is limited by the fact that DSA were assessed locally, with consequent variations between assays and definitions, and DSA sampling was incomplete with information on DQ antibodies missing. However, the similarity in rates of antibody-mediated rejection between treatment groups is a robust observation.
The finding that there was no increased risk for de novo DSA or antibody-mediated rejection in patients randomized to everolimus with reduced CNI contrasts with data suggesting that de novo DSA may be more frequent in patients given everolimus in a CNI-free regimen.36
Renal function, assessed by eGFR, was stable in the everolimus group over the course of the study and was virtually identical to the MPA group. Other randomized studies of everolimus with reduced-exposure CNI have also observed comparable renal function versus standard therapy with up to 2 years’ follow-up.32,35,37 Exposure to tacrolimus or CsA was near or above target range during the study, with up to 44% of tacrolimus-treated patients having a trough concentration above target at any one point, such that the between-group difference in exposure was less than planned. A post hoc analysis of results from the large randomized A2309 trial recently showed that higher tacrolimus concentrations are associated with a significantly increased risk for low eGFR and decreased eGFR at month 12 after kidney transplantation in everolimus-treated individuals.38 In that analysis, tacrolimus trough concentrations above 4 ng/ml were associated with inferior renal outcomes, with a further increase in risk above 6 ng/ml. Here, the reduction in CNI exposure in the everolimus/reduced tacrolimus group versus controls in this study (4.1 ng/ml versus 6.9 ng/ml for tacrolimus trough concentration at month 12) was not found to confer a renal benefit, consistent with experience from the A twelve-month, multicenter, open-label, randomized Study of the Safety, tolerability and Efficacy of Certican™ with IL-2 receptor antagonist, corticosteroids and two different exposure levels of Tacrolimus in de novo renal transplant recipients (ASSET) study.39 Unexpectedly, there was a small increase in eGFR in the control arm during the course of the study, which has not been observed with MPA plus conventional CNI regimens in recent studies.16,33,40,41 Mean eGFR at month 12 was 53−54 ml/min per 1.73 m2 in both treatment arms, similar to that seen in the MPA+tacrolimus group of the SYMPHONY study (54 ml/min per 1.73 m2 by MDRD formula). Given that 30% of the deceased-donor recipients received a graft from expanded criteria donor recipients in this trial compared with only 18% in the MPA+tacrolimus arm of SYMPHONY, a sustained eGFR>50 ml/min per 1.73 m2 can be regarded as satisfactory.
In terms of safety, everolimus targeting a trough concentration of 3–8 ng/ml avoided the increased rates of lymphocele seen previously,33 although wound healing complications were slightly more frequent overall. Other adverse events often associated with mTOR inhibitor therapy, including proteinuria and dyslipidemia, rarely led to everolimus discontinuation. The risk of proteinuria is higher under mTOR inhibition in patients receiving kidney transplants.42 This effect is a dose-dependent effect when everolimus is used de novo43 and may be related to podocyte injury44 and inhibition of vascular endothelial growth factor signaling.45 Discontinuation of study drug due to adverse events was twice as frequent in the everolimus group versus controls, possibly partly due to the high number of participating centers (n=189), many of which were unfamiliar with use of everolimus-based protocols. Conversely, dose changes or temporary interruptions were twice as frequent in the MPA group, raising the possibility that in this open-label study some case investigators may have been more reluctant to discontinue the conventional regimen than to switch patients from the everolimus protocol to standard therapy.
CMV infection occurred in fewer than a third as many patients under everolimus compared with MPA. Although steroid doses were higher in the MPA group, and MPA dosing was relatively high, this observation is consistent with previous experience in kidney transplantation.13 A minimum of 6 months’ CMV prophylaxis with valganciclovir, instead of the minimum of 3 months recommended here, has been shown to be advantageous46 and may be particularly relevant for patients receiving non-mTOR inhibitor regimens. BK virus infections were also less frequent with everolimus, a point of potential interest. There has been mixed evidence in published studies for an effect of mTOR inhibition on the risk of BK infection after kidney transplantation.47 Some initial studies have suggested that BK viral load may be reduced after initiation of everolimus therapy after onset of infection.48,49
TRANSFORM is one of the first trials to apply a novel combined end point which incorporates both acute rejection and graft function at 1 year, an approach recognized as helpful for improving long-term survival.11,12 The study benefited from the largest population of de novo kidney transplant patients included in a randomized trial to date. It involved 186 centers, with widely differing degrees of experience in using everolimus-based immunosuppression, which may have contributed to the variable adherence to CNI exposure targets in patients treated with everolimus. Local determinations of tacrolimus concentrations, with known discordance of results between assay types,50 may also have contributed. CsA concentrations were relatively high and above target range in a relatively high proportion of patients. The resulting narrowing of the planned difference in exposure between treatment groups is a limitation of the trial.
Additionally, graft biopsy specimens were read locally at each center, and although graded according to Banff 2009 criteria30 this inevitably introduces center-specific variation. It should also be noted that the study specifically excluded patients at high risk for rejection and included only approximately 4% of black patients, so the findings should not be extrapolated to individuals at high immunologic risk. Furthermore, follow-up to month 12 post-transplant does not encompass the long-term effects of the treatment regimens on renal function or parameters such as development of DSA or antibody-mediated rejection.
In conclusion, in this large population of de novo kidney transplant patients at mild-to-moderate immunologic risk, a regimen of everolimus with reduced tacrolimus from the time of transplant was noninferior to MPA plus conventional CNI for a binary end point assessing both immunosuppressive efficacy and preservation of graft function. Renal function remained stable in both groups during the first year post-transplant despite a high proportion of expanded criteria donor recipients, and rates of tBPAR were low in patients given tacrolimus with either everolimus or MPA. A significant reduction in the incidence of clinically important viral infections was observed with everolimus. Patients are now being followed to 2 years post-transplant.
Disclosures
J.P. has received consulting honoraria from Novartis and travel grants from Novartis and Chiesi, and his institution has received research grants from Novartis, Astellas, Chiesi, and Amgen. S.P.B. has received consulting honoraria and travel grants form Novartis, Astellas, and Chiesi. His institution has received research grants from Astellas and Novartis. O.W. has received research funds and/or honoraria from Alexion, Astellas, Bristol-Myers Squibb, Chiesi, Janssen-Cilag, MSD, Novartis, Pfizer, Roche, and Shire. H.T. has received consulting honoraria and travel grants from Novartis, Pfizer, BMS, and Roche, and his institution has received research grants from Novartis, Pfizer, BMS, Roche, Veloxis, and Teraclone. S.M. has received research grants from Novartis and Astellas. Y.Q. has received speaker’s honoraria from Alexion, Mallenckrodt, and Relypssa. S.C. has received research funding, travel support, or consulting honoraria from Novartis, Astellas, and Alexion. F.O. has received consulting honoraria and travel grants from Novartis, Astellas, Pfizer, BMS, and Roche, and his institution has received research grants from Novartis and Astellas. C.S.’s institution has received research grants from Novartis and Astellas. R.O. has received speaker’s honoraria from Novartis. Y.W. has received speaker’s honoraria from Novartis, Astellas, Chugai, and Roche, and travel grants from Roche. C.L. has received speaker’s honoraria from Alexion and Novartis, and travel grants from Alexion, Novartis, and Amgen. F.C. has received consulting honoraria and travel grants from Novartis, Astellas, Pfizer, and Bristol Myers Squibb. M.H. has received consulting honoraria from Novartis. T.R.S. has received consulting honoraria from Novartis, and grants from Astellas, Bristol Myers Squibb, and Novartis. W.-L.L., A.M., and P.B. are employees of Novartis. F.V. has received research grants from Novartis, Astellas, Genentech, Alexion, and Bristol Myers.
Supplementary Material
Acknowledgments
A medical writer (Caroline Dunstall), was funded by Novartis Pharma AG, Basel, Switzerland.
The Advancing renal TRANSplant eFficacy and safety Outcomes with an eveRoliMus-based regimen study is funded by Novartis Pharma AG, Basel, Switzerland. Trial registry: ClinicalTrials.gov identifier NCT01950819.
J.P., H.T., S.C., F.O., C.S., R.O., Y.W., C.L., F.C., M.H., T.R.S., and F.V. comprised the scientific steering committee and designed the study with input from the sponsor, Novartis Pharma AG. All of the scientific steering committee members, S.P.B., O.W., S.M., and Y.Q. recruited patients and collected data. W.-L.L. undertook the statistical analyses. A.M. and P.B. provided medical input. All authors had access to the study data, assessed the analyses, critically reviewed the manuscript, and approved the final version for publication.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Transformation in Immunosuppression: Are We Ready for it?,” on pages 1791–1792.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010009/-/DCSupplemental.
Contributor Information
Collaborators: Alan Jardine, Tim Friede, Rafael Maldonado, Pablo Massari, Silvina Aleman, Silvia Maurich, Luis E. Gaite, Pablo Raffaele, Nora Imperiali, Scott Campbell, Steven Chadban, Peter Hughes, Ashley Irish, John Kanellis, Wai Lim, Philip J O’Connell, Graeme Russ, Zoltan Endre, Peter Mount, Paul Hengster, Peter Neudorfer, Rainer Oberbauer, Johann Pratschke, Dirk Kuypers, Jean-Louis Bosmans, Emine N. Broeders, Laurent Weekers, Helio Tedesco Silva, Jr., Elias D. Neto, Valter D. Garcia, Emil P. Dimitrov, Alvaro Kompatzki, Carlos Benavides, Johanna Schweineberg, Nikolina Basic Jukic, Mladen Knotek, Sanjin Racki, Ondrej Viklicky, M.A. Bakr, Christophe Legendre, Elisabeth Cassuto, Vincent Pernin, Vincent Vuiblet, Mathias Buchler, Claudia Sommerer, Peter Weithofer, Thomas Rath, Oliver Witzke, Markus van der Giet, Wolfgang Arns, Lutz Renders, Antje Habicht, Daniel Seehofer, Bernhard Banas, Frank Lehner, Johann Pratschke, Ioannis Boletis, Dimitrios Goumenos, Vasileios Papanikolaou, Spyros Drakopoulos, Dinesh Khullar, Veerbhadra Guptha, Shibu Jacob, Alan Fernandes Almeida, Eytan Mor, Richard Nakache, Mario Carmellini, Paolo Rigotti, Giacomo Colussi, Giuseppe Tisone, Paola Todeschini, Luigi Biancone, Franco Citterio, Vincenzo Cantaluppi, Loreto Gesualdo, Umberto Maggiore, Yoshihiko Watarai, Naotake Akutsu, Takashi Kenmochi, Duck Jong Han, Myoung Soo Kim, Sung Joo Kim, Torki AlOtaibi, Dania Chelala, Hilal Abou Zeinab, Khalil Jaber, Ghazali Ahmad Kutty, Hin Seng Wong, Francisco Javier Monteon Ramos, J.W. de Fijter, Stefan P. Berger, F.J. Bemelman, A.D. van Zuilen, L. Hilbrands, M.H.L. Christiaans, Anders Hartmann, Romina Danguilan, Angel Joaquin Amante, Kazimierz Ciechanowski, Maciej Glyda, Marek Karczewski, Alicja Debska-Slizien, Fernando Nolasco, Jose Guerra, Joana Santos, Patricia Joao Matias, Arnaldo Figueiredo, Yan G. Moysyuk, Aleksey V. Pinchuk, Ilya V. Aleksandrov, Vladimir E. Zagainov, Elena I. Boretskaya, Vladimir L. Medvedev, Ashraf Attia, Wael Habhab, Meteb Bugami, Neven Vavic, Igor Mitic, Goran Paunovic, Terence Kee, Tatiana Baltesova, Eva Lackova, Zuzana Zilinska, Ivana Dedinska, Miha Arnol, Elmi Muller, Julio Pascual, Federico Oppenheimer, Asuncion Sancho, Alex Gutierrez Dalmau, Domingo Marrero, Josep M. Cruzado, Amado Andres Belmonte, Juan Carlos Ruiz San Millan, Antonio Osuna, Ana Fernandez, Lars Wennberg, Bengt von Zur Muhlen, Bengt Gustafsson, Uyen Huynh-Do, Meng-Kun Tsai, Ming Ju Wu, Tsung Ching Chou, Prajej Ruangkanchanasetr, Sakarn Bunnag, Atiporn Ingsathit, Aydin Turmen, Ahmet V. Celik, Huseyin Kocak, Alexander Wiseman, Phillippe Gauthier, Fuad Shihab, Stevenson Bynon, Bernard Fischbach, Goran B. Klintmalm, Richard Knight, Kenneth L. Brayman, Jason Wellen, Stanley J. Jordan, Yasir Qazi, Ronald Cotton, Venkat Peddi, David Leeser, Mohamed E. Akoad, Shamkant Mulgaonkar, Martha Pavlakis, Reginald Gohh, Charles Bratton, Nahel Elias, Debra Sudan, Mary Waybill, Johnny Hong, Silas Norman, Ivo Tzvetanov, Dean Kim, Mitchell Henry, Jeffrey Rogers, Chandrasekar Santhanakrishnan, Nicolae Leca, Tomasz Kozlowski, Flavio Vincenti, Enver Akalin, Clifton E. Kew, David Shaffer, Liise K Kayler, Steven Steinberg, Stuart M. Flechner, Donald Hricik, Michael de Vera, and Didier Mandelbrot
Appendix 1
The TRANSFORM Data Monitoring Committee
Alan Jardine, BHF Cardiovascular Research Centre, Glasgow, United Kingdom; Tim Friede, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
The TRANSFORM Investigators
Argentina: Rafael Maldonado, Pablo Massari, Silvina Aleman, Silvia Maurich, Luis E. Gaite, Pablo Raffaele, Nora Imperiali; Australia: Scott Campbell, S.C., Peter Hughes, Ashley Irish, John Kanellis, Wai Lim, Philip J O’Connell, Graeme Russ, Zoltan Endre, Peter Mount; Austria: Paul Hengster, Peter Neudorfer, R.O., Johann Pratschke; Belgium: Dirk Kuypers, Jean-Louis Bosmans, Emine N. Broeders, Laurent Weekers; Brazil: H.T. Jr., Elias D. Neto, Valter D. Garcia; Bulgaria: Emil P. Dimitrov; Chile: Alvaro Kompatzki; Colombia: Carlos Benavides, Johanna Schweineberg; Croatia: Nikolina Basic Jukic, Mladen Knotek, Sanjin Racki; Czech Republic: Ondrej Viklicky; Egypt: M.A. Bakr; France: C.L., Elisabeth Cassuto, Vincent Pernin, Vincent Vuiblet, Mathias Buchler; Germany: C.S., Peter Weithofer, Thomas Rath, O.W., Markus van der Giet, Wolfgang Arns, Lutz Renders, Antje Habicht, Daniel Seehofer, Bernhard Banas, Frank Lehner, Johann Pratschke; Greece: Ioannis Boletis, Dimitrios Goumenos, Vasileios Papanikolaou, Spyros Drakopoulos; India: Dinesh Khullar, Veerbhadra Guptha, Shibu Jacob, Alan Fernandes Almeida; Israel: Eytan Mor, Richard Nakache; Italy: Mario Carmellini, Paolo Rigotti, Giacomo Colussi, Giuseppe Tisone, Paola Todeschini, Luigi Biancone, F.C., Vincenzo Cantaluppi, Loreto Gesualdo, Umberto Maggiore; Japan: Y.W., Naotake Akutsu, Takashi Kenmochi; Korea, Republic of: Duck Jong Han, Myoung Soo Kim, Sung Joo Kim; Kuwait: Torki AlOtaibi; Lebanon: Dania Chelala, Hilal Abou Zeinab, Khalil Jaber; Malaysia: Ghazali Ahmad Kutty, Hin Seng Wong; Mexico: Francisco Javier Monteon Ramos; Netherlands: J.W. de Fijter, S.P.B., F.J. Bemelman, A.D. van Zuilen, L. Hilbrands, M.H.L. Christiaans; Norway: Anders Hartmann; Philippines: Romina Danguilan, Angel Joaquin Amante; Poland: Kazimierz Ciechanowski, Maciej Glyda, Marek Karczewski, Alicja Debska-Slizien; Portugal: Fernando Nolasco, Jose Guerra, Joana Santos, Patricia Joao Matias, Arnaldo Figueiredo; Russia: Yan G. Moysyuk, Aleksey V. Pinchuk, Ilya V. Aleksandrov, Vladimir E. Zagainov, Elena I. Boretskaya, Vladimir L. Medvedev; Saudi Arabia: Ashraf Attia, Wael Habhab, Meteb Bugami; Serbia: Neven Vavic, Igor Mitic, Goran Paunovic; Singapore: Terence Kee; Slovakia: Tatiana Baltesova, Eva Lackova, Zuzana Zilinska, Ivana Dedinska; Slovenia: Miha Arnol; South Africa: Elmi Muller; Spain: J.P., F.O., Asuncion Sancho, Alex Gutierrez Dalmau, Domingo Marrero, Josep M. Cruzado, Amado Andres Belmonte, Juan Carlos Ruiz San Millan, Antonio Osuna, Ana Fernandez; Sweden: Lars Wennberg, Bengt von Zur Muhlen, Bengt Gustafsson; Switzerland: Uyen Huynh-Do; Taiwan: Meng-Kun Tsai, Ming Ju Wu, Tsung Ching Chou; Thailand: Prajej Ruangkanchanasetr, Sakarn Bunnag, Atiporn Ingsathit; Turkey: Aydin Turmen, Ahmet V. Celik, Huseyin Kocak; United States: T.R.S., Alexander Wiseman, Phillippe Gauthier, Fuad Shihab, Stevenson Bynon, Bernard Fischbach, Goran B. Klintmalm, Richard Knight, Kenneth L. Brayman, Jason Wellen, Stanley J. Jordan, Y.Q., Ronald Cotton, Venkat Peddi, David Leeser, Mohamed E. Akoad, S.M., Martha Pavlakis, Reginald Gohh, Charles Bratton, Nahel Elias, Debra Sudan, Mary Waybill, Johnny Hong, Silas Norman, Ivo Tzvetanov, Dean Kim, M.H., Jeffrey Rogers, Chandrasekar Santhanakrishnan, Nicolae Leca, Tomasz Kozlowski, F.V., Enver Akalin, Clifton E. Kew, David Shaffer, Liise K Kayler, Steven Steinberg, Stuart M. Flechner, Donald Hricik, Michael de Vera, Didier Mandelbrot.
References
- 1.Gondos A, Döhler B, Brenner H, Opelz G: Kidney graft survival in Europe and the United States: Strikingly different long-term outcomes. Transplantation 95: 267–274, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al.: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Halloran PF, Chang J, Famulski K, Hidalgo LG, Salazar ID, Merino Lopez M, et al.: Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J Am Soc Nephrol 26: 1711–1720, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stegall MD, Cornell LD, Park WD, Smith BH, Cosio FG: Renal allograft histology at 10 years after transplantation in the tacrolimus era: Evidence of pervasive chronic injury. Am J Transplant 18: 180–188, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Einecke G, Reeve J, Halloran PF: Hyalinosis lesions in renal transplant biopsies: Time-dependent complexity of interpretation. Am J Transplant 17: 1346–1357, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Chapman JR: Chronic calcineurin inhibitor nephrotoxicity-lest we forget. Am J Transplant 11: 693–697, 2011 [DOI] [PubMed] [Google Scholar]
- 7.McDonald S, Russ G, Campbell S, Chadban S: Kidney transplant rejection in Australia and New Zealand: Relationships between rejection and graft outcome. Am J Transplant 7: 1201–1208, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Park WD, Larson TS, Griffin MD, Stegall MD: Identification and characterization of kidney transplants with good glomerular filtration rate at 1 year but subsequent progressive loss of renal function. Transplantation 94: 931–939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasiske BL, Israni AK, Snyder JJ, Skeans MA; Patient Outcomes in Renal Transplantation (PORT) Investigators : The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis 57: 466–475, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Schnitzler MA, Johnston K, Axelrod D, Gheorghian A, Lentine KL: Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation 91: 1347–1356, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) Office of New Drugs (OND) Division of Transplant and Ophthalmology Products (DTOP): Surrogate endpoints in clinical trials of kidney transplantation. Available at: https://www.fda.gov/downloads/Drugs/NewsEvents/UCM470429.pdf. Accessed May 15, 2017
- 12.Committee for Medicinal Products for Human Use (CHMP): European Medicines Agency. Guideline on clinical investigation of immunosuppressants for sold organ transplantation. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003593.pdf. Accessed May 15, 2017
- 13.Brennan DC, Legendre C, Patel D, Mange K, Wiland A, McCague K, et al.: Cytomegalovirus incidence between everolimus versus mycophenolate in de novo renal transplants: Pooled analysis of three clinical trials. Am J Transplant 11: 2453–2462, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Vitiello D, Neagoe PE, Sirois MG, White M: Effect of everolimus on the immunomodulation of the human neutrophil inflammatory response and activation. Cell Mol Immunol 12: 40–52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouëffic Y, Potter-Perigo S, Chan CK, Johnson PY, Braun K, Evanko SP, et al.: Sirolimus blocks the accumulation of hyaluronan (HA) by arterial smooth muscle cells and reduces monocyte adhesion to the ECM. Atherosclerosis 195: 23–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WQ, Zhong L, Zhang L, Ji XP, Zhang M, Zhao YX, et al.: Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. Br J Pharmacol 156: 941–951, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy D, Sin SH, Lucas A, Venkataramanan R, Wang L, Eason A, et al.: mTOR inhibitors block Kaposi sarcoma growth by inhibiting essential autocrine growth factors and tumor angiogenesis. Cancer Res 73: 2235–2246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ: Role of mTOR in solid tumor systems: A therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev 26: 611–621, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa T, Takaoka M, Ohara T, Tomono Y, Hao H, Bao X, et al.: Antiproliferative effect of a novel mTOR inhibitor temsirolimus contributes to the prolonged survival of orthotopic esophageal cancer-bearing mice. Cancer Biol Ther 14: 230–236, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim WH, Russ GR, Wong G, Pilmore H, Kanellis J, Chadban SJ: The risk of cancer in kidney transplant recipients may be reduced in those maintained on everolimus and reduced cyclosporine. Kidney Int 91: 954–963, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Yanik EL, Gustafson SK, Kasiske BL, Israni AK, Snyder JJ, Hess GP, et al.: Sirolimus use and cancer incidence among US kidney transplant recipients. Am J Transplant 15: 129–136, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Yanik EL, Siddiqui K, Engels EA: Sirolimus effects on cancer incidence after kidney transplantation: A meta-analysis. Cancer Med 4: 1448–1459, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharnidharka VR, Schnitzler MA, Chen J, Brennan DC, Axelrod D, Segev DL, et al.: Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: A national study. Transpl Int 29: 1226–1236, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groth CG, Bäckman L, Morales JM, Calne R, Kreis H, Lang P, et al.; Sirolimus European Renal Transplant Study Group : Sirolimus (rapamycin)-based therapy in human renal transplantation: Similar efficacy and different toxicity compared with cyclosporine. Transplantation 67: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al.: Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation 69: 1252–1260, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA: Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: A systematic review of the evidence. Transplantation 82: 1153–1162, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Mulay AV, Hussain N, Fergusson D, Knoll GA: Calcineurin inhibitor withdrawal from sirolimus-based therapy in kidney transplantation: A systematic review of randomized trials. Am J Transplant 5: 1748–1756, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Su L, Tam N, Deng R, Chen P, Li H, Wu L: Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: A systematic review and meta-analysis. Int Urol Nephrol 46: 2035–2044, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al.: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Eckardt KU, Berns JS, Rocco MV, Kasiske BL: Definition and classification of CKD: The debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis 53: 915–920, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Tedesco Silva H Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, et al.: Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 10: 1401–1413, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al.; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Flechner SM, Gurkan A, Hartmann A, Legendre CM, Russ GR, Campistol JM, et al.: A randomized, open-label study of sirolimus versus cyclosporine in primary de novo renal allograft recipients. Transplantation 95: 1233–1241, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR, et al.: Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant 17: 1358–1369, 2017 [DOI] [PubMed] [Google Scholar]
- 36.de Fijter JW, Holdaas H, Øyen O, Sanders JS, Sundar S, Bemelman FJ, et al.; ELEVATE Study Group : Early conversion from calcineurin inhibitor- to everolimus-based therapy following kidney transplantation: Results of the randomized ELEVATE trial. Am J Transplant 17: 1853–1867, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Cibrik D, Silva HT Jr, Vathsala A, Lackova E, Cornu-Artis C, Walker RG, et al.: Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 95: 933–942, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Shihab F, Qazi Y, Mulgaonkar S, McCague K, Patel D, Peddi VR, et al.: Association of clinical events with everolimus exposure in kidney transplant patients receiving low doses of tacrolimus. Am J Transplant 17: 2363–2371, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langer RM, Hené R, Vitko S, Christiaans M, Tedesco-Silva H Jr, Ciechanowski K, et al.: Everolimus plus early tacrolimus minimization: A phase III, randomized, open-label, multicentre trial in renal transplantation. Transpl Int 25: 592–602, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Budde K, Zeier M, Witzke O, Arns W, Lehner F, Guba M, et al.; HERAKLES Study Group : Everolimus with cyclosporine withdrawal or low-exposure cyclosporine in kidney transplantation from Month 3: A multicentre, randomized trial. Nephrol Dial Transplant 32: 1060–1070, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Uchida K, Yoshimura N, Takahara S, Teraoka S, Teshima R, et al.: Efficacy and safety of concentration-controlled everolimus with reduced-dose cyclosporine in Japanese de novo renal transplant patients: 12-month results. Transplant Res 2: 14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diekmann F, Andrés A, Oppenheimer F: mTOR inhibitor-associated proteinuria in kidney transplant recipients. Transplant Rev (Orlando) 26: 27–29, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Wiseman AC, McCague K, Kim Y, Geissler F, Cooper M: The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcomes. Am J Transplant 13: 442–449, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Cinà DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, et al.: Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol 23: 412–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ko HT, Yin JL, Wyburn K, Wu H, Eris JM, Hambly BD, et al.: Sirolimus reduces vasculopathy but exacerbates proteinuria in association with inhibition of VEGF and VEGFR in a rat kidney model of chronic allograft dysfunction. Nephrol Dial Transplant 28: 327–336, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Humar A, Limaye AP, Blumberg EA, Hauser IA, Vincenti F, Jardine AG, et al.: Extended valganciclovir prophylaxis in D+/R- kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: Two-year results of the IMPACT study. Transplantation 90: 1427–1431, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Mallat SG, Tanios BY, Itani HS, Lotfi T, McMullan C, Gabardi S, et al.: CMV and BKPyV infections in renal transplant recipients receiving an mTOR inhibitor-based regimen versus a CNI-based regimen: A systematic review and meta-analysis of randomized, controlled trials. Clin J Am Soc Nephrol 12: 1321–1336, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belliere J, Kamar N, Mengelle C, Allal A, Sallusto F, Doumerc N, et al.: Pilot conversion trial from mycophenolic acid to everolimus in ABO-incompatible kidney-transplant recipients with BK viruria and/or viremia. Transpl Int 29: 315–322, 2016 [DOI] [PubMed] [Google Scholar]
- 49.Yen CL, Tian YC, Wu HH, Weng CH, Chen YC, Tu KH, et al.: Conversion to mTOR-inhibitors with calcineurin inhibitor elimination or minimization reduces urinary polyomavirus BK load in kidney transplant recipients. J Formos Med Assoc 115: 539–546, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Agrawal YP, Cid M, Westgard S, Parker TS, Jaikaran R, Levine DM: Transplant patient classification and tacrolimus assays: More evidence of the need for assay standardization. Ther Drug Monit 36: 706–709, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.