Summary
Background
MLN0128 is a first-in-class, dual mTOR inhibitor with potential to outperform standard rapalogs through inhibition of TORC1 and TORC2. This phase II study was designed to assess antitumor activity of MLN0128 in metastatic castration-resistant prostate cancer (mCRPC).
Methods
Eligible patients had mCRPC previously treated with abiraterone acetate and/or enzalutamide. Five patients started MLN0128 at 5 mg once daily, subsequently dose reduced to 4 mg because of toxicity. Four subsequent patients started MLN0128 at 4 mg daily. Primary endpoint was progression-free survival at 6 months.
Results
Nine patients were enrolled and median time on treatment was 11 weeks (range: 3–30). Best response was stable disease. All patients had a rise in PSA on treatment, with a median 159% increase from baseline (range: 12–620%). Median baseline circulating tumor cell count was 1 cell/mL (range: 0–40); none had a decrease in cell count posttreatment. Grade ≤ 2 adverse events included fatigue, anorexia, and rash. The most common serious adverse events were grade 3 dyspnea and maculopapular rash. Eight patients discontinued treatment early because of radiographic progression (n = 1), grade 3 toxicity (n = 5), or investigator discretion (n = 2). Four patients had immediate PSA decline following drug discontinuation, suggesting MLN0128 could cause compensatory increase of androgen receptor (AR) activity. Correlative studies of pretreatment and posttreatment biopsy specimens revealed limited inhibition of AKT phosphorylation, 4EBP1 phosphorylation, and eIF4E activity.
Conclusions
Clinical efficacy of MLN0128 in mCRPC was limited likely due to dose reductions secondary to toxicity, PSA kinetics suggesting AR activation resulting from mTOR inhibition, and poor inhibition of mTOR signaling targets.
Keywords: mTOR, Prostate cancer, MLN0128
Introduction
The mechanistic target of rapamycin (mTOR) is a critical kinase that links extracellular signal transduction with metabolic processes that control cell growth. mTOR is a downstream component in the phosphoinositide 3-kinase (PI3K) signaling pathway, which is deregulated in 42% of locally advanced prostate cancers and nearly 100% of advanced prostate cancers [1]. The mTOR protein can form two distinct kinases depending on the macromolecular complex it assembles with co-associated proteins. These are named mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [2]. The primary targets of mTORC1 are the translation initiation inhibitors 4EBP1, 4EBP2, and 4EBP3, as well as S6 kinase 1 and S6 kinase 2 [3]. This arm of mTOR signaling is vital for the regulation of mRNA translation and protein synthesis [4]. The most well-characterized substrate of mTORC2 is the oncogenic kinase AKT. mTOR hyperactivation as well as deregulation of downstream protein synthesis is necessary for tumor formation and metastasis in mouse prostate cancer models [5, 6]. Given the frequency of its deregulation in advanced prostate cancer, targeting the PI3K pathway and in particular mTOR kinase has been a high priority.
The first mTOR inhibitors available for clinical trials in prostate cancer were rapamycin and its analogs (also known as rapalogs). These mTOR inhibitors function in an allosteric fashion by first binding to the immunophilin FKBP12 (an FK506-binding protein); the resulting complex can then directly inhibit mTORC1 function [7, 8]. In vivo studies demonstrated significant antitumor efficacy in tissue-specific prostate cancer mouse models [9]. However, three separate clinical trials using rapamycin and rapalogs demonstrated little to no antitumor effects in patients with advanced stage prostate cancer [10–12]. Similarly, a phase 1/2 combination trial with rapamycin and gefitinib in metastatic castration-resistant prostate cancer (CRPC) also did not result in significant antitumor activity [13]. One potential mechanism for the lack of efficacy of rapamycin and similar drugs is incomplete inhibition of oncogenic mTOR kinase activity. Rapamycin poorly inhibits the phosphorylation of 4EBPs and AKT [14], both of which are critical downstream effectors. Further, rapamycin administration can lead to paradoxical feedback activation of the PI3K signaling pathway [15].
These findings led to the development of ATP active site inhibitors of mTOR such as MLN0128. Unlike allosteric inhibitors, these small molecules can selectively target the ATP binding site of mTOR [16, 17], resulting in a significant decrease in both mTORC1 and mTORC2 kinase activity. In animal models, ATP active site inhibitors of mTOR consistently outperform allosteric inhibitors [6, 17]. For example, whereas rapamycin and associated analogs predominantly decrease the phosphorylation of the mTORC1 substrates S6 kinase 1/2, ATP site inhibitors also target 4EBP and AKT phosphorylation, demonstrating potent inhibition of both mTORC1 and mTORC2 [18]. Moreover, MLN0128 has demonstrated superior antitumor efficacy in a mouse model of prostate cancer driven by PI3K pathway hyperactivation [6]. Given these in vitro and in vivo findings, we hypothesized that ATP site inhibitors of mTOR such as MLN0128 could more effectively inhibit mTOR kinase signaling in patients with metastatic CRPC, leading to a clinical response. In this phase II study, we tested the therapeutic efficacy of MLN0128 in patients with metastatic CRPC.
Materials and methods
This registered phase II study (clinicaltrials.gov NCT02091531) was approved by the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board. All patients enrolled on the study provided written informed consent. Patient’s were accrued between 3/14/2014 and 11/18/2015. Overall, 21 patients with progressive CRPC were anticipated to be enrolled in this open label, interventional clinical trial at MSK.
Patient eligibility
To be eligible for inclusion, patients had to have histologically confirmed metastatic CRPC with evidence of disease progression defined by one or more of these criteria: a) rising PSA levels, with a minimum of 3 consecutive rising levels obtained more than 1 week apart; b) new or progressive soft-tissue masses on transaxial imaging (computed tomography or magnetic resonance imaging scan); or c) at least 2 new metastatic lesions on radionuclide bone scan. Standard physical and laboratory eligibility requirements included adequate bone marrow reserve, adequate liver and kidney function, castrate levels of testosterone, and a Karnofsky Performance Status ≥70%. Patients must have received enzalutamide or abiraterone, but have no prior exposure to PI3K/mTOR pathway inhibitors. Previous docetaxel treatment was permitted.
Study design
The primary objective was to assess the efficacy of MLN0128 in patients with metastatic CRPC who had received prior enzalutamide or abiraterone acetate. Patients were to receive a fixed oral daily dose of 5 mg of MLN0128 based off of Phase I results. However, the first 5 patients on study required a dose reduction to 4 mg or less; 4 more patients were subsequently enrolled and received a fixed oral daily dose of 4 mg of MLN0128.
Efficacy was to be assessed by the proportion of patients with progression-free survival (PFS) at 6 months from the start of treatment. Progression was defined per Prostate Cancer Working Group 2 guidelines [19]. A two-stage design differentiating between 6-month PFS rates of 0.30 and 0.50 was used; if 7 or more patients (of 21) achieved the primary endpoint of 6-month PFS, an additional 21 patients would be enrolled. However, the study was stopped early because of toxicity and lack of activity.
Patient evaluation
Study participants were assessed for safety weekly in cycles 1 and 2, biweekly in cycles 3 and 4, and monthly in cycle 5 and beyond, based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (NCI-CTCAE v4.0). Cycles were 4 weeks. Safety evaluations were based on medical review of adverse event reports and the results of vital sign measurements, physical examinations, electrocardiograms, and clinical laboratory tests throughout the conduct of the study.
Secondary endpoints
PSA kinetics (at 8 weeks) and radiographic response (FDG-PET imaging at 4 weeks) were secondary endpoints that were tracked and correlated with disease progression. These measures were not part of the definition of disease progression. Response and progression were evaluated using a combination of the Response Evaluation Criteria in Solid Tumors (RECIST) [20], modified for prostate cancer, and the guidelines for prostate cancer endpoints developed by the Prostate Cancer Clinical Trials Working Group (PCWG2).
Exploratory endpoints
Circulating tumor cells (CTCs) were enumerated through molecular analysis on the Epic Sciences platform. Tumor biopsy specimens taken before and 4 weeks after therapy initiation were analyzed for markers of PI3K pathway signaling by immunohistochemistry to explore the protein level of PTEN (phosphatase and tensin homolog) and the phosphorylation status of rpS6, 4EBP1, and AKT. In order to measure the effects of MLN0128 on protein synthesis, a proximity ligation assay was used to test for activity of the eukaryotic translation initiation factor 4E (eIF4E).
Sequencing data
Specific genetic alterations in tumor samples were elucidated using MSK-IMPACT (Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets), a proprietary hybridization capture-based next-generation sequencing assay for targeted deep sequencing of all exons and selected introns of 468 key cancer genes in formalin-fixed, paraffin-embedded tumors (FFPE) [21].
Immunofluorescence
4 μm sections from FFPE specimens were deparaffinized in xylene (Sigma-Aldrich, St. Louis, MO), rehydrated in graded ethanol, and rinsed in distilled water. Antigen retrieval was performed using a citrate buffer (10 mM, pH 6.0; Vector Laboratories, Burlingame, CA) and the heat-induced epitope retrieval method. Subsequent normal serum block was done using donkey serum (Sigma-Aldrich), and Phospho-S6 Ribosomal Protein (Ser240/244) (D68F8, #5364; Cell Signaling Technology, Danvers, MA), Phospho-4E-BP1 (Thr37/46) (236B4, #2855, Cell Signaling), and Phospho-Akt (Ser473) (D9E, #4060, Cell Signaling) primary antibodies were incubated overnight at 4 °C. The sections were then incubated with secondary antibodies (IgG anti-rabbit or IgG anti-mouse conjugated with Alexa Fluor 488 or Alexa Fluor 594, Invitrogen/Thermo Fisher Scientific, Waltham, MA) for 90 min at room temperature. They were then washed in a PBS buffer, rinsed in distilled water, dehydrated in graded ethanol, and mounted with ProLong Gold Antifade Mountant with DAPI (Invitrogen/Thermo Fisher Scientific).
Immunohistochemistry
PTEN immunohistochemistry was performed using a genetically validated protocol as previously described [22]. Briefly, the protocol uses the Ventana automated staining platform (Ventana Discovery Ultra, Ventana Medical Systems, Tucson, AZ) and a rabbit anti-human PTEN antibody (Clone D4.3 XP, #9188; Cell Signaling).
Proximity ligation assay
The in situ proximity ligation assay (PLA) was optimized to detect interactions between eIF4E and eIF4G (eIF4E–eIF4G) in human biopsy samples. The detection efficiency was validated using a previously described mouse model [22]. PLAs were performed on FFPE biopsy samples obtained before and after treatment with MLN0128. Following deparaffinization and rehydration of tissue sections, antigen retrieval was performed in a decloaking chamber at 95 °C for 30 min in Tris-EDTA buffer, pH 9.0. The PLA protocol was followed according to the manufacturers’ instructions (Sigma-Aldrich), with incubation of the primary antibodies at 4 °C overnight. The antibodies were used at the following concentrations: 1:250 for eIF4E (mouse, clone A-10, #SC-271480, Santa Cruz Biotechnology, Dallas, TX); 1:250 for eIF4G (rabbit, #2498; Cell Signaling). PLA minus and PLA plus probes were added and incubated for 1 h at 37 °C. The two hybridized oligonucleotides were joined in a closed circle using a ligase. The DNA was then amplified using rolling circle amplification, and detection of the amplicons was carried out using the Duolink In Situ Brigthfield kit (Sigma-Aldrich). The first results were visualized by brightfield microscopy (Nikon E8100). To perform high-throughput analysis of the whole tissue, slides were scanned (magnification 40X) using the Aperio CSO (Leica Biosystems, Wetzlar, Germany), and the number of PLA signals per cell was counted in the entire neoplastic tissue by semi-automated image analysis (HALO, Indica Labs, Corrales, NM).
Results
Patient characteristics
Overall, 9 patients with progressive CRPC were treated with MLN0128 at MSK between April 2014 and September 2015. Baseline characteristics are summarized in Table 1. The median age was 67 (range: 52–79), media PSA was 271.26 ng/mL (range: 3.94–655.12) and median CTC count was 1 cell/mL of blood (range: 0–40 cells). All patients had been previously treated with at least 1 second-generation androgen receptor (AR)-targeted therapy: 9 had received enzalutamide and 8 had received abiraterone. Four patients (44%) had previously received chemotherapy with docetaxel.
Table 1.
Baseline patient characteristics
| Characteristic | |
|---|---|
| Patients accrued, n | 9 |
| Patients evaluable, n | 9 |
| Age, y | |
| Median | 67 |
| Range | 52–79 |
| Baseline PSA, ng/mL | |
| Median | 271.26 |
| Range | 3.94–655.12 |
| Primary Gleason score | |
| Median | 8.5 |
| Range | 7–10 |
| Acid Phosphatase | |
| Median | 50.9 |
| Range | 1.3–250.4 |
| CTCs, cells/mL | |
| Median | 1 |
| Range | 0–40 |
| Race, n | |
| Black | 1 |
| White | 8 |
| Prior Treatment, n | |
| Enzalutamide | 9 |
| Abiraterone | 8 |
| Docetaxel | 4 |
PSA prostate-specific antigen, CTCs circulating tumor cells
Toxicity
Table 2 lists common toxicities for all 9 patients. The most common grade 2 or higher toxicities were rash in 4 patients, which led to 1 patient discontinuing treatment early; fatigue in 3 patients; mucositis in 3 patients; and dyspnea in 3 patients, causing 2 of them to discontinue treatment early. There were no episodes of grade 4 toxicity. Grade 3 toxicities included mucositis (1 patient), rash (1 patient), pain (1 patient), dyspnea (2 patients), and delirium (1 patient). The first 5 patients were treated with MLN0128 5 mg daily [23]. All 5 patients were dose reduced from 5 mg daily to 4 mg daily due to toxicity. One patient required an additional dose reduction to 3 mg daily. The protocol was subsequently amended to change the starting dose to 4 mg daily. At this starting dose, no dose reductions were necessary.
Table 2.
Selected toxicities for all patients
| Toxicity | Common toxicity criteria by grade, no. (%) | |||
|---|---|---|---|---|
|
| ||||
| 1 | 2 | 3 | 4 | |
| Urinary Frequency | 8 (89) | 0 | 0 | 0 |
| Fatigue | 9 (100) | 3 (33) | 0 | 0 |
| Anorexia | 6 (67) | 1 (11) | 0 | 0 |
| Mucositis | 4 (44) | 2 (22) | 1 (11) | 0 |
| Rash | 6 (67) | 3 (33) | 1 (11) | 0 |
| Constipation | 4 (44) | 1 (11) | 0 | 0 |
| Nausea | 3 (33) | 1 (11) | 0 | 0 |
| Diarrhea | 5 (56) | 2 (22) | 0 | 0 |
| Pain | 9 (100) | 1 (11) | 1 (11) | 0 |
| Dyspnea | 5 (56) | 1 (11) | 2 (22) | 0 |
| Edema | 2 (22) | 2 (22) | 0 | 0 |
| Vomiting | 3 (33) | 0 | 0 | 0 |
| Dizziness | 1 (11) | 1 (11) | 0 | 0 |
| Delirium | 0 | 0 | 1 (11) | 0 |
Patient outcomes
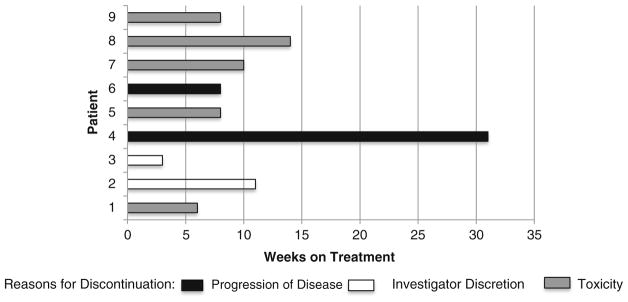
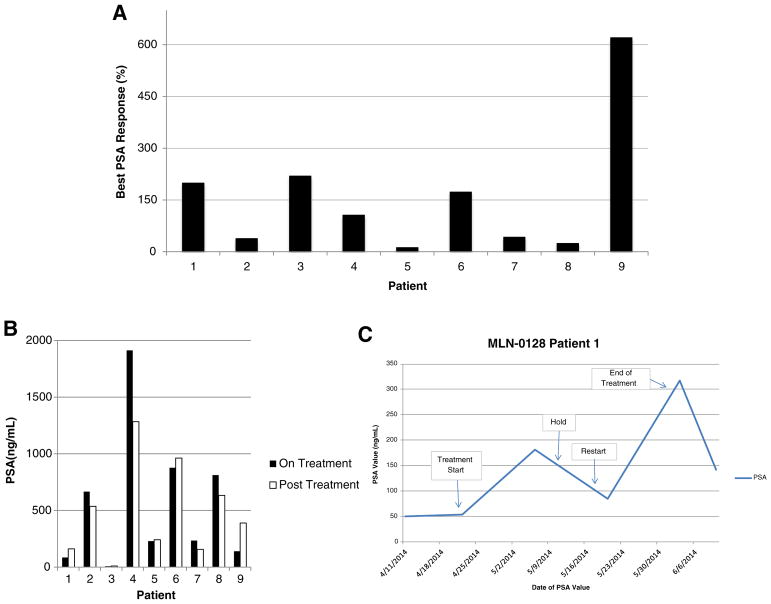
Eight of 9 patients (89%) discontinued treatment before the scheduled 6-month trial endpoint. Median time on treatment was 11 weeks. The time on study and the reasons for discontinuation (progression, toxicity, or investigator discretion) are shown in Fig. 1. Of the 8 patients who discontinued treatment early, 5 did so because of toxicity, 1 had radiographic progression, and 2 left at investigator discretion. All patients experienced a rise in PSA on treatment (Fig. 2A). The median PSA rise at the end of treatment was 159% from baseline (range: 12–620%). Four of 9 patients experienced a decrease in PSA following discontinuation of MLN0128 (Fig. 2B). One example of the inhibitory effect of mTOR on AR function is shown in Fig. 2C, where MLN0128 was held for toxicity and then restarted. PSA levels declined when MLN0128 was held, and increased again when MLN0128 was restarted.
Fig. 1.
Time on study and reasons for discontinuation in patients with advanced prostate cancer (n = 9). Nine patients with castration-resistant prostate cancer were treated during the study. Median time in the study was 11 weeks. Eight patients discontinued treatment before the study endpoint was reached because of unacceptable toxicity (5 patients), radiographic progression (1 patients), and investigator discretion (2 patients)
Fig. 2.
A) Maximal percent PSA change from baseline. The greatest percent PSA change from baseline for each patient at any time during the study is shown. This constituted a PSA rise for all 9 patients (range: 12%–620%). B) Upon withdrawal of MLN0128, 4 of 9 patients exhibited a PSA decline after >1 week. C) PSA kinetics with initiation and withdrawal of MLN0128 in patient 1
Circulating tumor cells
Using the Epic Sciences platform, CTCs were evaluated at baseline and 4 weeks after discontinuation of the drug [24]. No patient had a decrease in CTC count.
Tumor sequencing
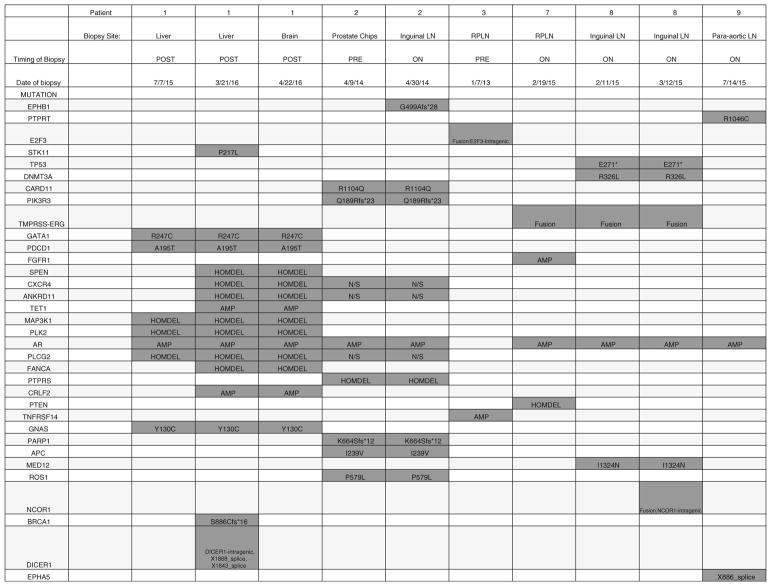
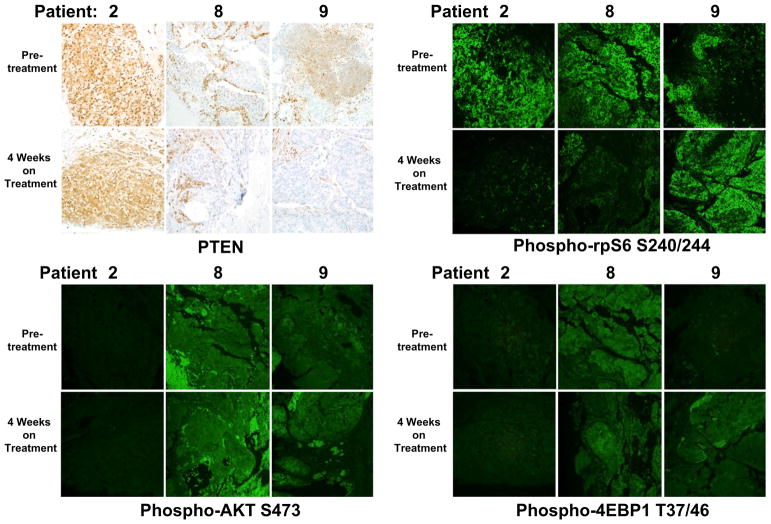
Six of 9 patients had biopsy samples sequenced using MSK-IMPACT, the in-house proprietary targeted genomic sequencing test. One sample was a prostate sample and the rest were metastatic biopsy samples. All samples had genetic alterations, including amplifications, fusions, deletions, or point mutations, ranging from 2 to 13 per tumor. Only one patient exhibited a homozygous deletion of PTEN and an additional patient exhibited a presumed loss of function mutation of PIK3R3 (Fig. 3). There were no other mutations detected in PI3K pathway genes by MSK-IMPACT. At the protein level using IHC, 2 out of 3 evaluable patients were negative for PTEN (Fig. 4).
Fig. 3.
Genetic alterations in biopsy specimens from patients with metastatic CRPC. Six of 9 patients had biopsy samples sequenced using the in-house proprietary targeted genomic sequencing test MSK-IMPACT. Biopsy site, temporal relationship to treatment, and specific genetic alterations are shown. * = stop codon
Fig. 4.
Effects of MLN0128 therapy on downstream signaling targets. Immunohistochemical and immunofluorescence analysis was done on biopsy specimens for patients who had available specimens from before treatment and after 4 weeks on treatment. Tissues were stained for PTEN, the mTORC1 targets rpS6 and 4EBP1, and the mTORC2 substrate AKT. rpS6 phosphorylation was decreased in 2 of 3 patients posttreatment. However, AKT and 4EBP1 did not display any decrease in phosphorylation in the posttreatment setting. All representative images were taken at 20× magnification
Effect on downstream signaling
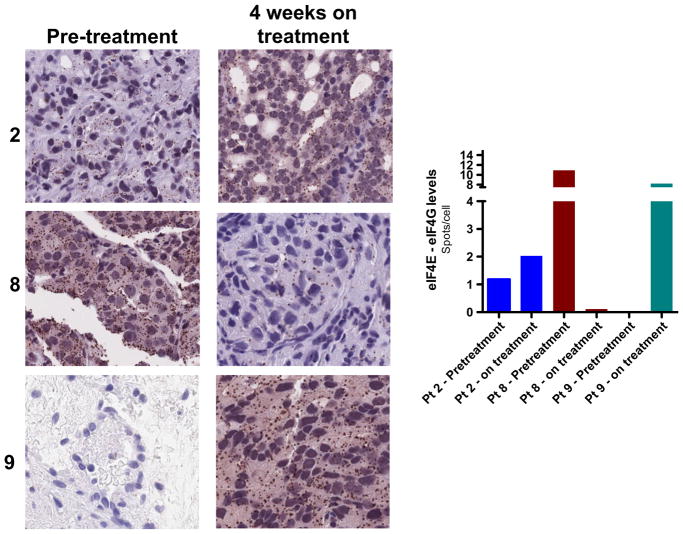
Six patients had an evaluable baseline biopsy, and 3 of the 6 went on to have a week-4 posttreatment biopsy. In order to assess the effects of MLN0128 on downstream signaling targets, we analyzed pre- and posttreatment tissues from the 3 evaluable patients, focusing on the phosphorylation status of mTORC1 targets rpS6 (ribosomal protein S6) and 4EBP1, as well as the mTORC2 substrate AKT. Immunofluorescence analysis showed that rpS6 phosphorylation was decreased in 2 of the 3 patients posttreatment, though we found no decrease in phosphorylation of AKT or 4EBP1 (Fig. 4). The mTOR kinase regulates protein synthesis by phosphorylating 4EBPs, which leads to an increase in eIF4E activity and mRNA translation initiation. We measured eIF4E activity in pre- and posttreatment tissue samples from the 3 evaluable patients using a proximity ligation assay. Surprisingly, samples from 2 patients displayed a significant increase in eIF4E activity (Fig. 5). Overall, these findings suggest that MLN0128 had little impact on downstream signaling in tumor tissues and resulted in a paradoxically increased level of eIF4E activity.
Fig. 5.
The proximity ligation assay (PLA) was used to assess eIF4E activity in pretreatment and posttreatment biopsy tissues. eIF4E activity is elevated in posttreatment tissues of 2 patients (out of 3). All representative images were taken at 20× magnification
Discussion
This paper reports on the clinical effects of MLN0128, a potent and orally bioavailable dual inhibitor of the mTOR kinase, in a phase II clinical trial of patients with heavily pretreated CRPC. The rationale for the study was based on the frequent deregulation of the oncogenic PI3K signaling pathway in CRPC and promising preclinical studies suggesting the effectiveness of dual mTOR inhibitors in prostate cancer [6, 9, 25]. In this patient cohort, 8 of 9 discontinued the drug before the trial endpoint because of toxicity, disease progression, or at the investigator’s discretion. None of the patients had a decrease in their PSA levels or CTC counts while on the study drug.
There are several potential explanations for the lack of clinical efficacy of MLN0128 in patients with CRPC. The first 5 patients started at the established 5 mg daily dose but had to be dose reduced because of toxicities, and the remaining 4 patients were started at 4 mg daily. Even with this reduced dose, the median time on treatment was less than 12 weeks, with 5 patients discontinuing treatment early because of unacceptable side effects. Therefore, it is possible that the dose needed for maximum therapeutic effect was not achieved. This is supported by our data, which indicate poor inhibition of downstream signaling targets. For example, while rpS6 phosphorylation was inhibited in 2 of 3 evaluable patients, the mTOR substrates 4EBP1 and AKT did not display any decreased phosphorylation (Fig. 4). Moreover, 2 of the 3 patients exhibited a paradoxical increase in eIF4E activity after only 4 weeks on treatment (Fig. 5). These findings closely mimic a neoadjuvant study of rapamycin, the allosteric inhibitor of mTOR, in men with intermediate- to high-risk localized prostate cancer treated before radical prostatectomy. Although inhibition of rpS6 phosphorylation was observed, there were no effects on tumor cell proliferation, induction of apoptosis, PSA levels, or posttreatment tumor grade or stage [12]. It has been shown that eIF4E hyperactivity is necessary for tumor formation and progression in mouse models of cancer, whereas rpS6 phosphorylation is dispensable [18, 22]. In our study, MLN0128 inhibited rpS6 phosphorylation but did not sufficiently inhibit potentially more important tumorigenic downstream signaling in patient tumors, leading to maintained oncogenic protein synthesis. Although our data suggests that MLN0128 has limited ability to affect downstream activity, this interpretation is constrained by our small sample size as well as potential variability in pre- and post-treatment tissues analyzed. Additional studies are warranted.
Although this study was negative for a clinical response, it is possible that patients would have experienced positive clinical outcomes if mTOR were more potently and specifically targeted. More recent trials are exploring the use of intermittent high-dose strategies that could potentially improve efficacy by potently inhibiting mTOR for short durations [26, 27]. Recently, a new class of third-generation linked mTOR inhibitors has been reported with significantly more specificity and excellent preclinical responses [28, 29]. In the future, these linked compounds may provide a therapeutic window to efficiently target downstream mTOR signaling in prostate cancer.
Most prostate cancer remains reliant on AR signaling throughout its evolution, and androgen deprivation therapies have been a mainstay of treatment for mCRPC. However, responses to these drugs is often short lived. Therefore, there has been increased focus on studying alternative signaling pathways in prostate cancer, including the PI3K-AKT-mTOR pathway. However, all patients in our cohort experienced a PSA increase after initiating MLN0128 therapy. This is consistent with prior work demonstrating significant crosstalk between the PI3K and AR signaling pathways [30–32]. In our study, in addition to a rise in PSA after MLN0128 initiation, 4 patients had decreases in their PSA after stopping therapy (Fig. 2), suggesting a relief of PI3K-AKT-mTOR potentiating effects on AR signaling. Although the patients in this study were previously treated with enzalutamide or abiraterone, these were not ongoing during this trial. To address the issue of increased AR activity, a new phase I/II study is currently testing the clinical efficacy of CC-115, a dual mTOR/ATP site inhibitor, with enzalutamide in patients with CRPC (NCT02833883).
Our study highlights the need for molecular biomarkers to enrich for patients who may respond to PI3K-AKT-mTOR pathway inhibitors. It is interesting that in our population of 9 patients, 3 (33%) exhibited alterations to PTEN. More research is needed into the predictive value of PTEN deletions in response to PI3K pathway inhibitors. Indeed, it was recently shown that CRPC patients who were negative for PTEN by immunohistochemical analysis were more likely to respond to the AKT inhibitor ipatasertib [33]. Other biomarkers may also be considered, including readouts for eIF4E activity (using the proximity ligation assay) or downstream targets of eIF4E [34]. Moreover, the ideal tissue sampling method to ascertain these biomarkers requires further optimization. As highlighted by the lack of tumor in several of our biopsy specimens, sampling of metastatic disease can be difficult, and not all sites have the same diagnostic yield. In addition, as disease progresses and new treatments are considered, original tumor tissue may not accurately represent the new molecular events that lead to recurrence or progression. Indeed, this is suggested by our sequencing data, in which tumors from the same patient at different time points as well as different locations sometimes had disparate genetic profiles (Fig. 3). In the future, new technologies such as targeted sequencing of cell-free DNA and circulating tumor DNA may help overcome the issues of sampling bias and tumor heterogeneity [35].
This study presents evidence that MLN0128 had limited clinical efficacy on a cohort of unselected patients with metastatic CRPC. Correlative studies indicate that downstream mTOR substrates were poorly inhibited and a reciprocal increase in AR activity was observed. Studies are currently under way to co-target the mTOR signaling pathway and the AR. New third-generation mTOR inhibitors, which may have more favorable side effect profiles, should be considered for clinical testing.
Acknowledgments
Funding support D.E.R. received funding and drug supply from Millenium Pharmaceuticals to support this trial. K.B. is a recipient of an American Society of Clinical Oncology Young Investigator Award and is funded by a National Cancer Institute training grant (T32CA009515) and a Pilot and Feasibility Studies Program grant funded by the Co-operative Center for Excellence in Hematology. B.S.C. is funded by 1R01 CA182503-01A1. T.L.L. was funded in part by a Transformative Impact Award from the CDMRP (W81XWH-12-PCRP-TIA). A.C.H. is a V Foundation Scholar and is funded by a NextGen Grant for Transformative Cancer Research from the American Association for Cancer Research, a Fred Hutchinson Cancer Research Center/University of Washington Cancer Center Support Grant, a National Institutes of Health Career Development Award (1K08CA175154–01), and the Burroughs Wellcome Fund. D.E.R., Y.C., and H.I.S. received support through the NIH/NCI Cancer Center Support Grant P30 CA008748. B.S.C, Y.C, A.C.H., and D.E.R. are recipients of a Movember-Prostate Cancer Foundation Challenge Award. NCI P50CA092629 (B.S.C., Y.C., H.I.S., D.E.R), NCI P30CA008748 (B.S.C., Y.C., H.I.S., D.E.R).
Footnotes
Compliance with ethical standards
Conflict of interest D.E.R. is a consultant for Janssen (uncompensated) and receives research funding from Astellas, Astra-Zeneca, Celgene, Genentech, Janssen, Medivation, Novartis, Taiho, Tracon. L.G. declares that she has no conflicts of interest. K.B. declares that he has no conflicts of interest. A.T. declares that she has no conflicts of interest. B.S.R. declares that he has no conflicts of interest., Y.C. declares that he has no conflicts of interest. K.P. declares that she has no conflicts of interest. G.S. declares that he has no conflicts of interest. H.I.S. declares that he has no conflicts of interest. T.L.L. declares that she has no conflicts of interest. A.C.H. declares that he has no conflicts of interest.
Research involving human participants and/or animals Informed consent was obtained from all individual participants included in the study.
References
- 1.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed]
- 2.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh AC, Truitt ML, Ruggero D. Oncogenic AKTivation of translation as a therapeutic target. Br J Cancer. 2011 doi: 10.1038/bjc.2011.241. [DOI] [PMC free article] [PubMed]
- 4.Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl. 2014 doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed]
- 5.Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP. Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal. 2009 doi: 10.1126/scisignal.2000189. [DOI] [PMC free article] [PubMed]
- 6.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012 doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed]
- 7.Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci U S A. 1994;91(26):12574–8. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013 doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed]
- 9.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004 doi: 10.1038/nm1052. [DOI] [PubMed]
- 10.Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, Gautschi O, Borner M, Fechter E, Stenner F, Winterhalder R, Muller B, Schiess R, Wild PJ, Ruschoff JH, Thalmann G, Dietrich PY, Aebersold R, Klingbiel D, Gillessen S Swiss Group for Clinical Cancer Research (SAKK) Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08) Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed]
- 11.Amato RJ, Jac J, Mohammad T, Saxena S. Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer. 2008 doi: 10.3816/CGC.2008.n.015. [DOI] [PubMed]
- 12.Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood DP, Creel PA, Mundy K, Davis SL, Wang T, Albadine R, Schultz L, Partin AW, Jimeno A, Fedor H, Febbo PG, George DJ, Gurganus R, De Marzo AM, Carducci MA. A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0124. [DOI] [PMC free article] [PubMed]
- 13.Rathkopf DE, Larson SM, Anand A, Morris MJ, Slovin SF, Shaffer DR, Heller G, Carver B, Rosen N, Scher HI. Everolimus combined with gefitinib in patients with metastatic castration-resistant prostate cancer: phase 1/2 results and signaling pathway implications. Cancer. 2015 doi: 10.1002/cncr.29578. [DOI] [PMC free article] [PubMed]
- 14.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed]
- 15.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65(16):7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 16.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009 doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed]
- 17.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009 doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed]
- 18.Hsieh AC, Costa M, Zollo O, Davis C, Feldman ME, Testa JR, Meyuhas O, Shokat KM, Ruggero D. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.01.021. [DOI] [PMC free article] [PubMed]
- 19.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008 doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed]
- 21.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015 doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed]
- 22.Hsieh AC, Nguyen HG, Wen L, Edlind MP, Carroll PR, Kim W, Ruggero D. Cell type-specific abundance of 4EBP1 primes prostate cancer sensitivity or resistance to PI3K pathway inhibitors. Sci Signal. 2015 doi: 10.1126/scisignal.aad5111. [DOI] [PMC free article] [PubMed]
- 23.Infante JR, Tabernero J, Cervantes A, Jalal S, Burris HA, Macarulla T, Perez-Fidalgo JA, Neuwirth R, Patel C, Gangolli E, Brake R, Sturm J, Westin EH, Gordon M. Abstract C252: a phase 1, dose-escalation study of MLN0128, an investigational oral mammalian target of rapamycin complex 1/2 (mTORC1/2) catalytic inhibitor, in patients (pts) with advanced non-hematologic malignancies. Mol Cancer Ther. 2013:12. [Google Scholar]
- 24.Werner SL, Graf RP, Landers M, Valenta DT, Schroeder M, Greene SB, Bales N, Dittamore R, Marrinucci D. Analytical validation and capabilities of the epic CTC platform: enrichment-free circulating tumour cell detection and characterization. J Circ Biomark. 2015 doi: 10.5772/60725. [DOI] [PMC free article] [PubMed]
- 25.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65(7):2825–31. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 26.Burris HA, 3rd, Kurkjian CD, Hart L, Pant S, Murphy PB, Jones SF, Neuwirth R, Patel CG, Zohren F, Infante JR. TAK-228 (formerly MLN0128), an investigational dual TORC1/2 inhibitor plus paclitaxel, with/without trastuzumab, in patients with advanced solid malignancies. Cancer Chemother Pharmacol. 2017 doi: 10.1007/s00280-017-3343-4. [DOI] [PubMed]
- 27.Ghobrial IM, Siegel DS, Vij R, Berdeja JG, Richardson PG, Neuwirth R, Patel CG, Zohren F, Wolf JL. TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: a phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenstrom’s macroglobulinemia. Am J Hematol. 2016 doi: 10.1002/ajh.24300. [DOI] [PubMed]
- 28.Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E, Barratt DG, Cosulich S, Klinowska T, Rosen N, Shokat KM. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016 doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed]
- 29.Fan Q, Aksoy O, Wong RA, Ilkhanizadeh S, Novotny CJ, Gustafson WC, Truong AY, Cayanan G, Simonds EF, Haas-Kogan D, Phillips JJ, Nicolaides T, Okaniwa M, Shokat KM, Weiss WA. A kinase inhibitor targeted to mTORC1 drives regression in glioblastoma. Cancer Cell. 2017;31(3):424–435. doi: 10.1016/j.ccell.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011 doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed]
- 31.Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, Plaisier S, Garraway IP, Huang J, Graeber TG, Wu H. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011 doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed]
- 32.Schwartz S, Wongvipat J, Trigwell CB, Hancox U, Carver BS, Rodrik-Outmezguine V, Will M, Yellen P, de Stanchina E, Baselga J, Scher HI, Barry ST, Sawyers CL, Chandarlapaty S, Rosen N. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. 2015 doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed]
- 33.de Bono JS, De Girogi U, Massard C, Bracarda S, Nava Rodrigues D, Kocak I, et al. PTEN loss as a predictive biomarker for the Akt inhibitor ipatasertib combined with abiraterone acetate in patients with metastatic castration-resistant prostate cancer (mCRPC) Ann Oncol: Official J Eur Soc Med Oncol/ESMO. 2016;(suppl_6):7180. [Google Scholar]
- 34.Sheridan CM, Grogan TR, Nguyen HG, Galet C, Rettig MB, Hsieh AC, Ruggero D. YB-1 and MTA1 protein levels and not DNA or mRNA alterations predict for prostate cancer recurrence. Oncotarget. 2015;6(10):7470–80. doi: 10.18632/oncotarget.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, Foye A, Lloyd P, Nykter M, Beer TM, Alumkal JJ, Thomas GV, Reiter RE, Rettig MB, Evans CP, Gao AC, Chi KN, Small EJ, Gleave ME. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017 doi: 10.1093/jnci/djx118. [DOI] [PMC free article] [PubMed]