Abstract
Background Fibroblast growth factor-23 (FGF-23) has been hypothesized to play a role in the increased risk of cardiovascular disease in patients with CKD.
Methods We identified prospective studies reporting associations between FGF-23 concentration and risk of cardiovascular events. Maximally adjusted risk ratios (RRs) were extracted for each outcome and scaled to a comparison of the top versus bottom third of the baseline FGF-23 concentration, and the results aggregated.
Results Depending on the assay used, median FGF-23 concentrations were 43–74 RU/ml and 38–47 pg/ml in 17 general population cohorts; 102–392 RU/ml in nine cohorts of patients with CKD not requiring dialysis; and 79–4212 RU/ml and 2526–5555 pg/ml in eight cohorts of patients on dialysis. Overall, comparing participants in the top and bottom FGF-23 concentration thirds, the summary RRs (95% confidence intervals [95% CIs]) were 1.33 (1.12 to 1.58) for myocardial infarction, 1.26 (1.13 to 1.41) for stroke, 1.48 (1.29 to 1.69) for heart failure, 1.42 (1.27 to 1.60) for cardiovascular mortality, and 1.70 (1.52 to 1.91) for all-cause mortality. The summary RR for noncardiovascular mortality, calculated indirectly, was 1.52 (95% CI, 1.28 to 1.79). When studies were ordered by average differences in FGF-23 concentration between the top and bottom thirds, there was no trend in RRs across the studies.
Conclusions The similarly-sized associations between increased FGF-23 concentration and cardiovascular (atherosclerotic and nonatherosclerotic) and noncardiovascular outcomes, together with the absence of any exposure–response relationship, suggest that the relationship between FGF-23 and cardiovascular disease risk may be noncausal.
Keywords: cardiovascular disease, chronic kidney disease, dialysis, fibroblast, heart failure, FGF23
Cardiovascular disease risk increases as kidney function declines and this elevated risk is apparent even in early CKD.1–3 Cardiovascular disease in people with CKD is characterized particularly by arterial stiffening and left ventricular hypertrophy, which becomes increasingly marked as CKD progresses.4–6 People with CKD are also at increased risk of atherosclerotic heart disease. It has been suggested that some of the excess cardiovascular risk in CKD may be mediated through disordered calcium-phosphate metabolism due to reduced kidney function. 7–9
Blood fibroblast growth factor-23 (FGF-23) concentration rises early in CKD, and increases exponentially in relation to eGFR, functioning to maintain phosphate homeostasis as the capacity for urinary phosphate excretion declines.10 FGF-23 possesses an atypical heparin-binding domain, which results in a low binding affinity to most FGF receptors.11 Its physiologic actions may therefore be limited to the parathyroid glands and kidney, where the coreceptor Klotho is abundantly expressed. In the kidney, FGF-23 downregulates renal proximal tubular sodium-phosphate cotransport function, enhancing urinary phosphate excretion, and reduces vitamin D 1-α hydroxylation, leading to less intestinal calcium and phosphate absorption.12 However, FGF-23 could have Klotho-independent actions in other tissues, including the heart,13 and may contribute to the etiology of structural heart disease in patients with CKD.14,15 If so, interventions targeting FGF-23 might hold therapeutic potential.
We conducted a systematic review and meta-analysis of the evidence from prospective studies for associations between FGF-23 and the risk of different cardiovascular diseases. We compared the evidence for associations among cohorts of people unselected for CKD (general population cohorts) with those in patients with CKD who were not receiving dialysis at the time of recruitment (nondialyzed CKD cohorts) and in patients on dialysis. We assessed for evidence of an exposure–response relationship both within and across each of these three separate populations.
Methods
Search Strategy/Selection Strategy
A systematic and comprehensive search for English language publications with mention of FGF-23 or equivalent terms was performed in MEDLINE (1948–April 2017) and EMBASE (1974–April 2017, see Supplemental Table 1 for terms). Abstracts were reviewed and cohort studies in adults were selected for inclusion in the meta-analysis if (1) FGF-23 was a key exposure of interest, (2) at least one clinical cardiovascular disease outcome was assessed, and (3) outcomes were ascertained prospectively. Cardiovascular outcomes of interest included myocardial infarction, stroke, heart failure, and peripheral arterial disease as well as mortality attributed to cardiovascular disease. Full texts of publications that appeared to meet inclusion criteria were reviewed. Duplicate studies and those that included <200 participants were excluded. The quality of remaining studies was assessed using the Newcastle–Ottawa scale,16 and studies excluded if their results were at moderate-to-high risk of bias (score of <6/9). A study of terminal heart failure was excluded post hoc as the population was at exceedingly high risk.
Data Extraction
Three authors (A.M., K.D., and C.M.R.) extracted the following data from full-text articles: study and study population characteristics, FGF-23 assay type (C-terminal, reported in relative units per milliliter [RU/mL], or intact, reported in picograms per milliliter [pg/mL]), measures of FGF-23 distribution, details of statistical models, covariates used for multivariate adjustments, follow-up duration, and hazard ratios/risk ratios (RRs) for relevant cardiovascular outcomes for all reported models and, where reported, all-cause and cardiovascular mortality. Where necessary, further data were requested from study investigators.
Statistical Analyses
To assess the FGF-23 associations across the wide range of FGF-23 concentrations encountered in different populations, meta-analysis was prespecified to be performed overall and within three study population types: (1) general population (unselected individuals), (2) patients with nondialyzed CKD (defined as an eGFR<60 ml/min per 1.73 m2), and (3) patients on dialysis.
For each study, we aimed to extract from the primary publication, for each outcome, the hazard ratio or RR yielded by the model that included the greatest number of covariates. These covariates included incrementally: basic demographics (+); cardiovascular risk factors including diabetes, body mass index, and smoking (++); kidney function (+++); and markers of CKD–mineral bone disorder (++++). Because of the usually skewed nature of FGF-23 distributions, studies reported associations for top versus bottom quintile, quartile, or third of the FGF-23 distribution, or less frequently, per SD or a unit increase in log-transformed FGF-23. To enable comparisons and synthesis of data across the studies, these associations were converted (where necessary) to a measure of association corresponding to the top versus bottom third of the baseline FGF-23 concentration using established methods (see Supplemental Methods, Supplemental Table 2 for more detail).17,18 Where noncardiovascular mortality was not reported, RRs were derived indirectly from cardiovascular and all-cause mortality results assuming that on the natural logarithm scale, the RR for all-cause mortality is an inverse-variance weighted average of the RRs for cardiovascular and noncardiovascular mortality.
The heterogeneity between studies (both within each population and overall) was summarized. Random-effects meta-analytical methods19 were used to combine the RRs for the top versus bottom third of baseline FGF-23 concentration in each study, yielding a summary RR for all studies.
As the median baseline FGF-23 concentration correlated strongly with interquartile range, standard tests for linear trend (on a log scale) across studies ordered by median (or, if not reported, mean) baseline FGF-23 concentration (within each population and across all the individual studies) were used to assess whether larger absolute differences in FGF-23 concentration between top and bottom third were associated with larger RRs. Trend tests were also performed across population-specific summary RRs after meta-analysis of RRs from the contributing studies. In sensitivity analyses, to allow for a potentially different relationship in dialysis patients, the trend tests across individual studies were repeated after excluding dialysis population studies.
Primary analyses of disease associations did not take account of whether studies used C-terminal or intact assays, which is equivalent to the assumption that the results between the two assays are approximately comparable. However, this assumption may not necessarily hold as, for example, intraperson biologic variability of intact FGF-23 may be higher than C-terminal FGF-23.20 To investigate the sensitivity of results to this assumption, analyses were performed repeating trend tests, firstly after converting intact FGF-23 concentration to an approximately equivalent C-terminal concentration using the formula intact FGF-23=0.110×C-terminal FGF-23+32.2, developed from a small healthy general population,21 and secondly, after excluding all studies that only reported intact FGF-23. To further assess whether associations in individual studies could have been affected by within-person FGF-23 variability, regression dilution ratios were calculated from individual studies that had repeat FGF-23 measurements,22–24 using the McMahon nonparametric quintile method.25 RRs for cardiovascular and noncardiovascular outcomes were compared by heterogeneity tests.26 Analyses were performed using R version 3.2.1 (www.R-project.org) with the “metafor” package v1.
Results
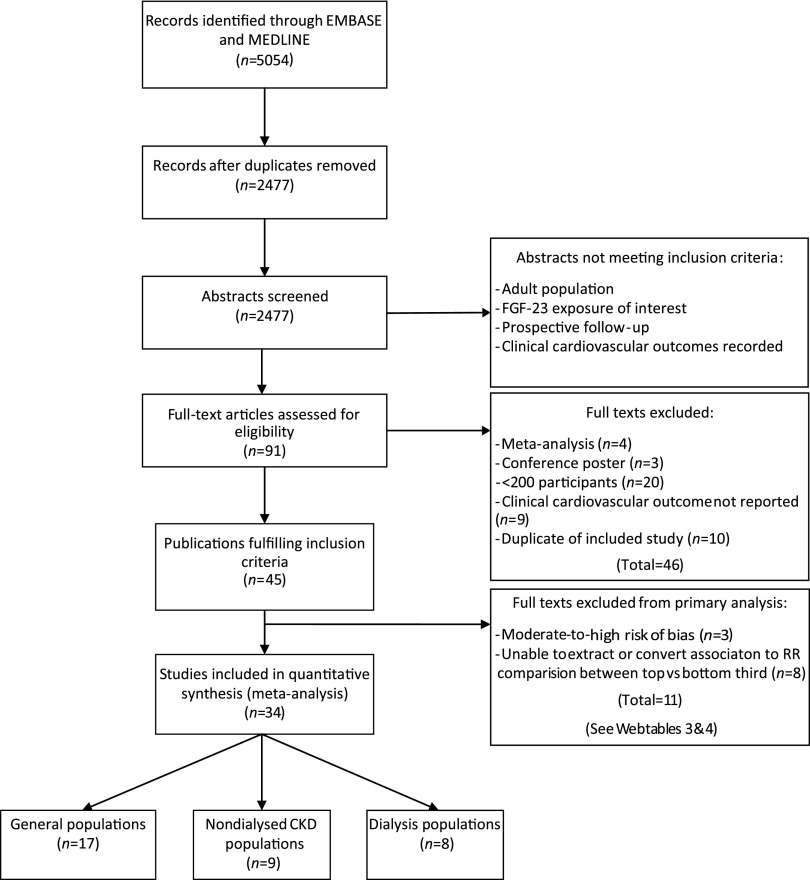
Our literature search (Supplemental Table 1) identified 2477 abstracts, of which 45 met the inclusion criteria (Figure 1). Three studies were excluded after a standard assessment for bias (Supplemental Table 3).27–29 Eight studies reported associations that could not be extracted or reliably expressed as RRs comparing the top versus bottom third of baseline FGF-23 concentration (see Supplemental Table 4 for results from these and the other excluded studies).30–37 Of 34 studies included in primary analyses, 17 were in predominantly general population cohorts,22,38–53 nine were in patients with CKD not on dialysis,23,54–61 and eight in patients on dialysis24,62–68 (Figure 1). For patients on dialysis, a single large trial (EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events [EVOLVE], n=2985) provided all the data on myocardial infarction, stroke, and heart failure (outcomes that were all confirmed by clinician adjudicators).24
Figure 1.
Study selection flowchart.
Table 1 describes the characteristics of included studies. Most of the studies (26 out of 34) measured FGF-23 concentrations using a C-terminal assay, with the remainder (eight out of 34) using an intact assay. Measures (median or, if unavailable, mean) of FGF-23 concentration were lowest in general population cohorts (43–74 RU/ml and 38–47 pg/ml for the respective assays); higher in nondialyzed CKD cohorts (102–392 RU/ml), and substantially higher in dialysis cohorts (79–4212 RU/ml and 2526–5555 pg/ml; Table 1).
Table 1.
Study and participant characteristics by population type
| Publication Author, Study Acronym | Study Location | No. of Participants;Follow-Up Duration | Baseline Demographics | Baseline Comorbidity Prevalences | FGF-23 Assay Type | Average FGF-23 Concentration |
| General population studies | ||||||
| Ärnlöv et al.,39 ULSAM | Uppsala, Sweden | 727; Median: 9.7 yr (range, 0.3–12.9) | Age: 78 yr | DM: 13% | Intact | Median 44 pg/ml (range, 9–162) |
| Men: 100% | eGFR: 74 (17) | |||||
| CVD: 27% | ||||||
| Ärnlöv et al.,38 PIVUS | Uppsala, Sweden | 1003; Median: 5.1 yr (range, 4.8–5.8) | Age: 70 yr | DM: 12% | Intact | Mean 47 pg/ml (SD 24) |
| Men: 50% | eGFR: 80 (14) | |||||
| White: 100% | CVD: 16% | |||||
| Brandenburg et al.,40 LURIC | Germany | 2974; Median: 9.9 yr | Age: 63 (10) yr | DM: 40% | C-terminal | Median 54 RU/ml (IQR, 40–78) |
| Men: 69% | eGFR<60: 14% | |||||
| White: 100% | CAD: 78% | |||||
| Deo et al.,41 CHS | USA | 3244; Mean: 8.1 yr (SD 3.2) | Age: 78 (5) yr | DM: 15% | C-terminal | Median 70 RU/ml (IQR, 53–99) |
| Men: 40% | eGFR: 71 (19) | |||||
| Black: 16% | HF: 9% MI: 11% | |||||
| di Giuseppe et al.,42 EPIC-Potsdam | Germany | 1443; Mean 8 yr (SD 2.2) | Age: 52 | DM: 7% | C-terminal | Median 48 RU/ml (IQR, NR) |
| Men: 44% | eGFR: NR | |||||
| White: NR | CAD: 10.8% | |||||
| di Giuseppe et al.,22 EPIC-Germany | Germany | 2908; Mean: 8.2 yr | Age: 52 | DM: 6% | C-terminal | Median 54 RU/ml (IQR, 38–72)a |
| Men: 50% | eGFR: 108 | |||||
| White: NR | Excluded MI and ST | |||||
| Garimella et al.,43 CHS | USA | 3143; Median: 9.8 yr | Age: NR | DM: NR | C-terminal | Median 71 RU/ml (IQR, 54–100) |
| Men: NR | eGFR: NR | |||||
| White: NR | CVD: NR | |||||
| Ix et al.,44 CHS | USA | 3107; Median: 10.5 yr (IQR, 5.9–11.5) | Age: 78 (5) yr | DM: 15% | C-terminal | Median 70 RU/ml (IQR, 53–99) |
| Men: 40% | eGFR: 71 (19) | |||||
| Black: 16% | CVD: 29% HF: 9% | |||||
| Kestenbaum et al.,45 MESA | USA | 6547; Median: 8.5 yr (IQR, 7.7–8.6) | Age: 62 yr | DM: 12% | Intact | Median 38 pg/ml (IQR, 31–46) Mean: 40 pg/ml (SD 15) |
| Men: 47% | eGFR: 84 (eGFR<60: 16%) | |||||
| White: 39% | CVD: 0% | |||||
| Lutsey et al.,46 ARIC | USA | 11,638; Median: 18.6 yr (maximum, 20.9) | Age: 57 yr | DM: 13% | Intact | Mean 44 pg/ml (SD 16) |
| Men: 43% | eGFR: 92 (eGFR<60: 3%) | |||||
| Black: 25% | CVD: 0% | |||||
| Masson et al.,47 PREDICTOR | Lazio, Italy | 1835; Mean: 3.8 yr | Age: 73 (5) yr | DM: 17% | C-terminal | Median 74 RU/ml (IQR, 58–97) |
| Men: 53% | Creatinine: 1.0 (0.3) mg/dl | |||||
| White: NR | CVD: 29% | |||||
| Panwar et al.,48 REGARDS | USA | 1551 (615 cases); Follow-up: NR | Age: 65 yr | DM: 21% | C-terminal | Median 70.5 RU/ml (IQR, 53–100) |
| Men: 45% | eGFR: 86.5 | |||||
| Black: 40% | CVD: 16% | |||||
| Parker et al.,49 HSS | San-Francisco, USA | 833; Median: 6.0 yr | Age: 67 (11) yr | DM: 27% | C-terminal | Median 43 RU/ml (IQR, 29–72) |
| Men: 81% | eGFR<60: 22% | |||||
| White: 60% | CVD: 100% | |||||
| Souma et al.,53 NOMAS | USA | 2525; Median: 14 yr | Age: 69 (10) yr | DM: 21% | C-terminal | Median 57 RU/ml (IQR, 44–81) |
| Men: 36% | eGFR: 80 (22) | |||||
| White: 21% | CVD: NR (no STs) | |||||
| Speer et al.50 | Saarland, Germany | 859; Median: 2.3 yr (IQR, 0.98–2.93) | Age: 64 yr | DM: 25% | C-terminal | Median 65 RU/ml (IQR, 45–115) |
| Men: 69% | Creatinine: 1.2 mg/dl (SD 0.8) | |||||
| White: NR | CAD: 43% HF: 86% | |||||
| Westerberg et al.,51 MrOS | Sweden | 2838; Mean: 4.5 yr | Age: 75.5 (3) yr | DM: 9% | Intact | Median 44 pg/ml (IQR, 32–58) |
| Men: 100% | eGFR: 72 (20) | |||||
| White: NR | CVD: 19% | |||||
| Wright et al.,52 NOMAS | USA | 2525; Mean: 12 yr (SD 5) | Age: 69 (10) yr | On glycemic agents: 15% | C-terminal | Median 57 RU/ml (IQR, 44–81) |
| Men: 36% | eGFR: 80 (22) | |||||
| White: 21% | CVD: NR (no STs) | |||||
| Nondialyzed CKD population studies | ||||||
| Alderson et al.,60 CRISIS | Salford, UK | 463; Median: 3.8 yr (IQR, 1.8–5.8) | Age: 64 (14) yr | DM: 31% | C-terminal | Median 209 RU/ml (IQR, 128–470) |
| Men: 62% | eGFR: 29 (15) | |||||
| White: 96% | CVD: 29% HF: 18% | |||||
| Baia et al.54 | Groningen, The Netherlands | 593; Median: 7.0 yr (IQR, 6.2–7.5) | Age: 52 (12) yr | DM: 18% | C-terminal | Median 140 RU/ml (IQR, 95–219) |
| Men: 54% | eGFR: 47 (16) | |||||
| White: 95% | CVD: NR | |||||
| Bouma-de Krijger et al.,23 MASTERPLAN | The Netherlands | 439; Follow-up: 2 yr | Age: 62 (12) yr | DM: 23% | C-terminal | Median 149 RU/ml (IQR, 87–241) |
| Men: 71% | eGFR: 36 (15) | |||||
| White: 93% | CVD: 27% | |||||
| Isakova et al.,55 CRIC | USA | 3879; 3.5 yr (IQR, 2.5–4.4) | Age: 58 (11) yr | DM: 48% | C-terminal | Median 146 RU/ml (IQR, 96–239) |
| Men: 55% | eGFR: 43 (14) | |||||
| Black: 42% | CAD: 22% HF: 10% | |||||
| Kendrick et al.,56 HOST | USA | 1099; Median: 2.9 yr Mean: 2.8 yr (SD 1.1) |
Age: 69 (11) yr | DM: 55% | C-terminal | Median 392 RU/ml (IQR, 216–945) |
| Men: 98% | eGFR: 18 (6) | |||||
| Black: 26% | CVD: 57% | |||||
| Levin et al.,57 CanPREDDICT | Canada | 2402; Median: 1 yr | Age: 68 (13) yr | DM: 48% | C-terminal | Median 237 RU/ml (IQR, 150–432) |
| Men: 63% | eGFR: 28 (9) | |||||
| White: 89% | CVD: NR | |||||
| Munoz-Mendoza et al.,61 CRIC | USA | 3875; Median: 6.9 yr (IQR, 4.2–8.2) | Age: 58 yr | DM: 48% | C-terminal | Median 146 RU/ml (IQR, 96–239) |
| Men: 55% | eGFR: 44 (15) | |||||
| Black: 42% | CAD: 22% HF: 10% | |||||
| Scialla et al.,58 CRIC | USA | 3860; Median: 3.7 yr (IQR, 2.5–4.7) | Age: 58 (11) yr | DM: 49% | C-terminal | Median 146 RU/ml (IQR, 96–239) |
| Men: 55% | eGFR: 44 (15) | |||||
| White: 42% | CVD: 31% | |||||
| Black: 41% | ||||||
| Seiler et al.,59 CARE FOR HOME | Hamburg, Germany | 444; Median: 2.6 yr (IQR, 1.4–3.6) | Age: 65 (12) yr | DM: 38% | C-terminal | Median 102 RU/ml (IQR, 64–164) |
| Men: 60% | eGFR: 45 (16) | |||||
| White: NR | Prevalent CVD: 30% | |||||
| Dialysis population studies | ||||||
| Chonchol et al.,62 HEMO | USA | 1340; Mean: 2.8 yr (SD 1.7) | Age: 57 (14) yr | Hemodialysis: 100% | Intact | Median 3118 pg/ml (IQR, 726–12,928) |
| Men: 45% | DM: 44% | |||||
| Black: 64% | CVD: 79% | |||||
| Jean et al.63 | France | 219; Median: 1.9 yr | Age: 67 (14) yr | Hemodialysis: 100% | C-terminal | Median 2740 RU/ml (IQR, 1192–8667) Mean: 7060 (SD 13,500) |
| Men: 57% | DM: 35% | |||||
| White: NR | CAD: 19% | |||||
| Kim et al.64 | South Korea | 205; Mean: 3.5 yr | Age: 47 (14) yr | PD: 100% | C-terminal | Median 79 RU/ml (IQR, 34–155) |
| Men: 60% | DM: 31% | |||||
| White: NR | CAD: 7% HF: 8% | |||||
| Moe et al.,24 EVOLVE | International | 2985; Median: 4.2 yr (IQR, 1.0–5.0) | Age: 54 yr | Hemodialysis: 100% | Intact (Millipore) | Median 5555 pg/ml (Q10–Q90, 580–19540) |
| Men: 59% | DM: 32% | |||||
| White: 58% | CVD: 95% HF: 23% | |||||
| Montford et al.,65 HOST | USA | 654; Median: 2.9 yr | Age: 60 (11) yr | Hemodialysis: 100% | C-terminal | Median 4212 RU/ml (IQR, 1411–13,816) |
| Men: 98% | DM: 41% | |||||
| White: 38% | CVD: 52% | |||||
| Nowak et al.66 | Germany | 239; Median: 2.5 yr (IQR, 2.0–2.7) | Age: 68 (14) yr | Hemodialysis: 100% | C-terminal | Mean 883 RU/ml (SD 1940) |
| Men: 64% | DM: 38% | |||||
| White: NR | CAD: 31% | |||||
| Olauson et al.67 | Sweden | 229; Median: 1.9 yr (range, 0.1–5) | Age: 55 yr | Hemodialysis: 41% | Intact | Median 2526 pg/ml (Q10–Q90, 431–19,495) |
| (IQR, 33–68) | PD: 54% | |||||
| Men: 65% | DM: 34% (as cause of ESRD) | |||||
| White: NR | CVD: 41% | |||||
| Scialla et al.,68 CHOICE | USA | 466; Median 3.4 yr (IQR, 1.8–5.9) | Age: 58 (15) yr | Hemodialysis: 100% | C-terminal | Median 1577 RU/ml (IQR, 818–4946) |
| Men: 55% | DM: 57% | |||||
| Black: 36% | CVD: 56% | |||||
Age and eGFR are mean (SD). ULSAM, Uppsala Longitudinal Study of Adult Men; DM, diabetes mellitus; CVD, cardiovascular disease; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors study; LURIC, Ludwigshafen Risk and Cardiovascular Health study; IQR, interquartile range; CAD, coronary artery disease; CHS, The Cardiovascular Health Study; HF, heart failure; MI, myocardial infarction; EPIC, European Prospective Investigation into Cancer and Nutrition; NR, not reported; ST, stroke; MESA, Multi-Ethnic Study of Atherosclerosis; ARIC, Atherosclerosis Risk in Communities Study; PREDICTOR, Valutazionedella PREvalenza di DIsfunzione CardiacaasinTOmatica e di scompensocaRdiaco; REGARDS, Reasons for Geographic and Racial Differences in Stroke; HSS, Heart and Soul Study; NOMAS, Stroke-free North Manhattan Study; MrOS, multicenter prospective Osteoporotic Fractures in Men study; CRISIS, Chronic Renal Insufficiency Standards Implementation Study; MASTERPLAN, Multifactorial approach and superior treatment efficacy in renal patients with the aid of nurse practitioners; CRIC, Chronic Renal Insufficiency Cohort; HOST, Homocysteine in Kidney and End Stage Renal Disease study; CanPREDDICT, Canadian study of prediction of death, dialysis and interim cardiovascular events; CARE FOR HOME, Cardiovascular And REnal outcome in CKD stage 2–4 patients—The FOuRth HOMburg evaluation; HEMO, The Hemodialysis Study; PD, peritoneal dialysis; EVOLVE, Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events; Q10, 10th percentile; Q90, 90th percentile; CHOICE, Choices for Healthy Outcomes in Caring for ESRD.
Approximated from median (IQR) of two mid quartiles.
Across these three populations, the estimated absolute difference in mean FGF-23 concentrations between the top and the bottom third of the FGF-23 distributions ranged from 72 RU/ml in general population studies, to 433 RU/ml in nondialyzed CKD studies, to 8644 RU/ml in dialysis population studies (C-terminal studies only).
It was notable that the ten general population cohorts had a mean age of 65 years or above (Table 1). The estimated crude mortality rates were, on average, high in all populations, with evidence of higher mortality with reduced kidney function. For example, the average all-cause mortality ranged from 1.9% to 5.3% per annum (p.a.) across the general populations, 2.0% to 14.2% p.a. in nondialyzed CKD populations, and 2.0% to 21.0% p.a. in dialysis populations.
Association between FGF-23 and Risk of Cardiovascular Events
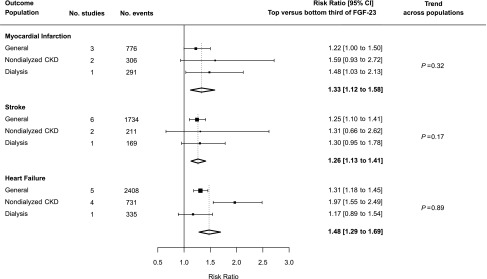
Six studies assessed the association between FGF-23 and risk of myocardial infarction (three in general populations,22,44,49 two in patients with CKD not on dialysis,44,56 and one in patients on dialysis24). Overall, comparing participants in the top versus bottom third of baseline FGF-23 concentration, there was a 33% increased risk of myocardial infarction (summary RR, 1.33; 95% confidence interval [95% CI], 1.12 to 1.58), but no evidence of linear trend across the different populations studied (trend P=0.32; Figure 2).
Figure 2.
Association between FGF-23 and risk of cardiovascular disease event by population type. Heterogeneity tests across the summary RRs for the three outcomes: all populations combined, P=0.23; general populations, P=0.59; nondialyzed CKD populations, P=0.75; and dialysis populations, P=0.47.
For the studies reporting an interquartile range of baseline FGF-23 concentrations, there was good correlation between median baseline FGF-23 concentration and the interquartile range (correlation coefficient, 0.99), so ordering studies by increasing baseline FGF-23 concentration effectively orders the studies by increasing absolute difference between the means of FGF-23 concentrations in the top versus bottom third of each study’s FGF-23 distribution. Tests for linear trend in the RRs for myocardial infarction across the ordered studies were nonsignificant both within the three separate populations and across all individual studies (trend across all individual studies P=0.22; Supplemental Figure 1).
Associations between FGF-23 and risk of stroke of any type were reported in nine studies, including six in general populations,22,44,45,48,49,52 two in nondialyzed CKD populations,44,56 and one in dialysis populations.24 Overall, comparing those in the top versus the bottom third of baseline FGF-23 concentration, there was a 26% increased risk of stroke (RR, 1.26; 95% CI, 1.13 to 1.41). This increase in risk was consistent between populations (trend P=0.17; Figure 2), and there was no significant trend toward larger RRs with higher median FGF-23 difference both within each population considered separately (where relevant) and across all studies (trend across all individual studies P=0.95; Supplemental Figure 2).
Four general population studies (n=1251 events)22,45,48,52 and a small nondialyzed CKD study (n=43 events)56 reported ischemic stroke events. Overall, no significant association between FGF-23 and risk of ischemic stroke was observed for the top versus the bottom third of baseline FGF concentration (RR, 1.08; 95% CI, 0.92 to 1.27; Supplemental Figure 3).
Associations between FGF-23 and risk of heart failure were reported in ten studies, including five in a general population,42,44–46,49 four in patients with nondialyzed CKD,23,44,58,59 and one in patients on dialysis.24 Overall, comparing patients in the top versus the bottom third of baseline FGF-23 concentration, there was a 48% increased risk of heart failure (RR, 1.48; 95% CI, 1.29 to 1.69). There was no evidence of trend across populations (trend P=0.89; Figure 2) and no clear trend toward larger RRs with higher median FGF-23 difference both within each population considered separately and overall (trend across all individual studies P=0.76; Supplemental Figure 4). There was also no good evidence that FGF-23 was more strongly associated with heart failure than myocardial infarction or stroke, overall (heterogeneity P=0.23) or in any of the three separate populations (Figure 2).
Associations between FGF-23 and risk of peripheral artery disease and some other noted cardiovascular outcomes are provided in Supplemental Table 5.
Association between FGF-23 and Mortality
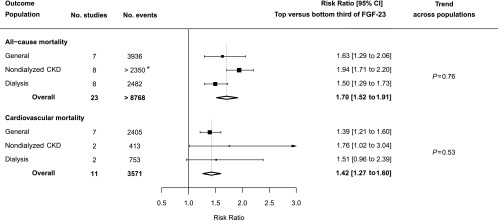
Twenty-three studies reported associations between FGF-23 and all-cause mortality: seven in a general population,39,40,44,47,49,51,53 eight in a nondialyzed CKD population,23,44,54,56,57,59–61 and eight in a dialysis population.24,62–68 Overall, comparing participants in the top versus bottom third of baseline FGF-23 concentration, there was an increased risk of death from all causes (RR, 1.70; 95% CI, 1.52 to 1.91). There was no good evidence of a trend across the three populations (trend P=0.76; Figure 3) or toward larger RRs with higher median FGF-23 at baseline (trend across all individual studies P=0.97; Supplemental Figure 5).
Figure 3.
Association between FGF-23 and risk of all-cause and cardiovascular mortality by population type. *Number of events not reported for one study.
Eleven studies reported associations between FGF-23 level and cardiovascular mortality (seven studies in general populations,39,40,44,46,47,51,53 two in nondialyzed CKD populations,44,54 and two in dialysis populations24,68). Overall, comparing patients in the top versus bottom third of the baseline FGF-23 concentration, there was a 42% increased risk of cardiovascular mortality (RR, 1.42; 95% CI, 1.27 to 1.60) with no evidence of trend across populations (trend P=0.53; Figure 3) and no trend toward larger RRs with higher median FGF-23 (trend across all individual studies P=0.49; Supplemental Figure 6).
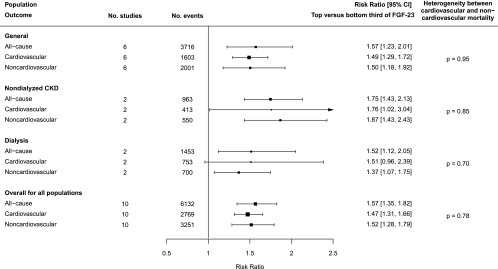
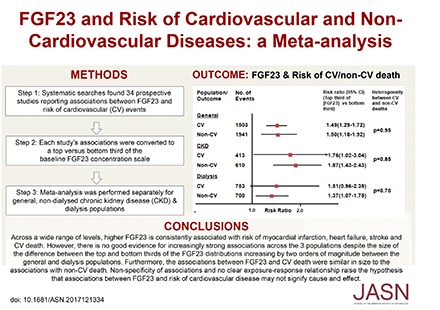
Among patients on dialysis in the EVOLVE trial,24 comparing patients in the top versus the bottom third of the baseline FGF-23 concentration, there was a 27% (RR, 1.27; 95% CI, 1.02 to 1.58) increased risk of noncardiovascular mortality (n=514 deaths), which was similar to the RR for cardiovascular mortality (RR, 1.26; 95% CI, 1.00 to 1.57; n=607 deaths). Only one of the other nine studies (a general population cohort) reported RRs for cardiovascular mortality (RR, 1.76; 95% CI, 1.34 to 2.32; n=474) as well as for noncardiovascular mortality (RR, 1.47; 95% CI, 1.17 to 1.85; n=612 deaths).53 For the remaining eight studies, RRs for noncardiovascular mortality were derived indirectly, using associations for cardiovascular and all-cause mortality.39,40,44,46,47,51,54,68 The overall combined RR for all studies for noncardiovascular mortality for the top versus the bottom third of the baseline FGF-23 concentration was 1.52 (95% CI, 1.28 to 1.79), with results suggesting that for each of the separate populations, the RRs for cardiovascular and noncardiovascular mortality were comparable (Figure 4).
Figure 4.
Association between FGF-23 concentration and risk of cause-specific mortality overall and by population type.
Sensitivity Analyses and Assessment for Publication Bias
The results of trend tests remained nonsignificant after exclusion of studies on patients on dialysis (Supplemental Figures 1, 2, and 4– 6), after exclusion of studies that only reported intact FGF-23 concentrations, and after using a formula for interassay conversion.21 Repeat measurements within groups of FGF-23 were highly correlated in all three types of populations studied (regression dilution ratios all >0.8, Supplemental Table 6),22–24 so adjustment for regression-dilution bias was not performed. All-cause and cardiovascular mortality associations were not substantially affected by adjustment for other markers of CKD–mineral bone disorder (Supplemental Figure 7).40,46,49,54,55,59,66
Funnel plots of associations between FGF-23 and all-cause mortality by type of population suggested evidence for publication bias for the general population cohorts (Egger regression test P=0.005) and that RRs for all-cause mortality may be slight overestimates (Supplemental Figures 5 and 8). There was no important heterogeneity between studies with respect to other outcomes (Supplemental Figures 1– 4 and 6).
Discussion
This systematic review and meta-analysis assessed the epidemiologic associations between FGF-23 concentration and cardiovascular outcomes, as well as associations with cardiovascular and all-cause mortality in populations with and without known kidney disease. Overall, we found that, irrespective of a population’s level of kidney function, a difference in FGF-23 concentration corresponding to that between top and bottom thirds of baseline FGF-23 concentration was associated with about 30% increased risk of myocardial infarction and stroke, 40% increased risk of cardiovascular mortality, and 50% increased risk of heart failure. In the studies where it was possible to estimated effects on both cardiovascular and noncardiovascular mortality, we found that the strength of the association between FGF-23 and these categories of deaths was approximately similar.
The Bradford Hill criteria for causality of a disease risk factor includes the presence of epidemiologic associations that are both consistent and specific for that disease, evidence of a biologic gradient (i.e., greater exposure leads to increased effect, which we refer to as exposure-response), temporality (i.e., the cause precedes the effect), and biologic plausibility.69
In support of raised FGF-23 concentration being a cause of cardiovascular disease, our study found consistent moderate associations between FGF-23 and disease risks. FGF-23 concentration also rises before any other marker of CKD–mineral bone disorder,10 so it temporally mirrors the rise in cardiovascular risk as CKD progresses.1 In addition, there is biologic plausibility because cardiac myocytes exposed to FGF-23 become hypertrophied and develop electrophysiologic disturbances (sometimes referred to as “off-target” effects as they appear to be Klotho-independent).13–15
We also observed that FGF-23 was strongly associated with noncardiovascular causes of death, reflecting a lack of specificity of the associations between raised FGF-23 concentration and disease risk. This observation could reflect the pleiotropy of FGF-23 in disease causation. It has previously been reported that raised FGF-23 concentration is associated with a higher risk of ESRD,55 AKI (RR for top versus bottom quartile, 1.99; 95% CI, 1.04 to 3.80),70 fractures (RR, 1.56; 95% CI, 1.11 to 2.20),71 and serious infection (RR, 1.59; 95% CI, 1.14 to 2.22).62 There is emerging evidence that FGF-23 may promote inflammation through direct effects on hepatocytes,72 and predispose to infection through downregulation of monocytic expression of 1,25 dihydroxycholecalciferol73 or other effects.74 A mechanistic study has also suggested that FGF-23 may promote progression of prostate cancer.75
An alternative, more plausible explanation for the observed nonspecificity of associations across a range of disease outcomes is residual confounding. This may arise because of imprecise or incomplete measurement of baseline prognostic factors other than FGF-23. Examples of such factors include level of kidney function (which is measured with greater error at high eGFR), duration of CKD, and risk factors that correlate with low kidney function.
Furthermore, we found no evidence for a log-linear exposure-response relationship such as that which is commonly observed for known causes of cardiovascular disease (e.g., LDL cholesterol76,77 and BP78). Indeed, the RRs corresponding to a difference between top and bottom thirds of FGF-23 distribution were of similar magnitude in each of the three populations despite the absolute difference in FGF-23 varying by two orders of magnitude across these populations. Such a pattern could potentially be explained by a “log-log” relationship with flattening of the exposure-response curve at high FGF-23 concentration. But this shape of association would imply that, if FGF-23 is a cause of cardiovascular disease, therapeutic agents designed to reduce FGF-23 would need to achieve large absolute reductions in FGF-23 in those with high levels to achieve worthwhile risk reductions.
A limitation of this meta-analysis is that we were, for the most part, restricted to published summary data. The availability of individual participant-level data from all eligible studies could allow for more granular estimation of associations and perhaps a more sensitive analysis of any exposure-response relationship using a standardized method with fewer assumptions. It would also allow for the inclusion of the studies that could not be reliably converted onto a top versus bottom third scale. However, the studies excluded because of inability to convert associations showed positive associations between FGF-23 and disease risks that were similar in size to those observed by the included studies (Supplemental Table 4).30–37 Furthermore, given the lack of trends across the three population types despite a two-fold increase in the absolute difference in FGF-23 concentration, it is unlikely that individual participant data would identify an important log-linear trend missed by our tabular meta-analysis. Individual participant-level data would also not overcome residual confounding, which is the main limitation of this meta-analysis. Finally, not all relevant studies reported associations for all outcomes of interest (which may have introduced bias) and there was a lack of detailed data on noncardiovascular causes of death, so it was not possible to examine whether there were deaths (e.g., from cancer) that were particularly strongly associated with FGF-23.
In summary, this systematic review and meta-analysis has demonstrated that across a wide range of levels of kidney function, higher FGF-23 concentration was consistently associated with modest increased risks of myocardial infarction, heart failure, stroke, and cardiovascular death. However, higher FGF-23 was also associated with an increased risk of noncardiovascular causes of death. Our findings suggest that associations between FGF-23 and particular diseases, both in populations with CKD and those without known disease, may not signify cause and effect.
Disclosures
The Medical Research Council Population Health Research Unit and Clinical Trial Service Unit and Epidemiological Studies Units, which are part of the Nuffield Department of Population Health, University of Oxford, have a staff policy of not accepting honoraria or consultancy fees except for reimbursement of expenses to attend scientific meetings. R.H. reports grants from Novartis Pharma AG. D.C.W. reports honoraria and/or consultant fees from Amgen, Akebia, Boehringer Ingelheim, Johnson and Johnson, and Vifor Fresenius. S.M.M. reports grants from the National Institutes of Health, Veterans Administration, and Chugai pharmaceuticals outside the submitted work and a grant and position (scientific advisory committee) from Amgen. M.J.L. reports grants from UK Medical Research Council, British Heart Foundation, Cancer Research UK, National Institute for Health Research, UK Biobank, Wyeth, Novartis, National Health Institute Blood and Transplantation, and Merck outside the submitted work. C.B. reports grants from UK Medical Research Council, John Wyeth & Brother Ltd. (now Pfizer), Novartis, Bayer Germany, Boehringer Ingelheim, British Heart Foundation, and Cancer Research UK outside the submitted work. W.G.H. reports grants from the British Heart Foundation and Boehringer Ingelheim outside the submitted work. J.Y. is an Amgen employee. A.M., K.D., C.M.R., L.H., R.d.G., A.B.-d.K., and B.M. have nothing to disclose. The EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) study provided new analyses for this manuscript and was funded by Amgen.
Supplementary Material
Acknowledgments
We thank Dr. Bastian Dehmel (Amgen Inc.) for supporting re-analysis of the EVOLVE data, Dr. Serge Masson (Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy, on behalf of the investigators of the PREDICTOR [Valutazione della PREvalenza di DIsfunzione Cardiaca asinTOmatica e di scompenso caRdiaco conclamato in un campione di popolazione di età ≥ 65 anni nel Lazio] study), and Prof. Joachim Ix for providing previously unreported results.
Study concept: B.M., W.G.H., and R.H.; literature search: C.M.R., A.M., B.M., K.D., and L.H.; provision of data: D.C.W., S.M.M., R.d.G., and A.B.-d.K.; statistical analysis specification: B.M., W.G.H., and A.M.; statistical analyses: A.M., K.D., J.Y., and B.M.; first draft of manuscript: W.G.H., B.M., and A.M.; revision: all authors.
This study was supported by the Medical Research Council UK, which provides support for the Medical Research Council Population Health Research Unit at the University of Oxford, and by the British Heart Foundation. W.G.H. is supported by a Medical Research Council and Kidney Research UK Professor David Kerr Clinician Scientist Award.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121334/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, et al. : Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. ; Chronic Kidney Disease Prognosis Consortium: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE: Long-term evolution of cardiomyopathy in dialysis patients. Kidney Int 54: 1720–1725, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Wheeler DC, London GM, Parfrey PS, Block GA, Correa-Rotter R, Dehmel B, et al. ; EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) Trial Investigators: Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: The EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc 3: e001363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, et al. : Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. : Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 9.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al. : Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, et al. : Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int 89: 140–150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wahl P, Wolf M:FGF23 in chronic kidney disease. In: Endocrine FGFs and Klothos, edited by Kuro-o M, Georgetown, TX, Landes Bioscience and Springer Science, 2012, pp 107–125 [Google Scholar]
- 13.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. : FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, et al. : Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant 31: 1088–1099, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutiérrez OM: Connecting the dots on fibroblast growth factor 23 and left ventricular hypertrophy. Nephrol Dial Transplant 31: 1031–1033, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. : The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Third Symposium on Systematic Reviews: Beyond the Basics. Oxford, July 2000. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 30, 2018
- 17.Danesh J, Collins R, Appleby P, Peto R: Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA 279: 1477–1482, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Mafham M, Emberson J, Landray MJ, Wen CP, Baigent C: Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: A meta-analysis. PLoS One 6: e25920, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper H, Hedges LV, Valentine JC, editors: The Handbook of Research Synthesis and Meta-Analysis, New York, Russell Sage Foundation, 2009 [Google Scholar]
- 20.Smith ER, Cai MM, McMahon LP, Holt SG: Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab 97: 3357–3365, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 22.di Giuseppe R, Kühn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, et al. : Plasma fibroblast growth factor 23 and risk of cardiovascular disease: Results from the EPIC-Germany case-cohort study. Eur J Epidemiol 30: 131–141, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Bouma-de Krijger A, Bots ML, Vervloet MG, Blankestijn PJ, Ter Wee PW, van Zuilen AD, et al. : Time-averaged level of fibroblast growth factor-23 and clinical events in chronic kidney disease. Nephrol Dial Transplant 29: 88–97, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Moe SM, Chertow GM, Parfrey PS, Kubo Y, Block GA, Correa-Rotter R, et al. ; Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial Investigators: Cinacalcet, fibroblast growth factor-23, and cardiovascular disease in hemodialysis: The Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Circulation 132: 27–39, 2015 [DOI] [PubMed] [Google Scholar]
- 25.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. : Blood pressure, stroke, and coronary heart disease. Part 1, prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 335: 765–774, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Cochran WG: Some methods for strengthening the common c2 tests. Biometrics 10: 417–451, 1954 [Google Scholar]
- 27.Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O’Donnell TF, et al. : Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol 63: 2421–2428, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano C, Hamano T, Fujii N, Obi Y, Matsui I, Tomida K, et al. : Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone 50: 1266–1274, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Prié D, Forand A, Francoz C, Elie C, Cohen I, Courbebaisse M, et al. : Plasma fibroblast growth factor 23 concentration is increased and predicts mortality in patients on the liver-transplant waiting list. PLoS One 8: e66182, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. : Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC: Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J 161: 956–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba RD, Fink JC, Sun K, Cappola AR, Dalal M, Crasto C, et al. : Serum fibroblast growth factor-23 and risk of incident chronic kidney disease in older community-dwelling women. Clin J Am Soc Nephrol 7: 85–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JE, Gohda T, Walker WH, Skupien J, Smiles AM, Holak RR, et al. : Risk of ESRD and all cause mortality in type 2 diabetes according to circulating levels of FGF-23 and TNFR1. PLoS One 8: e58007, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuñón J, Cristóbal C, Tarín N, Aceña Á, González-Casaus ML, Huelmos A, et al. : Coexistence of low vitamin D and high fibroblast growth factor-23 plasma levels predicts an adverse outcome in patients with coronary artery disease. PLoS One 9: e95402, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Söderholm M, Engström G: Fibroblast growth factor 23 and incidence of subarachnoid hemorrhage: Nested case-control study. Stroke 46: 3260–3262, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Fyfe-Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, et al. : Serum fibroblast growth factor-23 and incident hypertension: The Atherosclerosis Risk in Communities (ARIC) study. J Hypertens 34: 1266–1272, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langsford D, Tang M, Cheikh Hassan HI, Djurdjev O, Sood MM, Levin A: The Association between biomarker profiles, etiology of chronic kidney disease, and mortality. Am J Nephrol 45: 226–234, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, et al. : Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin J Am Soc Nephrol 8: 781–786, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, et al. : Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 83: 160–166, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Brandenburg VM, Kleber ME, Vervloet MG, Tomaschitz A, Pilz S, Stojakovic T, et al. : Fibroblast growth factor 23 (FGF23) and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis 237: 53–59, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Deo R, Katz R, de Boer IH, Sotoodehnia N, Kestenbaum B, Mukamal KJ, et al. : Fibroblast growth factor 23 and sudden versus non-sudden cardiac death: The Cardiovascular Health Study. Am J Kidney Dis 66: 40–46, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.di Giuseppe R, Buijsse B, Hirche F, Wirth J, Arregui M, Westphal S, et al. : Plasma fibroblast growth factor 23, parathyroid hormone, 25-hydroxyvitamin D3, and risk of heart failure: A prospective, case-cohort study. J Clin Endocrinol Metab 99: 947–955, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Garimella PS, Ix JH, Katz R, Chonchol MB, Kestenbaum BR, de Boer IH, et al. : Fibroblast growth factor 23, the ankle-brachial index, and incident peripheral artery disease in the Cardiovascular Health Study. Atherosclerosis 233: 91–96, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, et al. : Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, et al. : Fibroblast growth factor-23 and cardiovascular disease in the general population: The Multi-Ethnic Study of atherosclerosis. Circ Heart Fail 7: 409–417, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, et al. : Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: The Atherosclerosis Risk in Communities study. J Am Heart Assoc 3: e000936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masson S, Agabiti N, Vago T, Miceli M, Mayer F, Letizia T, et al. ; Investigators of the PREDICTOR study: The fibroblast growth factor-23 and Vitamin D emerge as nontraditional risk factors and may affect cardiovascular risk. J Intern Med 277: 318–330, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Panwar B, Jenny NS, Howard VJ, Wadley VG, Muntner P, Kissela BM, et al. : Fibroblast growth factor 23 and risk of incident stroke in community-living adults. Stroke 46: 322–328, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, et al. : The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speer T, Groesdonk HV, Zapf B, Buescher V, Beyse M, Duerr L, et al. : A single preoperative FGF23 measurement is a strong predictor of outcome in patients undergoing elective cardiac surgery: A prospective observational study. Crit Care 19: 190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westerberg PA, Tivesten Å, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, et al. : Fibroblast growth factor 23, mineral metabolism and mortality among elderly men (Swedish MrOs). BMC Nephrol 14: 85, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MS, et al. : Plasma FGF23 and the risk of stroke: The Northern Manhattan Study (NOMAS). Neurology 82: 1700–1706, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souma N, Isakova T, Lipiszko D, Sacco RL, Elkind MS, DeRosa JT, et al. : Fibroblast growth factor 23 and cause-specific mortality in the general population: The Northern Manhattan study. J Clin Endocrinol Metab 101: 3779–3786, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baia LC, Humalda JK, Vervloet MG, Navis G, Bakker SJ, de Borst MH; NIGRAM Consortium: Fibroblast growth factor 23 and cardiovascular mortality after kidney transplantation. Clin J Am Soc Nephrol 8: 1968–1978, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Group: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kendrick J, Cheung AK, Kaufman JS, Greene T, Roberts WL, Smits G, et al. ; HOST Investigators: FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol 22: 1913–1922, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levin A, Rigatto C, Barrett B, Madore F, Muirhead N, Holmes D, et al. ; CanPREDDICT Investigators: Biomarkers of inflammation, fibrosis, cardiac stretch and injury predict death but not renal replacement therapy at 1 year in a Canadian chronic kidney disease cohort. Nephrol Dial Transplant 29: 1037–1047, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, et al. ; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators: Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol 25: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seiler S, Rogacev KS, Roth HJ, Shafein P, Emrich I, Neuhaus S, et al. : Associations of FGF-23 and sKlotho with cardiovascular outcomes among patients with CKD stages 2-4. Clin J Am Soc Nephrol 9: 1049–1058, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alderson HV, Ritchie JP, Middleton R, Larsson A, Larsson TE, Kalra PA: FGF-23 and osteoprotegerin but not fetuin-A are associated with death and enhance risk prediction in non-dialysis chronic kidney disease stages 3-5. Nephrology (Carlton) 21: 566–573, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Munoz Mendoza J, Isakova T, Cai X, Bayes LY, Faul C, Scialla JJ, et al. ; CRIC Study Investigators: Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int 91: 711–719, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK: Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol 27: 227–237, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, et al. : High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24: 2792–2796, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Kim HJ, Park M, Park HC, Jeong JC, Kim DK, Joo KW, et al. : Baseline FGF23 is associated with cardiovascular outcome in incident PD patients. Perit Dial Int 36: 26–32, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montford JR, Chonchol M, Cheung AK, Kaufman JS, Greene T, Roberts WL, et al. ; HOST Investigators: Low body mass index and dyslipidemia in dialysis patients linked to elevated plasma fibroblast growth factor 23. Am J Nephrol 37: 183–190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowak A, Friedrich B, Artunc F, Serra AL, Breidthardt T, Twerenbold R, et al. : Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in hemodialysis patients. PLoS One 9: e100688, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olauson H, Qureshi AR, Miyamoto T, Barany P, Heimburger O, Lindholm B, et al. : Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: Are gender and cardiovascular disease confounding the relationship? Nephrol Dial Transplant 25: 3033–3038, 2010 [DOI] [PubMed] [Google Scholar]
- 68.Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, et al. : Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 42: 25–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill AB: The environment and disease: Association or causation? Proc R Soc Med 58: 295–300, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown JR, Katz R, Ix JH, de Boer IH, Siscovick DS, Grams ME, et al. : Fibroblast growth factor-23 and the long-term risk of hospital-associated AKI among community-dwelling older individuals. Clin J Am Soc Nephrol 9: 239–246, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirza MA, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, Ljunggren O, et al. : Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res 26: 857–864, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. : Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 90: 985–996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nowak KL, Bartz TM, Dalrymple L, de Boer IH, Kestenbaum B, Shlipak MG, et al. : Fibroblast growth factor 23 and the risk of infection-related hospitalization in older adults. J Am Soc Nephrol 28: 1239–1246, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rossaint J, Unruh M, Zarbock A: Fibroblast growth factor 23 actions in inflammation: A key factor in CKD outcomes. Nephrol Dial Transplant 32: 1448–1453, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Feng S, Wang J, Zhang Y, Creighton CJ, Ittmann M: FGF23 promotes prostate cancer progression. Oncotarget 6: 17291–17301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. ; Prospective Studies Collaboration: Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 370: 1829–1839, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration: Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376: 1670–1681, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration: Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.