Abstract
Background Most patients on hemodialysis are treated thrice weekly even if they have residual kidney function, in part because uncertainty remains as to how residual function should be valued and incorporated into the dialysis prescription. Recent guidelines, however, have increased the weight assigned to residual function and thus reduced the treatment time required when it is present. Increasing the weight assigned to residual function may be justified by knowledge that the native kidney performs functions not replicated by dialysis, including solute removal by secretion. This study tested whether plasma concentrations of secreted solutes are as well controlled in patients with residual function on twice weekly hemodialysis as in anuric patients on thrice weekly hemodialysis.
Methods We measured the plasma concentration and residual clearance, dialytic clearance, and removal rates for urea and the secreted solutes hippurate, phenylacetylglutamine, indoxyl sulfate, and p-cresol sulfate in nine patients on twice weekly hemodialysis and nine patients on thrice weekly hemodialysis.
Results Compared with anuric patients on thrice weekly dialysis with the same standard Kt/Vurea, patients on twice weekly hemodialysis had lower hippurate and phenylacetylglutamine concentrations and similar indoxyl sulfate and p-cresol sulfate concentrations. Mathematical modeling revealed that residual secretory function accounted for the observed pattern of solute concentrations.
Conclusions Plasma concentrations of secreted solutes can be well controlled by twice weekly hemodialysis in patients with residual kidney function. This result supports further study of residual kidney function value and the inclusion of this function in dialysis adequacy measures.
Keywords: hemodialysis, uremia, urea modelling
The majority of patients on hemodialysis receive treatment thrice weekly. There is growing interest, however, in the efficacy of twice weekly hemodialysis for patients with residual kidney function.1–5 Observational studies have provided evidence for the value of residual function. An early study showed that an increase in urea clearance provided by residual function conveyed a greater survival advantage than the same increase in urea clearance provided exclusively by peritoneal dialysis.6 Studies of hemodialysis have recently focused on initiating treatment twice weekly, when residual function is still present. These studies suggest that such incremental treatment is associated with no greater mortality and possibly better quality of life than thrice weekly treatment.5,7–9
Residual function provides several benefits that may allow twice weekly hemodialysis to be effective.10–12 Residual function facilitates control of extracellular fluid volume and inorganic ion concentrations. It may also limit the accumulation of uremic waste solutes. Residual function may be particularly important for removal of waste solutes that are normally cleared by tubular secretion, a function which is not replicated by hemodialysis but can remain active in the residual kidney.13–18
One impediment to prescribing twice weekly treatment for patients with residual function is uncertainty as to how residual function should be incorporated into the dialysis prescription. However, the 2015 Update of the Kidney Disease Outcomes Quality Initiative (KDOQI) Guideline for Hemodialysis Adequacy19 assigned increased weight to residual function in calculating the adequacy index standard Kt/Vurea (stdKt/Vurea), and thus reduced the treatment time required when it is present. Increasing the weight assigned to residual function may be justified by knowledge that the native kidney performs functions not replicated by dialysis, including solute removal by secretion. This study tested the hypothesis that plasma concentrations of such solutes are as well controlled in patients with residual function receiving twice weekly hemodialysis as in anuric patients receiving thrice weekly hemodialysis.
Methods
Informed consent was obtained from nine patients with residual function maintained on twice weekly hemodialysis and nine anuric patients maintained on thrice weekly hemodialysis. Local nephrologists were asked to identify patients for whom they were prescribing twice weekly hemodialysis. Recruitment was on the basis of twice weekly treatment without regard to the extent of residual kidney function or calculated adequacy measures. Eleven patients were identified, of whom nine agreed to participate. Thrice weekly patients were recruited if they were anuric and stably maintained on treatment providing adequate single-pool Kt/Vurea (spKt/Vurea) per guidelines. The patients’ regular prescriptions were not altered. The study was approved by the Stanford Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
The twice weekly patients received treatment on Monday/Friday or Tuesday/Saturday. A post-treatment blood sample was collected at the end of a Friday or Saturday session and urine was collected over the interdialytic interval until the next session. Pre- and post-treatment blood samples and waste dialysate were collected at that session. In the anuric thrice weekly patients, pre- and post-treatment blood samples and waste dialysate were collected during a midweek session. Five of these patients had been included in a prior study.17 In both groups, the entire spent dialysate volume was collected and post-treatment blood samples were collected by standard methods used for adequacy measurements. Concentrations of the secreted solutes hippurate, phenylacetylglutamine, indoxyl sulfate, and p-cresol sulfate were measured by liquid chromatography–tandem mass spectrometry, as previously described.17 Urea was measured by an enzymatic assay. Free, unbound solute fractions were calculated as the concentrations in plasma ultrafiltrate divided by the concentrations in total plasma.
Calculations
Values for spKt/Vurea and other urea kinetic parameters were calculated with the web-based Solute-Solver program developed by Daugirdas et al.20 Inputs included the pre- and post-treatment plasma urea concentrations, pre- and post-treatment weights, treatment schedule, and the treatment time, blood flow, dialysate flow, and dialyzer mass transfer area coefficient. Two values for the adequacy parameter stdKt/Vurea were obtained to reveal differences in the weight assigned to residual function in assessing dialysis adequacy.21 The first of these is denominated the “2015 KDOQI stdKt/Vurea.” It is the stdKt/Vurea value provided by Solute-Solver and incorporates the increased weight assigned to residual function in the 2015 Update of the KDOQI Guideline. The second of these is denominated the “original stdKt/Vurea” and was calculated as described in the Supplemental Material. It represents the value for stdKt/Vurea originally defined by Gotch22 and incorporated in the 2006 KDOQI Guideline.23
Residual solute clearances were calculated as the amount of solute in the interdialytic urine divided by the average of the plasma concentrations at the beginning and end of the collection period. The dialytic clearances for the secreted solutes were calculated as the amount in the dialysate divided by the logarithmic mean of the pre- and post-treatment plasma concentrations. Generation rates for the secreted solutes were obtained by mathematical modeling, assuming constant generation and residual clearance rates. Input values were the dialysis schedule and the values for residual clearance, dialytic clearance, pretreatment plasma concentration, and volume of distribution for each solute. Volumes of distribution for the secreted solutes were estimated as the amount of solute removed in the dialysate divided by the difference between the pre- and post-treatment plasma concentrations. Modeling was performed using Matlab (v2016b).
The contribution of residual function to the control of plasma concentrations of urea and the secreted solutes was further assessed by modeling. The fraction of solute removed by residual clearance was calculated as the urinary removal rate divided by the generation rate. The urinary removal rate for each solute was calculated as the residual clearance multiplied by the time-averaged concentration (TAC). TAC values for the secreted solutes were obtained using the model described above. Because measured pretreatment plasma solute concentrations are affected by the day on which samples are collected, the model was also used to obtained average pretreatment concentrations. The treatment times required for each of two weekly sessions to achieve a stdKt/Vurea of 2.2, using the 2015 and 2006 formulations of stdKt/Vurea, were also obtained by modeling as further described in the Supplemental Material.
Statistical Methods
Comparisons between twice weekly and thrice weekly values were performed using the Mann–Whitney U test. The ratios of the residual solute clearance to residual urea clearance and the ratio of the dialytic solute clearance to dialytic urea clearance were compared using the Wilcoxon signed rank test. The fractions of each solute removed by residual clearance in the twice weekly group were compared with ANOVA, with significance of differences between the secreted solutes and urea assessed by Dunnett test. Statistical analysis was performed with SPSS (v24).
Results
Characteristics of the patients are summarized in Table 1. Dialysis vintage was lesser in the twice weekly patients than the thrice weekly patients (1.3±1.0 versus 3.6±1.9 years). The twice weekly and thrice weekly patients had been on their respective treatment schedules since initiating dialysis. Monthly laboratory values for potassium, phosphorus, albumin, and hemoglobin obtained from unit records were similar in the two groups.
Table 1.
Patient characteristics
| Characteristic | Twice Weekly | Thrice Weekly | P Value |
|---|---|---|---|
| Age, yr | 74±10 | 66±11 | 0.14 |
| Dialysis vintage, yr | 1.3±1.0 | 3.6±1.9 | <0.01 |
| No. of DM | 7 | 5 | — |
| Sex (men/women) | 5/4 | 9/0 | — |
| Weight, kg | 73±12 | 84±20 | 0.39 |
| Potassium, mEq/L | 4.8±0.3 | 4.9±0.5 | 0.93 |
| Phosphorus, mg/dl | 5.9±0.9 | 6.2±2.1 | 1.0 |
| Albumin, g/dl | 3.8±0.6 | 3.8±0.4 | 0.73 |
| Hemoglobin, g/dl | 10.8±0.9 | 10.9±1.1 | 0.61 |
Results are mean±SD. DM, diabetes mellitus; —, no data.
Residual function, prescription parameters, and measures of dialysis adequacy are summarized in Table 2. The twice weekly patients had an average residual urea clearance of 2.8±1.5 ml/min and an average urine output of 832±422 ml/d. Their weekly ultrafiltration volume was less than that of the thrice weekly patients and their average weekly treatment time was less than two thirds that of the thrice weekly patients. The single treatment spKt/Vurea values were similar in the two groups despite the lower dialysate flow in the twice weekly group. Values for both the 2015 KDOQI and original stdKt/Vurea were also similar in the two groups. In the twice weekly patients; however, the 2015 KDOQI stdKt/Vurea values were significantly higher than the original stdKt/Vurea values (2.53±0.53 versus 2.25±0.39; P<0.01), reflecting the increased weight given to residual clearance in the 2015 Update of the KDOQI Guideline.
Table 2.
Residual function, dialysis prescription parameters, and kinetic measures
| Parameter | Twice Weekly | Thrice Weekly | P Value |
|---|---|---|---|
| Kru, ml/min | 2.8±1.5 | — | — |
| Urine output, ml/d | 832±422 | — | — |
| Weekly ultrafiltration, L/wk | 4.2±2.6 | 7.2±2.5 | 0.02 |
| Dialysis time, min/wk | 369±22 | 605±81 | <0.001 |
| spKt/Vurea | 1.51±0.21 | 1.58±0.19 | 0.39 |
| 2015 KDOQI stdKt/Vurea | 2.53±0.53a | 2.41±0.14 | 0.49 |
| Original stdKt/Vurea | 2.25±0.39 | 2.41±0.14 | 0.11 |
| Blood flow, ml/min | 367±66 | 400 | 0.44 |
| Dialysate flow, ml/min | 589±93 | 713±98 | 0.01 |
Results are mean±SD. 2015 KDOQI stdKt/Vurea and original stdKt/Vurea values were obtained as described in the Methods and Supplemental Material. Urine output was estimated from a 3 day collection period. The weekly ultrafiltration was the sum of the ultrafiltration for treatments during the week of sample collection. Dialyzers used were Revaclear/Revaclear 300/Revaclear Max/F160/F180 with frequency 4/1/0/3/1 and 6/0/3/0/0 in the twice weekly and thrice weekly groups, respectively. Kru, residual urea clearance; —, no data.
P<0.05, 2015 KDOQI versus original stdKt/Vurea.
The pretreatment plasma concentrations of urea and the secreted solutes along with their residual and dialytic clearances and generation rates are summarized in Table 3. Of note, average generation rates were similar in the two groups for each of the solutes. Despite a similar generation rate, one less treatment per week, and a lower dialytic clearance, the plasma urea concentration was not significantly higher in the twice than the thrice weekly patients. Results were notably different for hippurate and phenylacetylglutamine. The plasma concentrations of these solutes were much lower in the twice weekly patients than the thrice weekly patients despite the application of a similar dialytic clearance for a much lower total weekly treatment time. Values for indoxyl sulfate and p-cresol sulfate followed a different pattern. Both residual and dialytic clearances of these solutes were markedly lower than the corresponding values for hippurate and phenylacetylglutamine because of plasma protein binding. The plasma concentrations for indoxyl sulfate and p-cresol sulfate were however no higher in the twice weekly patients with residual function than in the thrice weekly patients treated with similar dialytic clearances for a much longer total treatment time.
Table 3.
Plasma concentrations, residual clearances, dialytic clearances, and solute generation rates
| Solute Measurement | Twice Weekly | Thrice Weekly | P Value |
|---|---|---|---|
| Urea | |||
| Pretreatment plasma concentration, mg/dl | 67±18 | 51±23 | 0.11 |
| Residual clearance, ml/min | 2.8±1.5 | — | — |
| Dialytic clearance, ml/min | 247±31 | 274±12 | <0.01 |
| Estimated generation, g/d | 7±2 | 7±4 | 0.73 |
| HIPP | |||
| Pretreatment plasma concentration, mg/dl | 2.7±2.7 | 5.5±2.6 | 0.02 |
| Residual clearance, ml/min | 14±8 | — | — |
| Dialytic clearance, ml/min | 122±33 | 124±12 | 0.89 |
| Estimated generation, mg/d | 500±264 | 400±231 | 0.47 |
| PAG | |||
| Pretreatment plasma concentration, mg/dl | 2.3±2.4 | 4.3±2.9 | 0.03 |
| Residual clearance, ml/min | 10±8 | — | — |
| Dialytic clearance, ml/min | 160±29 | 178±22 | 0.24 |
| Estimated generation, mg/d | 313±173 | 364±279 | 0.82 |
| IS | |||
| Pretreatment plasma concentration, mg/dl | 1.9±0.9 | 2.3±0.8 | 0.39 |
| Residual clearance, ml/min | 1.7±1.4 | — | — |
| Dialytic clearance, ml/min | 28±10 | 31±7 | 0.34 |
| Estimated generation, mg/d | 57±21 | 58±29 | 0.73 |
| PCS | |||
| Pretreatment plasma concentration, mg/dl | 4.2±2.1 | 4.3±1.9 | 1.0 |
| Residual clearance, ml/min | 0.7±0.6 | — | — |
| Dialytic clearance, ml/min | 21±8 | 23±5 | 0.49 |
| Estimated generation, mg/d | 90±63 | 90±57 | 0.67 |
Results are mean±SD. The free fractions of the secreted solutes were not different in the two groups (twice versus thrice weekly: HIPP, 55%±17% versus 52%±17%; PAG, 104%±26% versus 95%±9%; IS, 7.0%±2.4% versus 7.0%±2.4%; PCS, 5.7%±2.0% versus 6.0%±1.9%). In one patient whose pretreatment HIPP concentration fell below the assay range, a pretreatment HIPP concentration half the lower limit of the assay range was imputed. HIPP clearances were not calculated for this patient and for another patient whose post-treatment plasma concentration fell below the assay range. PAG clearances were not calculated for a patient whose post-treatment plasma concentration fell below the assay range. —, no data; HIPP, hippurate; PAG, phenylacetylglutamine; IS, indoxyl sulfate; PCS, p-cresol sulfate.
The contribution of residual kidney function to the control of solute concentrations in patients receiving twice weekly as compared with thrice weekly hemodialysis was further assessed as summarized in Table 4. The dialytic clearance of each solute was first compared with the dialytic clearance of urea. In accord with prior reports, the dialytic clearances of the normally secreted solutes were lower than the dialytic clearance of urea, with the highest relative value for the unbound solute phenylacetylglutamine and progressively lower values associated with increasing protein binding for hippurate, indoxyl sulfate, and p-cresol sulfate. The residual clearance of each solute was next compared with the residual clearance of urea. The pattern for residual clearances was strikingly different than that for dialytic clearances. The residual clearances for hippurate and phenylacetylglutamine were significantly higher than the residual clearance of urea, and the residual clearances for indoxyl sulfate and p-cresol sulfate were only slightly lower. Comparison of residual solute clearances with estimated residual glomerular filtration confirmed that this pattern could be explained by continued active solute secretion in the residual kidney, as summarized in Supplemental Table 1. For each of the secreted solutes, the ratio of its residual clearance to urea’s residual clearance was much higher than the ratio of its dialytic clearance to urea’s dialytic clearance. As a result, residual function removed a larger fraction of these solutes than of urea in the patients receiving twice weekly treatment, providing control of their plasma concentrations despite the lesser treatment frequency and time. Modeled values for their average pretreatment concentrations provided a measure of the control of plasma solute concentrations. Values in the twice weekly patients were not significantly different for urea, significantly lower for hippurate and phenylacetylglutamine, which had the highest residual clearance rates relative to urea, and not significantly different for indoxyl sulfate and p-cresol sulfate. Values for the TAC followed a similar pattern, as shown in Table 4. As in previous studies, generation rates of the secreted solutes varied widely among individual patients, so that plasma solute levels were not correlated with residual clearance rates. The dependence of plasma concentrations on residual clearances became apparent only when plasma concentrations were modeled by imputing the same solute generation and maintaining the clearance values and other parameters observed in individual patients, as illustrated in Figure 1.
Table 4.
Residual clearances relative to dialytic clearances and their effect on plasma solute concentrations
| Solute | Twice Weekly | APC | APC2×/APC3× | TAC | TAC2×/TAC3× | ||||
|---|---|---|---|---|---|---|---|---|---|
| Kd,solute/Kd,urea | Kr,solute/Kr,urea | Fraction of Solute Removed by Residual Clearance | Twice Weekly, mg/dl | Thrice Weekly, mg/dl | Twice Weekly, mg/dl | Thrice Weekly, mg/dl | |||
| Urea | — | — | 0.27±0.11 | 71±19 | 53±24 | 1.3 | 50±13 | 36±16 | 1.4 |
| HIPP | 0.52±0.11 | 8.0±7.2a | 0.75±0.18b | 3.1±3.1 | 6.3±3.0c | 0.50 | 2.4±2.1 | 4.1±2.0 | 0.58 |
| PAG | 0.67±0.07 | 4.5±3.4a | 0.63±0.19b | 2.6±2.6 | 4.9±3.3c | 0.53 | 1.8±1.6 | 3.0±2.1 | 0.60 |
| IS | 0.12±0.03 | 0.67±0.47a | 0.56±0.24b | 2.1±1.1 | 2.6±0.9 | 0.80 | 1.7±0.8 | 2.2±0.9 | 0.78 |
| PCS | 0.09±0.03 | 0.28±0.16a | 0.44±0.22 | 4.6±2.3 | 5.0±2.2 | 0.92 | 3.9±1.9 | 4.2±1.8 | 0.91 |
Results are mean±SD. In the twice weekly group, the residual and dialytic clearances and the fraction removed by residual clearance could not be calculated for PAG in one patient and for HIPP in one patient because their post-treatment plasma concentrations fell below the assay range. APC values could therefore not be estimated in these patients. —, no data; APC, average pretreatment concentration; Kd,solute/Kd,urea, ratio of the dialytic solute clearance to dialytic urea clearance; Kr,solute/Kr,urea, ratio of the residual solute clearance to residual urea clearance; APC2×/APC3×, ratio of the APC in the twice weekly compared with the thrice weekly group; TAC2×/TAC3×, the ratio of TAC in twice weekly to thrice weekly patients; HIPP, hippurate; PAG, phenylacetylglutamine; IS, indoxyl sulfate; PCS, p-cresol sulfate.
P<0.05, Kd,solute/Kd,urea versus Kr,solute/Kr,urea.
P<0.05, values for the secreted solutes compared with urea.
P<0.05, twice versus thrice weekly.
Figure 1.
Pretreatment plasma solute levels modeled imputing the same solute generation rate for all patients decline with increasing residual clearance. Patients with residual function on twice weekly hemodialysis are represented by blue circles and anuric patients on thrice weekly hemodialysis are represented by red circles. Modeling was performed assuming that all patients had the average solute generation rate for the study population as well as maintaining the observed values for solute clearances and other parameters in individual patients. When equal solute production was assumed, modeled plasma levels for the secreted solutes in the twice weekly patients were closely related to their residual clearances (hippurate: r=0.89, P=0.008; phenylacetylglutamine: r=0.80, P=0.02; indoxyl sulfate: r=0.87, P=0.002; p-cresol sulfate: r=0.71, P=0.03). There was a lesser association for plasma urea levels and residual clearance (r=0.63, P=0.07). Residual clearances could not be calculated for hippurate in two patients and for phenylacetylglutamine in one patient because post-treatment plasma concentrations fell below the assay range.
Discussion
The vast majority of patients on hemodialysis in the United States receive dialysis thrice weekly, whether they have residual native kidney function or not.24–26 Thrice weekly treatment was already the most common prescription when the Medicare ESRD Program legislation was passed in 1973.12,27 The National Cooperative Dialysis Study (NCDS) later compared the effect of shorter and longer treatment times and of targeting higher and lower urea levels in patients on thrice weekly treatment.28 Its planning committee considered an additional comparison of twice and thrice weekly treatment, but decided this would make the study too complicated.29 Subsequent analysis of the NDCS data led to the adoption of Kt/Vurea to measure adequacy, further establishing thrice weekly treatment as the standard of care.30
Although thrice weekly hemodialysis remains routine, there has been renewed interest in twice weekly treatment for patients with residual kidney function. KDOQI Guidelines allow for twice weekly treatment when residual urea clearance is >3 ml/min.19,23 Under these guidelines, most patients could presumably begin hemodialysis with two treatments per week.7,24,31 Better preservation of residual function with twice rather than thrice weekly treatment may confer advantages beyond reduction in the number of trips to the dialysis unit.3,7,11
Several factors now likely restrict the prescription of twice weekly hemodialysis. No trial has yet compared twice weekly and thrice weekly treatment and so physicians cannot be sure that twice weekly treatment provides equal or superior outcomes in patients with residual function. Twice weekly treatment complicates scheduling for providers. In some patients, accumulation of fluid or inorganic ions may discourage an attempt at twice weekly treatment even when residual function is present. The chance to skip a treatment could motivate improved dietary compliance and allow adequate volume and inorganic ion control with twice weekly treatment in some of these patients, but this remains to be seen. Another impediment to twice weekly treatment may be the concern that long treatment times are required. However, the 2015 Update of the KDOQI Guideline increased the weight assigned to residual function in calculating stdKt/Vurea.19 This had the effect of reducing the time required to meet adequacy targets with twice weekly treatment, as illustrated in Supplemental Figure 1.
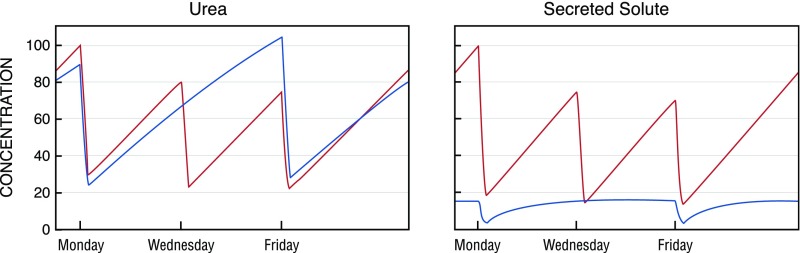
Residual function was assigned increased weight in the most recent KDOQI Guideline based largely on the consideration that it operates continuously.21 Our results provide a further potential justification for assigning increased weight to residual function. Incorporating residual function into measures of dialysis adequacy presents us with a question of exchange rate. Further, the use of urea in calculating adequacy has tended to maximize the value of dialysis treatment relative to residual function. Urea has the highest dialytic clearance measured for any solute because it diffuses out of red cells as blood passes through the dialyzer.32 In the native kidney, by contrast, urea is reabsorbed by the tubules whereas many other solutes are actively secreted.13,15,17 These processes can continue in the residual kidney of patients on dialysis. The clearance of secreted solutes relative to urea is thus much higher in the residual kidney than in the dialyzer, as shown in Table 4. The residual kidney thus removes a greater portion of these solutes than of urea, as further shown in the table. Also, if a solute’s residual secretory clearance is sufficiently high relative to that of urea, its plasma levels will be lower in patients receiving twice weekly hemodialysis than in anuric patients receiving thrice weekly hemodialysis providing the same stdKt/Vurea, as illustrated in Figure 2. This possibility is exemplified by our finding of lower plasma levels for hippurate and phenylacetylglutamine in the twice weekly patients with residual function than in thrice weekly patients without residual function.
Figure 2.
The contribution of residual clearance to the control of plasma levels of each solute depends heavily on the ratios of the solute's clearance relative to urea in the native kidney and the dialyzer and on the value of Kru. Modeled plasma solute levels for urea (left panel) and a representative secreted solute (right panel) in an anuric patient on thrice weekly hemodialysis (red lines) and a patient of the same size with Kru 2.5 ml/min on twice weekly hemodialysis (blue lines) are illustrated. The prescription was adjusted to achieve an spKt/Vurea 1.3 in the anuric patient and an equivalent stdKt/Vurea 2.2 in the patient with residual function. Solute generation rates were set the same in both patients. The secreted solute was assumed to have a dialytic clearance 0.5 times that of urea, a residual clearance eight times that of urea, and a volume of distribution 0.3 times that of urea. Plasma levels for both solutes are normalized to 100% of the average peak concentrations on thrice weekly treatment.
The finding that residual function contributes importantly to the removal of secreted solutes is similar to previous findings that residual function contributes importantly to the removal of low-molecular-weight proteins, as represented by β2 microglobulin.33,34 As with secreted solutes, low-molecular-weight proteins are cleared at a higher rate relative to urea by residual kidney function than by hemodialysis. The relative contribution of residual function to the removal of low-molecular-weight proteins declined with the introduction of high-flux dialysis, but residual function remains an important determinant of β2 microglobulin levels in patients on dialysis along with an as yet incompletely characterized, nonrenal, nondialytic clearance mechanism.33,35
A notable difference between residual clearance of secreted solutes and low-molecular-weight proteins is that residual secretory clearances may be preserved to varying degrees for different solutes. Variably impaired secretion of both natural solutes and medications has previously been observed in patients with chronic renal insufficiency.14,36,37 Presumably, this may reflect reduced activity of transport proteins or competition for transport among accumulating uremic solutes. In this study, the average fractional clearance of hippurate was similar in patients on dialysis and controls, as summarized in Supplemental Table 1. The value in patients on dialysis was more variable, however, than that observed in controls. However, the fractional clearances of phenylacetylglutamine, indoxyl sulfate, and p-cresol sulfate were significantly reduced in the residual kidney, with the most marked reduction in the fractional clearance of p-cresol sulfate. Of note, impaired secretion of indoxyl sulfate and p-cresol sulfate was apparent only when clearances were calculated using the free, unbound solute concentration. A large increase in the free fraction of these solutes in patients on dialysis maintained fractional clearance values expressed in terms of total solute concentrations close to normal. Experimental error may also have contributed to the variability in the residual clearance values we calculated. Diurnal variation in solute levels, which may be occasioned by food intake, has been observed for several of the secreted solutes.38 Such variation would cause error in residual clearance values calculated from the solute recovery in interdialytic urine collections and post- and pre-treatment plasma concentrations, with the magnitude of the error potentially greater with higher residual solute clearances.
Our study has limitations. Most importantly, we did not assess clinical outcomes and cannot tell to what extent twice weekly treatment is compatible with health. A crossover study comparing twice and thrice weekly treatment in patients with residual function could assess what portion of patients can be successfully switched to twice weekly treatment, and whether twice weekly treatment provides quality of life equal to or better than that provided by thrice weekly treatment. A larger trial would be required to assess the effect of twice weekly treatment on the preservation of residual function and on mortality and other long-term outcomes. Other limitations of our study include its small size and the measurement of a limited number of solutes. Knowledge of the effect of twice weekly treatment on these solutes does not allow us to predict its effect on other solutes. Finally, twice weekly treatment in the patients we studied was not prescribed to achieve a target stdKt/Vurea. Treatment was instead made on the basis of the patients’ preferences and their nephrologists’ impressions that the treatment was adequate.
In conclusion, plasma concentrations of secreted solutes can be well controlled by twice weekly hemodialysis in patients with residual kidney function. This result demonstrates a biologic value of residual function, and motivates further study of the incorporation of residual function in calculations of dialysis adequacy. It should be noted that alternate measures of dialysis adequacy have been proposed that assign even greater weight to residual function than the 2015 Update of the KDOQI Guideline.39,40 Ultimately, we hope that trials will be performed to assess the clinical effect of twice weekly hemodialysis.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the assistance of the staff of the Veterans Affairs Palo Alto dialysis unit, and Dr. Brigitte Schiller and the staff of the Satellite Healthcare dialysis units.
S.C.L., T.W.M., and T.L.S. designed the study, carried out the experiments, analyzed the data, and drafted the manuscript. N.S.P. carried out the experiments and analyzed the data. J.N.S. and A.T. developed the mathematical modeling. All authors approved the final version of the manuscript.
T.L.S. was supported by a Veterans Affairs Career Development Award (CX001036-01A1).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018010081/-/DCSupplemental.
References
- 1.Rhee CM, Ghahremani-Ghajar M, Obi Y, Kalantar-Zadeh K: Incremental and infrequent hemodialysis: A new paradigm for both dialysis initiation and conservative management. Panminerva Med 59: 188–196, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangvoraphonkchai K, Davenport A: Increasing haemodialytic clearances as residual renal function declines: An incremental approach. Blood Purif 44: 217–226, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Davenport A: Will incremental hemodialysis preserve residual function and improve patient survival? Semin Dial 28: 16–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghahremani-Ghajar M, Rojas-Bautista V, Lau WL, Pahl M, Hernandez M, Jin A, et al.: Incremental hemodialysis: The university of California irvine experience. Semin Dial 30: 262–269, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile C, Casino FG, Kalantar-Zadeh K: Is incremental hemodialysis ready to return on the scene? From empiricism to kinetic modelling. J Nephrol 30: 521–529, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Bargman JM, Thorpe KE, Churchill DN; CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Golper TA: Incremental dialysis: Review of recent literature. Curr Opin Nephrol Hypertens 26: 543–547, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Obi Y, Eriguchi R, Ou SM, Rhee CM, Kalantar-Zadeh K: What is known and unknown about twice-weekly hemodialysis. Blood Purif 40: 298–305, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew A, Obi Y, Rhee CM, Chen JL, Shah G, Lau WL, et al.: Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int 90: 1071–1079, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Bargman JM, Golper TA: The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant 20: 671–673, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K: Preservation of residual kidney function in hemodialysis patients: Reviving an old concept. Kidney Int 90: 262–271, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toth-Manikowski SM, Shafi T: Hemodialysis prescription for incident patients: Twice seems nice, but is it incremental? Am J Kidney Dis 68: 180–183, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowenstein J, Grantham JJ: Residual renal function: A paradigm shift. Kidney Int 91: 561–565, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Suchy-Dicey AM, Laha T, Hoofnagle A, Newitt R, Sirich TL, Meyer TW, et al.: Tubular secretion in CKD. J Am Soc Nephrol 27: 2148–2155, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigam SK, Wu W, Bush KT, Hoenig MP, Blantz RC, Bhatnagar V: Handling of drugs, metabolites, and uremic toxins by kidney proximal tubule drug transporters. Clin J Am Soc Nephrol 10: 2039–2049, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW: Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 25: 615–622, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marquez IO, Tambra S, Luo FY, Li Y, Plummer NS, Hostetter TH, et al.: Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol 6: 290–296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Kidney Foundation : KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 66: 884–930, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Daugirdas JT, Depner TA, Greene T, Silisteanu P: Solute-solver: A web-based tool for modeling urea kinetics for a broad range of hemodialysis schedules in multiple patients. Am J Kidney Dis 54: 798–809, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Chin AI, Depner TA, Daugirdas JT: Assessing the adequacy of small solute clearance for various dialysis modalities, with inclusion of residual native kidney function. Semin Dial 30: 235–240, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Gotch FA: The current place of urea kinetic modelling with respect to different dialysis modalities. Nephrol Dial Transplant 13[Suppl 6]: 10–14, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Hemodialysis Adequacy 2006 Work Group : Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis 48[Suppl 1]: S2–S90, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Unruh M, Zager PG, Kovesdy CP, Bargman JM, Chen J, et al.: Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis 64: 181–186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obi Y, Streja E, Rhee CM, Ravel V, Amin AN, Cupisti A, et al.: Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: A cohort study. Am J Kidney Dis 68: 256–265, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Crowley ST, Beddhu S, Chen JLT, Daugirdas JT, Goldfarb DS, et al.: Renal replacement therapy and incremental hemodialysis for veterans with advanced chronic kidney disease. Semin Dial 30: 251–261, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scribner BH, Cole JJ, Ahmad S, Blagg CR: Why thrice weekly dialysis? Hemodial Int 8: 188–192, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Lowrie EG, Laird NM, Parker TF, Sargent JA: Effect of the hemodialysis prescription of patient morbidity: Report from the National Cooperative Dialysis Study. N Engl J Med 305: 1176–1181, 1981 [DOI] [PubMed] [Google Scholar]
- 29.Burton BT: Adequacy of dialysis. Introduction: Purpose of the conference. Kidney Int 2 [Suppl 1]: 1975 [PubMed] [Google Scholar]
- 30.Gotch FA, Sargent JA: A mechanistic analysis of the National Cooperative Dialysis Study (NCDS). Kidney Int 28: 526–534, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Saran R, Li Y, Robinson B, et al.: US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 67: S1–S434, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneditz D, Platzer D, Daugirdas JT: A diffusion-adjusted regional blood flow model to predict solute kinetics during haemodialysis. Nephrol Dial Transplant 24: 2218–2224, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Roumelioti ME, Nolin T, Unruh ML, Argyropoulos C: Revisiting the middle molecule hypothesis of uremic toxicity: A systematic review of beta 2 microglobulin population kinetics and large scale modeling of hemodialysis trials in silico. PLoS One 11: e0153157, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odell RA, Slowiaczek P, Moran JE, Schindhelm K: Beta 2-microglobulin kinetics in end-stage renal failure. Kidney Int 39: 909–919, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Ward RA, Greene T, Hartmann B, Samtleben W: Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 69: 1431–1437, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Cheung KWK, Hsueh CH, Zhao P, Meyer TW, Zhang L, Huang SM, et al.: The effect of uremic solutes on the organic cation transporter 2. J Pharm Sci 106: 2551–2557, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Hsueh CH, Yoshida K, Zhao P, Meyer TW, Zhang L, Huang SM, et al.: Identification and quantitative assessment of uremic solutes as inhibitors of renal organic anion transporters, OAT1 and OAT3. Mol Pharm 13: 3130–3140, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Rivara MB, Zelnick LR, Hoofnagle AN, Newitt R, Tracy RP, Kratz M, et al.: Diurnal and long-term variation in plasma concentrations and renal clearances of circulating markers of kidney proximal tubular secretion. Clin Chem 63: 915–923, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casino FG, Basile C: How to set the stage for a full-fledged clinical trial testing ‘incremental haemodialysis’ [published online ahead of print July 21, 2017]. Nephrol Dial Transplant doi: 10.1093/ndt/gfx225 [DOI] [PubMed] [Google Scholar]
- 40.Casino FG, Basile C: The variable target model: A paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant 32: 182–190, 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.