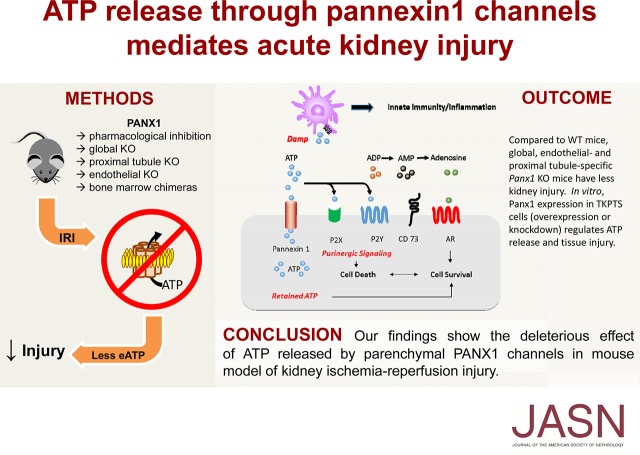
Abstract
Background Pannexin1 (Panx1), an ATP release channel, is present in most mammalian tissues, but the role of Panx1 in health and disease is not fully understood. Panx1 may serve to modulate AKI; ATP is a precursor to adenosine and may function to block inflammation, or ATP may act as a danger-associated molecular pattern and initiate inflammation.
Methods We used pharmacologic and genetic approaches to evaluate the effect of Panx1 on kidney ischemia-reperfusion injury (IRI), a mouse model of AKI.
Results Pharmacologic inhibition of gap junctions, including Panx1, by administration of carbenoxolone protected mice from IRI. Furthermore, global deletion of Panx1 preserved kidney function and morphology and diminished the expression of proinflammatory molecules after IRI. Analysis of bone marrow chimeric mice revealed that Panx1 expressed on parenchymal cells is necessary for ischemic injury, and both proximal tubule and vascular endothelial Panx1 tissue-specific knockout mice were protected from IRI. In vitro, Panx1-deficient proximal tubule cells released less and retained more ATP under hypoxic stress.
Conclusions Panx1 is involved in regulating ATP release from hypoxic cells, and reducing this ATP release may protect kidneys from AKI.
Keywords: acute renal failure, ischemia-reperfusion, purinergic signaling, ATP
AKI is accompanied by release of ATP due to cell damage, and ATP can act as a damage-associated molecular pattern (DAMP) and further exacerbate injury through activation of innate immunity.1 Extracellular ATP is degraded by ectonucleotidases (CD39 and CD73) to ADP, AMP, and adenosine; ATP and ADP can bind to and activate P2 purinergic receptors to aggravate injury.2,3 Alternatively, adenosine can act on P1 purinergic receptors (A1, A2A, A2B, and A3) to attenuate inflammation and protect kidneys from ischemia-reperfusion injury (IRI).4–7
Pannexin1 (Panx1) is a transmembrane protein that belongs to a small family of proteins exhibiting a structural homology to gap junction-forming invertebrate innexins.8 In vivo, they form large, nonselective transmembrane channels that efficiently release ATP to the extracellular space.9 Panx1 is the most ubiquitously expressed of the three pannexins and has been found in neurons, muscle, and immune cells.10–12 It is also present in polarized epithelial cells in lung and kidney, which are a rich source of extracellular ATP.13,14 In the kidney, Panx1 expression was found in apical membranes of proximal tubules (PTs), thick descending limbs, and collecting ducts as well as in renal vasculature.14 Panx1 is required for the release of intracellular ATP from renal epithelial cells.14 Extracellular ATP helps govern renal hemodynamics and tubular transport.15,16 The reduction of blood flow to the kidney in AKI results in endothelial activation, epithelial dysfunction, and cell death, as well as neutrophil and macrophage migration to the injured site, all of which are in part mediated or affected by P2 purinergic receptors.17 Few studies have examined the importance of P2 receptors in AKI; however, inhibition of P2X7R attenuates inflammation and IRI.18,19 Overall, ATP is implicated in several physiologic and pathologic settings in the kidney.
We hypothesize that Panx1 plays a critical role in regulating the microenvironment in AKI. By controlling release of ATP, inhibition or genetic deletion of Panx1 may reduce extracellular ATP and have a protective effect in renal IRI. Decreased release of ATP may limit precursors for CD39/CD73, thereby decreasing tissue levels of adenosine and exacerbating AKI. Retention of ATP may provide energy equivalence to maintain cell viability in sublethally injured kidney cells.
Methods
Mice
Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the University of Virginia Animal Care and Use Committee. Male mice between 8 and 10 weeks of age were used.
Panx1 knockout (KO; Panx1−/−) and Panx1-floxed mice (Panx1f/f or B6;129-Casp4del Panx1tm1Vshe/J)20 as well as VeCadCreERT2+Panx1f/f mice21,22 were characterized previously. Panx2 and Panx3 mRNA was undetectable in kidneys of Panx1−/− mice (data not shown). PepckCre+/+ mice were obtained from Volker Haase (Vanderbilt University).23 We previously generated a PepckCre-YFP reporter mouse and found that YFP is expressed in tubules in kidney cortex in PepckCreRosa26-YFP/wt mice.24 Panx1−/− mice were crossed with C57BL/6J wild-type (WT) mice (Jackson Laboratories) to generate Panx1+/− heterozygotes, and by crossing the heterozygotes, a WT littermate control group was obtained. After primary characterization of the injury model, progeny-derived controls were used. PepckCre+/+ and VeCadCreERT2+ mice were bred with Panx1f/f mice to generate PT- and vascular endothelial–specific KOs of Panx1, respectively. Age-matched littermate controls were used in all experiments using mice with tissue-specific Panx1 deletion. Mice were maintained in standard vivarium housing on a chow diet with freely available water. Genotyping primer sequences and genotyping strategies for each strain were as previously described20,21,23 (Supplemental Figure 1, A and B). PCR of DNA extracted from organs of PepckCrePanx1f/f mice showed specificity of recombination (Supplemental Figure 1C).
Mouse Surgery and Drug Treatments
Mice were anesthetized with an intraperitoneal injection of a ketamine (120 mg/kg) and xylazine (120 mg/kg) mixture and placed on a warm pad to maintain body temperature at 34.5°C–35.5°C. In our experience, this is an optimal temperature range for ischemia-reperfusion surgeries; maintenance of mouse body temperature of 37°C leads to early mortality and less meaningful results, because other confounding factors could contribute to kidney injury independent of ischemia-reperfusion. Bilateral flank incisions were performed, and blood flow through both renal arteries and veins was interrupted by clamping for 26 minutes. Clamps were released, and kidneys were allowed to reperfuse for a period of 24 hours. This ischemic period was anticipated to cause a significant amount of irreversible injury in the control mice, resulting in a rise in plasma creatinine, acute tubular necrosis (ATN), and an increase in proinflammatory molecule expression. For sham-operated mice, only flank incisions were performed after anesthesia, and renal blood vessels were not clamped. Surgical wounds were closed, and buprenorphine (0.15 mg/kg intraperitoneally) was administered as an analgesic. During the reperfusion period of 24 hours, mouse cages were put under a heating lamp to enhance recovery from the procedure.
Carbenoxolone (CBX; C4790; Sigma-Aldrich) was prepared in a vehicle of normal saline, and it was administered (245 mg/kg intraperitoneally) 1 hour before IRI (Supplemental Figure 2A). Tamoxifen (T5648; Sigma-Aldrich) was dissolved in 5% EtOH in USP corn oil, and it was administered (40 mg/kg per day intraperitoneally) to VeCadCreERT2+Panx1f/f and control mice on 5 successive days as described elsewhere,25 with the last dose given 6 days before IRI as shown in Supplemental Figure 2C.
Assessment of Kidney Function and Histology
After 24 hours of reperfusion, mice were anesthetized with an intraperitoneal injection of a ketamine (120 mg/kg) and xylazine (120 mg/kg) mixture. Blood was collected from the retro-orbital sinus, and plasma creatinine (milligrams per deciliter) was determined with an enzymatic method using a slightly modified manufacturer’s protocol (using double the sample volume; Diazyme Laboratories) that has been verified by liquid chromatography/mass spectrometry.26 Mice were euthanized by cervical dislocation, and kidneys were dissected and decapsulated. Kidneys were cut transversely, and pieces were either flash frozen in liquid nitrogen for RNA extraction, fixed in 4% paraformaldehyde/0.1 M phosphate buffer (pH 7.4), and further processed for hematoxylin and eosin (H&E) staining or fixed in 1% periodate-lysine-paraformaldehyde/0.1 M phosphate buffer (pH 7.4), frozen in O.C.T. compound (Fisher Scientific), and further processed for immunofluorescence labeling. H&E staining of kidney sections and subsequent scoring of ATN were performed as described earlier.26 The following stereologic parameters were defined: counting frame, 400×400 mm; sample grid, 800×800 mm; and grid spacing, 85 mm. These values were determined empirically, such that adequate numbers of sample sites were visited and adequate numbers of markers (of tubular injury) were acquired, in keeping with accepted counting rules for stereology. A total of 76±4 (mean±SEM) grid sites were evaluated per section; the sampling fraction was 25% of a total average area of 1.59±0.07×106 μm2 for each kidney section.
Immunofluorescence labeling was performed on 5 μM frozen kidney sections. Briefly, sections were incubated in 10% horse serum and 0.03% Triton X-100 in PBS followed by Alexa Fluor 555 rat anti-mouse neutrophil antibody (Clone 7/4; 7 μg/ml; Cedarlane). Photomicrographs were acquired using an Axiovert 200M microscope (Zeiss).
Quantitative Real-Time PCR
TriReagent was used to obtain RNA from kidney according to the manufacturer’s protocol (Molecular Research Center Inc.), and RNA was transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad). Primers were designed using the Primer-BLAST tool (primer sequences are in Supplemental Table 1), and iTaq Universal SYBR Green Supermix was used as a source of PCR reaction components. The reaction protocol was as follows: initial denaturation at 95°C for 3 minutes; the denaturation, annealing, and elongation program repeated 35 times at 95°C for 45 seconds, 52°C for 60 seconds, and 72°C for 60 seconds; and a melting curve analysis to confirm single product. To validate absence of Panx1 mRNA in the kidney, TaqMan probes (Applied Biosystems) were used as described elsewhere.20 Samples were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) or hypoxanthine phosphoribosyl transferase 1 (Hprt).
Bone Marrow Chimera Generation
Progeny-derived Panx1−/− and Panx1+/+ mice (6–8 weeks old) as well as C57BL/6 mice possessing the CD45.1 pan-leukocyte marker allele (B6.SJL-Ptprca Pepcb/BoyJ; Jackson Laboratory) were used to generate bone marrow (BM) chimeras as described elsewhere.6 Briefly, recipient mice were lethally irradiated, and they were immediately reconstituted with intravenous injection of 107 donor BM cells. The resulting chimeric mice were housed in the vivarium for 9 weeks before experimentation. CD45.1 mice were used as donor in the CD45.1→Panx1+/+ (WT) group to test irradiation and reconstitution efficiencies. Irradiation and reconstitution efficiencies were assessed by flow cytometry. Kidney cell suspensions were obtained by decapsulating the organ, cutting it into small pieces, and incubating it in a suspension of DNAse1 and collagenase for 30 minutes at 37°C. Cells were pushed through a 40-μm filter using a syringe plunger and spun down in a centrifuge, and the pellet was suspended in red blood cell lysis buffer for 5 minutes. Buffer was neutralized with PBS plus 0.1% BSA, and cells were used for cytometry. Splenocytes were obtained by pushing the spleen through a 70-μm filter, centrifuging, and treating with red blood cell lysis buffer as above. BM was obtained by dissecting two long bones of a hind leg, cutting the epiphyses, and washing the marrow out using 10 ml saline and a syringe. BM cells were washed through a 40-μm filter, and the suspension was centrifuged. Supernatant was discarded, and cells were resuspended in 1 ml PBS plus 0.1% BSA (cytometry buffer); 100 μl of the cell suspension was used in flow cytometry analysis by staining with anti-CD45.1 and CD45.2 antibodies for 1 hour, washing, and resuspending in 100 μl of cytometry buffer. 7-AAD was added 15 minutes before analysis to discriminate live from dead cells. Samples were analyzed using the FACSCalibur flow cytometer (BD Scientific) with Cytek eight-color upgrade, and data analysis was with FlowJo v.10 software (FlowJo LLC).
CD45-positive cells were analyzed by discerning the live population of single cells with positive fluorescence signal on the basis of compensation matrix and negative controls. Reconstitution efficiency of >90% in the kidney, spleen, and BM was shown by flow cytometry (Supplemental Figure 3); 9 weeks after reconstitution, mice were subjected to IRI.
Cell Culture
The transformed murine proximal tubule cell line (TKPTS) was generously provided by Dr. Elsa Bello-Reuss (Texas Tech University Health Sciences Center).27 TKPTS cells were cultured in DMEM/F12 medium with HEPES and l-glutamine (Invitrogen) and supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified incubator with 5% CO2 atmosphere. To obtain Panx1 knockdown (KD) and control TKPTS lines, CRISPR/cas9-based double-nickase plasmid (sc-424967-NIC; Santa Cruz Biotechnology) or a scrambled sequence control plasmid (sc-437281; Santa Cruz Biotechnology) was used in concentrations recommended by the manufacturer’s protocol. Cells were seeded in an antibiotic-free medium, and after reaching 50% confluence, they were transfected using Lipofectamine2000 reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. After 2 days, puromycin (2 μg/ml in growth medium) was used to select for successfully transfected cells. KD was confirmed by quantitative PCR (qPCR) (Supplemental Figure 4).
To generate cells with transient overexpression of Panx1, cells were seeded in an antibiotic-free medium, and after reaching 50% confluence, they were transfected using Lipofectamine2000 reagent and an overexpression plasmid according to the manufacturer’s protocol. Experiments were conducted 24–36 hours after transfection.
Hypoxia-reoxygenation (H/R) studies were performed by using the oil coverage method. TKPTS cells (50,000 per well) were grown to confluence in 96-well tissue culture plates. Briefly, medium from a confluent cell monolayer was replaced with mineral oil (Sigma-Aldrich) to induce hypoxia, and after 1 or 6 hours, cultures were then refreshed with medium for reoxygenation.
Extracellular and intracellular ATP concentrations were measured after 15 or 60 minutes of reoxygenation using luminescent CellTiter-Glo reagent (Promega) according to the manufacturer’s instructions. Briefly, the culture medium was removed from each well and centrifuged. Extracellular ATP was measured in the resulting supernatant, which was mixed 1:1 with CellTiter-Glo reagent. For intracellular ATP, the cells in each well (after removing culture medium) were lysed directly by addition of CellTiter-Glo reagent and subsequently diluted 1:1 with fresh medium before reading luminescence. Whole-cell lysates used for RNA extraction were harvested after 1 hour of reoxygenation and processed; qPCR was performed as described for kidney samples.
In some experiments, cells were grown to confluence, and the cell culture media were removed and replaced with DMEM/F12 medium with HEPES and l-glutamine containing 20 μM antimycin A (Sigma-Aldrich). After 10, 60, 120, or 240 minutes, intracellular ATP concentration was assessed as above.
In experiments involving cisplatin, cells were grown to confluence, and the cell culture media were removed and replaced with DMEM/F12 medium with HEPES and l-glutamine containing 0, 20, or 40 μM cisplatin (Fresenius Kabi) for 16 hours. Cells were lysed using RIPA buffer (Sigma-Aldrich), and protein was isolated. Protein concentration was measured with the Pierce BCA Protein Assay Kit. To perform western blot, 20 μg protein or 3 μl prestained protein ladder (Fisher) was loaded onto 15% polyacrylamide gel and run at 100 V for 60 minutes. Transfer to nitrocellulose membrane was performed at 100 V for 60 minutes. Membranes were blocked using Odyssey Blocking Buffer (Licor) for 60 minutes and incubated overnight with primary antibodies diluted in blocking buffer (caspase3, 1:1000; Cell Signaling and β-tubulin, 1:500; Santa Cruz Biotechnology). After washing and 60 minutes of incubation with secondary antibodies (1:20,000; LI-COR), membranes were photographed using the Odyssey Infrared Imaging (LI-COR) system, and fluorescence intensity was analyzed with ImageStudio software (LI-COR). In some experiments, cells were grown to confluence, and the cell culture media were removed and replaced with DMEM/F12 medium with HEPES and l-glutamine containing 0, 5, 10, or 20 μM cisplatin. After 18 hours, intracellular ATP concentration was assessed as above.
Statistical Analyses
A normality test was used to determine if the data fit a Gaussian distribution. When comparing two normally distributed datasets with equal variance, a t test was used. For data with unequal variance, a t test with Welch correction was used. For non-Gaussian distributed data, a Mann–Whitney test was performed to determine statistical significance. A two-way ANOVA was used to compare means between groups with two independent variables. The Tukey post hoc analysis was used. Data were analyzed using Prism 7.03 (GraphPad Software Inc.). Results are displayed containing all replicated experiments, and values shown are mean only or mean±SEM. P values<0.05 were considered statistically significant.
Results
CBX Protects Kidneys from IRI
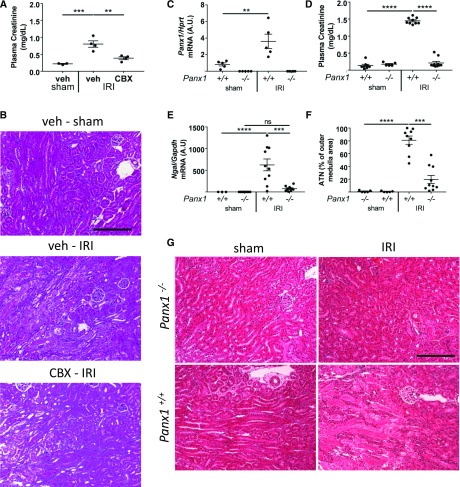
To assess the role of Panx1 in kidney IRI, we determined the effect of pharmacologic inhibition of Panx1. We used CBX, an inhibitor of Panx1 activity.8 CBX is an effective, albeit broad, gap junction inhibitor. CBX is a commonly used Panx1 activity blocker, and it acts through interaction with the first extracellular loop of Panx1.28 Plasma creatinine was elevated in vehicle-treated C57BL/6 mice after ischemia-reperfusion, but it was attenuated by a single injection of CBX (245 mg/kg intraperitoneally) (Figure 1A) given 1 hour before IRI (Supplemental Figure 2A). Substantial ATN in kidneys of vehicle-treated mice was attenuated by CBX (Figure 1B). The results show that pharmacologic inhibition of gap junctions, including Panx1, attenuates renal IRI.
Figure 1.
Pharmacologic inhibition or genetic deletion of pannexin1 (Panx1) protects mice against renal ischemia-reperfusion injury (IRI). (A and B) Mice were treated with vehicle (saline; n=7) or carbenoxolone (CBX; 245 mg/kg intraperitoneally; n=4) 1 hour before IRI. Kidneys of C57BL/6 mice were subjected to 26 minutes of bilateral ischemia or sham operation followed by 24 hours of reperfusion, and mice were then euthanized. Experimental design for A and B is in Supplemental Figure 2A. (A) Plasma creatinine (milligrams per deciliter) values. (B) Representative hematoxylin and eosin–stained kidney sections (outer medulla area). Scale bar, 100 μm. (C–G) Panx1+/+ and Panx1−/− mice were subjected to 26 minutes of bilateral IRI (n=9 and n=10, respectively) or sham operation (n=5 and n=5, respectively), and they were euthanized after 24 hours of kidney reperfusion. (C) Validation of Panx1 knockout by quantitative PCR and effect of IRI on Panx1 mRNA expression. Panx1 expression levels in whole-kidney tissue lysates were normalized to hypoxanthine-guanine phosphoribosyltransferase (Hprt) and presented as fold change relative to sham-operated Panx1+/+ mice. (D) Plasma creatinine (milligrams per deciliter) values. (E) Expression of neutrophil gelatinase–associated lipocalin (Ngal) in kidney relative to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). (F) Quantification of acute tubular necrosis (ATN; percentage) in hematoxylin and eosin–stained kidney sections (outer medulla area), which is (G) illustrated in representative fields. n=5 per group. Error bars represent mean±SEM. Scale bar, 100 μm. **P<0.01; ***P<0.001; ****P<0.001.
Panx1−/− Mice Are Protected from Renal IRI
We next investigated genetic deletion of Panx1 to exclude off-target effects of the drug. We used Panx1−/− mice that lack exon 3, which encodes one of the transmembrane domains of the Panx1 protein.20 Panx1−/− mice and both littermate- and progeny-derived age-matched WT controls (Panx1+/+) were subjected to IRI. Panx1 mRNA expression was undetectable in Panx1−/− mice (Figure 1C). Panx1 mRNA in whole-kidney tissue lysates increased in Panx1+/+ mice after IRI (0.83 versus 3.61; P<0.01). This suggests that Panx1 mRNA is regulated by IRI, similar to the effects of hypoxia on other cell types.10 Littermate- and progeny-derived WT control mice had comparable increases in plasma creatinine after IRI (1.5±0.05 and 1.4±0.03 mg/dl for littermates and progeny-derived mice, respectively; P value was not significant) and comparable plasma creatinine values after sham surgery (0.11±0.01 and 0.17±0.1 mg/dl for littermates and progeny-derived mice, respectively; P value was not significant); progeny-derived controls were, therefore, used for all future experiments. After IRI, the increases in plasma creatinine (Figure 1D) and kidney neutrophil gelatinase–associated lipocalin (Ngal) mRNA (Figure 1E) observed in WT mice were markedly reduced in Panx1−/− mice. Assessment of kidneys of WT mice showed a significant amount of ATN (by H&E staining) primarily in the outer medulla (Figure 1, F and G). These results show that global Panx1 deficiency protects kidneys from IRI.
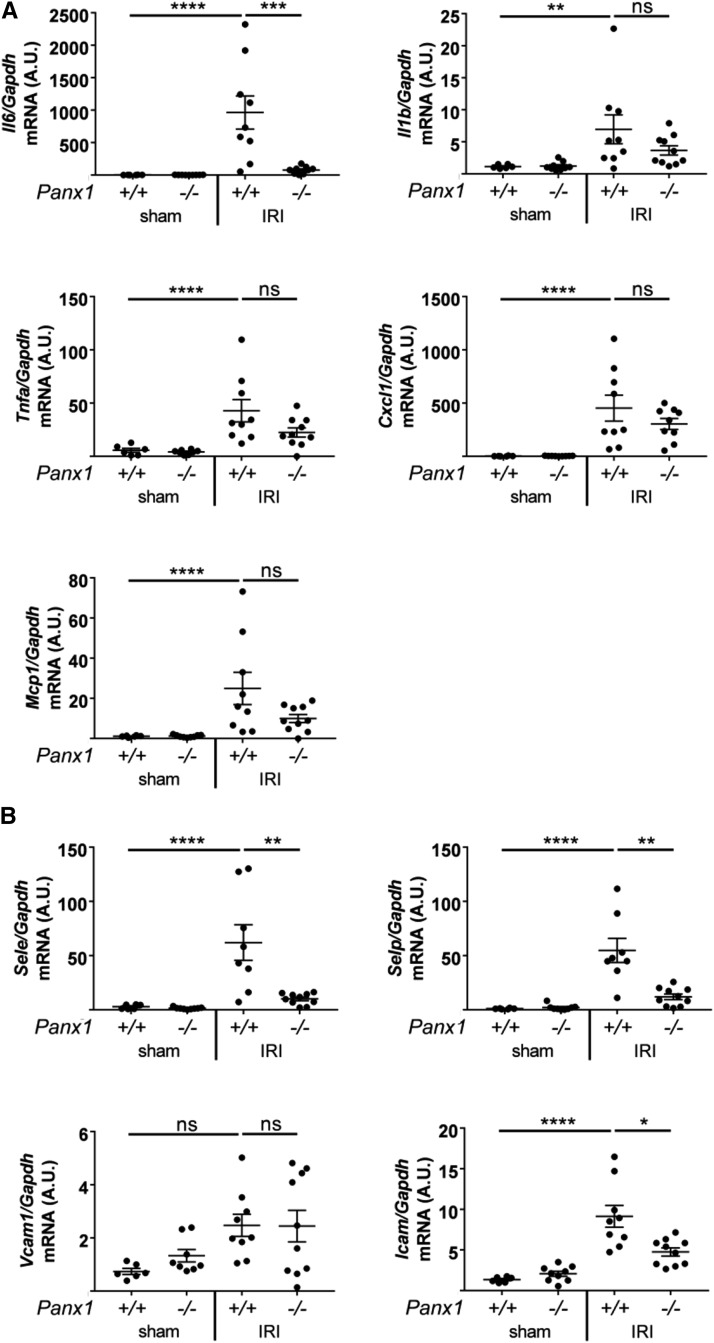
Panx1 Deficiency Is Associated with Reduced Cytokine, Chemokine, and Adhesion Molecule Expression in Kidneys after IRI
We examined expression of proinflammatory molecules in whole-kidney tissue lysates of ischemic kidneys of Panx1+/+ and Panx1−/− mice by qPCR (Figure 2A). After IRI, the increase in Il6 observed in Panx1+/+ was significantly reduced in Panx1−/− mice. Other proinflammatory cytokines/chemokines were increased in Panx1+/+ and reduced in Panx1−/− mice, although the decrease was not significant. Similarly, the increase in adhesion molecules selectin E, selectin P, and intercellular adhesion molecule 1 observed in Panx1+/+ was significantly reduced in Panx1−/− mice (Figure 2B). These data suggest that deletion of Panx1 protects against IRI by blocking expression of specific proinflammatory molecule and endothelial activation markers.
Figure 2.
Global deletion of pannexin1 (Panx1) attenuates ischemia-reperfusion injury (IRI)–induced increase in transcript levels of markers of inflammation and injury. Levels of (A) proinflammatory cytokine (Il6, Il1b, and Tnfa) and chemokine (macrophage chemoattractant protein 1 [Mcp1] and hemokine [C-X-C motif] ligand 1 [Cxcl1]) and (B) leukocyte adhesion molecule (intercellular adhesion molecule 1 [Icam], selectin E [SelE], selectin P [SelP], and vascular cell adhesion protein 1 [Vcam]) mRNA in whole-kidney lysates (same mice as in Figure 1, C–G) as measured by quantitative real-time PCR. Results are normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and presented as fold change relative to sham-operated Panx1+/+ mice. *P<0.05; **P<0.01; ***P<0.001; ****P<0.001.
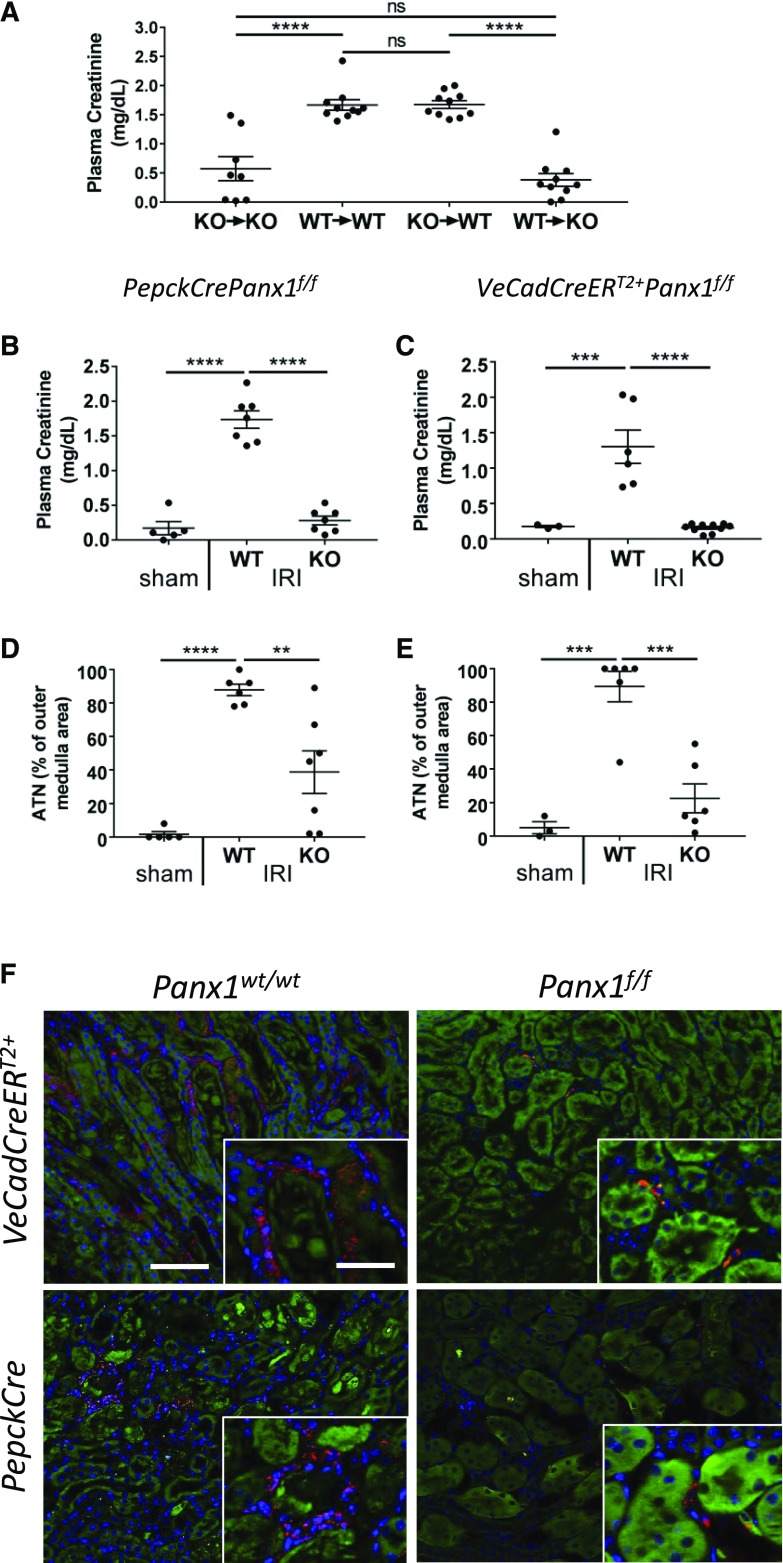
Panx1 Deficiency in Parenchymal Cells and Not in BM-Derived Cells Is Primarily Responsible for Protection against IRI in Panx1−/− Mice
Panx1 is nearly ubiquitously expressed in mammalian tissues, including a variety of immune and parenchymal cells. To investigate whether Panx1 on hematopoietic cells or kidney parenchyma is important in the development of injury, we generated BM chimeras as we have done previously.6 After IRI, Panx1+/+ mice with BM that was reconstituted with Panx1+/+ BM (WT→WT) had a significant rise in plasma creatinine that was attenuated in Panx1−/− mice receiving Panx1−/− BM (KO→KO). More importantly, although the plasma creatinine was significantly elevated in KO→WT mice, mice with WT BM cells reconstituted into Panx1−/− mice (WT→KO) showed decreased plasma creatinine. These data show the importance of Panx1 expression in parenchymal cells of the kidney in mediating injury during IRI (Figure 3A).
Figure 3.
Proximal tubule– and endothelial-specific pannexin1−/− (Panx1−/−) mice are protected against renal ischemia-reperfusion injury (IRI). (A) Bone marrow chimeras show that nonhematopoietic cells are responsible for protection against IRI in Panx1−/− mice. Plasma creatinine values in bone marrow chimeras of Panx1+/+ (wild type [WT]) and Panx1−/− (knockout [KO]) mice subjected to 26 minutes of bilateral IRI and 24 hours of reperfusion are shown (n=8–10 per group). ****P<0.001. (B–F) PepckCrerePanx1f/f (KO; n=7), PepckCcrePanx1wt/wt (WT; n=7; [B, D, and F] proximal tubule–specific KO and WT controls), VeCadCreERT2+Panx1f/f (KO; n=10), and VeCadCreERT2+Panx1wt/wt (WT; n=6; [C, E, and G] tamoxifen-inducible endothelial-specific KO and WT controls) mice were subjected to 26 minutes of bilateral IRI or sham operation and 24 hours of reperfusion. Sham operations were performed using PepckCrePanx1wt/wt or PepckCrePanxf/wt littermates. Experimental design for B, D, and F is in Supplemental Figure 2B, and experimental design for C, E, and F is in Supplemental Figure 2C. (B and C) Plasma creatinine values of proximal tubule and endothelial Panx1−/− mice, respectively. ***P<0.001; ****P<0.001. (D and E) Quantification of acute tubular necrosis (ATN; percentage of outer medulla area) in hematoxylin and eosin–stained kidney sections of proximal tubule and endothelial Panx1−/− mice, respectively. **P<0.01; ***P<0.001; ****P<0.001. (F) Neutrophil infiltration is decreased in Panx1 tissue-specific KO mice. After IRI, kidneys of PepckCrePanx1f/f, VdCadCreERT2+Panx1f/f, and appropriate control mice were immunolabeled for the presence of neutrophils (red; 7/4 immunoreactivity) as described in Methods. Representative micrographs of outer medulla area are presented. Blue indicates 4′,6-diamidino-2-phenylindole-labeled nuclei. Green indicates kidney autofluorescence adjusted to reveal tubule architecture. Scale bars, 100 μm; 25 μm in insets.
Kidneys of Mice Deficient in Panx1 in Either PT or Vascular Endothelial Cells Are Protected from IRI
On the basis of the BM chimera experiments that Panx1 on parenchymal cells is important in kidney injury, we generated mice with tissue-specific deletions of Panx1. We used constitutive PT-specific Panx1 KO mice (PT Panx1KO; achieved by crossing PepckCre mice with Panx1f/f mice) and appropriate littermate controls (WT; PepckCre mice crossed with Panx1wt/wt mice).29 After IRI (the protocol is in Supplemental Figure 2), plasma creatinine increased in WT mice, but it was significantly attenuated in the PT Panx1KO mice (Figure 3B). By H&E staining, kidneys of PT Panx1KO had less ATN compared with WT mice (Figure 3D, Supplemental Figure 5). Given the importance of the endothelium in AKI30,31 and that endothelial Panx1 controls microvascular inflammation,21 we next examined the role of Panx1 deletion in endothelial cells. For these experiments, we used inducible VeCadCreERT2+Panx1f/f mice that we treated with tamoxifen (Methods) to induce Cre recombinase expression, leading to deletion of endothelial Panx1 (Supplemental Figure 2C); littermate control mice (WT; VeCadCreERT2+Panx1wt/wt) also received tamoxifen. Endothelial Panx1-deficient mice (Endo Panx1KO; VeCadCreERT2+Panx1f/f) were also protected from IRI. After ischemia-reperfusion, the rise in plasma creatinine observed in WT mice was attenuated in endothelial Panx1KO mice (Figure 3C). Histologically, by H&E staining, kidneys of endothelial Panx1KO had less ATN compared with WT mice (Figure 3E, Supplemental Figure 5). Neutrophil infiltration after IRI in kidneys of WT mice was markedly reduced in kidneys of both endothelial Panx1KO and PT Panx1KO mice (Figure 3F). Together, these data suggest that tissue-specific deletion of Panx1 from either vascular endothelial or PT cells protects kidneys from IRI and reduces immune cell infiltration, which is consistent with a pattern of reduced inflammation observed in Panx1−/− mice. Using stereology of H&E sections, the degree of protection relative to injury in rank order of protection (greatest to least) was Panx1−/− > Endo Panx1 KO > PT Panx1 KO mice.
ATP Release from Kidney Epithelial Cells Is Dependent on Panx1 Expression
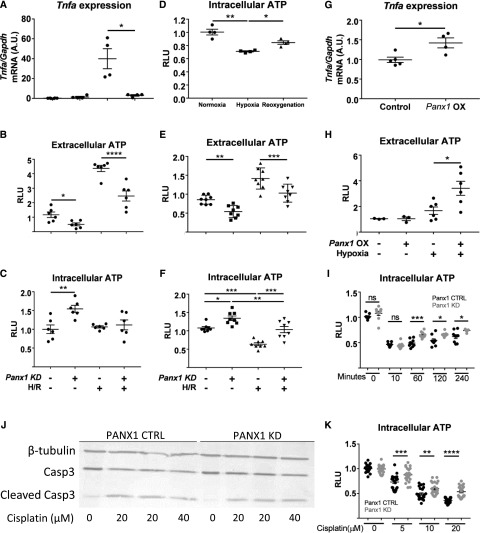
To study the relationship between ATP release, Panx1, and hypoxic injury, we used an in vitro H/R approach.32 Via the CRISPR/Cas9 system, we generated TKPTS (murine proximal tubule epithelial) cell lines with guide RNAs targeting Panx1 to generate a KD cell line (Panx1 KD or Panx1-deficient cells) or a scrambled guide RNA control (Panx1 control) (Supplemental Figure 4A). After mild H/R (60 minutes of hypoxia and 15 minutes of reoxygenation) of TKPTS cells, Tnfa, the most commonly used marker of tubular injury in this in vitro model, was increased in control TKPTS cells but not in Panx1-deficient cells (Figure 4A). Under normoxic conditions, intracellular ATP levels were higher and extracellular ATP levels were lower in Panx1-deficient compared with control TKPTS cells (Figure 4, B and C). After mild H/R, extracellular ATP was significantly increased in control TKPTS cells compared with Panx1-deficient cells (Figure 4B), but intracellular ATP levels were similar in Panx1-deficient and control TKPTS cells (Figure 4C). In control TKPTS cells with a longer period of hypoxia alone (360 minutes), intracellular ATP levels decreased, which is considered a hallmark of hypoxia, and during reoxygenation (60 minutes), intracellular ATP levels partially recovered (Figure 4D). After severe hypoxia and reoxygenation (360 and 60 minutes, respectively), extracellular ATP levels increased in control and Panx1-deficient cells relative to normoxia, but they were lower in Panx1-deficient cells than in control cells after H/R. Reciprocal changes were observed in intracellular ATP; levels decreased in control and Panx1-deficient cells relative to normoxia, but they were higher in Panx1-deficient cells than in control cells after H/R. (Figure 4, E and F).
Figure 4.
ATP release and/or intracellular retention in kidney tubule epithelial cells are key factors in protection from injury. (A–F) Pannexin1 (Panx1)-deficient (Panx1 knockdown [KD], +) and scrambled control (Panx1 KD, −; referred to as Panx1 control in Results) murine proximal tubule cell line (TKPTS) clones were created using the CRISPR/Cas9 system. Hypoxia-reoxygenation (H/R) injury was simulated in vitro by exposing cells to (A–C, G, and H) 60 minutes or (D–F) 360 minutes of hypoxia under mineral oil (or culture medium for sham conditions) followed by a period of reoxygenation in culture medium. Extracellular and intracellular ATP content was measured after a (B and C) 15-minute or (D–F) 60-minute reoxygenation period, and it is expressed as fold change compared with sham control cells (relative light units [RLUs]). (C) Tnfa mRNA levels were measured in cell lysates after 60 minutes of reoxygenation as an indicator of H/R-induced injury. (D) Intracellular ATP during normoxia, after 360 minutes of hypoxia alone, and after 360 minutes of hypoxia followed by 60 minutes of reoxygenation. (G and H) Transient overexpression of Panx1 causes cells to release more ATP in stress conditions. TKPTS cells were transfected using lipofectamine (mock transfection, control; −) or lipofectamine and plasmid to observe Panx1 overexpression (OX; +). (G) Tnfa mRNA levels were measured in cell lysates after 60 minutes of reoxygenation as an indicator of H/R-induced injury. (H) Extracellular ATP content was measured after a 15-minute reoxygenation period, and it is expressed as fold change compared with sham control cells. (I) Intracellular ATP decreased after addition of antimycin A but recovered more rapidly in Panx1-deficient cells. Antimycin A (20 μm) was added to cells, and intracellular ATP was measured 0, 10, 60, 120, or 240 minutes later. (J) Western blot of KD and control TKPTS cells treated with 20 or 40 μM cisplatin for 16 hours showing expression of caspase3 and the amount of cleaved caspase3, with β-tubulin used as loading control. Quantification is in Supplemental Figure 4C. (K) Decrease in intracellular ATP after incubation with cisplatin is smaller in Panx1-deficient cells. Treatment of cells with 5–20 μM cisplatin for 18 hours. Values are shown as fold change in RLU, with each group compared with their respective nontreated control. CTRL, control; Gapdh, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01; ***P<0.001; ****P<0.001.
Next, Panx1 was overexpressed in TKPTS cells. After mild H/R (as above), Tnfa was significantly greater in Panx1-overexpressing TKPTS cells (Figure 4G). Baseline extracellular ATP concentrations were similar in WT and Panx1-overexpressing TKPTS cells. However, after H/R, extracellular ATP increased significantly in Panx1-overexpressing TKPTS cells compared with control TKPTS cells (Figure 4H).
Two additional models of cellular injury were used. In the first model, intracellular ATP levels were reduced by one half 10 minutes after the addition of 20 μm antimycin A and recovered more rapidly in Panx1-deficient cells than in control cells over a period of 6 hours (Figure 4I). In the second model, the cisplatin-induced increase in the ratio of cleaved caspase3 to caspase3 was smaller in Panx1-deficient cells than in control cells. The ratios of cleaved to uncleaved caspase3 were 65%, 35%, and 16% lower in Panx1 KD cells compared with Panx1 control cells for 0, 20, and 40 μm cisplatin, respectively (Figure 4J, Supplemental Figure 4, B and C). A dose-dependent decrease in intracellular ATP was observed in control cells treated with 5–20 μM cisplatin over an 18-hour period, but this ATP depletion was significantly less in Panx1 KD cells (Figure 4K).
These in vitro studies suggest that extracellular ATP released by Panx1 and/or the loss of intracellular ATP can contribute to kidney injury by increasing inflammation through cytokine release and expression of adhesion molecules.
Discussion
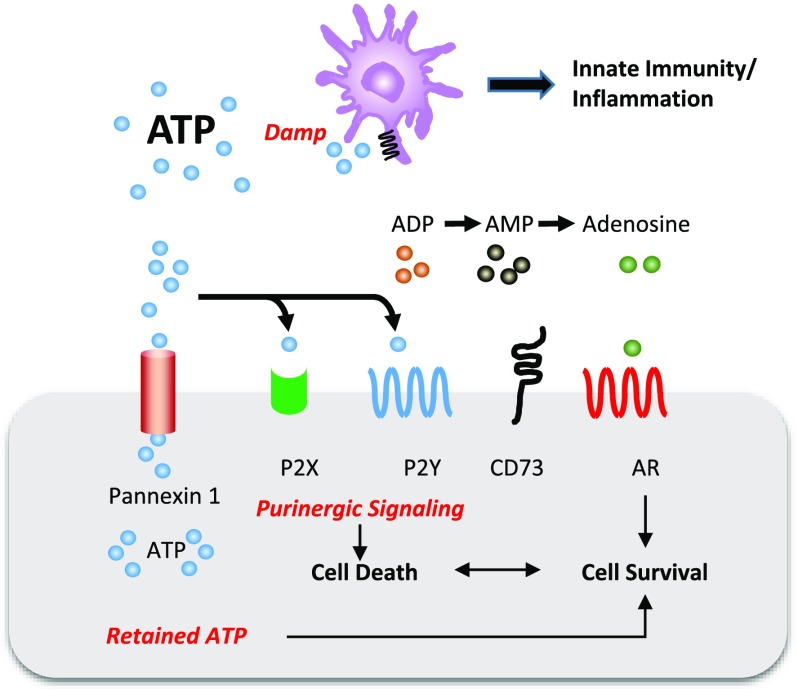
We identified an important role for Panx1 in kidney IRI. Pharmacologic inhibition and genetic ablation of this channel protected kidneys from ischemic injury. BM chimeras showed that Panx1 expression on parenchymal rather than hematopoietic cells is necessary for IRI, which suggests the importance of renal parenchymal Panx1 channels in propagation of injury. Consistent with BM chimeras, we observed protection from kidney injury in mice with tissue-specific deletion of Panx1 in PT or vascular endothelial cells, supporting the requirement for parenchymal Panx1. Protection observed in Panx1-deficient mice was associated with reduced inflammation as evidenced by decreased cytokine expression, adhesion molecule expression, and neutrophil infiltration. Panx1 KD PT cells were more resistant to cell death than control cells, and Panx1 deficiency was associated with greater intracellular and lower extracellular ATP. Two different mechanisms of Panx1 KO–mediated protection are proposed (Figure 5). One is decreased ATP (DAMP) release to the microenvironment and hence, attenuated inflammation, whereas the second may involve preservation of intracellular ATP stores to avoid cell death. Taken together, these results strongly suggest an important role of Panx1 in regulating epithelial and endothelial ATP flux and AKI; Panx1 inhibition may serve as an important target for the prevention of AKI.
Figure 5.
Pannexin1 (Panx1) deficiency protects mouse kidneys from ischemia-reperfusion injury (IRI). ATP is efficiently released from cells through Panx1 channels. During injury, released ATP may (1) act as a damage-associated molecular pattern (DAMP) and activate innate immunity and inflammation or (2) serve as a substrate for sequential dephosphorylation with ATP/ADP acting on P2X/P2Y receptors, leading to cell death or further downstream dephosphorylation by CD39 (not depicted) and CD73 to adenosine, which may lead to cell survival through adenosine receptors (ARs). Endothelial cells and epithelial cells that are deficient of Panx1 may (1) decrease extracellular ATP and attenuate DAMPs-mediated inflammation or decrease purinergic signaling–induced cell death of either endothelial cells or epithelial cells or (2) retain ATP, leading to cell survival.
The renal interstitial microenvironment is a unique immunologic milieu that is situated between the basement membranes of epithelial cells and consists of interstitial fibroblasts, pericytes, endothelial cells, and mononuclear phagocytes.33 Under normal homeostatic conditions, the interstitium is involved in sensing and processing filtered antigens by immune cells as well as managing reabsorbed solute and fluids.34 Fibroblasts have a regulatory function and produce substances, such as adenosine and erythropoietin, to maintain kidney homeostasis.35 After kidney injury, the fibroblasts become the main producers of extracellular matrix and a driving force behind fibrosis in progression to CKD.36 Tubular cells, normally having a versatile role in kidney function, can also activate innate and adaptive immune responses after IRI by signaling to interstitial immune cells. One such mechanism works through the release of proinflammatory DAMPs, such as ATP, heat shock proteins, double-stranded DNA, and others. These can, in turn, bind to receptors present on dendritic cells, and thereby, they can activate innate immunity and also help in the resolution of injury.1,37
Purinergic signaling in the kidney regulates kidney homeostasis as well as disease response.38 Adenosine is a purine nucleoside produced from ATP by sequential enzymatic dephosphorylation in tissues under stress, such as ischemia, hypoxia, and inflammation. In the kidney, adenosine is an important regulator of metabolic cellular activity.39 After released into the interstitial microenvironment, adenosine can bind to different types of adenosine receptors (i.e., A1, A2A, A2B, and A3R) expressed on various innate immune cells, such as neutrophils, macrophages, dendritic cells, and natural killer cells, but also, the tubular epithelium,40,41 and it can also modulate cellular responses.42–44 Adenosine production is largely dependent on enzyme activity. CD73 is a cell surface 5′-ectonucleotidase responsible for breakdown of AMP to adenosine,45 and it is highly expressed in fibroblasts or PTs, where it has been shown to promote protection against IRI.46 ATP infusion had a protective effect on renal regeneration after ischemic insult47; however, it may also function as the source of adenosine.48 In vitro injury studies by Kribben et al.49 and Nakagawa et al.50 identified ATP as a proregenerative factor in isolated rat PT culture.
Although released ATP serves as a source of adenosine, our study shows that ATP may act as a DAMP. It is known that purinergic P2 receptors, which can induce proinflammatory molecular pathways, are present both throughout the renal tissue3 and across immune cell populations.51 In the context of AKI, the most likely mediators of the effects of Panx1-released ATP are P2X7 receptors. Studies using A438079, a P2X7-specific receptor antagonist, showed that P2X7 receptors could be necessary for IRI,18 and BM chimeras of WT and P2X7 KO mice showed that immune cell P2X7 receptors are responsible for the inflammatory response in IRI.52 Taken together, the data show that, in IRI, ATP released from injured tissue (as seen in our studies) can activate an immune response through P2X7 receptors and that disruption of ATP-P2X7 signaling may be a novel therapeutic strategy in AKI treatment.
Panx1 is a large, nonselective transmembrane channel that efficiently releases ATP to the extracellular space on activation.9 The cellular localization of Panx1 in the kidney is unknown, and other isoforms and Panx2 and Panx3 mRNA were undetectable in whole-kidney tissue lysates by qPCR and use of TaqMan probes (data not shown). There is a limited number of studies in the kidney. (1) Panx1 was localized to the renal artery, and Panx3 was localized to the juxtaglomerular apparatus and cortical arterioles.53 (2) Panx1 was expressed in the kidney epithelium.14 In this latter study, Panx1 was found along the apical membrane of almost all of the nephron, excluding distal convoluted tubule.
On comparing the degree of protection in the three models, we observed a rank order of protection (greatest protection to least protection)—global Panx1 KO > endothelial Panx1 > epithelial Panx1. We base this conclusion on a rigorous stereologic morphometric analysis (Methods) previously described by us,26 which is the gold standard. Given the critical role played by different tissues in kidney IRI, such as endothelium, PTs, and immune cells, it is not surprising that an intervention at any of these targets can lead to marked protection. Although plasma creatinine may not have shown differences in the degree of protection in these models, plasma creatinine may not necessarily provide a sensitive method to detect differences between groups, and urinary or tissue biomarkers have not been validated in these genetic constructs to provide a sensitive or specific measure of tissue injury. Several different mechanisms may be involved in the protective effect in the global Panx1-deficient mice. The effect of ATP release by vascular Panx1 on inflammatory cells has been described.21 Using intravital microscopy, decreased leukocyte adhesion and emigration in endothelial Panx1 KO mice after topical administration of TNFα to the cremaster muscle was observed. In these studies, kidneys of epithelial Panx1 KO, endothelial Panx1 KO, and global Panx1 KO mice were more resistant to IRI than the control groups. In kidneys of global Panx1 KO mice, there was marked suppression of IL-6, an inflammatory marker, as well as adhesion molecules and neutrophil infiltration. Although we observed a decrease in a number of other proinflammatory cytokine transcripts, the difference in expression was not significant (perhaps due to the measurement of transcripts at a late 24-hour time point). The differences in Tnfa that were observed between homogenous populations of control and Panx1 KD TKPTS cells in vitro were not evident in kidneys, where a heterogeneous population of cells, only some of which produce TNF, contributes to whole-kidney lysate; lack of changes in Tnfa expression in vivo could, therefore, be due to limitations in sensitivity of detection and later time points. These data indicate that global Panx1 deficiency in comparison with WT mice is associated with reduced kidney inflammation after IRI. Panx1 is often found on polarized cells13; luminal ATP is derived from filtered nucleotides as well as ATP released from epithelial cells54 and reaches greater concentrations in PTs compared with more distal segments.55 The effect of ATP, released from cells after a hypoxic event, on surrounding tissue can only be extrapolated from studies mentioned earlier in the context of leukocytes in purinergic signaling.56,57 Further studies will be needed to assess the effect of P2 receptors on injury caused by extracellularly released ATP. Higher intracellular ATP levels in cells deficient of Panx1 may be important in providing necessary energy to maintain oxidative phosphorylation to minimize hypoxic damage. Thus, protection from IRI may be due in part to maintenance of intracellular ATP and reduced extracellular ATP minimizing local DAMP-mediated activation of the immune response. Future studies will examine the intracellular distribution of ATP associated with Panx1 deficiency, because it may govern ATP permeability between intracellular compartments and not just at the cell membrane.58
In summary, we show the role of Panx1 channel as a defining factor in kidney response to IRI. Released ATP may act as a DAMP on surrounding tissue and infiltrating immune cells, allowing propagation of inflammation and injury. In a reciprocal manner, reduced intracellular ATP in Panx1-sufficient cells leads to reduced energy equivalence and renders cells susceptible to injury. We show that disrupting Panx1-mediated ATP release protects kidney function and histology and may prove to be an effective therapeutic or preventive strategy in AKI.
Disclosures
M.D.O. has a research grant from Pfizer Inc./AM-Pharma B.V. and has equity in Adenosine Therapeutics, LLC.
Supplementary Material
Acknowledgments
We thank the University of Virginia Research Histology Core for assistance in preparation of histology slides. We thank Dr. Elsa Bello-Reuss (Texas Tech University Health Sciences Center) for providing transformed murine proximal tubule cell line cells and all members of the laboratory of M.D.O.
Research conducted for this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health awards F32DK108563 (to H.M.P.), American Society of Nephrology Ben Lipps Fellowship Program (to H.M.P.), K01DK91444, R03DK107941, and Carl W. Gottschalk Research Scholar Grant of the ASN Foundation for Kidney Research (to A.B.), P01HL120480 (to K.S.R. and B.E.I.), R01DK062324 (to M.D.O.), R01DK085259 (to M.D.O.), R01DK105133 (to M.D.O.), and T32DK72922 (to M.D.O.) and support via Training in the Pharmacological Sciences grant 5T32GM007055 (to C.B.M.). The stereology data described here were gathered on an “MBF Bioscience and Zeiss microscope system for stereology and tissue morphology” funded by National Institutes of Health grant 1S10RR026799-01 (to M.D.O.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017121306/-/DCSupplemental.
References
- 1.Rosin DL, Okusa MD: Dangers within: DAMP responses to damage and cell death in kidney disease. J Am Soc Nephrol 22: 416–425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idzko M, Ferrari D, Eltzschig HK: Nucleotide signalling during inflammation. Nature 509: 310–317, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solini A, Usuelli V, Fiorina P: The dark side of extracellular ATP in kidney diseases. J Am Soc Nephrol 26: 1007–1016, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HT, Jan M, Bae SC, Joo JD, Goubaeva FR, Yang J, et al.: A1 adenosine receptor knockout mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol 290: F1367–F1375, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Okusa MD, Linden J, Macdonald T, Huang L: Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol 277: F404–F412, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Day Y-J, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, et al.: Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK: Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H: Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100: 13644–13649, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao L, Locovei S, Dahl G: Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Cisneros-Mejorado A, Gottlieb M, Cavaliere F, Magnus T, Koch-Nolte F, Scemes E, et al.: Blockade of P2X7 receptors or pannexin-1 channels similarly attenuates postischemic damage. J Cereb Blood Flow Metab 35: 843–850, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong F, Yang XJ, Jiang TB, Chen Y: Ischemia triggered ATP release through Pannexin-1 channel by myocardial cells activates sympathetic fibers. Microvasc Res 104: 32–37, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, et al.: Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal 1: ra6, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M: Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J: Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol 303: F1454–F1459, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inscho EW: ATP, P2 receptors and the renal microcirculation. Purinergic Signal 5: 447–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praetorius HA, Leipziger J: Intrarenal purinergic signaling in the control of renal tubular transport. Annu Rev Physiol 72: 377–393, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Y, Bai J, Zhou X, Tang J, Jiang C, Tolbert E, et al.: P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol 308: C463–C472, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabadi M, Kim M, Li H, Han SJ, Choi Y, D’Agati V, et al.: ATP induces PAD4 in renal proximal tubule cells via P2X7 receptor activation to exacerbate ischemic AKI. Am J Physiol Renal Physiol 314: F293–F305, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, et al.: Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507: 329–334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohman AW, Leskov IL, Butcher JT, Johnstone SR, Stokes TA, Begandt D, et al.: Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat Commun 6: 7965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good ME, Eucker SA, Li J, Bacon HM, Lang SM, Butcher JT, et al.: Endothelial cell Pannexin1 modulates severity of ischemic stroke by regulating cerebral inflammation and myogenic tone. JCI Insight 3: pii:96272, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, et al.: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feil S, Krauss J, Thunemann M, Feil R: Genetic inducible fate mapping in adult mice using tamoxifen-dependent Cre recombinases. Methods Mol Biol 1194: 113–139, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Inoue T, Abe C, Sung SS, Moscalu S, Jankowski J, Huang L, et al.: Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through α7nAChR+ splenocytes. J Clin Invest 126: 1939–1952, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernest S, Bello-Reuss E: Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Michalski K, Kawate T: Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J Gen Physiol 147: 165–174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwiebert EM: ATP release mechanisms, ATP receptors and purinergic signalling along the nephron. Clin Exp Pharmacol Physiol 28: 340–350, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Perry HM, Huang L, Ye H, Liu C, Sung SJ, Lynch KR, et al.: Endothelial sphingosine 1‑phosphate receptor‑1 mediates protection and recovery from acute kidney injury. J Am Soc Nephrol 27: 3383–3393, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molitoris BA, Sutton TA: Endothelial injury and dysfunction: Role in the extension phase of acute renal failure. Kidney Int 66: 496–499, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, Harken AH: A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res 99: 288–293, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Kaissling B, Le Hir M: The renal cortical interstitium: Morphological and functional aspects. Histochem Cell Biol 130: 247–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okusa MD, Li L: Dendritic cells in acute kidney injury: Cues from the microenvironment. Trans Am Clin Climatol Assoc 123: 54–62, 2012 [PMC free article] [PubMed] [Google Scholar]
- 35.Souma T, Suzuki N, Yamamoto M: Renal erythropoietin-producing cells in health and disease. Front Physiol 6: 167, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeisberg M, Kalluri R: Physiology of the renal interstitium. Clin J Am Soc Nephrol 10: 1831–1840, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim MG, Boo CS, Ko YS, Lee HY, Cho WY, Kim HK, et al.: Depletion of kidney CD11c+ F4/80+ cells impairs the recovery process in ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant 25: 2908–2921, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Menzies RI, Tam FW, Unwin RJ, Bailey MA: Purinergic signaling in kidney disease. Kidney Int 91: 315–323, 2017 [DOI] [PubMed] [Google Scholar]
- 39.Hansen PB, Schnermann J: Vasoconstrictor and vasodilator effects of adenosine in the kidney. Am J Physiol Renal Physiol 285: F590–F599, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J: International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- 41.Welch WJ: Adenosine, type 1 receptors: Role in proximal tubule Na+ reabsorption. Acta Physiol (Oxf) 213: 242–248, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Okusa MD: Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J: Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203: 2639–2648, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gessi S, Varani K, Merighi S, Ongini E, Borea PA: A(2A) adenosine receptors in human peripheral blood cells. Br J Pharmacol 129: 2–11, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar V, Sharma A: Adenosine: An endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol 616: 7–15, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Sung SJ, Li L, Huang L, Lawler J, Ye H, Rosin DL, et al.: Proximal tubule CD73 is critical in renal ischemia-reperfusion injury protection. J Am Soc Nephrol 28: 888–902, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegel NJ, Glazier WB, Chaudry IH, Gaudio KM, Lytton B, Baue AE, et al.: Enhanced recovery from acute renal failure by the postischemic infusin of adenine nucleotides and magnesium chloride in rats. Kidney Int 17: 338–349, 1980 [DOI] [PubMed] [Google Scholar]
- 48.Mandel LJ, Takano T, Soltoff SP, Murdaugh S: Mechanisms whereby exogenous adenine nucleotides improve rabbit renal proximal function during and after anoxia. J Clin Invest 81: 1255–1264, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kribben A, Feldkamp T, Horbelt M, Lange B, Pietruck F, Herget-Rosenthal S, et al.: ATP protects, by way of receptor-mediated mechanisms, against hypoxia-induced injury in renal proximal tubules. J Lab Clin Med 141: 67–73, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa S, Omura T, Yonezawa A, Yano I, Nakagawa T, Matsubara K: Extracellular nucleotides from dying cells act as molecular signals to promote wound repair in renal tubular injury. Am J Physiol Renal Physiol 307: F1404–F1411, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Di Virgilio F, Vuerich M: Purinergic signaling in the immune system. Auton Neurosci 191: 117–123, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Koo TY, Lee JG, Yan JJ, Jang JY, Ju KD, Han M, et al.: The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int 92: 415–431, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Lohman AW, Billaud M, Straub AC, Johnstone SR, Best AK, Lee M, et al.: Expression of pannexin isoforms in the systemic murine arterial network. J Vasc Res 49: 405–416, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwiebert EM, Kishore BK: Extracellular nucleotide signaling along the renal epithelium. Am J Physiol Renal Physiol 280: F945–F963, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Vekaria RM, Unwin RJ, Shirley DG: Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Junger WG: Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11: 201–212, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob F, Pérez Novo C, Bachert C, Van Crombruggen K: Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal 9: 285–306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Penuela S, Lohman AW, Lai W, Gyenis L, Litchfield DW, Isakson BE, et al.: Diverse post-translational modifications of the pannexin family of channel-forming proteins. Channels (Austin) 8: 124–130, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.