Abstract
The central nervous system (CNS) is believed to use the abundant degrees of freedom of muscles and joints to stabilize a particular task variable important for task success, such as footpath during walking. Stroke survivors often demonstrate impaired balance and high incidences of falls due to increased footpath variability during walking. In the current study, we use the uncontrolled manifold (UCM) approach to investigate the role of motor abundance in stabilizing footpath during swing phase in healthy individuals and stroke survivors. Twelve stroke survivors and their age- and gender-matched controls walked over-ground at self-selected speed, while electromyographic and kinematic data were collected. UCM analysis partitioned the variance of muscle groups (modes) across gait cycles into “good variance” (i.e., muscle mode variance leading to a consistent or stable footpath) or “bad variance” (i.e., muscle mode variance resulting in an inconsistent footpath). Both groups had a significantly greater “good” than “bad” variance, suggesting that footpath is an important task variable stabilized by the CNS during walking. The relative variance difference that reflects normalized difference between “good” and “bad” variance was not significantly different between groups. However, significant differences in muscle mode structure and muscle mode activation timing were observed between the two groups. Our results suggest that though the mode structure and activation timing are altered, stroke survivors may retain their ability to explore the redundancy within the neuromotor system and utilize it to stabilize the footpath.
Keywords: Stroke, Locomotion, Footpath variability, Uncontrolled manifold hypothesis
Introduction
Foot placement during walking may be actively controlled by the central nervous system (CNS) in neurologically intact individuals (Winter 1992; Bauby and Kuo 2000). Ivanenko et al. (2002) found that, at different walking speeds and gravity loads simulated by varying the body weight support, healthy individuals demonstrated significant changes of muscle activity patterns but limited changes in their foot trajectory. Thus, this suggests that reproducibility of foot trajectory during walking may be achieved by intermuscular coordination. Furthermore, Winter (1992) hypothesized that foot trajectory is precisely controlled by the CNS, evidenced by the fact that small changes in joint angles of the lower extremity account for large changes in toe clearance during the swing phase of walking. In addition, studies have shown significant improvements in functional walking capacity following a gait training that emphasizes on tracking a prescribed foot trajectory pattern in stroke survivors (Krishnan et al. 2012, 2013a; Srivastava et al. 2014). A recent case study based on a single stroke survivor showed that a gait training focusing on foot placement can improve muscle coordination during walking (Krishnan et al. 2012). These previous findings indicate that foot position may be an important task variable during walking in neurologically intact individuals and stroke survivors.
It has been suggested previously that the CNS stabilizes specific task variables important for task success instead of controlling individual joints or muscles during a movement (Scholz and Schoner 1999). However, following a neurological injury the ability to stabilize a task variable may be altered (Reisman and Scholz 2003; Black et al. 2007). Stroke has been shown to be associated with greater step-to-step variability in gait parameters (Balasubramanian et al. 2009). Therefore, stroke survivors may also exhibit increased variability in their footpaths during walking. Previous studies have examined the variability of the vertical and anterior–posterior (AP) foot position measured as foot clearance and step length, respectively (Begg et al. 2007; Balasubramanian et al. 2009). However, there is a lack of information regarding the neural mechanisms involved in stabilizing the footpath during walking. It was suggested that emphasizing the stabilization of a task variable may be more important than improving the coordination of muscles or joints following a neurological injury (Ivanenko et al. 2009). Therefore, determining whether the footpath is an important task variable stabilized by the CNS during walking and whether the stabilization strategy is altered following stroke can further assist in designing adequate rehabilitation techniques for individuals with stroke.
A neurologically intact CNS has an abundant number of degrees of freedom (DOF) available at the level of joints or muscles to perform a task (Bernstein 1967). The CNS is believed to use the available flexibility to covary muscles and joint motions in stabilizing a variable important for task success. Recent studies suggested that the CNS does not control individual muscles. Instead, the muscles are controlled in groups, called muscle modes (Krishnamoorthy et al. 2004; Ting and Macpherson 2005). Thus, muscle modes covary to control the stabilization of a task variable termed as muscle mode synergy in this context. In the current study, we used the computational method of the uncontrolled manifold (UCM) approach to understand the control of this synergy and determined how the CNS uses the variability of muscle modes’ activation across cycles to stabilize a task variable (Gelfand and Tsetlin 1966; Scholz and Schoner 1999). According to the UCM approach, the variance of muscles across gait cycles can be divided into two components. The variance component that leads to a stable footpath is termed as “good variance” or VUCM, whereas the variance component that results in a variable footpath is termed as “bad variance” or VORT. A task variable is considered stabilized when the value of VUCM is significantly greater than the value of VORT, indicating that a larger portion of the muscle mode variance across cycles is contributing toward a consistent footpath. The normalized difference between “good” and “bad” variance termed relative variance difference determines the strength of the synergy to stabilize the task variable.
There is a lack of understanding regarding how the CNS stabilizes the footpath in the vertical and AP directions during human walking. The UCM approach has been used previously to understand the stabilization of a task variable in the stroke population and neurologically intact individuals (Reisman and Scholz 2003; Papi et al. 2015). These studies suggested that the CNS can stabilize a task variable during reaching or walking even following stroke, evidenced by a significantly greater “good” variance in comparison with “bad” variance. The purpose of the current study was to identify the muscle mode synergy for the control of footpath. Specifically, we would like to examine the distribution of “good” and “bad” muscle mode variances used to stabilize the vertical and AP footpath across gait cycles during the swing phase in the stroke survivors and their age- and gender-matched healthy controls. Based on the previous literature, we hypothesized that the good variance (VUCM) would be larger than the bad variance (VORT) in both healthy individuals and stroke survivors. Furthermore, we expected that strength of the synergy would be weaker following neurological injury. Stroke survivors usually have greater step-to-step movement variability in comparison with neurologically intact individuals. Thus, we expected to see that the total amount of variability rendered by the stroke survivors would be larger than the total variability rendered by the healthy controls.
Materials and methods
Twelve stroke survivors (9 males and 3 females) who had sustained a stroke more than 3 months prior to the study were recruited. Demographic details of the stroke survivors are listed in Table 1. Subjects were excluded if they had evidence of multiple strokes, chronic white matter disease on MRI, congestive heart failure, peripheral artery disease with intermittent claudication, cancer, pulmonary or renal failure, unstable angina, uncontrolled hypertension (>190/110 mmHg), dementia (mini–mental state examination <22) (Cockrell and Folstein 2002), severe aphasia, orthopedic conditions affecting the legs or the back, or cerebellar signs (e.g., ataxia). Gender- and age-matched (±5 years), neurologically intact subjects were recruited for each of the stroke survivors. Control subjects were included only if they were free from any musculoskeletal, vascular, or neurological disorder that can significantly affect their walking ability. All subjects gave written informed consent to participate in the study, approved by the university’s institutional review board.
Table 1.
Demographic detail of the stroke survivors
| Subject ID | Age (years) | Duration post-stroke (months) |
Side affected | Sex | LE Fugl- Meyer assessment |
Self-selected speed (m/s) |
Number of modes |
|---|---|---|---|---|---|---|---|
| S1 | 56 | 95 | Left | M | 24 | 1.04 | 4 |
| S2 | 80 | 53 | Left | M | 25 | 0.75 | 4 |
| S3 | 60 | 3 | Right | F | 11 | 0.15 | 3 |
| S4 | 43 | 3 | Right | M | 21 | 0.53 | 4 |
| S5 | 67 | 20 | Left | M | 12 | 0.29 | 4 |
| S6 | 70 | 149 | Left | F | 28 | 0.78 | 4 |
| S7 | 58 | 17 | Left | M | 24 | 0.55 | 4 |
| S8 | 48 | 11 | Right | F | 27 | 0.67 | 4 |
| S9 | 75 | 14 | Right | M | 17 | 0.16 | 3 |
| S10 | 54 | 12 | Left | M | 17 | 0.54 | 4 |
| S11 | 59 | 35 | Left | M | 24 | 0.90 | 4 |
| S12 | 59 | 3 | Right | M | 17 | 0.55 | 4 |
Data acquisition and analysis
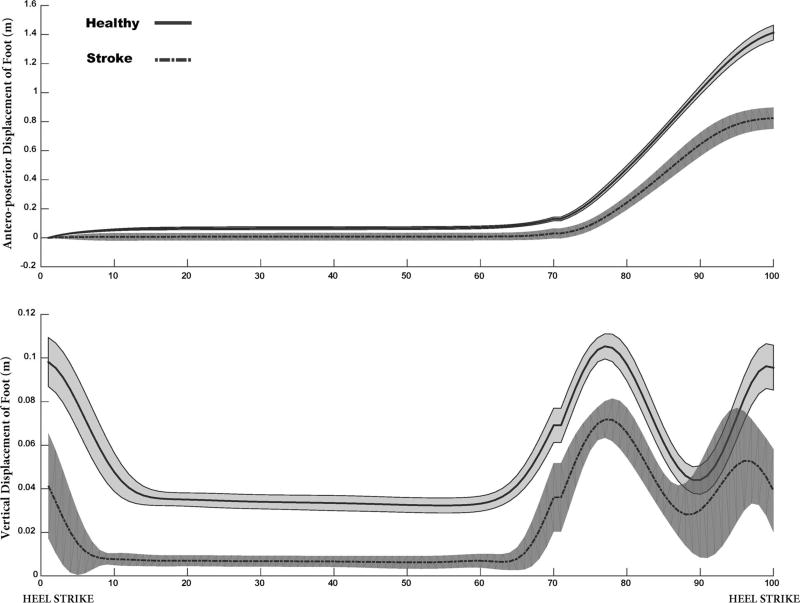
Stroke survivors and healthy controls were asked to walk at their self-selected over-ground walking speed. Kinematic data were recorded and sampled at 120 Hz using an eight-camera motion capture system (Qualisys Gothenburg, Sweden). Electromyographic (EMG) data from ten muscles were recorded using a 16-channel EMG system (MA-416-003 Motion Lab System Baton Rouge, LA) and sampled at 1200 Hz with a 16-bit resolution. Kinematic and EMG data were recorded from the paretic extremity of the stroke survivors and the corresponding extremity of the matched control. Disposable self-adhesive surface electrodes were attached on the muscle belly of the biceps femoris longus (BF), vastus lateralis (VL), vastus medialis (VM), rectus femoris (RF), gluteus medius (GM), soleus (SO), medial head of gastrocnemius, (MG) lateral head of gastrocnemius (LG), medial hamstrings (MH), and tibialis anterior (TA). EMG signals were high-pass filtered with a cutoff frequency of 20 Hz, rectified, and then low-pass filtered with a cutoff frequency of 6 Hz using a second-order Butterworth filter. EMG from each muscle was normalized to its peak amplitude across all gait cycles. Footpath (Fig. 1) was computed based on the coordinates of the reflective marker attached on the top of the fifth metatarsal of the foot. Marker trajectories were low-pass filtered with a cutoff frequency of 6 Hz. The EMG data and the foot marker coordinates during the swing phase were used for the UCM analysis.
Fig. 1.
Antero-posterior and vertical foot displacement of a representative stroke survivor and the matched healthy control averaged across gait cycles. The shaded area represents standard deviation across gait cycles
Step 1: Nonnegative matrix factorization
Nonnegative matrix factorization (NMF) was used to compute muscle modes (Ting and Macpherson 2005). The linear envelopes of EMG data from all gait cycles were concatenated for computing muscle modes across time. NMF factorizes the concatenated original EMG data (EMGO) into two matrices. The matrix W corresponds to the mode structure and specifies the relative contribution of each muscle to a muscle mode, whereas the matrix H is the activation timing of each muscle mode across a gait cycle such that (W × H) is the reconstructed EMG data (EMGR). The number of adequate modes required for reconstructing the original EMG (EMGO) after data reduction was based on the variability accounted for (VAF). VAF was defined as the ratio of sum of squared errors between the original and reconstructed EMG data (EMGO − EMGR)2 to the sum of squared original EMG data (EMGO)2 (Clark et al. 2010):
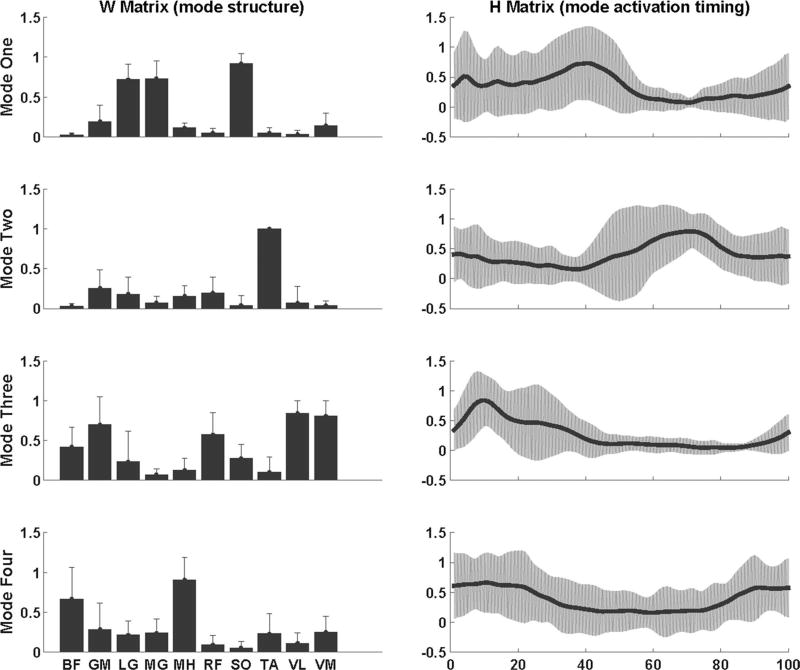
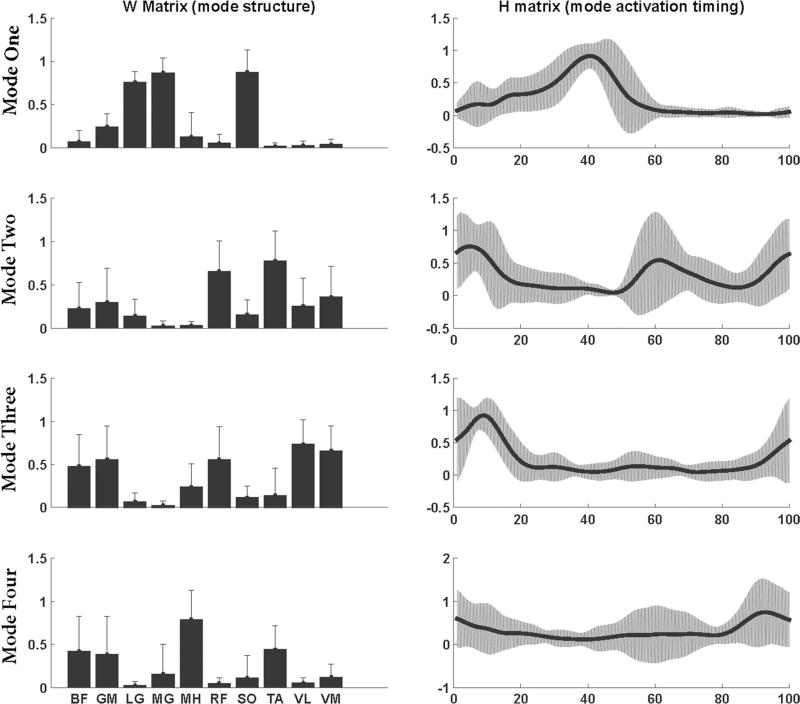
The analysis starts with the assumption that only one mode is sufficient to reconstruct the EMG data. The number of modes was increased until a VAF ≥ 90 % was achieved for each of the ten muscles (Clark et al. 2010). To align muscle modes of all subjects in the same order, the mode structures were matched based on the dot products between the W matrices normalized to their lengths. A dot product with value one would represent a perfect match. To determine the mode order, a representative healthy subject was selected. Dot products between the mode structures of the representative subject and each healthy individual were computed. The modes were ordered such that the dot products of each corresponding mode were closest to one. For stroke survivors, the order of modes was based on the average W matrices of healthy individuals. Mode1 primarily consisted activity from SO, MG, and LG, Mode2 consisted activity from TA and RF, Mode3 consisted activity from GM, VL, VM, and RF, and Mode4 consisted activity from BF and MH (Figs. 2, 3).
Fig. 2.
Averaged (across subjects) muscle mode structure (W matrices) and mode activation timing (H matrices) for stroke survivors during over-ground walking. Error bars represent the standard deviation across subjects
Fig. 3.
Averaged (across subjects) muscle mode structure (W matrices) and mode activation timing (H matrices) for neurologically intact individuals during over-ground walking. Error bars represent the standard deviation across subjects
Step 2: Linear regression
The Jacobian matrix obtained by the regression analysis represents how changes in the modes’ activation timing (H) are related to the changes in the footpath (Foot) during the swing phase. The regression equation for the changes in the vertical footpath during swing phase related to changes in the muscle modes’ activation timing is given as:
and the equation for the AP position of foot is given as:
The coefficients were computed at each sample across the gait cycles to obtain the Jacobian matrix (J) = [C1, C2, C3, C4]. Here C1, C2, C3, C4 are the regression coefficients from the two equations in case of four modes; therefore, we will obtain a (2 × 4) matrix for vertical and AP directions together at each sample.
Step 3: Uncontrolled manifold analysis
The Jacobian matrix (J) was used to compute how much of the muscle mode variance led to the footpath variability (VORT) or reflected flexible combinations of the muscle modes that produced a consistent footpath (VUCM) using the uncontrolled manifold (UCM) approach. Details of the UCM approach can be found elsewhere (Scholz and Schoner 1999). Briefly, at every time point of the swing phase, we computed the null space of J matrix which is a set of solution such that J. ε = 0. This null space of the Jacobian represents the basis vectors in muscle modes space or the uncontrolled manifold (UCM) subspace. Within this UCM space, all combinations of muscle modes lead to a consistent footpath. The space orthogonal to the UCM subspace represents the space where combinations of muscle modes result in an inconsistent footpath. To compute these two subspaces, the mean magnitudes for each muscle mode were computed across all trials and subtracted from the mode data. At every time point of the swing, for n number of modes and d number of footpath axes, the mean free mode data (ΔH) were projected into the null space of the Jacobian, i.e., the UCM space spanned by a set of (n − d) basis vectors, ε:
and the space orthogonal to the null space, i.e., orthogonal space:
The variance of the muscle modes that did not affect the footpath (VUCM) was computed as the average of squared fUCM across steps (N) and normalized by the DOFs within the UCM subspace (n − d):
The variance that affects the footpath (VORT) was normalized by the DOFs in the orthogonal subspace (d) and computed as:
The relative variance difference (ΔV) between the two variance components was computed as:
The relative variance difference reflects the normalized difference between “good” (VUCM) and “bad” (VORT) variance and determines the strength of the muscle mode synergy to stabilize a task variable. A value close to positive one indicates a stronger synergy of muscle modes to stabilize the footpath during walking.
The total variance (VTOT) for all the subjects during the swing phase is given as:
Statistical analysis
For statistical analyses, VUCM, VORT, ΔV, and VTOT were averaged across the swing phase for each subject. A mixed-design ANOVA was performed with a within-subject factor (i.e., variance components, VUCM and VORT) and a between-subject factor (i.e., group, healthy individuals, and stroke survivors). Independent t tests were used to compare ΔV and VTOT between groups, and paired t test was used to compare the differences between VUCM and VORT within stroke and healthy groups. Spearman’s correlation was used to assess the relationship between Fugl-Meyer (FMA) scores and VTOT in stroke survivors. The number of modes for all the subjects is either three or four (dichotomous variable). Therefore, point biserial correlation was used to assess the relationship between the number of muscle modes and FMA scores of stroke survivors. For comparisons of mode structure and activation timing between the stroke survivors and healthy controls, NMF was performed using four modes for all the stroke survivors. Pearson’s correlation was used to quantify the similarity of the mode structure (Clark et al. 2010) and activation timing (Tropea et al. 2013; Coscia et al. 2015) between stroke survivors and matched controls. We performed one-sample t test to determine whether stroke survivors’ correlation coefficients are significantly different from the healthy controls. To derive the reference value of correlation coefficient in healthy controls, we used bootstrapping method with 1000 iterations for the Pearson’s correlation and compared one representative healthy subject with the mean mode structures and mode activation timings of all healthy controls. We then averaged the coefficients obtained from each of the iterations for the “W” and “H” matrices of each mode. The averaged coefficient for the mode consisting primarily activity of SO, MG, and LG (Mode1) was 0.92 for the W and H matrices. For all the other modes, the values of coefficients were 0.73. Therefore, we used 0.92 for Mode1 and 0.73 for rest of the modes as the reference values of healthy controls. The significance level was set at p < 0.05. Statistical analyses were performed in SPSS version 21 (IBM Co., Somers, NY) or MATLAB, version R2014b (The Mathworks Inc., Redmond, WA).
Results
Mode analysis
There was no significant correlation between FMA and VTOT (r = −0.170, p = 0.59) or between FMA and the number of muscle modes (r = 0.529, p = 0.077) for the stroke survivors. However, previous literature has demonstrated a significant correlation between the functional impairment levels and number of muscle modes in stroke survivors (Clark et al. 2010). A smaller sample size in the current study may have led to the difference between our results and previous literature. Results from one-sample t test revealed that correlation coefficient for the mode structure of Mode1, Mode2, Mode3, and mode activation timing of Mode3 was not significantly different from the reference values obtained from healthy populations. Therefore, stroke survivors had three modes with a structure similar to healthy controls and one mode that had activation timing similar to healthy controls. The mode structure and the activation timing for the remaining modes in stroke survivors were significantly different from healthy controls (Table 2).
Table 2.
Mode structure quality and mode timing quality of stroke survivors compared with healthy controls
| Modes | Correlation coefficient for mode structure and activation |
p values (difference from healthy) |
|---|---|---|
| Mode structure | ||
| Mode1 | 0.85 ± 0.17 | 0.250 |
| Mode2 | 0.62 ± 0.20 | 0.100 |
| Mode3 | 0.62 ± 0.26 | 0.207 |
| Mode4 | 0.32 ± 0.45 | 0.010 |
| Mode activation timing | ||
| Mode1 | 0.61 ± 0.36 | 0.013 |
| Mode2 | 0.32 ± 0.26 | <0.001 |
| Mode3 | 0.72 ± 0.20 | 0.506 |
| Mode4 | 0.25 ± 0.33 | 0.006 |
Means ± standard deviations are listed for each measure
Bold indicates p values that are not significantly different from healthy, suggesting similarity of structure or activation timing between stroke survivors and healthy controls
Regression analysis
All the modes together explained significant amount of variance of the AP foot position (healthy R2 = 0.95 ± 0.01; stroke R2 = 0.80 ± 0.14) and vertical foot position (healthy R2 = 0.96 ± 0.01; stroke R2 = 0.93 ± 0.03) for all the subjects (p < 0.001 for all healthy and stroke survivors). The variance explained by each mode for the foot position in the vertical and AP directions varied through the swing phase in stroke survivors and healthy controls. In general, Mode4 accounted for at least 80 % of the variance with an increase of ~1 to 5 % in the variance accounted for with inclusion of each additional mode to the regression model using stepwise regression.
Each mode is a significant predictor of foot position in the AP and vertical directions for at least 70 % of the swing phase in all healthy subjects (p < 0.05). Out of the ten stroke survivors who had four modes, seven subjects had all four modes as significant predictors of foot position for at least 70 % of the swing phase in the AP direction and for at least 60 % in the vertical direction. One subject out of these ten stroke survivors had three modes as significant predictor of AP foot position but all four modes as significant predictors of vertical foot position. Two subjects had three modes that significantly predicted foot position in vertical direction and four modes that significantly predicted AP foot position. In all these subjects, Mode3 that consisted activity from GM, VL, VM, and RF did not significantly predict the foot position in either AP or vertical directions. All modes were significant predictors of the foot position in the AP and vertical directions for at least 60 % of the swing phase in the two stroke survivors who had three modes (p < 0.05).
UCM analysis
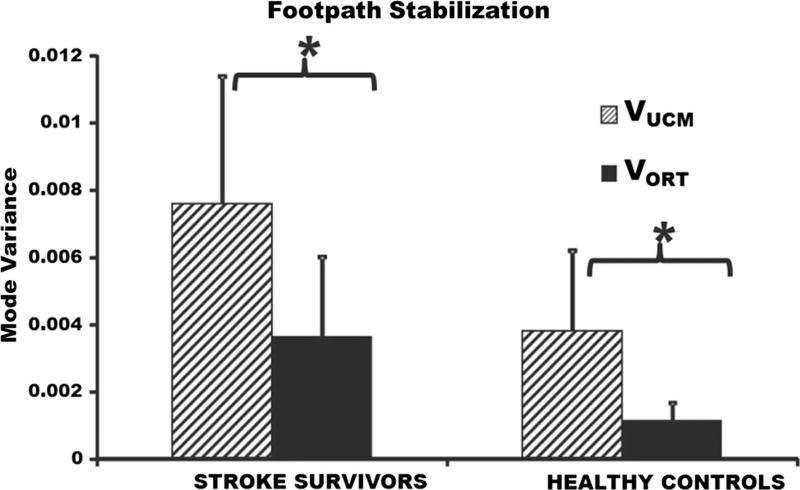
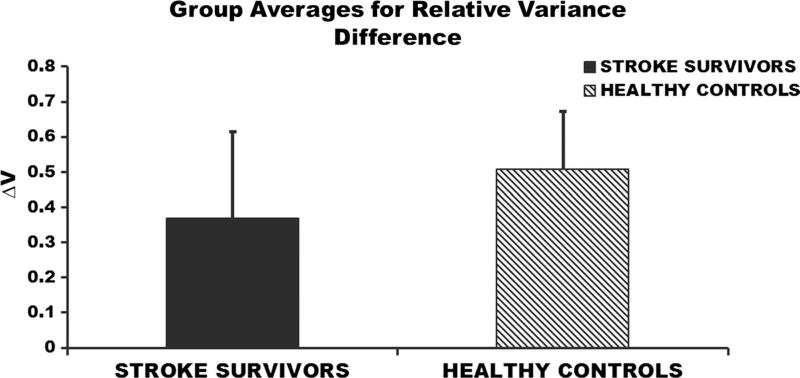
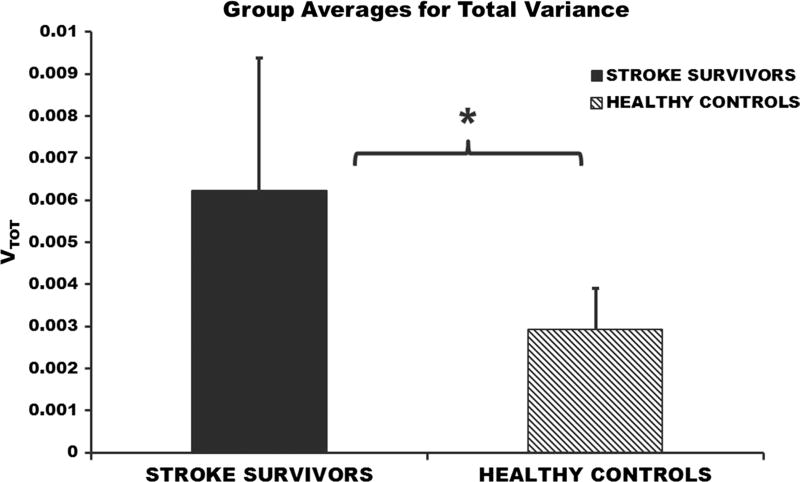
The differences in the two variance components (VUCM and VORT) were not significant between healthy individuals and stroke survivors (p = 0.08); however, a larger sample size may be needed to completely understand between group differences of the two variance components. VUCM was significantly greater than VORT within stroke population (p = 0.001; VUCM = 0.0077 ± 0.0037; VORT = 0.0035 ± 0.0023) and within healthy individuals (p = 0.001; VUCM = 0.0039 ± 0.0014; VORT = 0.0012 ± 0.0005) (Fig. 4), suggesting that footpath was stabilized during the swing phase of walking. There were no significant differences in the relative variance difference between stroke survivors and their healthy age- and gender-matched controls (p = 0.37; healthy = 0.50 ± 0.17; stroke = 0.38 ± 0.24) (Fig. 5). The total variance was significantly greater in stroke survivors (p = 0.001; healthy = 0.0031 ± 0.0009; stroke = 0.0062 ± 0.0031) compared to the healthy individuals (Fig. 6).
Fig. 4.
Variance components averaged across swing phase of walking. The hatched bars represent mode variance that leads to a stable footpath (VUCM) averaged across stroke subjects (left), healthy subjects (right). The solid bars represent mode variance resulting in an inconsistent footpath averaged across stroke (left) and healthy (right) subjects. Error bars represent the standard deviation across subjects. *p < 0.05
Fig. 5.
Averaged (across subjects) relative variance difference (ΔV) during the swing phase of walking. Error bars represent the standard deviation across subjects
Fig. 6.
Total variance during the swing phase of walking averaged across stroke subjects and healthy matched controls. Error bars represent the standard deviation across subjects. *p < 0.05
Discussion
In the current study, we used the UCM approach to determine whether or not the CNS stabilizes the footpath during walking. The footpath was stabilized by muscle mode synergy during the swing phase of walking; i.e., healthy individuals and stroke survivors were able to utilize the motor abundance to stabilize their footpath. In addition, our results showed that stroke survivors had a greater total variance (VTOT) than the age- and gender-matched healthy controls. However, the relative variance difference was not significantly different between the stroke survivors and healthy individuals.
Muscle mode synergies stabilize the footpath during swing phase in stroke survivors and healthy people
The results of the current study support our hypothesis demonstrating that VUCM was significantly larger than the VORT in both healthy individuals and stroke survivors. This suggests that footpath is stabilized by the CNS using abundant degrees of freedom at the level of muscle modes, meaning that footpath is an important task variable during the swing phase of walking. Our results are consistent with the previous findings, demonstrating that the CNS uses motor abundance to stabilize a task variable in healthy individuals (Danna-dos-Santos et al. 2007; Krishnan et al. 2013b). Studies involving various activities such as anticipatory postural adjustments, walking, stepping, and pointing have demonstrated that multiple DOFs at the level of joints or muscle modes covary across trials to stabilize a task variable important for successful accomplishment of the task (Krishnamoorthy et al. 2003; Tseng et al. 2003; Wang et al. 2006; Krishnan et al. 2013b). Furthermore, it was shown previously that individuals retain the ability to use motor abundance in stabilizing a task variable even after a neurological injury (Reisman and Scholz 2003; Black et al. 2007; Papi et al. 2015). The current study provides further evidence to support this result, here specifically for stabilization of the footpath during walking.
Stroke survivors had greater total variance compared to healthy controls
The stroke survivors had a larger VTOT in comparison with the healthy individuals. Balasubramanian et al. (2009) showed that the variability of gait parameters in stroke survivors’ paretic limb is greater compared to their non-paretic limb and the healthy individuals. In addition, previous reaching studies also showed an increase in the trial-to-trial variability of hand path movement and movement timing to reach a target following stroke in comparison with healthy people (Reisman and Scholz 2003; Freitas et al. 2011). The results from the current study are consistent with previous findings, showing that stroke survivors are more variable in comparison with healthy individuals.
It has been shown that the total joint variance increases when stabilizing the task variable during a more challenging reaching task (De Freitas et al. 2007). In the current study, a greater muscle mode variance across cycles observed in stroke subjects compared to healthy controls may be due to the fact that the neuromuscular impairments following stroke make walking more challenging for the stroke survivors (Pohl et al. 2002).
No significant differences were seen between the healthy individuals and stroke survivors in the relative variance difference
We expected that the relative variance difference (ΔV) during walking would be significantly greater in the healthy individuals in comparison with the stroke subjects because it was expected that strength of muscle modes synergy to stabilize the footpath across gait cycles would be altered post-stroke. However, there was no significant difference in the ΔV between the groups in the current study. These results suggest that motor impairment following stroke may not significantly deteriorate the synergy strength of stroke survivors to stabilize task variables (Reisman and Scholz 2003; Papi et al. 2015).
A value of the relative variance difference closer to positive one would suggest stronger synergy of footpath stabilization compared to a value away from positive one. The ΔV values for healthy individuals and stroke survivors in the current study were less closer to one (healthy = 0.50 ± 0.17; stroke = 0.38 ± 0.24) compared to the values reported for healthy people during reaching (Gera et al. 2010). This suggests that the healthy controls may not stabilize the footpath during walking as precisely as the fingertip positions during a reaching task which requires precision to the target. Therefore, as pointed out by Krishnan et al. (2013b), the CNS may not require control of the footpath during walking as precisely as the task variables stabilized during reaching or standing posture. Walking with reduced precision of footpath control (i.e., a smaller ΔV) may be an efficient strategy for healthy individuals.
Possible mechanism underlying footpath stabilization post-stroke
Coordinating the end effector during a movement is considered to be at the highest level of hierarchical control (Latash et al. 2008), for example footpath during walking. A similar level of hierarchy has also been observed in individuals following neurological injury where the CNS focuses on recovering a normal footpath instead of a normal angular motion of the lower limb segments and the pattern of muscles’ activity (Grasso et al. 2004). The CNS may try to conserve the patterns of the end effectors such as footpath during walking at the cost of reorganizing the joint motion and EMG patterns in healthy individuals and individuals following neurological injuries (Grasso et al. 1998, 2004). Our results suggest a similar hierarchical control where footpath stabilization may be prioritized in stroke and neurologically intact populations.
In the current study, healthy individuals and stroke survivors had a significantly greater VUCM than VORT, suggesting that both groups were able to stabilize their footpath during walking. Furthermore, there were no significant differences between the healthy individuals and stroke survivors’ muscle mode synergy strength, evidenced by their similar relative variance difference (ΔV). Nevertheless, differences were observed in one of the mode structures and most of the mode activation timing profiles between healthy individuals and stroke survivors. It was suggested that a damaged nervous system may develop new motor patterns instead of attempting to reactivate the normal motor patterns in order to stabilize an end effector during movements (Cirstea and Levin 2000; Ivanenko et al. 2009). Therefore, it is possible that stroke survivors may utilize motor abundance in stabilizing the end effector by altering muscle mode patterns following neurological injury.
Study limitations
In the current study, a trend toward negative correlation between the impairment levels and total variance was observed although the correlation was not significant. Therefore, future studies with greater number of subjects and more diverse levels of motor impairments post-stroke may be required to further understand these relationships. Although the results from the current study may help us gain a better understanding of the relationship between muscle activation and footpath control in healthy and post-stroke gait, the muscle activation of the contralateral leg may also play an important role in altering the footpath of ipsilateral leg during swing. Therefore, further investigations are needed to include muscles of the stance limb in the analysis to completely understand footpath control during walking. Moreover, the current analysis does not include the mediolateral footpath which is also an important variable controlled by the CNS during walking; thus, future studies are needed to understand the mediolateral footpath control by including adductor and abductor muscles.
It has been documented that NMF performs better than other often used factorization algorithm, i.e., principal component analysis in identifying muscle modes (Tresch et al. 2006). However, one of the limitations of NMF is that NMF cannot be applied on the detrended EMG data which includes both negative and positive values. Thus, without detrending the EMG data first, it is possible that the muscle modes extracted in the current study might be partly confounded by task-related or biomechanical constraints (Kutch and Valero-Cuevas 2012; Ranganathan and Krishnan 2012).
Conclusions
The overall goal of this study was to determine whether the CNS uses motor abundance to stabilize the footpath during walking and to identify the differences in footpath stabilization between stroke survivors and healthy individuals. Our results demonstrated that healthy individuals have the ability to use motor abundance for stabilizing the vertical and AP footpath during the swing phase of walking which is retained in individuals following stroke. In addition, stroke survivors had a greater total variance compared to healthy controls which is consistent with previous literature suggesting an increase movement variability following stroke. We also found that stroke survivors had similar synergy strength compared to the healthy controls. However, the mode structure and activation timing between healthy individuals and stroke survivors were significantly different. Thus, it is possible that though individuals post-stroke have altered muscle mode structure and activation timing, their footpath stabilization strategy may not be affected post-stroke.
Acknowledgments
The authors would like to thank the Delaware Rehabilitation Institute (DRI) research core in helping with recruitment, scheduling, and clinical evaluations of the subjects.
Funding This work was supported by Grant R01HD038582 from the National Institutes of Health (NIH).
Footnotes
Compliance with ethical standards
Ethical standard All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
Conflict of interest We have no conflicts of interest to disclose.
References
- Balasubramanian CK, Neptune RR, Kautz SA. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. 2009;29:408–414. doi: 10.1016/j.gaitpost.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Begg R, Best R, Dell’Oro L, Taylor S. Minimum foot clearance during walking: strategies for the minimisation of trip-related falls. Gait Posture. 2007;25:191–198. doi: 10.1016/j.gaitpost.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Bernstein NA. The co-ordination and regulation of movements. Pergamon Press; Oxford: 1967. [Google Scholar]
- Black DP, Smith BA, Wu J, Ulrich BD. Uncontrolled manifold analysis of segmental angle variability during walking: preadolescents with and without Down syndrome. Exp Brain Res. 2007;183:511–521. doi: 10.1007/s00221-007-1066-1. [DOI] [PubMed] [Google Scholar]
- Cirstea M, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-mental state examination. Geriatr Psychiatry. 2002;140 [Google Scholar]
- Coscia M, Monaco V, Martelloni C, Rossi B, Chisari C, Micera S. Muscle synergies and spinal maps are sensitive to the asymmetry induced by a unilateral stroke. J Neuroeng Rehabil. 2015;12:39. doi: 10.1186/s12984-015-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna-dos-Santos A, Slomka K, Zatsiorsky VM, Latash ML. Muscle modes and synergies during voluntary body sway. Exp Brain Res. 2007;179:533–550. doi: 10.1007/s00221-006-0812-0. [DOI] [PubMed] [Google Scholar]
- De Freitas SMSF, Scholz JP, Stehman AJ. Effect of motor planning on use of motor abundance. Neurosci Lett. 2007;417:66–71. doi: 10.1016/j.neulet.2007.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas SMSF, Gera G, Scholz JP. Timing variability of reach trajectories in left versus right hemisphere stroke. Brain Res. 2011;1419:19–33. doi: 10.1016/j.brainres.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand I, Tsetlin M. Models of the structural-functional organization of certain biological systems. Moscow Nauka. Cambridge MA: MIT Press; 1966. On mathematical modeling of the mechanisms of the central nervous system; pp. 9–26. in Russian, published in English in 1971 by. [Google Scholar]
- Gera G, Freitas S, Latash M, Monahan K, Schoner G, Scholz J. Motor abundance contributes to resolving multiple kinematic task constraints. Mot Control. 2010;14:83–115. doi: 10.1123/mcj.14.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80:1868–1885. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- Grasso R, Ivanenko YP, Zago M, et al. Distributed plasticity of locomotor pattern generators in spinal cord injured patients. Brain. 2004;127:1019–1034. doi: 10.1093/brain/awh115. [DOI] [PubMed] [Google Scholar]
- Ivanenko Y, Grasso R, Macellari V, Lacquaniti F. Control of foot trajectory in human locomotion: role of ground contact forces in simulated reduced gravity. J Neurophysiol. 2002;87:3070–3089. doi: 10.1152/jn.2002.87.6.3070. [DOI] [PubMed] [Google Scholar]
- Ivanenko Y, Poppele R, Lacquaniti F. Distributed neural networks for controlling human locomotion: lessons from normal and SCI subjects. Brain Res Bull. 2009;78:13–21. doi: 10.1016/j.brainresbull.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res. 2003;152:281–292. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle modes during shifts of the center of pressure by standing persons: effect of instability and additional support. Exp Brain Res. 2004;157:18–31. doi: 10.1007/s00221-003-1812-y. [DOI] [PubMed] [Google Scholar]
- Krishnan C, Ranganathan R, Kantak SS, Dhaher YY, Rymer WZ. Active robotic training improves locomotor function in a stroke survivor. J Neuroeng Rehabil. 2012;9:57. doi: 10.1186/1743-0003-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C, Kotsapouikis D, Dhaher YY, Rymer WZ. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Arch Phys Med Rehabil. 2013a;94:1202–1206. doi: 10.1016/j.apmr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Rosenblatt NJ, Latash ML, Grabiner MD. The effects of age on stabilization of the mediolateral trajectory of the swing foot. Gait Posture. 2013b;38:923–928. doi: 10.1016/j.gaitpost.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Kutch JJ, Valero-Cuevas FJ. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLoS Comput Biol. 2012;8:e1002434. doi: 10.1371/journal.pcbi.1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latash ML, Gorniak S, Zatsiorsky VM. Hierarchies of synergies in human movements. Kinesiology. 2008;40:29. [PMC free article] [PubMed] [Google Scholar]
- Papi E, Rowe PJ, Pomeroy VM. Analysis of gait within the uncontrolled manifold hypothesis: stabilisation of the centre of mass during gait. J Biomech. 2015;48:324–331. doi: 10.1016/j.jbiomech.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Pohl PS, Duncan PW, Perera S, Liu W, Lai SM, Studenski S, Long J. Influence of stroke-related impairments on performance in 6-minute walk test. J Rehabil Res Dev. 2002;39:439–444. [PubMed] [Google Scholar]
- Ranganathan R, Krishnan C. Extracting synergies in gait: using EMG variability to evaluate control strategies. J Neurophysiol. 2012;108:1537–1544. doi: 10.1152/jn.01112.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–2527. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. doi: 10.1007/s002210050738. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Kao PC, Kim SH, et al. Assist-as-needed robot-aided gait training improves walking function in individuals following stroke. IEEE Trans Neural Syst Rehabil Eng. 2014 doi: 10.1109/TNSRE.2014.2360822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting LH, Macpherson JM. A limited set of muscle synergies for force control during a postural task. J Neurophysiol. 2005;93:609–613. doi: 10.1152/jn.00681.2004. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol. 2006;95:2199–2212. doi: 10.1152/jn.00222.2005. [DOI] [PubMed] [Google Scholar]
- Tropea P, Monaco V, Coscia M, Posteraro F, Micera S. Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: a pilot study. J Neuroeng Rehabil. 2013;10:0003–0010. doi: 10.1186/1743-0003-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y-W, Scholz JP, Schöner G, Hotchkiss L. Effect of accuracy constraint on joint coordination during pointing movements. Exp Brain Res. 2003;149:276–288. doi: 10.1007/s00221-002-1357-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zatsiorsky VM, Latash ML. Muscle synergies involved in preparation to a step made under the self-paced and reaction time instructions. Clin Neurophysiol. 2006;117:41–56. doi: 10.1016/j.clinph.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Winter DA. Foot trajectory in human gait: a precise and multifactorial motor control task. Phys Ther. 1992;72:45–53. doi: 10.1093/ptj/72.1.45. [DOI] [PubMed] [Google Scholar]