Abstract
Background Osmosis drives transcapillary ultrafiltration and water removal in patients treated with peritoneal dialysis. Crystalloid osmosis, typically induced by glucose, relies on dialysate tonicity and occurs through endothelial aquaporin-1 water channels and interendothelial clefts. In contrast, the mechanisms mediating water flow driven by colloidal agents, such as icodextrin, and combinations of osmotic agents have not been evaluated.
Methods We used experimental models of peritoneal dialysis in mouse and biophysical studies combined with mathematical modeling to evaluate the mechanisms of colloid versus crystalloid osmosis across the peritoneal membrane and to investigate the pathways mediating water flow generated by the glucose polymer icodextrin.
Results In silico modeling and in vivo studies showed that deletion of aquaporin-1 did not influence osmotic water transport induced by icodextrin but did affect that induced by crystalloid agents. Water flow induced by icodextrin was dependent upon the presence of large, colloidal fractions, with a reflection coefficient close to unity, a low diffusion capacity, and a minimal effect on dialysate osmolality. Combining crystalloid and colloid osmotic agents in the same dialysis solution strikingly enhanced water and sodium transport across the peritoneal membrane, improving ultrafiltration efficiency over that obtained with either type of agent alone.
Conclusions These data cast light on the molecular mechanisms involved in colloid versus crystalloid osmosis and characterize novel osmotic agents. Dialysis solutions combining crystalloid and colloid particles may help restore fluid balance in patients treated with peritoneal dialysis.
Keywords: peritoneal dialysis, aquaporin-1, ultrafiltration, membrane permeability
Peritoneal dialysis (PD) is a renal replacement modality that applies the principle of osmosis through the peritoneal membrane to restore water balance in patients with ESRD. Fluid overload is associated with poor outcome in patients on dialysis,1 and achieving adequate ultrafiltration (UF) is key to successful PD, especially in patients with low residual renal function.2–6
Osmosis refers to the movement of water across a barrier that is permeable to water but restricts the transport of solutes—which therefore act as osmotic agents. In 1861, Graham was the first to introduce a distinction between osmotic agents on the basis of their physical properties: small-sized osmotic agents prone to crystallization and that easily diffuse across membranes were termed crystalloid, whereas noncrystalline substances retained by membranes because of their larger size were referred to as colloid (from the Ancient Greek κολλα, glue).7 Although glucose has been used as the prototypic crystalloid osmotic agent to drive water removal in PD for decades, its use carries major limitations. Because of their small size, glucose molecules easily diffuse across the peritoneal membrane, thereby limiting UF during long dwells. Furthermore, glucose leads to adverse metabolic effects,8 and its long-term use is associated with structural and functional changes of the peritoneal membrane that contribute to loss of UF capacity.9–11 These drawbacks have led to the development of alternative osmotic agents including icodextrin, a mixture of glucose polymers. Thanks to their larger hydrodynamic radius (RH), polymers of icodextrin are absorbed from the peritoneal cavity at a much slower rate than glucose, via the lymphatic circulation, making them particularly suitable for long dwells and sustained UF.12,13
The transport of water and solutes across the endothelium lining peritoneal capillaries during PD can be functionally described in terms of a three-pore model (TPM).14,15 On the basis of the TPM, numerous small pores located at interendothelial clefts mediate solute-coupled fluid transport, ultrasmall pores located in endothelial cells facilitate transcellular water transport, and a relatively small number of large pores account for transport of macromolecules but have a negligible role in water transport.14–17 The identification of aquaporin-1 (AQP1) water channels in the endothelium of peritoneal capillaries and studies of peritoneal transport in Aqp1 mouse models established AQP1 as the molecular counterpart of ultrasmall pores, playing a critical role in water removal during PD with glucose-based dialysis solutions.18–23 In contrast with crystalloid osmosis, the colloidal size of some glucose polymers and the absence of sodium sieving suggested that icodextrin induces transperitoneal UF by different mechanisms.12,24 In addition, computer-based predictions and scarce observations in patients undergoing PD suggested the potential advantage of combining icodextrin and glucose in the same dwell to potentiate UF.25,26 However, the pathways and mechanisms of colloid versus crystalloid osmosis across the peritoneal membrane, and the mechanism of action of combinations of osmotic agents, have not been investigated.
In this study, we combined experimental studies in mouse models of PD, biophysical studies, and advanced computer simulations to characterize the basic principles of colloid versus crystalloid osmosis across the peritoneal membrane and to investigate the pathways mediating water flow generated by the glucose polymer icodextrin. We also demonstrate how dialysis solutions combining crystalloid and colloid particles may improve the control of fluid volume in PD.
Methods
Full details of the methods can be found in the Supplemental Material.
Mouse TPM
The initial parameters of the TPM were adapted on the basis of osmotic conductance for glucose and peritoneal solute transport rate measured in independent experiments in Aqp1+/+ and Aqp1−/− mice; the αC parameter was adjusted from 0.02 to 0.04, to match both the difference between Aqp1+/+ and Aqp1−/− mice (using 3.86% glucose as osmotic agent) and the osmotic water transport for icodextrin (Supplemental Table 1). All relevant exchange parameters were rescaled by (body weight)0.75, corresponding to a scaling factor of approximately 336 from man to mouse, similar to that previously described.20
Animals
Sex-matched Aqp1 mouse littermates generated as previously described,27 aged 8–12 weeks, were used to assess the influence of the water channel AQP1. All animals had access to standard diet and tap water ad libitum. Experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and the Animal Ethics Committee of the UCL Medical School.
Peritoneal Transport Studies
Transport across the mouse peritoneum was investigated using a modified peritoneal equilibration test with different PD solutions (Baxter Healthcare, Belgium) (Table 1), as described previously.20,21,28,29 Baseline characteristics of the animals are presented in Supplemental Table 2. Intraperitoneal volume (IPV) versus time curves were performed using a fluorescent BSA conjugate as an indicator-dilution technique, as previously described,21 or by direct volumetry. Both techniques yielded values that were closely correlated, including when icodextrin was used as osmotic agent (Pearson r=0.98, P=0.003). Dialysate sodium was measured using the indirect ion selective electrode method.
Table 1.
Conventional and bimodal PD solutions: composition and osmolarity
| Variable | 1.36% Gluc. | 3.86% Gluc. | 1.1% A.A. | 7.5% Icod. | CIG 1.36% | CIG 3.86% |
|---|---|---|---|---|---|---|
| Sodium, mmol/L | 132 | 132 | 132 | 133 | 132 | 132 |
| Calcium, mmol/L | 1.25 | 1.25 | 1.25 | 1.75 | 1.75 | 1.75 |
| Magnesium, mmol/L | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Lactate, mmol/L | 40 | 40 | 40 | 40 | 40 | 40 |
| Chloride, mmol/L | 95 | 95 | 105 | 96 | 96 | 96 |
| Osmotic agent | ||||||
| Glucose, mmol/L | 75.49 | 214.25 | — | — | 75.49 | 214.25 |
| Glucose monohydrate, g/dl | 1.5 | 4.25 | — | — | 1.5 | 4.25 |
| Amino acids, mmol/L | — | — | 87 | — | — | — |
| Icodextrin, g/dl | — | — | — | 7.5 | 7.5 | 7.5 |
| Osmolarity (mOsm/L) | 344 | 483 | 365 | 284 | 358 | 497 |
| pH | 5.2 | 5.2 | 6.7 | 5.0–6.0 | 5.0–6.0 | 5.0–6.0 |
Gluc., glucose; A.A., amino acids; icod., icodextrin; —, not applicable.
Dynamic Light Scattering
Dynamic light scattering was used to determine the apparent RH and size distribution profile of the glucose polymer icodextrin in solution (Extraneal 7.5%). The measurements were performed on a Malvern CGS3 equipped with a HeNe laser (λ=632.8 nm) at a temperature of 37.3°C and an angle of 90°. The size distributions were obtained by a CONTIN analysis of the data.
Determination of Total Icodextrin, Icodextrin Metabolites, and Absorption of Carbohydrates
Total icodextrin in the dialysate was measured by enzymatic hydrolysis with amyloglucosidase (Sigma-Aldrich, Saint-Louis, MO), icodextrin metabolites were determined in the dialysate of mice exposed to 7.5% icodextrin using a weak anion-exchange column (BioBasic AX; 150×4.6 mm, 5 µm; Thermo Electron, Woburn, MA) and a refractive index detector, and total HMW fractions were estimated as the difference between total icodextrin and LMW G2-G7 fractions, as previously described.30–32 The amount of carbohydrates absorbed from the peritoneal cavity was calculated as the difference between the mass of carbohydrates (glucose and icodextrin) infused into the peritoneal cavity and the mass removed.
Statistical Methods
Data are presented as mean±SEM. Comparisons between results from different groups were performed using a t test, one-, or two-way ANOVA, as appropriate. Significance level was indicated in the legend of each figure (*P<0.05, **P<0.01, ***P<0.001).
Results
Osmosis Induced by Icodextrin Occurs Independently of Water Channels and Tonicity
We first assessed the relative contribution of transcellular and paracellular routes to osmotic water transport induced by various osmotic agents, using TPM-based modeling and a well established mouse model of PD with commercially available PD solutions (Table 1).
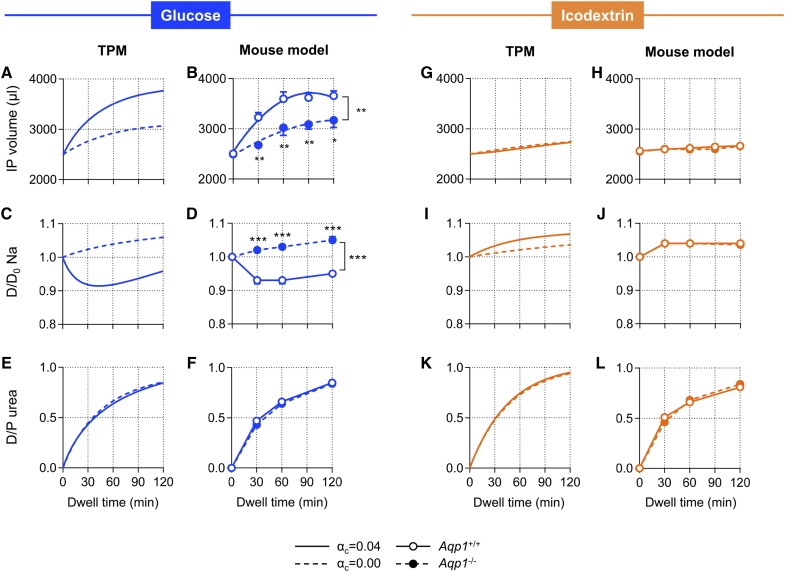
The original version of the TPM was adapted to the intrinsic permeability characteristics of the mouse peritoneal membrane (Supplemental Table 1), to allow crossvalidation between computer simulations and experimental data obtained in vivo and provide mechanistic insights into the physiology of water transport. We applied this mouse-adapted version of the TPM to simulate changes in IPV, dialysate sodium kinetics, and peritoneal solute transport over time, using hypertonic glucose and icodextrin as prototypic osmotic agents. We next performed the same procedure in vivo, using experimental PD on Aqp1 mice (Figure 1).
Figure 1.
Osmosis induced by icodextrin occurs independently of water channels and tonicity. Results from computer simulations on the basis of the TPM and from an experimental model of PD in Aqp1 mice showing intraperitoneal (IP) volume versus time curves (A, B, G, and H), kinetics of dialysate sodium (C, D, I, and J), and dialysate-to-plasma ratio (D/P) of urea (E, F, K, and L). The TPM applied a fractional Kf for ultrasmall pores (αc) of either 0.04 (solid line) or 0.00 (dotted line), and in vivo experiments were performed in Aqp1+/+ (open circles, solid line) and Aqp1−/− (close circles, dotted lines) mice during 2-hour dwells, using 3.86% glucose (blue) or 7.5% icodextrin (orange). Data in mice are mean±SEM; n=4–6/group. Na, sodium.
In both models, hypertonic glucose-based dialysate induced a rapid increase in IPV and a dialysate sodium sieving during the first part of the dwell in the presence of ultrasmall pores (water channels) (Figure 1, A–D). The absence of water channels was associated with a significant reduction in the osmotic water permeability of the peritoneal membrane, the abolition of sodium sieving, and an approximately 50% decrease in total net UF (Figure 1, A–D, Table 2), in line with previous studies.20,21 To the contrary, solutions containing icodextrin induced a slow and sustained UF, with no sodium sieving (Figure 1, G–J). The latter kinetics were essentially unchanged in absence of water channels (modified TPM or deletion of AQP1) (Figure 1, G–J). Peritoneal solute transport rate—expressed as the dialysate-to-plasma ratio (D/P) of urea—was not affected by the absence of water channels (Figure 1, E, F, K, and L). The osmotic effect of glucose was transient, with a decreased UF rate that paralleled dissipation of the osmotic gradient. In contrast, the water flow induced by icodextrin was constant over time, independent of the osmotic gradient, occurring despite the lower tonicity of the dialysate compared with that of plasma (Supplemental Figure 1). In these experiments, measured changes in IPV closely correlated with predictions from the TPM (Pearson r=0.98, P<0.001) and both techniques were in excellent agreement (Supplemental Figure 2), thereby validating the mouse-adapted version of the TPM. Longer dwell times (up to 4 hours) using 7.5% icodextrin in our model and in the adapted version of the TPM were associated with a progressive and linear increase in net UF (from 10.1±2.2 to 18.1±1.9 and 24.2±3.3 µl/g body wt for 2, 3, and 4-hour dwells in mice, respectively; P for linear trend <0.01). To the contrary, using hypertonic glucose as osmotic agent, IPV reached a plateau after 90–100 minutes, followed by a decrease.
Table 2.
Transport parameters in Aqp1 mice using various osmotic agents
| Solution | Genotype | Net UF (µl/g body wt) | MTAC Urea Garred (µl/min) | MTAC Urea Waniewski (µl/min) |
|---|---|---|---|---|
| 1.36% glucose | Aqp1+/+ | 0.4±1.5 | 37.9±3.2 | 37.7±3.1 |
| Aqp1−/− | −5.8±1.4a | 42.5±2.5 | 43.7±2.6 | |
| 3.86% glucose | Aqp1+/+ | 34.0±1.6 | 31.5±1.4 | 29.8±1.3 |
| Aqp1−/− | 11.6±1.2b | 40.5±1.8 | 39.2±1.7 | |
| 1.1% aminoacids | Aqp1+/+ | 6.0±0.7 | 32.4±1.9 | 31.9±1.8 |
| Aqp1−/− | −1.7±1.0b | 35.4±1.8 | 35.6±1.9 | |
| 7.5% icodextrin | Aqp1+/+ | 5.4±1.3 | 31.4±1.1 | 31.0±1.1 |
| Aqp1−/− | 5.9±0.7 | 35.0±1.4 | 34.4±1.4 |
MTAC urea calculated using an f value of 0.33. n=4–6 mice per group. MTAC, mass transfer area coefficient.
P<0.05 versus Aqp1+/+.
P<0.001 versus Aqp1+/+.
P<0.01 versus Aqp1+/+.
To investigate how variations in mouse peritoneal solute transport influence water removal induced by different osmotic agents, we used intraperitoneal exposure to LPS (10 mg/kg) to modulate solute transport. Twenty-four hours after exposure to LPS, the D/P urea increased by 22% and 18% in 3.86% glucose- and 7.5% icodextrin-based solutions, respectively (P=0.92) (Supplemental Figure 3A). Whereas the approximately 20% increase in solute transport was associated with a 47% decrease in net UF generated by glucose, water removal achieved by icodextrin-based solutions increased by +114% from the basal condition (P<0.001 between glucose and icodextrin) (Supplemental Figure 3B). When analyzing all experiments, there was a negative correlation between net UF and solute transport during glucose-based dwells (Pearson correlation coefficient, r=−0.85, P<0.001, n=15), whereas both parameters positively correlated when icodextrin was used (r=0.71, P=0.003, n=15) (Supplemental Figure 3C).
We next tested the influence of AQP1 on the osmotic water transport generated by other crystalloid dialysis solutions (Tables 1 and 2), by comparing the net UF obtained in Aqp1 mice after 2-hour dwells using 1.36% glucose, 1.1% amino acids, 3.86% glucose, or 7.5% icodextrin. As shown in Figure 2A, glucose and amino acids, but not icodextrin, required the presence of water channels to induce transperitoneal osmosis. Solute transport, evaluated by the mass transfer area coefficient for urea, was similar in all groups (Table 2). The amount of UF generated by crystalloid agents closely correlated with the osmotic gradient between plasma and dialysis solution at baseline (Pearson r=0.98, P<0.0001) (Figure 2B). These findings are in agreement with simulations from the TPM, highlighting the importance of transcellular ultrasmall pores for crystalloid osmotic agents (i.e., low RH) (Figure 2C).
Figure 2.
Critical role of water channels and tonicity for crystalloid osmosis. (A) Net UF generated at the end of 2-hour dwells with 2.5 ml of dialysate containing 1.36% glucose, 3.86% glucose, 1.1% aminoacids, or 7.5% icodextrin, in Aqp1+/+ (black boxes) and Aqp1−/− (red boxes) mice. Boxes and whiskers represent minimum to maximum values; n=6/group. (B) Correlation between net UF obtained in the mouse model and the transperitoneal osmotic gradient at baseline (dialysate-to-plasma ratio of osmolality, D/Posm) for crystalloid osmotic agents (glucose and aminoacids, black circles, red line). Values for icodextrin are provided (gray circles) for comparison; each circle represents a mouse. (C) Predictions from the TPM showing the relationship between osmotic pressure and RH for fractional UF coefficients for ultrasmall pores (αc) of 0.04 (red line), 0.02 (black), and 0.00 (gray). BW, body weight.
The modelized data, validated in vivo, substantiated predictions that icodextrin induces a slow but sustained transperitoneal water flow exclusively via a paracellular route, independently of the crystalloid osmotic gradient. Conversely, glucose and amino acids provide a fast but transient water transport both via paracellular and transcellular pathways; they require the presence of an osmotic gradient and endothelial water channels to generate UF.
Colloid Osmosis Is Induced by Large Icodextrin Fractions
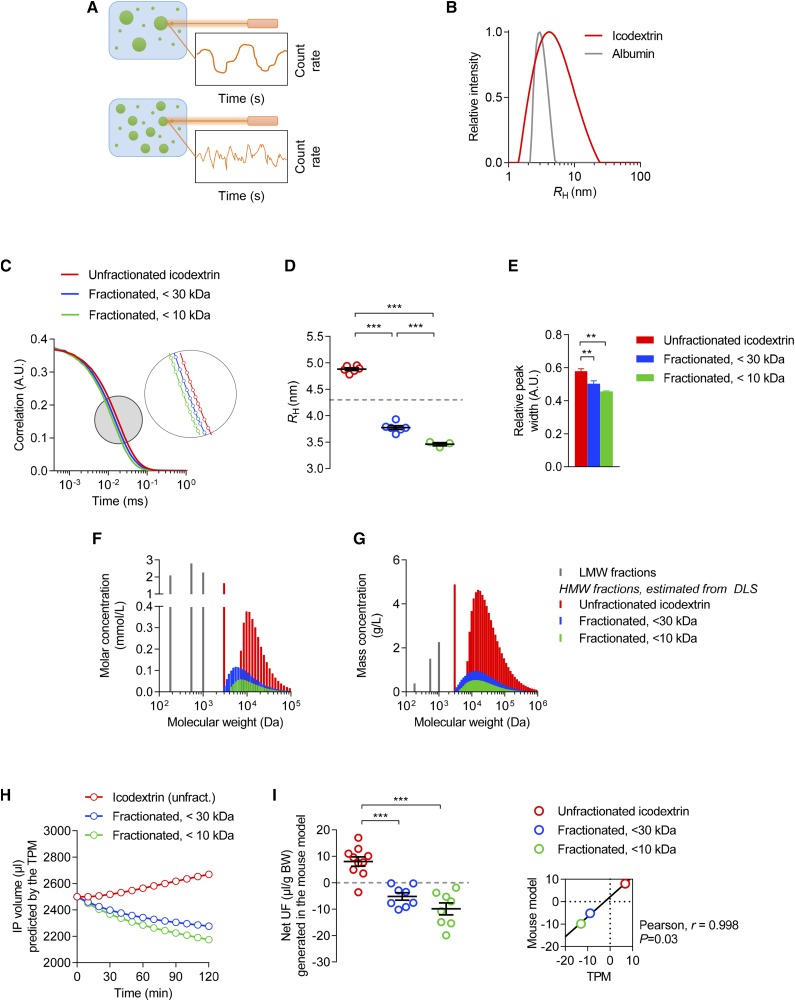
Because the ability of solutes to permeate the peritoneal membrane is primarily determined by their RH and the functional radius of small pores (approximately 4.0 nm),33 we assessed the RH of glucose polymers by dynamic light scattering (Figure 3A). Mean RH of icodextrin was 4.9 nm, with fractions ranging from 1.0 to 23.0 nm, versus 3.65 nm for albumin (Figure 3B). The presence of icodextrin fractions larger than albumin—the prototypic colloid osmotic agent—substantiated the fact that some icodextrin fractions induce colloid osmosis.12
Figure 3.
Colloid osmosis is induced by large icodextrin fractions. (A) Principle of dynamic light scattering (DLS). The sample is illuminated by a laser beam (λ=632.8 nm) and the fluctuations of the scattered light are detected at a known scattering angle by a fast photon detector. The particles in solution scatter the light, providing information about their Brownian motion, size, and distribution. (B) CONTIN size distribution of polymers in a 7.5% icodextrin solution (red line, mean of five independent measures) compared with human serum albumin (HSA) (gray line). The average apparent RH (Stokes–Einstein radius) of icodextrin is 4.9±0.04 nm—exceeding that of HSA (3.7 nm)—with fractions ranging between 1.0 and 23.0 nm. In comparison, RH of glucose (molecular mass, 180 D) is 0.37 nm. (C–E) (C) Correlation curves, (D) apparent RH, and (E) relative peak width obtained by DLS of icodextrin stock solution (red), and after UF using 30 kD molecular mass (blue) or 10 kD molecular mass (green) cut-off membranes. n=3–6 measures/solution. P<0.001 between curves. (F and G) (F) Osmolality and (G) mass spectra of unfractionated and fractionated icodextrin, with high-molecular-mass fractions directly derived from concentrations of icodextrin metabolites and from DLS. Although high-molecular-mass icodextrin fractions have an important contribution to the total mass of particles in solution, they only have a minimal effect on osmolality. (H) Intraperitoneal (IP) volume versus time curves predicted by the TPM using either unfractionated (red) or fractionated (<30 kD, blue; <10 kD, green) icodextrin. (I) Net UF measured at the end of 2-hour dwells performed with unfractionated (red) or fractionated (<30 kD, blue; <10 kD, green) icodextrin. Dots represent individual measures; bars are mean±SEM. Inlet, the relationship between mean values of net UF generated in vivo versus those predicted by the TPM. A.U., arbitrary units; BW, body weight; DLS, dynamic light scattering; Unfract., unfractionated.
To confirm the role of large fractions of glucose polymers in osmosis, we used UF membranes with different molecular mass cut-offs to selectively remove the largest particles. The use of a 30 kD cut-off membrane was predicted to remove polysaccharides with an RH>4.0 nm (i.e., the functional radius of small pores)14 and to abrogate transperitoneal water flow. UF of icodextrin stock solution with a 30 kD molecular mass cut-off membrane effectively decreased the concentration of total icodextrin from 75.0 to 24.8 mg/ml along with an approximately 60% reduction in high-molecular-mass metabolites. It also resulted in a shift toward lower RH values, reduced the size dispersity of glucose polymers (relative peak width 0.50±0.02 versus 0.58±0.01, P<0.01), and abrogated icodextrin-induced osmosis in vivo (net UF −5.2±1.4 versus 8.0±1.8 µl/g, P<0.001) (Figure 3, C–I). Using a 10 kD molecular mass cut-off membrane further reduced the concentration of total icodextrin (14.4 mg/ml), high-molecular-mass icodextrin fractions (−80%), size of polymers in solution (mean RH 3.4 nm), dispersity (relative peak width 0.46), and water transport (net UF −9.9±2.3 µl/g, P<0.001 versus stock 7.5% icodextrin) (Figure 3, C–I). To the contrary, removing the largest icodextrin fractions had no significant effect on the profile of low-molecular-mass metabolites (negligible amounts of G2, approximately 0.01 mg/ml; G3, G4, and G5 approximately 0.1 mg/ml; and G6 and G7 approximately 0.2–0.3 mg/ml), the concentration of electrolytes, and the transperitoneal osmotic gradient (Table 3). Using molecular mass distribution (derived from dynamic light scattering and concentrations of icodextrin metabolites) of unfractionated versus fractionated icodextrin, predictions from the TPM closely correlated with values obtained in vivo (Pearson r=0.998, P=0.03).
Table 3.
Effect of removing large icodextrin fractions on RH, solution dispersity, osmotic gradient, and water transport in vivo
| Variable | Icodextrin Polymers | ||
|---|---|---|---|
| All | <30 kD | <10 kD | |
| RH, nm | 4.9±0.02 | 3.8±0.03a | 3.4±0.00a |
| Relative peak width | 0.58±0.01 | 0.50±0.02b | 0.46±0.00a |
| AUC D/Posm | 125.5±1.4 | 122.6±0.4 | 119.9±0.8 |
| Net UF (µl/g BW) | 8.0±1.8 | −5.2±1.4a | −9.9±2.3a |
AUC, area under the curve; D/Posm, dialysate-to-plasma ratio of osmolality; BW, body weight.
P<0.001.
P<0.01.
Altogether, these data verified the hypothesis that transcapillary, paracellular water flow induced by icodextrin relies on the presence of colloidal particles in solution, with an RH exceeding that of the functional radius of interendothelial clefts and a high reflection coefficient.
Combinations of Crystalloid and Colloid Osmotic Agents Enhance Water Removal during PD
On the basis of the different mechanisms of osmotic water transport, we next tested the effect of combining glucose and icodextrin mixed in the same PD solution (Table 1) on transperitoneal water removal.
Predictions from the TPM suggested that combining 1.36% or 3.86% glucose with icodextrin (CIG) in the same dwell would enhance water transport via the paracellular route (Figure 4, A–F). These predictions were verified in the mouse model, where combinations of glucose and icodextrin yielded a net UF that exceeded the sum of UF generated by each of the osmotic agents used separately (Figure 4, G–I, Table 4). The effect on water removal was accompanied by a similar increase in transperitoneal sodium removal (Figure 4J), indicating enhanced water transport at the level of interendothelial junctions.
Figure 4.
Combinations of crystalloid and colloid osmotic agents enhance water and sodium removal. (A and B) Predictions from the TPM of the changes in intraperitoneal (IP) volume induced by (A) 1.36% or (B) 3.86% glucose and 7.5% icodextrin, used alone or in combination. (C and D) Contribution of transcellular and paracellular routes to transcapillary flow rate derived from TPM simulations. Simulations were performed for (C) 1.36% glucose, (D) 3.86% glucose, and combinations of icodextrin with (E) 1.36% glucose or with (F) 3.86% glucose. JvC denotes transcellular water transport across “ultrasmall” pores (blue line); JvS, paracellular water transport across “small pores” (green line); and the sum of JvC and JvS is represented in red. (G and H) Changes in IP volume over time in mice using (G) 1.36% or (H) 3.86% glucose alone or in combination with icodextrin. (I) Net UF generated in mice at the end of 2-hour dwells using 1.36% glucose (light blue bars), 3.86% glucose (dark blue), 7.5% icodextrin (orange), or a combination of osmotic agents (light and dark green, for CIG 1.36% and CIG 3.86%, respectively). (J) Sodium removal across the peritoneal membrane of mice using the same dialysis solutions. Data are mean±SEM; n=3–6/group. BW, body weight.
Table 4.
Mouse transport parameters using crystalloid and colloid osmotic agents, used alone or in combination
| Solution | Net UF (µl/g body wt) | D120/D0 Glucose | UF Efficiency (µl/g BW per mg CHO absorbed) |
|---|---|---|---|
| 1.36% glucose | 0.4±1.5 | 0.62±0.01 | 0.03±0.30 |
| CIG 1.36% | 29.2±1.3a | 0.60±0.04 | 2.65±1.13a |
| 3.86% glucose | 34.0±1.6 | 0.35±0.04 | 0.80±0.44 |
| CIG 3.86% | 51.2±1.3a | 0.38±0.02 | 2.06±0.95b |
| 7.5% icodextrin | 5.4±1.3 | NA | 0.32±0.20 |
Results are mean±SEM values; n=4–6 mice per group. D120/D0 glucose, dialysate glucose concentration at 120 min of the dwell/baseline dialysate glucose concentration; BW, body weight; CHO, carbohydrate; CIG, combination 7.5% icodextrin-glucose; NA, not applicable.
P<0.001 versus the sum of net UF provided by each osmotic agent used alone.
P<0.05.
In both models, the kinetics of IPV showed a fast movement of water across the peritoneum during the first part of the dwell, followed by a sustained osmosis over time (Figure 4, A, B, G, and H). As expected, water channels had a critical role in the crystalloid osmotic water flow generated during the initial part of the dwell (Supplemental Figure 4), whereas colloidal icodextrin fractions enhanced and prolonged small pore–mediated water transport (Figures 4, C–F and 5). It is noteworthy that the use of combined solutions was associated with a reduced absorption of carbohydrates, as compared with the sum of osmotic agents used alone (−19% and −11.5% of carbohydrates absorbed for CIG 3.86% and CIG 1.36%, respectively) (Table 4). Predictions from the TPM were in agreement with these observations, showing a 10%–15% reduction of carbohydrates absorbed during CIG dwells. As a result, combinations of glucose and icodextrin significantly increased UF efficiency—i.e., the amount of UF generated per amount of carbohydrate absorbed (Table 4). Altogether, these data validated predictions that crystalloid and colloid osmotic agents mixed in a same solution enhance UF. This striking effect is mediated by an increase in solute-coupled water transport across the paracellular route.
Figure 5.
Schematic representation of the mechanisms and pathways of crystalloid, colloid, and combined osmosis across the peritoneal membrane. Using crystalloid osmotic agents such as glucose or aminoacids (left panels), transcapillary UF occurs across small and ultrasmall (AQP1) pores and is directly related to the tonicity of the dialysis solution. Once the osmotic gradient has dissipated because of systemic absorption of the osmotic agent, reabsorption of fluid (backfiltration) occurs, mainly across the small pores. Large icodextrin subfractions (middle panels) generate an osmotic water transport independently of AQP1 water channels and tonicity, exclusively through the small pore system. The peritoneal coefficient of reflection for these large molecules is close to unity, indicating that they are not able to cross the membrane and induce a colloid osmosis. The slow absorption—via peritoneal lymphatics—and intraperitoneal metabolism of icodextrin molecules provide a sustained transcapillary UF making it suitable for long dwells, especially in patients with fast peritoneal solute rate. Combining glucose and icodextrin in the same dwell (right panels) enhances transcapillary UF, thanks to the added effects of initial AQP1-dependent free-water transport generated by glucose, and the prevention of backfiltration by large icodextrin molecules.
Metabolism of Icodextrin in the Mouse Peritoneal Cavity
Once in the peritoneal cavity, icodextrin undergoes some metabolization due to α-amylase.34,35 Because rodents, contrary to humans, have high plasma levels of α-amylase that may diffuse to the cavity and interfere with that metabolism, we measured α-amylase activity and icodextrin metabolites in plasma and dialysate in mice. Both Aqp1 wild-type and knockout mice showed elevated but similar circulating levels of the enzyme in basal conditions (2965±41 versus 3147±95 IU/L, in Aqp1+/+ and Aqp1−/− animals, respectively, P=0.26). Mean dialysate α-amylase activity was 176±16 UI/L at the end of 2-hour dwells in mice (corresponding to 5–7-hour dwells in patients36). The dialysate levels of α-amylase were similar for 3.86% glucose and 7.5% icodextrin (190±10 versus 140±35 IU/L, respectively, P=0.37), and they were not influenced by longer dwell duration (103±10 and 118±9 IU/L, for 3- and 4-hour dwells, respectively). The remaining concentration of total icodextrin (sum of high and low-molecular mass-fractions, 42.4±0.9 mg/ml) and the profile and relative concentration of low-molecular-mass icodextrin metabolites (G2–G7, Supplemental Figure 5) in the drained dialysate were similar to what has been observed in the effluent of patients receiving PD.34,35 The sum of G2–G4 metabolites represented 18% of the total glucose polymers in the dialysate, a proportion identical to that of patients at the end of their first icodextrin exchange.34 These data support the validity of the mouse model of PD to assess osmotic water transport induced by icodextrin.
Discussion
Our modelization, biophysical, and experimental studies in transgenic mouse models validate, for the first time, the fundamental differences between crystalloid and colloid osmosis across the peritoneal membrane. Whereas crystalloid agents such as glucose and amino acids require hypertonicity and the presence of AQP1, large icodextrin fractions generate a water flow that predominantly occurs across small pores of peritoneal capillaries and is independent of tonicity. Combinations of crystalloid and colloid osmotic agents enhance water transport via the paracellular route, potentiating sodium and water removal during PD.
The osmotic flow through an ideal semipermeable membrane (permeable to water but not to solutes) is mainly determined by the total number of particles in solution (i.e., osmolality), independently of their size—each 1 mM of solute exerting an osmotic pressure of 19.3 mm Hg according to van’t Hoff’s law. Conversely, the flow across a solute-permeable membrane—like most biologic membranes, including the peritoneum—depends upon the relatively nonpermeable solutes (Supplemental Figure 6). In continuous capillaries like those lining the peritoneal membrane, the protein-reflecting element, or small pore, has a functional radius of approximately 4.0 nm, and is located at the interendothelial clefts, covered by the glycocalyx.14,37–39 Because peritoneal capillaries—in contrast to the glomerular barrier—show no significant charge-selectivity,40–42 particles with an RH similar to or larger than the functional radius of small pores are expected to generate colloid osmosis during PD.
On the basis of albumin and its oncotic effect in the microvasculature, the glucose polymer icodextrin was developed to achieve UF during long dwells in patients receiving PD, while reducing the deleterious effects of glucose.12 Despite its global use as a dialysis solution for >20 years, the mechanism by which icodextrin induces water flow was never formally demonstrated. Our results demonstrate that large fractions of icodextrin are colloidal in nature and induce colloid osmosis. First, a significant proportion of the icodextrin glucose polymers have an RH larger than the functional radius of small pores (approximately 4 nm). Second, the selective removal of polymers with a RH>4 nm completely abrogates osmotic water flow across the mouse peritoneum, whereas it has no effect on the transperitoneal osmotic gradient. Third, biophysical and in vivo data are matched by computer simulations suggesting the effective osmolality mainly depends on high-molecular-mass fractions.25 We confirmed that large fractions of icodextrin have an important contribution to the total mass of particles in solution, but only a minimal effect on osmolality, whereas low-molecular-mass fractions significantly contribute to the tonicity of the solution. Our data validate predictions that solutes with a reflection coefficient close to unity induce colloid osmosis,43–45 and that approximately 25% of the particles in solution of icodextrin act as colloid.22,25 These specific properties provide a rationale supporting the effectiveness of icodextrin to achieve sustained UF during long dwells and restore fluid and sodium balance in patients receiving PD.46–48
Our studies also demonstrate that the pathways and fundamental mechanisms of osmosis differ between crystalloid and colloid agents. For the first time, we provide direct evidence that the water flow generated by colloid particles occurs across interendothelial clefts and is independent of the presence of water channels and tonicity (i.e., iso- or even hypotonic osmosis). Conversely, crystalloid agents such as glucose and amino acids require an osmotic gradient and the presence of endothelial water channels (AQP1) to promote osmotic water transport across the peritoneal membrane. In addition, we confirm in this model that faster peritoneal solute transport increases water removal induced by icodextrin whereas it decreases glucose-driven UF, in line with clinical observations.46–48 These studies substantiate the different and potentially complementary pathways of water transport during crystalloid versus colloid osmosis.
Our investigations of the potential benefit from combining crystalloid and colloid agents reveal that these combinations during the same dwell enhance osmotic water transport, sodium removal, and UF efficiency during PD. These results are in line with mathematical modeling both in mice and in patients,25 and data from clinical studies.49–53 The use of combined solutions led to a fast, glucose-induced, and AQP1-dependent osmotic water transport during the first part of the dwell, and a sustained UF thanks to the presence of large, colloidal fractions of icodextrin. Importantly, these large fractions enhanced solute-coupled water removal and prevented the backflow of water from dialysate to peritoneal capillaries after the dissipation of crystalloid osmotic gradient. In turn, the enhanced UF achieved using combinations of osmotic agents diluted the carbohydrates in the dialysis solution, thereby reducing their transperitoneal diffusion and systemic absorption. At the same time, fluid absorption rate from the cavity remained constant using either combined or separate solutions. Combining crystalloid and colloid osmosis offers the potential to enhance UF and sodium removal, and achieve euvolemia, especially in patients with fast peritoneal solute transport rate causing loss of UF capacity.
The translational relevance of our findings is supported by several points. Regarding the issue of scaling, 2-hour dwells in mice can be compared with approximately 5–7 hours dwells in humans, as a result of higher area-to-volume ratio and more rapid solute (approximately 3–4 times faster) and fluid (approximately 2.5 times) transport across the peritoneal membrane of small animals.36,54 Such dwell duration is close to the optimal duration of an icodextrin dwell to induce UF in patients receiving PD, which has been shown to be approximately 8 hours.12 The relatively low net UF achieved using 7.5% icodextrin as compared with 3.86% glucose-based dialysis solutions in our mouse model may be explained by time-scaling issues and potential additional factors, including differences in plasma osmolality, the absence of ESRD, intrinsic physiologic properties of the mouse peritoneal membrane (i.e., higher αC), and transperitoneal diffusion of α-amylase. Studies in rat models of PD have raised the issue of high intraperitoneal levels of α-amylase, potentially influencing the breakdown and metabolism of icodextrin.30–32,55–57 The α-amylase activity measured in the peritoneal effluent of our mouse models yielded values closer to those in patients receiving PD than in rats. In addition, the remaining icodextrin concentration in the dialysate (approximately 40 mg/ml) and the profile of low-molecular-mass icodextrin metabolites (with smaller polymers [G2 to G4] predominating over the larger ones [G5 to G7]) observed in mice at the end of the dwells were all similar to what is reported in patients receiving PD.34,56 It should be noted that the inclusion of the amylase activity in the TPM could potentially optimize predictions of both net UF and carbohydrate absorption in models using icodextrin.58 Altogether, these data support the usefulness and reliability of the mouse model of PD to assess icodextrin-induced water transport during PD.
These studies, on the basis of a multilevel approach, unravel essential features of crystalloid and colloid osmosis across biologic membranes. They formally demonstrate that large fractions of icodextrin induce colloid osmosis and elucidate how combining crystalloid and colloid particles enhances water and sodium transport across the peritoneal membrane. These insights provide a rationale for using combinations of osmotic agents to improve fluid balance in patients treated with PD.
Disclosures
B.L. and F.V. are employed by Baxter Healthcare.
Supplementary Material
Acknowledgments
We dedicate this work to the memory of Professor Bengt Rippe (Gothenburg, 1950–Lund, 2016), whose seminal work conceptualized the basis of fluid and solute transport across capillaries and, by extension, across the peritoneal membrane during peritoneal dialysis. The engaging and enthusiast personality of B.R. inspired a whole generation of physician-scientists and contributed to the diffusion of peritoneal dialysis worldwide. We thank Yvette Cnops, Ann-Christin Bragfors-Helin, Monica Eriksson, and Sebastien Druart for expert technical assistance.
This work was supported in part by Baxter Healthcare (extramural grant to J.M. and O.D.), the Fondation Saint-Luc (J.M.), the Fondation Horlait-Dapsens (J.M.), the Société Francophone de Dialyse (J.M.), the National Fund for Scientific Research (to J.M. and O.D.), and the Concerted Research Action (ARC16/21-074). A.S. was supported by the Special Research Fund of the Université Catholique de Louvain Medical School (Brussels, Belgium). Baxter Novum is the result of a grant from Baxter Healthcare to the Karolinska Institutet. C.-A.F. is a Senior Research Associate of the National Fund for Scientific Research.
J.M., B.R., C.M.O., and O.D. designed the study; J.M., A.S., C.-A.F., C.F., E.G.-L., B.L., E.G., and F.V. carried out experiments; B.R. and C.M.O. performed computer simulations; J.M., C.-A.F., B.L., E.G., C.M.O., and O.D. analyzed the data; J.M., C.M.O., and O.D. made the figures; J.M., C.M.O., and O.D. drafted and revised the paper; all authors approved the final version of the manuscript.
Footnotes
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017080828/-/DCSupplemental.
References
- 1.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, et al.: Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 28: 2491–2497, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D; The Canada-USA (CANUSA) Peritoneal Dialysis Study Group : Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. J Am Soc Nephrol 9: 1285–1292, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Brown EA, Davies SJ, Rutherford P, Meeus F, Borras M, Riegel W, et al.; EAPOS Group : Survival of functionally anuric patients on automated peritoneal dialysis: The European APD Outcome Study. J Am Soc Nephrol 14: 2948–2957, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Davies SJ: Longitudinal relationship between solute transport and ultrafiltration capacity in peritoneal dialysis patients. Kidney Int 66: 2437–2445, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG: Meta-analysis: Peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol 17: 2591–2598, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Lin A, Ni Z, Yao Q, Zhang W, Yan Y, et al.: Daily peritoneal ultrafiltration predicts patient and technique survival in anuric peritoneal dialysis patients. Nephrol Dial Transplant 25: 2322–2327, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Graham T: Liquid diffusion applied to analysis. Philos Trans R Soc Lond 151: 183–224, 1861 [Google Scholar]
- 8.Holmes CJ: Glucotoxicity in peritoneal dialysis--solutions for the solution! Adv Chronic Kidney Dis 14: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al.; Peritoneal Biopsy Study Group : Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 13: 470–479, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Davies SJ, Phillips L, Naish PF, Russell GI: Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol 12: 1046–1051, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Devuyst O, Margetts PJ, Topley N: The pathophysiology of the peritoneal membrane. J Am Soc Nephrol 21: 1077–1085, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Mistry CD, Mallick NP, Gokal R: Ultrafiltration with an isosmotic solution during long peritoneal dialysis exchanges. Lancet 2: 178–182, 1987 [DOI] [PubMed] [Google Scholar]
- 13.Mistry CD, Gokal R, Peers E: A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int 46: 496–503, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Rippe B, Stelin G, Haraldsson B: Computer simulations of peritoneal fluid transport in CAPD. Kidney Int 40: 315–325, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Rippe B, Rosengren BI, Venturoli D: The peritoneal microcirculation in peritoneal dialysis. Microcirculation 8: 303–320, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Krediet RT, Lindholm B, Rippe B: Pathophysiology of peritoneal membrane failure. Perit Dial Int 20[Suppl 4]: S22–S42, 2000 [PubMed] [Google Scholar]
- 17.Krediet RT: The physiology of peritoneal solute, water, and lymphatic transport. In: Nolph and Gokal’s Textbook of Peritoneal Dialysis, 3rd Ed., edited by Khanna R, Krediet RT, New York, Springer, 2009, pp 137–172 [Google Scholar]
- 18.Devuyst O, Nielsen S, Cosyns JP, Smith BL, Agre P, Squifflet JP, et al.: Aquaporin-1 and endothelial nitric oxide synthase expression in capillary endothelia of human peritoneum. Am J Physiol 275: H234–H242, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Folkesson HG, Yang J, Matthay MA, Ma T, Verkman AS: Reduced osmotic water permeability of the peritoneal barrier in aquaporin-1 knockout mice. Am J Physiol 276: C76–C81, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Ni J, Verbavatz JM, Rippe A, Boisdé I, Moulin P, Rippe B, et al.: Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 69: 1518–1525, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Morelle J, Sow A, Vertommen D, Jamar F, Rippe B, Devuyst O: Quantification of osmotic water transport in vivo using fluorescent albumin. Am J Physiol Renal Physiol 307: F981–F989, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Devuyst O, Rippe B: Water transport across the peritoneal membrane. Kidney Int 85: 750–758, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Morelle J, Devuyst O: Water and solute transport across the peritoneal membrane. Curr Opin Nephrol Hypertens 24: 434–443, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Ho-dac-Pannekeet MM, Schouten N, Langendijk MJ, Hiralall JK, de Waart DR, Struijk DG, et al.: Peritoneal transport characteristics with glucose polymer based dialysate. Kidney Int 50: 979–986, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Rippe B, Levin L: Computer simulations of ultrafiltration profiles for an icodextrin-based peritoneal fluid in CAPD. Kidney Int 57: 2546–2556, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Freida P, Wilkie M, Jenkins S, Dallas F, Issad B: The contribution of combined crystalloid and colloid osmosis to fluid and sodium management in peritoneal dialysis. Kidney Int Suppl 108: S102–S111, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS: Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels. J Biol Chem 273: 4296–4299, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Ni J, Moulin P, Gianello P, Feron O, Balligand JL, Devuyst O: Mice that lack endothelial nitric oxide synthase are protected against functional and structural modifications induced by acute peritonitis. J Am Soc Nephrol 14: 3205–3216, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Yool AJ, Morelle J, Cnops Y, Verbavatz JM, Campbell EM, Beckett EA, et al.: AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J Am Soc Nephrol 24: 1045–1052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-López E, Pawlaczyk K, Anderstam B, Qureshi AR, Kuzlan-Pawlaczyk M, Heimbürger O, et al.: Icodextrin metabolism and alpha-amylase activity in nonuremic rats undergoing chronic peritoneal dialysis. Perit Dial Int 27: 415–423, 2007 [PubMed] [Google Scholar]
- 31.García-López E, Werynski A, Heimbürger O, Filho JC, Lindholm B, Anderstam B: Rate of synthetic oligosaccharide degradation as a novel measure of amylase activity in peritoneal dialysis patients. Perit Dial Int 28: 296–304, 2008 [PubMed] [Google Scholar]
- 32.Davies SJ, Garcia Lopez E, Woodrow G, Donovan K, Plum J, Williams P, et al.: Longitudinal relationships between fluid status, inflammation, urine volume and plasma metabolites of icodextrin in patients randomized to glucose or icodextrin for the long exchange. Nephrol Dial Transplant 23: 2982–2988, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Kabanda A, Goffin E, Bernard A, Lauwerys R, van Ypersele de Strihou C: Factors influencing serum levels and peritoneal clearances of low molecular weight proteins in continuous ambulatory peritoneal dialysis. Kidney Int 48: 1946–1952, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Moberly JB, Mujais S, Gehr T, Hamburger R, Sprague S, Kucharski A, et al.: Pharmacokinetics of icodextrin in peritoneal dialysis patients. Kidney Int Suppl, 62[Suppl 81], S23–S33, 2002 [DOI] [PubMed] [Google Scholar]
- 35.García-López E, Lindholm B: Icodextrin metabolites in peritoneal dialysis. Perit Dial Int 29: 370–376, 2009 [PubMed] [Google Scholar]
- 36.Rippe B: How to assess transport in animals? Perit Dial Int 29[Suppl 2]: S32–S35, 2009 [PubMed] [Google Scholar]
- 37.Levick JR, Michel CC: Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Curry FE, Michel CC: A fiber matrix model of capillary permeability. Microvasc Res 20: 96–99, 1980 [DOI] [PubMed] [Google Scholar]
- 39.Rippe B: Free water transport, small pore transport and the osmotic pressure gradient three-pore model of peritoneal transport. Nephrol Dial Transplant 23: 2147–2153, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Krediet RT, Koomen GC, Koopman MG, Hoek FJ, Struijk DG, Boeschoten EW, et al.: The peritoneal transport of serum proteins and neutral dextran in CAPD patients. Kidney Int 35: 1064–1072, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Buis B, Koomen GC, Imholz AL, Struijk DG, Reddingius RE, Arisz L, et al.: Effect of electric charge on the transperitoneal transport of plasma proteins during CAPD. Nephrol Dial Transplant 11: 1113–1120, 1996 [PubMed] [Google Scholar]
- 42.Asgeirsson D, Axelsson J, Rippe C, Rippe B: Similarity of permeabilities for Ficoll, pullulan, charge-modified albumin and native albumin across the rat peritoneal membrane. Acta Physiol (Oxf) 196: 427–433, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Van’t Hoff JH: Die Rolle des osmotischen druckes in der analogie zwischen lösungen and gasen. Z Phys Chem 1: 481–508, 1887 [Google Scholar]
- 44.Kiil F: Mechanism of osmosis. Kidney Int 21: 303–308, 1982 [DOI] [PubMed] [Google Scholar]
- 45.Guyton AC, Hall JE: The body fluid compartments: Extracellular and intracellular fluids; interstitial fluid and edema. In: Guyton and Hall Textbook of Medical Physiology, 11th Ed., Philadelphia, Elsevier, 2005, pp 291–306 [Google Scholar]
- 46.Krediet RT, Ho-dac-Pannekeet MM, Imholz AL, Struijk DG: Icodextrin’s effects on peritoneal transport. Perit Dial Int 17: 35–41, 1997 [PubMed] [Google Scholar]
- 47.Johnson DW, Arndt M, O’Shea A, Watt R, Hamilton J, Vincent K: Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrol 2: 2, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies SJ, Woodrow G, Donovan K, Plum J, Williams P, Johansson AC, et al.: Icodextrin improves the fluid status of peritoneal dialysis patients: Results of a double-blind randomized controlled trial. J Am Soc Nephrol 14: 2338–2344, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Peers E: Icodextrin plus glucose combinations for use in CAPD. Perit Dial Int 17[Suppl 2]: S68–S69, 1997 [PubMed] [Google Scholar]
- 50.Faller B, Shockley T, Genestier S, Martis L: Polyglucose and amino acids: Preliminary results. Perit Dial Int 17[Suppl 2]: S63–S67, 1997 [PubMed] [Google Scholar]
- 51.Jenkins SB, Wilkie ME: An exploratory study of a novel peritoneal combination dialysate (1.36% glucose/7.5% icodextrin), demonstrating improved ultrafiltration compared to either component studied alone. Perit Dial Int 23: 475–480, 2003 [PubMed] [Google Scholar]
- 52.Dallas F, Jenkins SB, Wilkie ME: Enhanced ultrafiltration using 7.5% icodextrin/1.36% glucose combination dialysate: A pilot study. Perit Dial Int 24: 542–546, 2004 [PubMed] [Google Scholar]
- 53.Freida P, Galach M, Divino Filho JC, Werynski A, Lindholm B: Combination of crystalloid (glucose) and colloid (icodextrin) osmotic agents markedly enhances peritoneal fluid and solute transport during the long PD dwell. Perit Dial Int 27: 267–276, 2007 [PubMed] [Google Scholar]
- 54.Ni J, Cnops Y, Debaix H, Boisdé I, Verbavatz JM, Devuyst O: Functional and molecular characterization of a peritoneal dialysis model in the C57BL/6J mouse. Kidney Int 67: 2021–2031, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Flessner MF, Lofthouse J: Of mice and men: Species and age differences in dialysis with icodextrin. J Am Soc Nephrol 10: 226A, 1999 [Google Scholar]
- 56.de Waart DR, Zweers MM, Struijk DG, Krediet RT: Icodextrin degradation products in spent dialysate of CAPD patients and the rat, and its relation with dialysate osmolality. Perit Dial Int 21: 269–274, 2001 [PubMed] [Google Scholar]
- 57.McGEACHIN RL, Gleason JR, Adams MR: Amylase distribution in extrapancreatic, extrasalivary tissues. Arch Biochem Biophys 75: 403–411, 1958 [DOI] [PubMed] [Google Scholar]
- 58.Akonur A, Holmes CJ, Leypoldt JK: Predicting the peritoneal absorption of icodextrin in rats and humans including the effect of α-amylase activity in dialysate. Perit Dial Int 35: 288–296, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.