Abstract
Background:
Marine organisms produce a variety of compounds with pharmacological activities, including anticancer effects. They contain several secondary metabolites with interesting biological activities. This study attempted to find cytotoxicity of Hexane, Dichloromethane and Butanol partitions of Holothuria leucospilota and Echinometra mathaei.
Materials and Methods:
H. leucospilota and E. mathaei were collected from Persian Gulf. The animals were extracted by maceration with methanol-ethyl acetate (1:1). The H. leucospilota extract was partitioned by Kupchan method to hexane, dichloromethane, butanol, and water partitions. The cytotoxic activity of the extracts was investigated against HeLa (cervical cancer) and human umbilical vein endothelial cells cell lines by mitochondrial tetrazolium test assay after 72 h.
Results:
The cell survivals of HeLa cell were decreased by increasing the concentration of extracts. A significant reduction in cell viability at the doses of 30 (μg/ml) of dichloromethane (DCM) partition, 0.3, 3, and 30 (μg/ml) of ButOH partitions of sea cucumber, and 0.5 (μg/ml) of E. mathaei was observed. The median growth inhibitory concentration value of Hex, DCM, ButoH, and water partitions were 0.301, 0.21, 2.29, and 0.229 μg/ml, respectively.
Conclusion:
This study reveals that different partitions of H. leucospilota and total extract of E. mathaei have cytotoxic activity against cancer cell lines. More study is necessary to find the active metabolites in the more active partitions.
Keywords: Cancer, cytotoxic, echinometra, holothuria, Persian Gulf
Introduction
Cancer is a complicated disorder and one of the most causes of mortality worldwide, particularly in developed societies.[1] The number of patients catching this invasive disease is increasing with a rapid tilt. Fourteen million new clinical cases were reported only in the year 2010 that is going to increase to 22 million during the next two decades.[2]
Despite different studies in the field of cancer and especially in discovering of new drugs, there is not any effective and safe treatment for cancer. Hence, discovering new chemotherapeutic agents, especially from nature is a great wish for researchers. During the last two decades, about 50% of the drugs entered to the market were directly or indirectly derived from natural organisms. The marine litter is a unique resource of biologically active compounds with characteristic structural and chemical features.[3] Different organisms under the water such as seaweeds, sponges, corals, fungi, and ascidians have been analyzed for their biologic potentials, and also, active ingredients and several bioactive metabolites have been isolated from these marine resources showing different fields of bioactivities, such as anticancer, antibiotic, antiviral, antioxidant, anti-inflammatory, and antimalarial activities.[4,5,6] The dramatic marine pipeline of increasing the number of clinical and preclinical anticancer agents has matured during the last decade by the intense efforts of marine researchers in this field. Biological activities of these organisms are due to their metabolites such as terpenoid compounds, alkaloids, polyketides, peptides, shikimic acid derivatives, sugars, and steroids. In addition, the presence of halogen atoms such as chlorine and bromine is the main difference of these secondary metabolites.[7,8]
Novel structures and also novel mechanisms of action of marine metabolisms have led to new methods for treating cancer particularly for patients with solid tumors of the lung, breast, colon, or prostate.[9] Hence, an increasing search is needed for screening of marine organisms, especially in the field of cytotoxicity. In fact, the desire for this evolution has been stimulated by a great demand to develop more active and less toxic therapies for treatment of cancer.[10] It can generally be concluded that contemporary screening of marine natural products in the field of cancer seems necessary.[11] Iran has coastal lines about 1260 km along the Persian Gulf and the Oman Sea.[12] Despite the existence of a great extent of marine organisms in this region, there are only a few studies on the biological activities, especially cytotoxic screening of these interesting organisms. The lack of study in this area may result from difficulties of collection and identification of marine animals. In this study, we have analyzed different fractions of Holothuria leucospilota and Echinometra mathaei from Persian Gulf for their cytotoxic activities.
Materials and Methods
H. leucospilota and E. mathaei were collected from Bushehr, a southwest coastline of Persian Gulf in autumn 2013. Identification of both organisms was carried out kindly by Khoramshahr marine science and Technology University.
Preparation of the extracts
The animals (about 1.4 kg wet weight of H. leucospilota and 800 mg wet weight of E. mathaei) were cut into pieces, dried by freeze drier, and extracted four times with EtOAc-MeOH (1:1). The solvent was evaporated by rotary evaporator. Ten mg of E. mathaei extract was used for cytotoxic test. The total extract was partitioned to hexane, dichloromethane, butanol, and water by Kupchan partitioning method. First of all, the extract was partitioned between hexane and MeOH 90% and hexane and after that dichloromethane and MeOH 80%. Finally, MeOH solvent was removed completely, and the remaining water was partitioned with ButOH. The partitions were subjected to cytotoxic test.[13]
Cytotoxic test
After providing the partitions for cytotoxic screening, two cell lines, HeLa and human umbilical vein endothelial cell (HUVEC), were used. At the first, three cell lines were growing in the DMEM cell culture medium which supplemented with 10% fetal bovine serum. Penicillin and streptomycin were added to the media. All cell lines were cultured at 37°C in air/carbon dioxide (95:5) atmosphere.
At the second, different concentration of all partitions was tested for each cell line. Samples were dissolved in dimethyl sulfoxide (DMSO) and further diluted with cell culture medium. The final concentration used was 1% of total volume of medium in all treatments, including the control group.
For mitochondrial tetrazolium test (MTT) assay, 1 × 105 cells/well were plated into 96 well plates. The cells were incubated for 24 h to proliferate and reach their exponential phase of growth. The incubation time for each cell line was determined twice as long as the doubling time of each cell line. After that, 20 μl from each partition was added to the media.[14]
After 72 h of incubation, 30 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (5 mg/ml) in phosphate-buffered serum was added to each well. The plates were incubated for 4 h at 37°C. After incubation time, the medium was removed, and 100 μl cell culture grade DMSO was added. The formazan salts were analyzed by a microplate reader at 540 nm.
Cell viability was determined as a percentage of untreated cells (control value), and cytotoxicity value was calculated as IC50 (the concentration of a drug that is required for 50% inhibition) of the reagents comparing with control.
Results
The cytotoxic activities of various extracts of H. leucospilota were analyzed against HeLa cancerous cell line [Figure 1] and a normal cell line, HUVEC [Figure 2] by the MTT assay. The multiple concentration 0.03, 0.3, 3, 30 μg/ml of hexane, dichloromethane (DCM), butanol, and water extracts from H. leucospilota were used.
Figure 1.
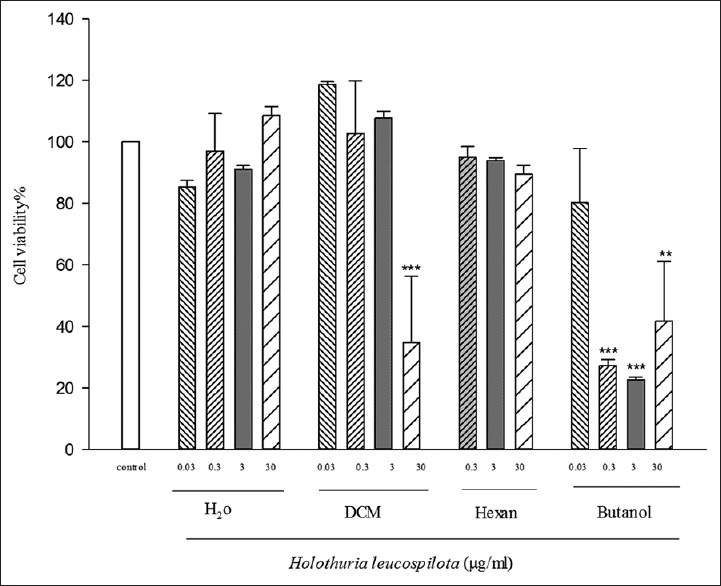
Cytotoxic activity of Holothuria leucospilota partitions on human umbilical vein endothelial cell line. Data represent the means ± standard error of the mean separate experiments (significant as compared to control ***P < 0.05). All tests were repeated three times. signifcant as compared to control **P < 0.05
Figure 2.
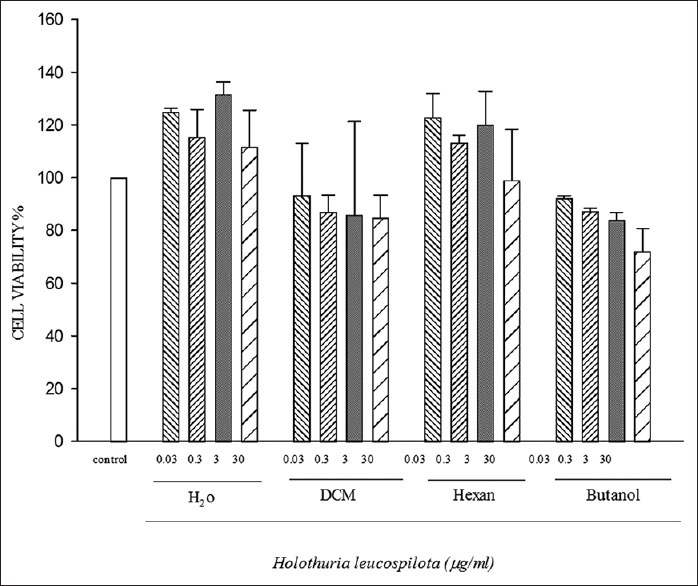
Cytotoxic activity of Holothuria leucospilota partitions on HeLa cell line. Data represent the means ± standard error of the mean separate experiments. All tests were repeated three times
Figure 1 shows the MTT test results for different H. leucospilota extract partitions with different concentrations on the HUVEC cell line after 72 h. We observed a significant reduction in cell viability at the doses of 30 (μg/ml) of DCM partition, 0.3, 3, and 30 (μg/ml) of ButOH partitions.
The results for different concentrations from H. leucospilota partitions on HeLa cell line, after 72 h, were shown in Figure 2.
The results for different concentrations from E. mathaei extract on HeLa cell line, after 72 h, were shown in Figure 3.
Figure 3.
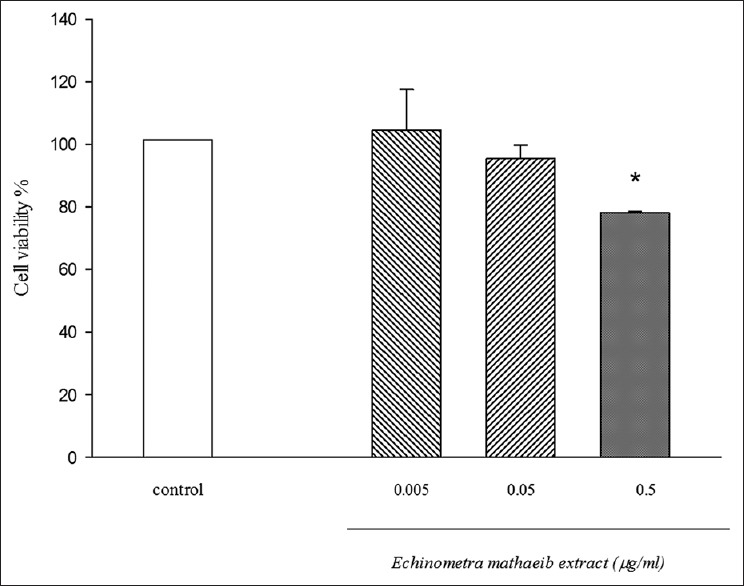
Cytotoxic activity of Echinometra mathaei extract on HeLa cell line. Data represent the means ± standard error of the mean separate experiments (significant as compared to control *P < 0.05). All tests were repeated three times. signifcant as compared to control *P < 0.05
Discussion
Currently, isolating bioactive compounds from natural food sources for producing pharmaceutical agents has become the focus of much attention. Secondary active metabolites in marine organisms such as algae, corals, sponges, mollusks, phytoplanktons, tunicates, echinoderms, and bacteria have shown various applications in pharmaceutical industries.[15,16]
The high number of medicinal compounds extracted from terrestrial herbs, structural diversity and uniqueness in marine organisms and lack of documentation about marine invertebrates have all stimulated more researches for drug discovery from marine sources.[17]
Isolation and purification of Dolastatins from the mollusk Dolabella auricularia or Halicondrins from marine Japanese sponge Halichondri aokadai has a wide effect in marine anticancer field.[18]
Similar to antitumor activity of marine invertebrates, echinoderms containing novel natural structures with a wide range of biological activities have provided a new vision in the development of new therapeutic anticancer agents.[19]
Holothurians are one of the most important organisms of Echinodermata that have been used for their anti-inflammatory and antidisease characteristics and also for treating different ailments in the south East Asia countries such as Korea, Japan, Indonesia, and China.[20,21] They are nutrient-rich invertebrates, similar to cucumber with a leathery skin and gelatinous body.[22]
Biological activities such as antioxidant, antiproliferative, anti-inflammatory, anticancer, anticoagulant, antifungal, and antibacterial activities of the secondary metabolites isolated from marine organisms, mainly from star-fish and sea cucumbers, have been examined in several researches previously.[20,21] Several biological and chemical studies carried out on various species of sea cucumbers indicated these marine organisms contain bioactive secondary metabolites, especially triterpene glycosides with cytotoxic activity. Triterpene glycosides isolated from Pseudocolochirus violaceus sea cucumber showed significant cytotoxic activity against MKN-45 and HCT-116 cell lines;[23] triterpene glycosides isolated from Mensamaria intercedens exhibited high cytotoxic activity in methanolic partition.[24]
Antiproliferative potential of three Malaysian sea cucumber species, H. scabra, H. leucospilota and Stichopus chloronotus, was analyzed and revealed that tested cell lines were much more sensitive to the water extract of H. leucospilota.[25] Evaluating the anticancer activity of the starfish Acanthaster planci along with Tamoxifen in human breast cancer cells indicated that the sea star extract (IC50 = 15.6 μg/ml) was active against cancer cells. A few studies have been reported about the Persian Gulf Echinodermata and their biological effects.[26] In a study by Andersson et al., the antibacterial and cytotoxic effect of saponin compounds of brittle star on leukemia cells was investigated.[27] Prabhu and Bragadeeswaran evaluated hemolytic and cytotoxic properties of Ophiocnemis marmorata[28] which is consistent with cytotoxic properties of E. mathaei extract in this study. In accordance with these results, our study revealed that butanol and dichloromethane extracts of H. leucospilota and crude extract of E. mathaei conducted cytotoxic effect on HeLa (cervical cancer) growth cells, demonstrating anticancer potential of echinoderms.
Conclusion
The study reveals that various partitions of H. leucospilota and also the total extract of E. mathaei have toxic activity against HeLa cell line. More research is necessary to isolate and identify the active compounds in the active partitions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.International Agency for Research on Cancer. France: WHO Press, IARC Publisher; 2014. World Cancer Report 2014 in Stewart and Wild Ed. [Google Scholar]
- 2.Gulland A. Global cancer prevalence is growing at “alarming pace,” says WHO. BMJ. 2014;348:g1338. doi: 10.1136/bmj.g1338. [DOI] [PubMed] [Google Scholar]
- 3.Schwartsmann G. Marine organisms and other novel natural sources of new cancer drugs. Ann Oncol. 2000;11(Suppl 3):235–43. doi: 10.1093/annonc/11.suppl_3.235. [DOI] [PubMed] [Google Scholar]
- 4.Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2:221–5. doi: 10.1016/s1470-2045(00)00292-8. [DOI] [PubMed] [Google Scholar]
- 5.Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR, et al. Marine natural products. Nat Prod Rep. 2007;24:31–86. doi: 10.1039/b603047p. [DOI] [PubMed] [Google Scholar]
- 6.Ghannadi A, Plubrukarn A, Zandi K, Sartavi K, Yegdaneh A. Screening for antimalarial and acetylcholinesterase inhibitory activities of some Iranian seaweeds. Res Pharm Sci. 2013;8:113–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 8.Yegdaneh A, Putchakarn S, Yuenyongsawad S, Ghannadi A, Plubrukarn A. 3-oxoabolene and 1-oxocurcuphenol, aromatic bisabolanes from the sponge Myrmekioderma sp. Nat Prod Commun. 2013;8:1355–7. [PubMed] [Google Scholar]
- 9.Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, et al. Environmental genome shotgun sequencing of the sargasso sea. Science. 2004;304:66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 10.Williams DH, Stone MJ, Hauck PR, Rahman SK. Why are secondary metabolites (natural products) biosynthesized? J Nat Prod. 1989;52:1189–208. doi: 10.1021/np50066a001. [DOI] [PubMed] [Google Scholar]
- 11.Firn RD, Jones CG. Natural products – A simple model to explain chemical diversity. Nat Prod Rep. 2003;20:382–91. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- 12.Sohrabipour J, Rabiei R. The checklist of green algae of the Iranian coastal lines of the Persian Gulf and Gulf of Oman. Iran J Bot. 2007;13:146–9. [Google Scholar]
- 13.Kupchan SM, Tsou G. Tumor inhibitors. LXXXI, structure and partial synthesis of fabacein. J Org Chem. 1973;38:178. [Google Scholar]
- 14.Mehdinezhad N, Ghannadi A, Yegdaneh A. Phytochemical and biological evaluation of some Sargassum species from Persian Gulf. Res Pharm Sci. 2016;11:243–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Yegdaneh A, Ghannadi A, Dayani L. Chemical constituents and biological activities of two Iranian Cystoseira species. Res Pharm Sci. 2016;11:311–7. doi: 10.4103/1735-5362.189307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameri A. Marine microbial natural products. Jundishapur J Nat Pharm Prod. 2014;9:e24716. doi: 10.17795/jjnpp-24716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobili S, Lippi D, Witort E, Donnini M, Bausi L, Mini E, et al. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365–78. doi: 10.1016/j.phrs.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Prado MP, Torres YR, Berlinck RG, Desiderá C, San-Chez MA, Craveiro MV, et al. Effects of marine organ-isms extracts on microtubule integrity and cell cycle pro-gression in cultured cells. J Exp Mar Bio Ecol. 2004;313:125–37. [Google Scholar]
- 19.Sheean PD, Hodges LD, Kalafatis N, Wright PF, Wynne PM, Whitehouse MW, et al. Bioactivity of extracts from gonadal tissue of the edible Australian purple sea urchin Heliocidaris erythrogramma. Sci Food Agric. 2007;87:694–701. [Google Scholar]
- 20.Aydın M, Sevgili H, Tufan B, Emre Y, Köse S. Proximate composition and fatty acid profile of three different fresh and dried commercial sea cucumbers from Turkey. International journal of food science & technology. 2011;46(3):500–8. [Google Scholar]
- 21.Conand C, Byrne M. A review of recent developments in the world sea cucumber fisheries. Oceanographic Lit Rev. 1995;7:570. [Google Scholar]
- 22.Weiss KR, McFarling UL, Loomis R. Plague of plastic chokes the seas. Los Angeles Times. 2006;2:2. [Google Scholar]
- 23.Zhang SY, Tang HF, Yi YH. Cytotoxic triterpene glycosides from the sea cucumber Pseudocolochirus violaceus. Fitoterapia. 2007;78:283–7. doi: 10.1016/j.fitote.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Zou ZR, Yi YH, Wu HM, Wu JH, Liaw CC, Lee KH, et al. Intercedensides A-C, three new cytotoxic triterpene glycosides from the sea cucumber mensamaria intercedens lampert. J Nat Prod. 2003;66:1055–60. doi: 10.1021/np030064y. [DOI] [PubMed] [Google Scholar]
- 25.Althunibat OY, Hashim RB, Taher M. In vitro antiox-idant and antiproliferative activities of three Malaysian sea cucumber species. Eur J Sci Res. 2009;37:376–87. [Google Scholar]
- 26.Mutee AF, Salhimi SM, Ghazali FC, Al-Hassan FM, Lim CH, Ibrahim K, et al. Apoptosis induced in human breast cancer cell line by Acanthaster planci starfish ex-tract compared to tamoxifen. Afr J Pharm Pharmacol. 2012;6:129–34. [Google Scholar]
- 27.Andersson L, Bohlin L, Iorizzi M, Riccio R, Minale L, Moreno-López W, et al. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon. 1989;27:179–88. doi: 10.1016/0041-0101(89)90131-1. [DOI] [PubMed] [Google Scholar]
- 28.Prabhu K, Bragadeeswaran S. Biological properties of brittle star Ophiocnemis marmorata collected from Para-ngipettai, Southeast coast of India. J Microbiol Antimicrob. 2013;5:110–8. [Google Scholar]


