Short abstract
An estimated 5.3 million Americans are living with a disability from a traumatic brain injury (TBI). There is emerging evidence of the detrimental effects from repeated mild TBIs (rmTBIs). rmTBI manifests its own unique set of behavioral and neuropathological changes. A subset of individuals exposed to rmTBI develop permanent behavioral and pathological consequences, defined postmortem as chronic traumatic encephalopathy. We have combined components of two classic rodent models of TBI, the controlled cortical impact model and the weight drop model, to develop a repeated mild closed head injury (rmCHI) that produces long-term deficits in several behaviors that correlate with neuropathological changes. Mice receiving rmCHI performed differently from 1-hit or sham controls on the elevated plus maze; these deficits persist up to 6 months postinjury (MPI). rmCHI mice performed worse than 1-hit and control sham mice at 2 MPI and 6 MPI on the Morris water maze. Mice receiving rmCHI exhibited significant atrophy of the corpus callosum at both 2 MPI and 6 MPI, as assessed by stereological volume analysis. Stereological analysis also revealed significant loss of cortical neurons in comparison with 1-hit and controls. Moreover, both of these pathological changes correlated with behavioral impairments. In human tau transgenic mice, rmCHI induced increases in hyperphosphorylated paired helical filament 1 tau in the hippocampus. This suggests that strategies to restore myelination or reduce neuronal loss may ameliorate the behavioral deficits observed following rmCHI and that rmCHI may model chronic traumatic encephalopathy in human tau mice.
Keywords: animal models, behavioral assessment, cognitive function, concussion, inflammation, traumatic brain injury, closed head injury
Introduction
In the United States, an estimated 5.3 million people are living with a disability that is directly related to a traumatic brain injury (TBI; Langlois and Sattin, 2005), resulting in direct and indirect costs of US$56 to 221 billion every year (Coronado et al., 2012). Adding to this astonishing figure, 1.7 million Americans a year suffer a TBI leading to hospitalization (Faul et al., 2010). While 75% of these patients sustain only a mild TBI (mTBI), also referred to as a concussion (Gerberding and Binder, 2003), 43% of patients discharged after acute TBI hospitalization develop a long-term disability (Rutland-Brown et al., 2006).
Concussion, or mTBI, is defined as a pathophysiological process affecting the brain, induced by direct or indirect biomechanical forces, that may lead to acute neurological deficits that typically resolve without structural injuries (Herring et al., 2011). Taking this definition into account, it is estimated that 1.4 to 3.8 million concussions annually in the United States do not lead to hospitalization (Faul et al., 2010; Laskowski et al., 2015). Compounding evidence suggests that single or repeated mild traumatic brain injuries (rmTBI) can lead to long-lasting cognitive and emotional deficits in individuals with mTBI or rmTBI (Bazarian et al., 2005; Bryant et al., 2010; Laskowski et al., 2015), as well as neuropathologies including chronic neuroinflammation and tauopathies such as chronic traumatic encephalopathy (CTE; Mckee et al., 2009). Clearly, rmTBI is a major health problem that lacks definitive incident reporting.
Primary injury associated with TBI can result in acute axonal swelling and shearing (Johnson et al., 2013a, 2013b), microglial activation (Ramlackhansingh et al., 2011; Johnson et al., 2013a, 2013b), neuronal loss (Raghupathi, 2004), hemorrhage, and blood–brain barrier disruption (Baskaya et al., 1997; Greve and Zink, 2009). Secondary injury cascades associated with these events include white matter atrophy, chronic and dynamic neuroinflammation, and neuronal loss. Of particular interest, disruptions of major white matter tracts following TBI have also been observed in both humans and rodents. In humans with a single TBI, 25% atrophy of the corpus callosum is observed chronically, concurrent with activated microglia within the corpus callosum (Johnson et al., 2013a). White matter abnormalities have also been observed in humans following multiple concussions or traumatic brain injuries, especially in athletes (Johnson et al., 2013b; Tremblay et al., 2014; Multani et al., 2016). In a rodent model of repeated mTBI, a sustained inflammatory response was observed in corpus callosum up to 7 weeks postinjury (Shitaka et al., 2011), and in a study at more chronic time points of 6 and 12 months postinjury (MPI), 10% to 20% corpus callosum thinning occurred (as measured by thickness in two-dimensional sections, not total volume). White matter thinning was observed in combination with increased astroglial and microglial activation up to 12 months post-rmTBI; however, elevated levels of ß-amyloid and abnormally phosphorylated tau (pTau) were not detected in C57Bl/6J mice (Mouzon et al., 2014).
Modeling TBI in rodents will always lack some components of the human condition, due to size differences, because rodents are lissencephalic, differences in rotational forces, and the wide diversity and range of mechanisms responsible for human injuries. One solution is the development and use of a range of animal models. In acute TBI, correlates of hippocampal neuropathology and memory performance have been reported (Hicks et al., 1993; Haus et al., 2016) in the rat. Some rodent models of rmTBI include impacting a mouse skull directly using a homemade or commercially available pneumatic device (Laurer et al., 2001; Uryu et al., 2002; Prins et al., 2010; Shitaka et al., 2011), dropping a weight down a tube onto a closed head of a suspended mouse on a KimWipe (Meehan et al., 2012) or aluminum foil (Kane et al., 2012), and a closed head impact model of engineered rotational acceleration (CHIMERA) focusing on precise control of injuries with unrestricted head movement allowing for coup–contrecoup injuries (Namjoshi et al., 2014). Models that do not resect the skin to impact the skull or dura directly are commonly referred to as repeated mild closed head injuries (rmCHI), a subset of rmTBI.
In 2001, McIntosh reported that mild head injury made the brain more vulnerable to a second concussive hit (Laurer et al., 2001), that repetitive TBI accelerates brain ß-amyloid accumulation, oxidative stress, and cognitive impairment (Uryu et al., 2002), and that there is a temporal window of vulnerability after rmTBI where a second concussion within 3 to 5 days can induce exacerbated axonal damage and greater behavioral dysfunction (Longhi et al., 2005). In rats, rmTBI 1 or 3 days apart results in significant tissue loss on magnetic resonance imaging compared with shams or a single injury, whereas extending the interval to 1 week between injuries resulted in no more injury than a single hit (Huang et al., 2013). Crawford compared the behavioral and pathological consequences in a mouse model of single versus repetitive injury (five injuries given at 48-hr intervals) administered by an electromagnetic controlled impactor, demonstrating a reproducible, simple, and noninvasive model with behavioral impairments after a single injury and increasing deficits after multiple injuries accompanied by increased focal and diffuse pathology (Mouzon et al., 2012, 2014).
Using mice expressing human tau (hTau) on a mouse tau knockout background (Andorfer et al., 2003), Ojo et al. (2016) showed that two injuries weekly over a period of 3 or 4 months resulted in impairments in cerebral blood flow and white matter damage, which was accompanied by a twofold increase in total tau levels and mild increases in tau oligomers/conformers and pTau (Thr231) species in brain gray matter; however, neurofibrillary astroglial tangles, neuropil threads, or perivascular foci of tau immunoreactivity were not observed. Comparing a single hit to repetitive mTBI by Burns found dendrite spine loss and chronic white matter inflammation in a mouse model of repetitive head trauma (Winston et al., 2016; Main et al., 2017). Further, Nedergaard and colleagues have reported impairment of glymphatic pathway function in the progression of tau pathology following TBI (Iliff et al., 2014). There are a number of excellent reviews that deal with the issues of modeling human repetitive TBI, and the even more difficult task of replicating the features of CTE in animal models (Abisambra and Scheff, 2014; Brody et al., 2015; Johnson et al., 2015; Ojo et al., 2016; Wojnarowicz et al., 2017).
In an effort to develop a robust, high-throughput rmCHI model that can be easily replicated across laboratories with precise control over injury parameters, we sought to use commercially available components and to determine if a model of closed head injury with components of acceleration–deceleration forces in an unrestrained head would produce white matter tract neuropathology representative of that observed in the clinical setting. Furthermore, we sought a model that demonstrates external validity, resulting in cognitive and behavioral deficits in variety of tasks that are sustained chronically (Henderson et al., 2013; Malkesman et al., 2013). We hypothesized that rmCHI, but not a single mild injury, would produce chronic behavioral changes and pathological abnormalities that would correlate with those behavioral changes. We predicted that the extent of behavioral impairment would correlate with the underlying extent of pathology.
To our knowledge, there are no reports correlating the relationship of white matter or cortical pathology with multiple measures of behavioral function in mice with rmCHI. Therefore, we focused on assessing corpus callosum neuropathology following rmCHI given the common finding of white matter damage in humans. We also assessed cortical neuron number below the site of impact. We report here on the association of robust, chronic behavioral changes and learning deficits with corpus callosum atrophy, long-term neuroinflammation, and cortical neuronal loss. Surprisingly, rmCHI behavioral impairments and associated pathology were not observed in immunodeficient non-obese diabetic (NOD)-scid gamma (NSGs) mice, whereas hyperphosphorylation of tau was observed in 10-month-old hTau mice exposed to rmCHI, but not in age-matched littermates.
Material and Methods
Animals and Injury Paradigm
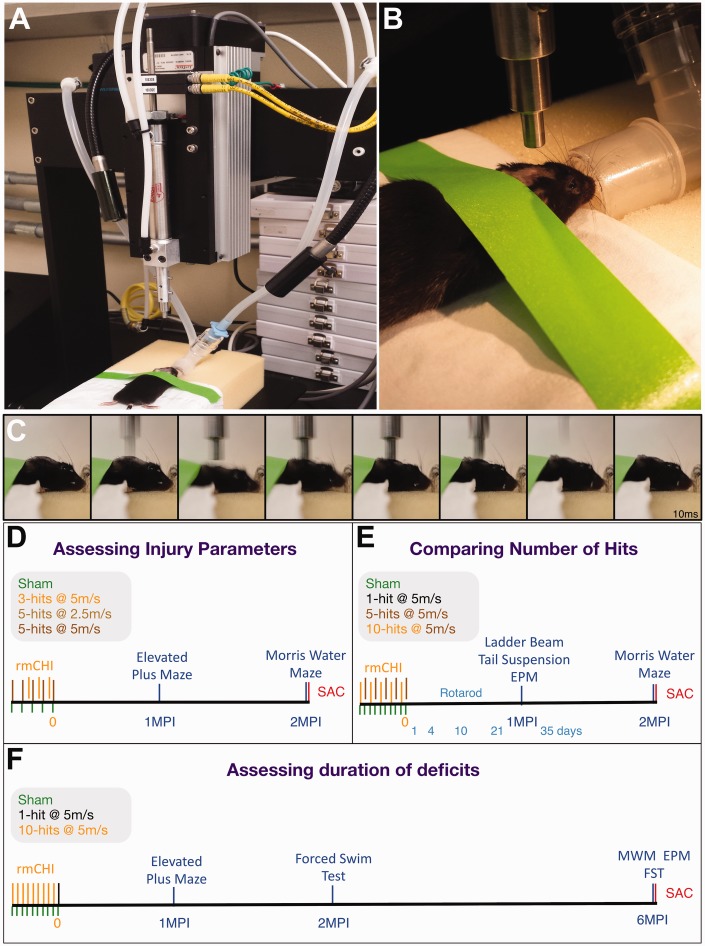
All procedures were carried out under the approval of the UCI IACUC committee (#2010-2945) in an AAALAC-accredited vivarium (#000238). All studies used 9- to 10-week-old male C57Bl/6J mice (Jackson Labs, Bar Harbor, ME) unless otherwise noted. Prior to the procedures, mice were acclimated for 1 week and handled for 3 days, 5 min per day. Components of both the controlled cortical impact (CCI) device (Figure 1(a); TBI-0310 Head Impactor, Precision Systems and Instrumentation, LLC, Fairfax Station, VA) and the weight drop model were combined. A foam bed modified from “Marmarou foam” (Type E Bed, Foam to Size, Inc., Ashland, VA) was modified to fit on the stage of the CCI device (5.25″ × 2.75″ × 17.25″), and a trench was cut out to position tubing and a nose cone to keep the animal under anesthesia for the duration of the procedure.
Figure 1.
Model of repeated mild closed head injury (rmCHI) used in long-term behavioral testing of mice. Following 4 min of isoflurane anesthesia exposure to shave and prepare the mouse head for impacts, mice are positioned atop Marmarou foam placed in the TBI-0310-controlled cortical impactor device (a). Lab tape is lightly applied to pin the ears back and flatten the head onto the foam (b) while positioning the piston directly above the dorsal aspect of the sagittal suture. After 7 min of initial knockdown in anesthesia, an injury is delivered to the mouse head. Stills from a time lapse video (10 frames per second) show the mouse receiving an impact, followed by depression and rotation into the foam, followed by a quick rebound toward the piston (c). Three main experiments were performed to (d) assess baseline injury parameters, comparing hits at a velocity of 2.5 m/s to 5 m/s and a 3-hit versus 5-hit paradigm; (e) compare the number of hits in a 1-, 5- and 10-hit paradigm; and (f) compare the behavioral and pathological deficits at 6 MPI with deficits observed at 2 MPI.
TBI = traumatic brain injury; MPI = months postinjury; EPM = elevated plus maze; FST = forced swim test; MWM = Morris water maze.
All animals spent 7 min under anesthesia, including shams; 2 min induction at 2.5% isoflurane, and 2 min to mark the tail, shave the head, and apply ophthalmic ointment. The animal was placed onto the modified Marmarou foam and positioned to breathe 1.5% isoflurane from a nose cone. Lab tape was lightly applied to the ears and fixed to the foam to position the head level and prevent movement during breathing (Figure 1(b)). A 5-mm diameter probe tip was aligned midline to the sagittal suture with the anterior edge of the tip parallel to a virtual line drawn across the posterior-most aspect of the eyes (roughly equivalent to a centerline impact 1 to 2 mm posterior to bregma). The mouse was at the CCI station for 3 min until an impact with speed 5.0 m/s, 1.0 mm depth, and 50 ms dwell time (slow-motion video of impact shows displacement of head into supporting foam pad, Figure 1(c)). In total, mice are under anesthesia for 7 min from knockdown to TBI impact. The isoflurane percentages were designed to minimize head movement from respiration while zeroing the piston and during impact while still keeping the animal unresponsive to a toe pinch reflex. Following impact, animals were moved to a recovery area where righting time was recorded. Sham animals underwent the same 7-min procedure with anesthesia over 5 or 10 days but were not impacted. In all experiments, mice were randomly assigned to groups, and observers were blind to group during all behavioral and histological evaluations.
Assessment of Injury Severity Parameters in rmCHI
To identify injury parameter thresholds that do or do not produce similar results to a baseline injury of 5 m/s, 5 hits over 5 days paradigm (ascertained during pilot experiments), we tested either half speed (2.5 m/s) or less hits (3 hits). Mice (n = 12/group) were divided into four groups: Shams, 3 hits at 5 m/s, 5 hits at 2.5 m/s, or 5 hits at 5 m/s (as a positive control). Mice hit 3 times were hit every other day, and mice hit 5 times were hit every 24 hr. Mice were tested on the elevated plus maze (EPM) at 1 month postinjury (MPI) and the Morris water maze (MWM) at 2 MPI. Animals were sacrificed at 9 weeks postinjury and perfused with 4% paraformaldehyde. Brains were excised, immersed in 30% sucrose/4% paraformaldehyde overnight, and snap-frozen in −50°C isopentane prior to histological analysis.
Comparison of 1-Hit, 5-Hit, and 10-Hit rmCHI Parameters
After determining injury severity parameters, we tested whether the injury response was exacerbated by 10 hits, expanded the battery of behavioral measures, and quantified the pathological response. Mice were grouped into four conditions: Shams (5 or 10 isoflurane exposures), a single impact (n = 11) on the last day, 5 hits over 10 days (once every 48 hr, n = 9), or 10 hits over 10 days (once every 24 hr, n = 13). The two sham groups received either 5 procedures over 10 days (once every 48 hr) or 10 sham procedures over 10 days. As there were no differences in any measures between the sham groups, shams were combined in all statistical analysis (n = 14). Single-impact animals received an impact on the final day of hits of both 5- and 10-hit groups to align all groups a common 0 days postinjury (DPI) start date. Mice were tested on the rotarod, ladder beam, EPM, MWM, and the tail suspension test (TST) and were sacrificed 2 MPI.
Are rmCHI Deficits Sustained 2 and 6 MPI?
Having observed deficits at 1 and 2 MPI, we tested if the deficits were sustained out to 6 MPI. Mice were grouped into three conditions: Sham (n = 7), a single impact (n = 11), or 10 hits over 10 days (once every 24 hr, n = 9). Mice were tested on EPM at 1 MPI and 6 MPI, the forced swim test (FST) at 2 MPI and 6 MPI, and MWM at 6 MPI. Two animals from each group were sacrificed at 2 MPI for pilot data for another experiment; the remaining mice were sacrificed at 6 MPI.
Are immunodeficient NSG Mice Comparable With C57Bl/6J Mice?
Can immunodeficient mice be used to test cell therapies in this model of rmCHI? Ten-week-old male NSG mice (Jackson Laboratories, catalog # 005557) were divided into four groups (n = 12/group): Shams, 1 hit at 5.0 m/s, 5 hits at 2.5 m/s, 5 hits at 5. 0 m/s. Following rmCHI, mice were tested on the EPM at 1 MPI and MWM at 2 MPI. Mice were sacrificed at 2 MPI.
Does rmCHI Exacerbate Tau Phosphorylation in hTau Mice?
Can we model CTE using mice expressing hTau? Seven-month-old male and female transgenic mice overexpressing human wild-type (WT) 4 repeat tau (hTau; Chabrier et al., 2014) were divided into four groups: Shams (n = 5 WT and 3 hTau) and 5 hits at 2.5 m/s (n = 6 WT and 5 hTau). Following rmCHI, mice were sacrificed at 3 MPI (10 months of age, when elevated hyperphosphorylated tau is not present in uninjured hTau mice). Group numbers were too low for behavioral testing; however, experimental conditions were randomized prior to injury so that histological and biochemical testing was performed blind.
Behavioral Assessments
Rotarod
Mice were tested for motor function, control, and balance using the rotarod task at 1, 4, 7, 14, 21, and 35 DPI. Mice were placed onto a rotarod device (Economex Rota-Rod, Columbus Instruments, Columbus, OH) for 1 min to acclimate, followed by 2 min of learning/acclimation at a steady rate of 5 revolutions per minute (rpm). Animals falling off the beam were immediately repositioned atop the beam during this 2-min learning phase. Following 2 min of acclimation to the task, two training trials were performed, followed by five testing trials. Daily latency to fall measurements were an average of the five testing trials. Both training and testing trials began at 3 rpm, accelerating at 2.0 rpm/s for the duration of each trial. Between each training and testing trial, any urine left on the beam was removed, and the beam was cleaned and dried.
Horizontal ladder beam
To assess motor function, mice were tested using the horizontal ladder beam. Foot faults, or missteps, were tabulated from video recordings of animals traversing the horizontal ladder with variable rung spacing (Cummings et al., 2007). Testing was performed in a dark room with a light source placed at the starting end of the ladder to motivate movement away from the light, into a closed dark cage at the end of the ladder. An enclosure, just bigger than the mouse, sits atop the ladder to allow a track for mice to walk in a line to the opposite end. Three successfully traversed trials in total were scored, using total forepaw missteps over three trials as the outcome measure.
Elevated plus maze
Anxiety and risk-taking behavior was assessed by comparing time spent in open arms, or by amount of entries into open arms. Animals were placed at the center point of the EPM apparatus (Noldus Information Technology, Leesburg, VA) 29″ off of the ground, facing an open arm extending 13.5″ from the center. Mice could move freely between unenclosed and enclosed closed arms, 7.5″ high walls. Trials lasted 5 min and were analyzed by EthoVision XT software (Version 8.0.5, Noldus Information Technology, Leesburg, VA). As indicated in the results, animals in were either tested under ambient light conditions or in a dark room with near-infrared illumination under the maze arms to allow for contrast and camera detection. Outcome measures gathered were total distance moved, entries into open and closed arms, and time spent in open/closed arms.
Morris water maze
The MWM is a well-characterized task to assess spatial learning and memory. The maze consists of a circular metal pool (44″ diameter) filled with water 3 inches from the top. White paint was used to dye the water and hide a platform (5″ diameter) 1 cm below the surface and out of the animal’s sightline. Water was maintained at 25°C to 30°C. Mice underwent 4 trials, 60 s max, per day over 5 days of testing. The platform remained stationary in a particular quadrant; however, the entry point changed with each trial, and the order changed each day. Mice used cues outside the maze (various poster-board shapes fixed to the testing room walls) to spatially navigate to the ‘hidden' platform. EthoVision XT software recorded each trial, quantifying latency to reach platform and time spent in each quadrant.
Forced swim test
The FST is a measure of depressive-like behavior. Mice were placed into a 2,000 ml glass beaker filled with 7 to 8 cm of water (25°C) for 6 min. Trials were video recorded and analyzed using EthoVision XT, allowing for simple quantification of time moving versus not moving, and assessment of relative ‘mobility'. Moving was determined by calculating the duration for which the center point of the animal was changing location with a start velocity set at 1.25 cm/s and a stop velocity set at 1.0 cm/s. Mobility was quantified using changes in pixel area of the subject between samples collected. Standard software thresholds were used to segment mobility into ‘immobile', ‘mobile', and ‘high mobility' levels, where the immobile threshold was set to 6%, and the high mobility threshold was set to 18%. Mice were tested on the FST in Experiment 2 at 2 and 6 MPI. A single exposure was used, not the 5-day repetitive variant of the FST, which has a learning component (Mul et al., 2016).
Tail suspension test
The TST is used to assess depression. Mice were hung from a beam raised 12″ off of a surface, affixed by a piece of tape at the tip of their tail. Mice tried to grab their tails and climb up to right themselves. Assessment of depression-like behavior was measured by scoring time immobile, or time not struggling to right themselves. More depressive-like behavior is indicated by more time immobile. A single 5-min trial was conducted under normal ambient light conditions.
Immunohistochemistry
Personnel blind to experimental group performed all histological analyses. Serial 30-μm coronal brain sections were collected from mice sacrificed at 9 weeks postinjury. Tissue was stained in free floating wells. Endogenous peroxide activity was quenched with hydrogen peroxide/methanol for 20 min, followed by cell permeabilization in 0.1% Triton-X. Tissue was blocked in fetal bovine serum and donkey serum for an hour prior to primary antibody incubation. Primary antibodies were incubated overnight at room temperature and included rat antimyelin basic protein (Millipore, #MAB386, 1:800), rabbit anti-Iba1 (Wako, #019-19741, 1:1000), rabbit antiglial fibrillary acidic protein (GFAP; Dako, #Z0334, 1:10,000), mouse anti-NeuN (Millipore, #MAB377, 1:1000), rabbit antineural/glial antigen 2 (NG2; Millipore, #AB5320, 1:800), mouse anti-CP13, and paired helical filament 1 (PHF-1; generous gift of Peter Davies, Albert Einstein College of Medicine). Tissue was incubated with secondary antibodies for an hour at room temperature. Secondary antibodies used include donkey anti-rabbit F(ab’)2 biotin conjugated (Jackson ImmunoResearch Laboratories, #711-066-152, 1:500), donkey anti-mouse F(ab’)2 biotin conjugated (Jackson, #715-066-151, 1:500), donkey anti-rat F(ab’)2 biotin conjugated (Jackson, #712-066-153, 1:500), and donkey anti-rabbit F(ab’)2 AF647 conjugated (Jackson, #711-606-152, 1:500) for NG2 immunofluorescent labeling. Following incubation with ABC Kit (Vector Labs, #PK-6100), DAB substrate was added to visualize antigens (Vector Labs, SK-4100). Tissue was mounted onto gelatin-coated slides and counterstained with methyl green to visualize nuclei. NG2 immunofluorescent sections were counterstained with Hoechst.
All images were captured on an Olympus BX60 microscope with a UPlanApo 10x/NA0.40 lens or Uplan FL20x/NA0.50 lens, and an Optronics Microfire A/R camera, with the exception of images in Figure 11 that were captured on an IX71 microscope and an Optronics PictureFrame. Scale bars were added using ImageJ and images of a stage micrometer using identical settings.
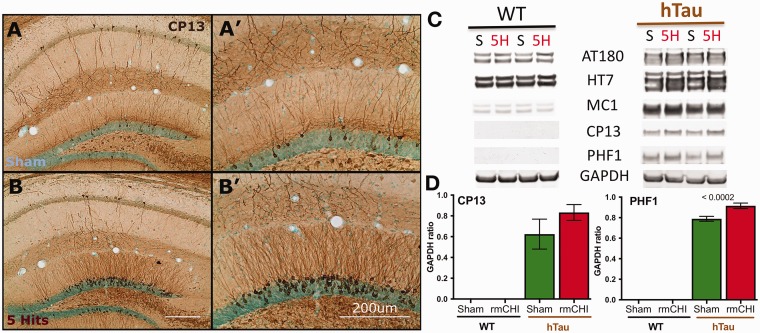
Figure 11.
Transgenic hTau mice show rmCHI induced increases in phosphorylated tau following within the hippocampus. Immunohistochemical analysis of (a) sham and (b) 5-hit rmCHI 10-month-old female hTau mice show increased staining of phosphorylated tau (CP13) 6 MPI. A majority of the increase was observed within the granule cell layer and apical dendrites of the dentate gyrus (a', b'). Male hTau mice exhibited a similar response to injury, whereas no CP13 staining was seen in wild-type (WT) mice regardless of injury status or gender (data not shown). (c and d) An ANOVA of Western blot protein levels demonstrated that phosphorylated tau (PHF-1) levels were significantly elevated in hTau mice compared with WT mice 3 months after the rmCHI (one-way ANOVA), F(3, 15) = 995.1, p < .001, post hoc Tukey’s p ≤ .0002 between sham and rmCHI hTau mice. Scale bars = 200 μm.
rmCHI = repeated mild closed head injury; MPI = months postinjury; ANOVA = analysis of variance; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; hTau = human tau.
Quantification
Thirty-micrometer sections were stained with cresyl violet for quantification of the corpus callosum. Contours of the corpus callosum were drawn using StereoInvestigator software v11.08.01 (MicroBrightField, Inc., Williston, VT) on every 12th section to evaluate estimated volume in a systematic, nonbiased, design-based approach. Anterior and posterior limits of corpus callosum were bound by sections that contained intact corpus callosum connecting the two hemispheres in the same coronal plane (Figure 5(b)). A 100-μm grid was laid down to assess corpus callosum volume; the coefficient of error for each animal was less than 0.10. The optical fractionator probe was used to quantify NeuN immunopositive cortical neurons in every 12th section. Anterior and posterior borders were determined by where corpus callosum was intact between both hemispheres. NeuN+ neurons were counted bilaterally, and borders were contoured based on anatomical landmarks as follows: A ventral limit was established by contouring a horizontal line form the most dorsal point of both thalami, extending to the most lateral aspect of the cortex. The corpus callosum was used as the ventral limit, until intersecting with the extended thalamic border. On each hemisphere, a medial limit was drawn extending from the point at which the cingulate gyrus extended into the cortex (Figure 6(b)). Using a 750 × 750 μm grid with a 30 × 30 μm counting frame, a 10-μm optical fractionator probe was used to count NeuN+ cells. A 100-μm grid was laid down to assess cortical volume using Cavalieri estimator. The optical fractionator probe was used to quantify NG2+ immunopositive oligodendrocyte precursors. From 30-μm serial coronal sections, every 12th section was assessed. Corpus callosum borders were drawn as previously mentioned. Using a 200 × 200 μm grid with a 50 × 50 μm counting frame, a 12-μm optical fractionator probe was used to count NG2+. Coefficient of error (CE) values are reported in the Results section.
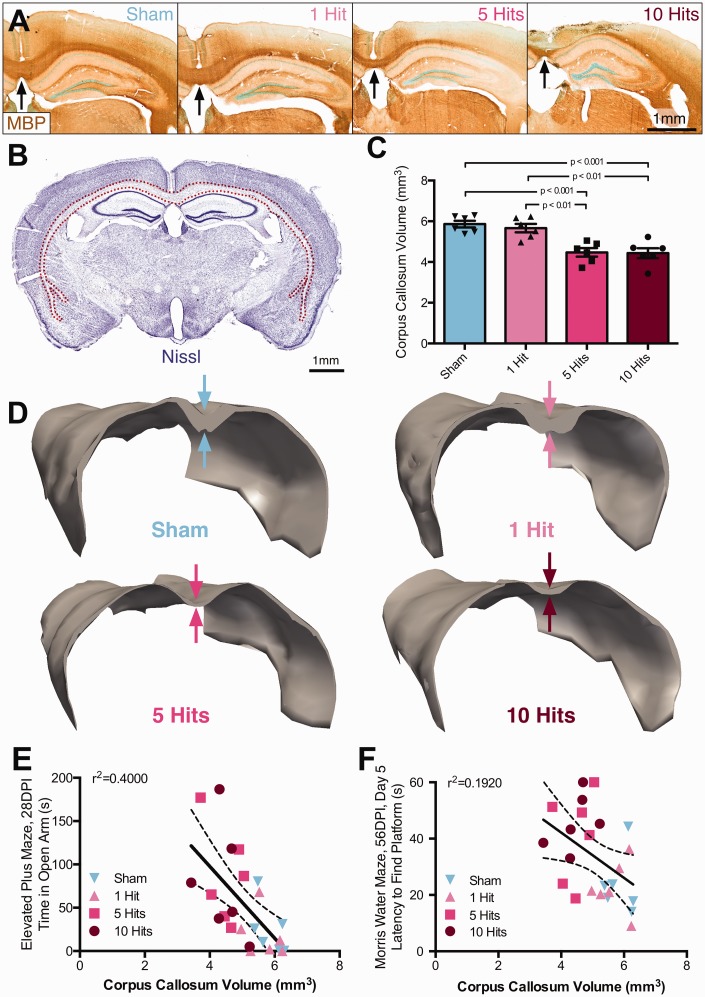
Figure 5.
Mice experiencing rmCHI have significant white matter damage and 31% atrophy of corpus callosum at 2 months postinjury. (a) Following 5 or 10 hits, mice developed white matter abnormalities and corpus callosum atrophy; immunocytochemistry for myelin basic protein (brown), black arrows denote corpus callosum thinning, scale bar = 1 mm. (b) Using principles of unbiased design-based stereology, corpora callosa volumes were estimated using a Cavalieri probe. Contours tracing the corpus callosum following cresyl violet staining (red dotted line) were made throughout the mouse brain at sections where corpus callosum was intact between the two hemispheres, scale bar = 1 mm. Sampling was conducted in a 1 in 12 series. (c) At 2 MPI, mice with rmCHI had a 31% reduction in corpus callosum volume compared with both sham and single hit groups (one-way ANOVA), F(3, 20) = 23.35, p < .0001. (d) Three-dimensional reconstructions of representative animals illustrate atrophy, especially in the posterior regions of the corpus callosum. (e) Corpus callosum volume negatively correlates with both elevated plus maze time in open arm (r = −.633, p = .0005), as well as (f) latency to reach platform on Day 5 of Morris water maze testing (r= −.438, p = .0161).
rmCHI = repeated mild closed head injury; DPI = days postinjury; MPI = months postinjury; ANOVA = analysis of variance.
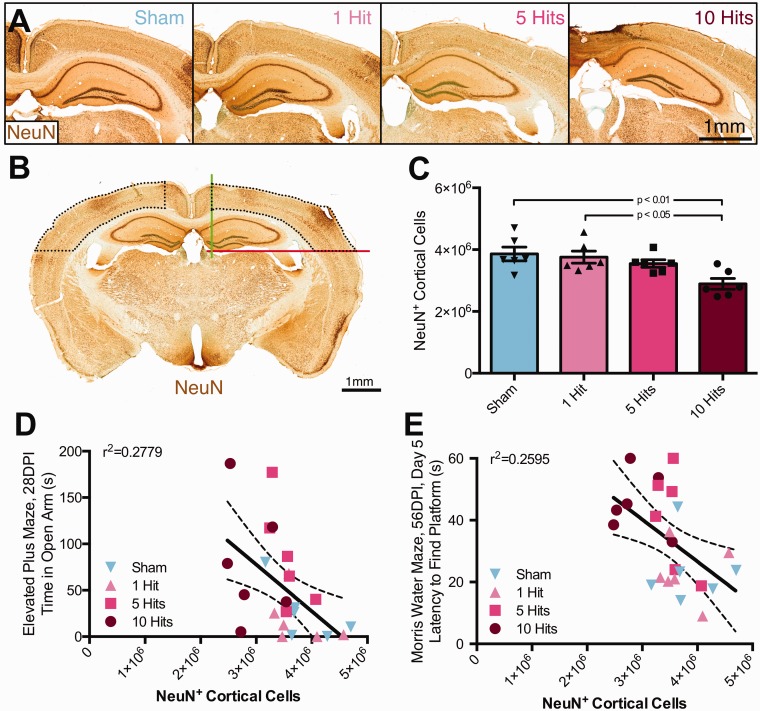
Figure 6.
rmCHI leads to neuronal loss in the cortex, negatively correlating with behavioral function. NeuN+ neuronal cell populations were estimated using principles of stereology. (a) Representative micrographs of NeuN+ staining from mice with 0, 1, 5, or 10 hits at 2 MPI (scale bar = 1 mm). (b) NeuN+ cells were quantified in cortex as defined by set borders. Within each hemisphere, a medial border was drawn at the apex of the cingulate gyrus of the corpus callosum (green line), and a ventral border was drawn at the apex of the thalamus (red line, scale bar = 1 mm). NeuN+ neurons were counted in the cortex bound by defined borders, as well as the corpus callosum. (c) At 2 MPI, there were less NeuN+ neurons in the cortex of 10-hit mice compared with both sham and 1-hit mice (one-way ANOVA), F(3, 20) = 5.693, p = .0055. (d) NeuN+ cortical neurons negatively correlate with both elevated plus maze time in open arm (r = −.527, p = .0041), as well as (e) latency to reach platform on Day 5 of Morris water maze testing (r = −.509, p = .0055).
rmCHI = repeated mild closed head injury; DPI = days postinjury; MPI = months postinjury; ANOVA = analysis of variance.
Western Blots
Following cardiac perfusion with phosphate-buffered saline, brains were removed and frozen on dry ice for biochemical analysis. Proteins were extracted in tissue protein extraction reagent buffer with protease/phosphatase inhibitors cocktail and analyzed on a sodium dodecyl sulfate polyacrylamide gel electrophoresis Western blots. The following primary antibodies were used: glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology, Dallas, TX), AT180 and HT7 (Pierce, Rockford, IL), MC1, CP13, and PHF-1 (kind gift from Peter Davies, Albert Einstein College of Medicine), followed by corresponding horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Cove, PA). The data are represented as mean values ± SEM.
Statistical Analyses
Prior to statistical analysis, and blind to treatment group, a Grubbs’ test was performed to identify potential outliers from each group (α = .05) and removed no more than a single identified outlier. Final n are shown in graphs as individual data points, or reported in the F statistic. For behavioral measures, one-way analysis of variance (ANOVA) was performed for single time point assessments, and repeated measures two-way ANOVA was performed for multiple time point assessments (rotarod and MWM tasks). Statistical significance was set at p < .05 on one-tailed t tests as a priori predictions were that injured would be worse than shams. Error bars in figures are SEM. Statistics were computed using Prism 6.
Results
Assessment of Injury Severity Parameters in rmCHI
To characterize a closed head TBI as mild or concussive, we investigated changes in both velocity and number of hits that would induce behavioral deficits and neuropathology associated with multiple head injuries to find the lower limit whereby a single hit produced negligible behavioral or pathological changes. Righting times using an impact force of 5 m/s were 43.4 s for shams (±1.9 SEM), 174.5 s for 1-hit (±35.8), 242.7 s for 5-hit (±19.0), and 186.5 s for 10-hit (±10.3) animals, comparable with other rmTBI models of concussion/mTBI in rodents (Prins et al., 2010, 2013; Namjoshi et al., 2014; Acabchuk et al., 2016; Nichols et al., 2016).
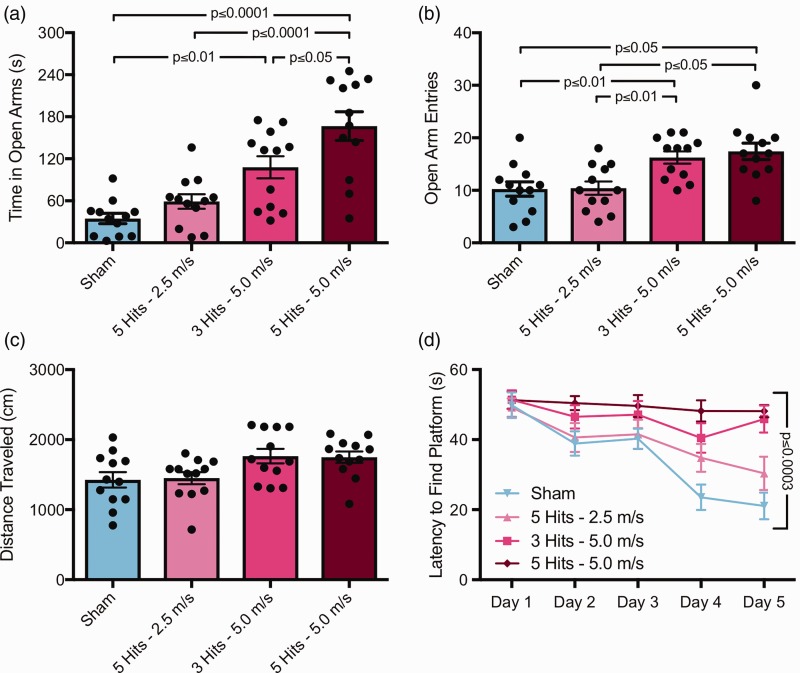
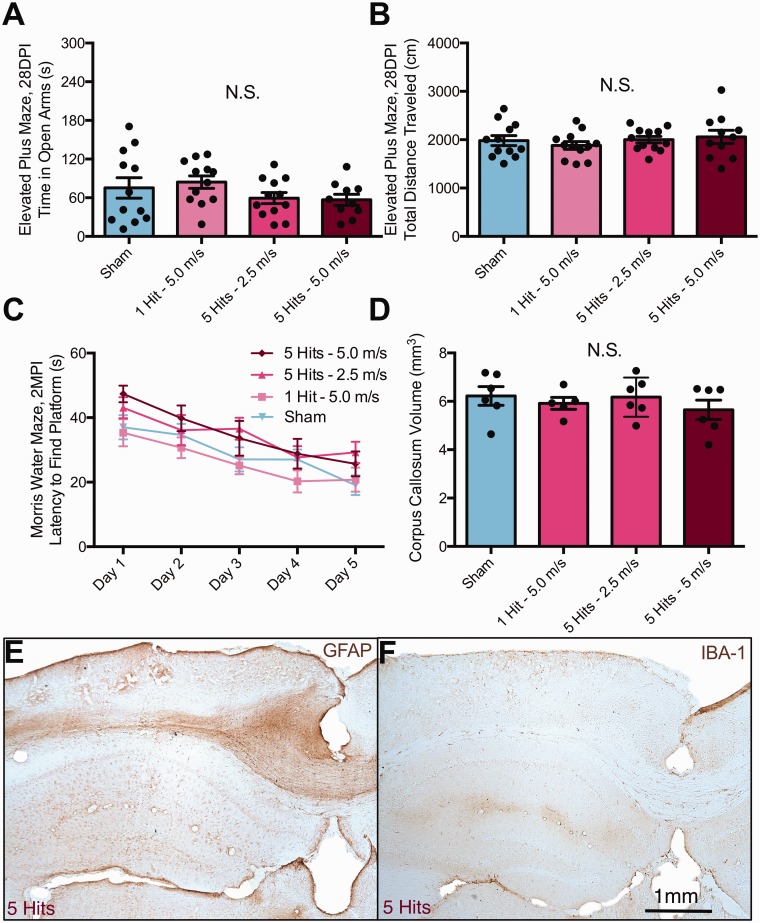
There was a significant effect of group on time spent in open arms (Figure 2(a)), F(3, 44) = 16.36, p < .0001. Sham mice spent less time in open arms compared with both 3 hits at 5 m/s (Tukey post hoc, p < .01) and 5 hits at 5.0 m/s (Tukey post hoc, p < .0001) mice, but not compared with 5 hits at 2.5 m/s mice. In addition, 5 hits at 5.0 m/s mice spent more time in open arms than both 5 hits at 2.5 m/s (Tukey post hoc, p < .0001) and 3 hits at 5.0 m/s (Tukey post hoc, p < .05). There was also a significant effect of group on open arm entries (Figure 2(b)), F(3, 44) = 7.887, p = .0003. Sham mice had less open arm entries compared with both 3 hits at 5 m/s (Tukey post hoc, p < .05) and 5 hits at 5.0 m/s (Tukey post hoc, p < .01) mice, but not compared with 5 hits at 2.5 m/s mice. In addition, 5 hits at 2.5 m/s mice had less open arm entries compared with both 3 hits at 5.0 m/s (Tukey post hoc, p < .05) and 5 hits at 5.0 m/s mice (Tukey post hoc, p < .01). In addition, there was a significant effect of group on total distance traveled (Figure 2(c)), F(3, 44) = 3.681, p = .0189, although Tukey post hoc testing revealed no differences between any groups.
Figure 2.
Increasing the velocity of rmCHI injuries results in behavioral deficits; reducing the number of hits at full velocity also induces deficits. (a) In the elevated plus maze test at 1 MPI, mice with 5 hits or 3 hits at 5.0 m/s showed increased time in open arms, F(3, 44) = 16.36, p < .0001. Sham mice spent less time in open arms compared with both 3 hits at 5 m/s (Tukey post hoc, p < .01) and 5 hits at 5.0 m/s (Tukey post hoc, p < .0001) mice. (b) rmCHI increased open arm entries compared with sham controls and mice with 5 hits at only 2.5 m/s, F(3, 44) = 7.887, p = .0003. (c) No changes in distance traveled in the elevated plus maze were observed across any of the groups via Tukey post hoc tests. (d) At 2 MPI, these same mice were tested for spatial learning using the Morris water maze. There was a significant interaction of group versus time, F(12, 176) = 3.278, p = .0003, and a graded behavioral profile was apparent. Sham mice were able to locate the platform quicker than both 3 hits at 5.0 m/s (Tukey post hoc, p < .0001) and 5 hits at 5.0 m/s mice (Tukey post hoc, p < .0001). In addition, 5 hits at 2.5 m/s mice were able to locate the hidden platform faster than both 3 hits at 5.0 m/s (Tukey post hoc, p < .01) and 5 hits at 5.0 m/s mice (Tukey post hoc, p < .01).
rmCHI = repeated mild closed head injury; MPI = months postinjury.
At 2 MPI, these same mice were tested for spatial learning using the MWM. There was a significant interaction of group versus time (Figure 2(d)), F(12, 176) = 3.278, p = .0003, where a graded behavioral profile became apparent. On Day 5 of testing, sham mice were able to locate the platform quicker than both 3 hits at 5.0 m/s (Tukey post hoc, p < .0001) and 5 hits at 5.0 m/s mice (Tukey post hoc, p < .0001). In addition, 5 hits at 2.5 m/s mice were able to locate the hidden platform faster than both 3 hits at 5.0 m/s (Tukey post hoc, p < .01) and 5 hits at 5.0 m/s mice (Tukey post hoc, p < .01).
Comparison of 1-Hit, 5-Hit, and 10-Hit rmCHI Parameters
Behavior
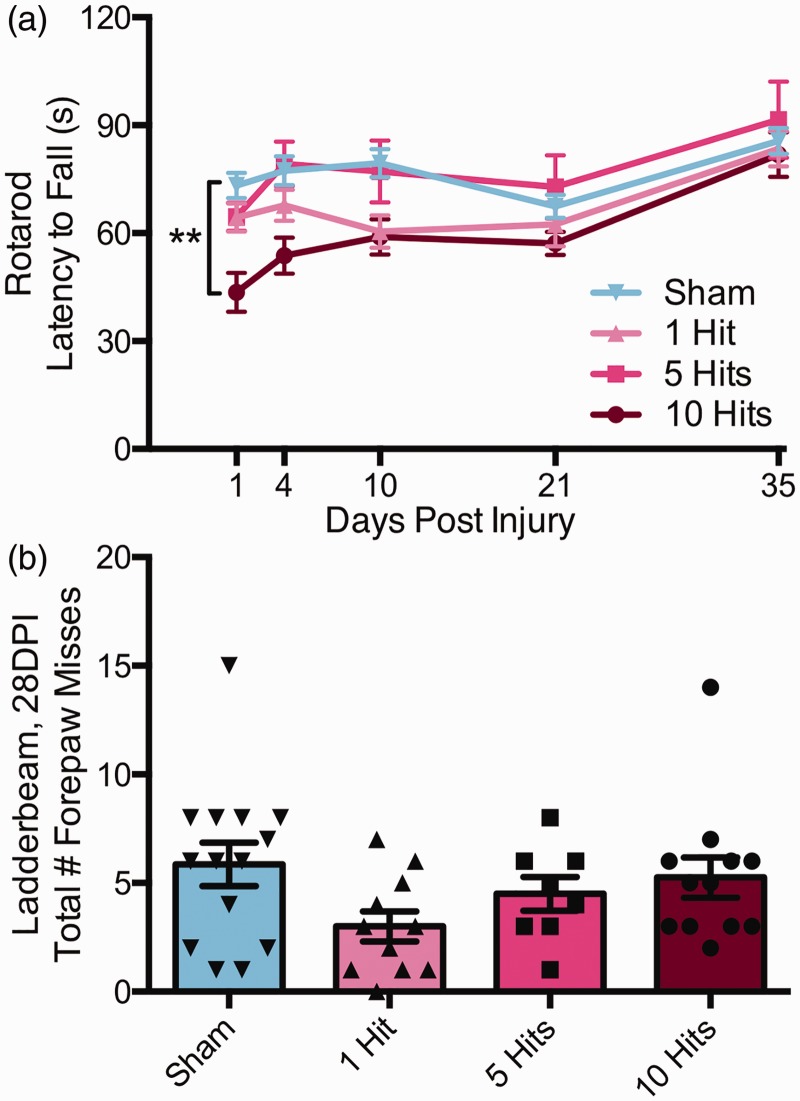
We assessed sham, 1-hit, 5-hit, and 10-hit animals on the rotarod up to Day 35 postinjury for motor performance on the rotarod. There was a significant interaction between injury group and latency to fall over time (two-way repeated measures ANOVA), F(12,172) = 2.354, p = .008; Figure 3(a). Specifically, mice that received 10 hits over 10 days showed significant impairments compared with sham animals at 1 DPI (Tukey post hoc, p < .01). There was a main effect of DPI on latency to fall, F(4, 172) = 26.81, p < .0001, as well as a main effect of group, F(3, 43) = 4.492, p = .0079. However, by 4 DPI, there were no significant differences between any group and shams.
Figure 3.
rmCHI results in minimal motor behavior deficits 1 DPI that return to 4 DPI. Following 0, 1, 5, or 10 rmCHIs, mice were tested on motor function using an accelerating rotarod at 1, 4, 10, 21, 28, and 35 DPI and on the horizontal ladder beam at 28 DPI (a). rmCHI resulted in rotarod performance deficits (two-way ANOVA, interaction, **p = .0080) such that mice with 10 hits fell quicker off the rotarod at 1 DPI compared with sham controls (Tukey post hoc, p < .01). No other time points of rotarod testing had significant differences between groups. Indeed, a learning effect is evidenced in the upward slop of all groups. (b) rmCHI had no effect on motor performance on the horizontal ladder beam as assessed by total forepaw misses over three trials (one-way ANOVA), F(3, 41) = 1.945, p = .1375.
rmCHI = repeated mild closed head injury; DPI = days postinjury; ANOVA = analysis of variance.
One-, 5-, and 10-hit mice also underwent testing on the horizontal ladder beam at 1 MPI to assess motor function and control. There were no significant effects of group on number of forepaw misses (one-way ANOVA), F(3, 41) = 1.945, p = .1375; Figure 3(b), as assessed by total number of forepaw foot faults or misses over three trials along beam rungs. Taken together, rotarod and ladder beam results suggest that group differences at 1 month or greater are not the result of CHI-induced chronic motor deficits.
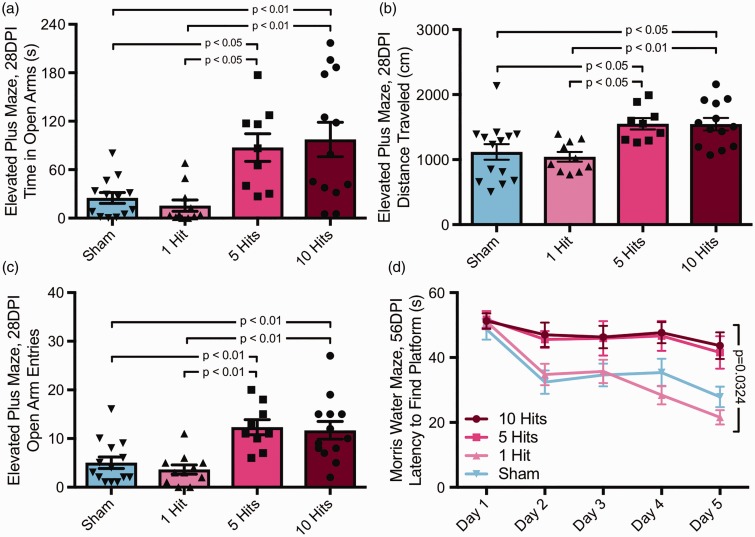
Anxiety-like behavior and risk-taking were assessed using the EPM task at 1 month postinjury. There was a significant effect of group on time spent in open arms (one-way ANOVA), F(3, 42) = 8.301, p = .0001; Figure 4(a). Specifically, 10-hit mice spent more time in open arms compared with both sham and 1-hit groups (Tukey post hoc, p < .01). Five-hit mice also spent significantly more time in open arms compared with sham and 1-hit mice (Tukey post hoc, p < .05). There was a significant effect of group on total distance traveled during the task (one-way ANOVA), F(3, 42) = 6.800, p = .0008; Figure 4(b). Specifically, 10-hit mice traveled more compared with both sham and 1-hit groups (Tukey post hoc, p < .01 and p < .05, respectively). Five-hit mice also traveled more compared with sham and 1-hit mice (Tukey post hoc, p < .05). Moreover, there was a significant effect of group on number of entries into open arms (one-way ANOVA), F(3, 43) = 9.327, p < .0001; Figure 4(c). Specifically, 10-hit mice had more entries into open arms compared with both sham and 1-hit groups (Tukey post hoc, p < .01). Five-hit mice also had more entries into open arms compared with sham and 1-hit mice (Tukey post hoc, p < .01). There was no significant effect of group on number of closed arm entries, F(3, 41) = 0.3840, p = .7651; data not shown. In addition to the raw numbers of greater time spent in open arms, we also observed that 5-hit and 10-hit animals would frequently extend their bodies out into open space beyond the sides of the open arms, whereas shams and 1-hit animals rarely if ever extended their bodies out over the sides of the maze. Depression-like behavior was assessed using the TST at 1 month postinjury. There was no significant difference between any group on time immobile during the task (one-way ANOVA), F(3, 43) = 2.350, p = .0857; data not shown.
Figure 4.
rmCHI alters cognitive function at 1 and 2 months postinjury. Following 0, 1, 5, or 10 rmCHIs, mice were tested on the elevated plus maze at 28 DPI (1 month) and the Morris water maze at 56 DPI (2 months). (a) rmCHI resulted in changes to performance on the elevated plus maze. Mice with 5 or 10 hits spent more time in the open arms compared with sham or 1-hit mice (one-way ANOVA), F(3, 42) = 8.301, p = .0002, and (b) rmCHI mice also traveled more distance during the task (one-way ANOVA), F(3, 42) = 6.800, p = .0008, demonstrating hyperactivity and no prolonged motor deficits. (c) In addition, mice with 5 or 10 hits exhibited more open arm entries than sham or 1-hit mice (one-way ANOVA), F(3, 42) = 9.327, p < .0001. (d) 2 months postinjury, mice were tested over 5 days on the Morris water maze. rmCHI mice that received either 5 or 10 hits were impaired in the ability to learn the location of the hidden platform (two-way ANOVA), F(12, 172) = 1.943, p = .0324 interaction. The p values on graphs represent Tukey post hoc differences.
rmCHI = repeated mild closed head injury; DPI = days postinjury; ANOVA = analysis of variance.
Finally, during initial comparison of rmCHI parameters, 1-, 5-, and 10-hit mice were tested on the MWM at 2 MPI to assess learning and memory. There was a significant interaction between group and latency to find the platform over 5 days of testing (two-way repeated measures ANOVA), F(12, 172) = 1.943, p = .0324; Figure 4(d). There was a main effect of time on time to reach platform, F(4, 172) = 17.42, p < .0001, as well as a main effect of group, F(3, 43) = 7.778, p = .0003. Mice receiving 10 hits exhibited a significantly longer latency to reach the platform on testing Day 4 compared with 1-hit mice (Tukey post hoc, p < .01) and on Day 5 compared with 1-hit (p < .001) and sham (p < .05) mice. Mice receiving 5 hits exhibited a significantly longer latency to reach the platform on testing Day 4 compared with 1-hit mice (p < .05) and on testing Day 5 compared with 1-hit mice (p < .01). On Day 6, a probe trial was completed without the platform in the tank (not shown). There was a significant effect of group on this probe task (one-way ANOVA), F(3, 43) = 5.898, p = .0018, where 10-hit mice spent less time in the target quadrant than 1-hit mice (Tukey post hoc, p < .01), and 5-hit mice spent less time in the target quadrant compared with 1-hit (Tukey post hoc, p < .01) and sham mice (Tukey post hoc, p < .05). There were no observed differences in swim speed between any groups, suggesting that there were no prolonged motor deficits that could account for group differences.
Quantification of pathology over injury severity at 2 MPI
Immunocytochemistry for myelin basic protein demonstrated that there were clear differences in corpus callosum thickness in response to rmCHI across injury severities (Figure 5(a)). To assess white matter integrity, volumetric analysis was performed using a Cavalieri probe (Figure 5(b)). There was a significant effect of injury condition on corpus callosum volume (one-way ANOVA), F(3, 20) = 23.35, p < .0001. Mice that received 10 hits and 5 hits had a significantly smaller corpora callosa compared with both sham and 1-hit mice (Tukey post hoc, p < .001 for all comparisons, Figure 5(c)). Specifically, 10-hit animals had 31% thinner corpora callosa than shams on average. Three-dimensional reconstructions were made using StereoInvestigator software to better visualize shape and size of the corpus callosum (Figure 5(d) and Supplemental Movie), where noticeable atrophy, especially in the splenium of the corpus callosum, was observed.
There were several relationships between corpus callosum volume and performance on behavioral tasks. Corpus callosum volume was negatively correlated with several measures on the EPM at 1 month postinjury: time spent in open arms (Pearson correlation, r2 = .400, p = .0009, Figure 5(e)); distance traveled (r2 = .406, p = .008); and open arm entries (r2 = .306, p = .005). Corpus callosum volume was also negatively correlated with Day 5 latency to platform in the MWM task (r2 = .192, p = .0322, Figure 5(f)) and probe platform crossings (r2 = .185, p = .036).
Neuronal loss within the cortex below the impact site was suggested by cresyl violet and NeuN immunocytochemistry for an antigen present within the nuclei of neurons (Figure 6(a)). To assess neuronal loss following rmCHI, an optical fractionator was employed to quantify NeuN+ neurons in the cortex bounded by anatomical landmarks (Figure 6(b)). There was a significant effect of group on NeuN+ cortical neurons (one-way ANOVA), F(3, 20) = 5.693, p = .0055; Figure 6(c). Specifically, 10-hit mice had significantly less NeuN+ neurons compared with both sham and 1-hit groups (Tukey post hoc, p < .01 and p < .05, respectively). NeuN+ neurons were reduced 25% in comparison with controls at 2 MPI. Using a Cavalieri probe to quantify cortical volume, a significant effect of group on cortical volume was also observed (one-way ANOVA), F(3, 20) = 9.514, p = .0004; data not shown. Ten-hit mice had smaller cortical volume compared with both sham and 1-hit controls (Tukey post hoc, p < .01). Similarly, five-hit mice had smaller cortical volumes compared with both sham and one-hit mice (Tukey post hoc, p < .01 and p < .05, respectively). Normalizing neuronal count to cortical volume reveals no significant effect of group on NeuN+ neurons per mm3 (one-way ANOVA), F(3, 20) = 2.384, p = .0996. NeuN+ neuronal count was negatively correlated with time spent in open arms during the EPM task at 1 month postinjury (Pearson correlation, r2 = .2779, p = .0041, Figure 6(d)) and also latency to platform on Day 5 of MWM testing at 2 MPI (r2 = .2595, p = .0055, Figure 6(e)).
Are Behavioral Deficits Sustained 1, 2, and 6 MPI?
In a separate cohort of mice, anxiety and risk-taking behavior was assessed on the EPM at 1 MPI and at 6 MPI. There was a significant effect of group on time spent in open arms (one-way ANOVA), F(2, 25) = 18.31, p < .0001; Figure 7(a), at 1 MPI. Specifically, 10-hit mice spent more time in open arms compared with both sham and 1 hit groups (Tukey post hoc, p < .0001). Moreover, there was a significant effect of group on number of entries into open arms (one-way ANOVA), F(2, 25) = 6.218, p = .0064. Specifically, 10-hit mice had more entries into open arms compared with both sham and 1-hit groups (Tukey post hoc, p < .05 and p < .01, respectively). There was also a significant effect of group on number of closed arm entries, F(2, 25) = 4.202, p = .0267. Ten-hit mice had fewer entries into closed arms compared with sham mice (Tukey post hoc, p < .05). There was no significant effect of group on total distance traveled during the task (one-way ANOVA), F(2, 25) = 1.932, p = .1658; Figure 7(b).
Figure 7.
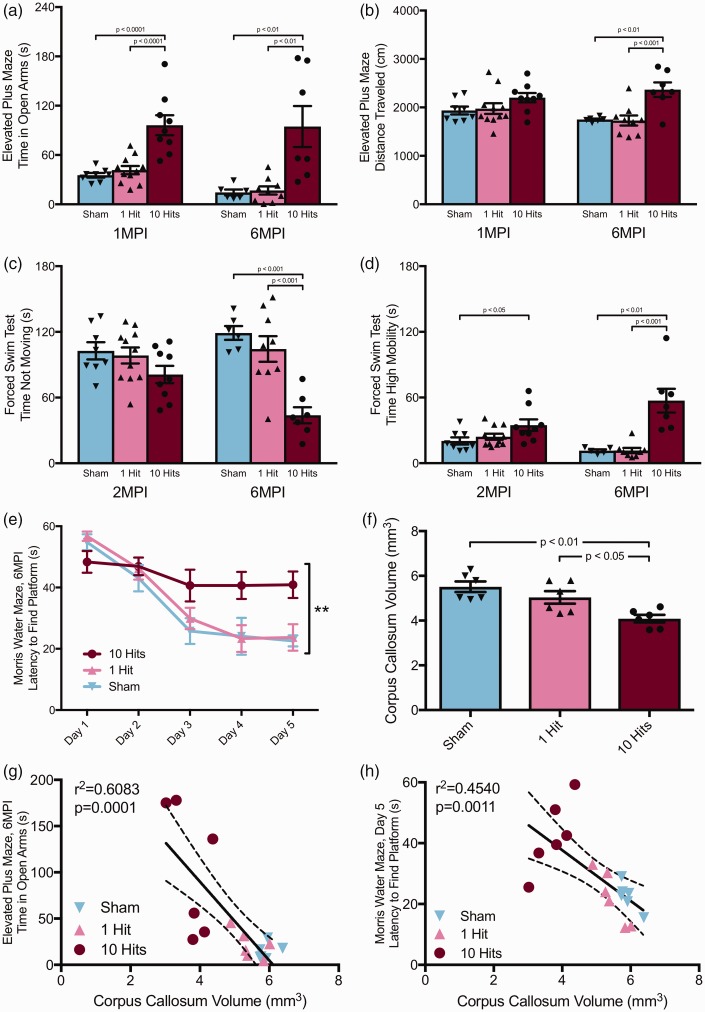
Sustained deficits in behavioral function and white matter atrophy persist up to 6 months postinjury. (a) Mice with rmCHI spent more time in open arms of the elevated plus maze at both 1 (one-way ANOVA), F(2, 25) = 18.31, p < .0001, and 6 MPI (one-way ANOVA), F(2, 19) = 9.960, p = .0011. (b) Mice with rmCHI were more active in the elevated plus maze at 6 MPI, but not at 1 MPI (6 MPI; one-way ANOVA), F(2, 19) = 10.26, p = .0010. (c) At 6 MPI, but not at 2 MPI, rmCHI mice spent less time not moving in the forced swim test compared with both 0- and 1-hit groups (one-way ANOVA), F(2, 19) = 15.62, p < .0001. (d) At both 2 MPI and 6 MPI, mice with rmCHI spent more time classified as “high mobility”: 2 MPI, one-way ANOVA, F(2, 25) = 3.542, p < .0442; 6 MPI, one-way ANOVA, F(2, 17) = 15.21, p = .0002. (e) Mice with 10 hits were unable to learn the location of the hidden platform in the Morris water maze at 6 MPI (two-way ANOVA), F(8, 76) = 2.858, p < .0078. (f) Mice with 10 hits had significant white matter atrophy compared with both sham and 1-hit mice (one-way ANOVA), F(2, 15) = 2.532, p < .01, and (g) corpus callosum volume measured at 6 MPI negatively correlated with latency to time in open arms during the elevated plus maze at 2 MPI (r= −.78, p = .0001). (h) Corpus callosum volume also negatively correlated with MWM Day 5 latency to find the platform at 6 MPI (r= −.674, p = .0011).
rmCHI = repeated mild closed head injury; MPI = months postinjury; ANOVA = analysis of variance; MWM = Morris water maze.
At 6 MPI, there was still a significant effect of group on time spent in open arms (one-way ANOVA), F(2, 19) = 9.960, p = .0011; Figure 7(a). Ten-hit mice spent more time in open arms compared with both sham and 1-hit groups (Tukey post hoc, p < .01). The significant effect of group on number of entries into open arms remained (one-way ANOVA), F(2, 18) = 26.48, p < .0001. Specifically, 10-hit mice had more entries into open arms compared with both sham and 1-hit groups (Tukey post hoc, p < .001 and p < .0001, respectively). However, there was no effect of group on number of closed arm entries, F(2, 19) = 0.2867, p = .7539. In contrast to observations at 1 MPI, there was a significant effect of group on total distance traveled during the task (one-way ANOVA), F(2, 19) = 10.26, p = .0010; Figure 7(b). Specifically, 10-hit mice traveled more during the task compared with both sham and 1-hit groups (Tukey post hoc, p < .01).
Mice were also tested on the FST to assess depressive-like behavior at 2 MPI and 6 MPI. There were no significant effects of group on time not moving (one-way ANOVA), F(2, 25) = 2.107, p = .1426, Figure 7(c); ‘immobile' time (one-way ANOVA), F(2, 25) = 3.375, p < .0504; or ‘mobile' time (one-way ANOVA), F(2, 25) = 2.114, p = .1418, at 2 MPI. However, there was a significant effect of group on ‘high mobility' time (one-way ANOVA), F(2, 25) = 3.542, p = .0442; Figure 7(d), where 10-hit mice spent more time classified as 'high mobility' compared with sham mice (Tukey post hoc, p < .05) at 2 MPI. In contrast to the 2 MPI data, at 6 MPI, a significant effect of group on time not moving (one-way ANOVA), F(2, 19) = 15.62, p < .0001; Figure 7(c), was observed, where 10-hit mice spent less time ‘not moving' than both sham and 1-hit groups (Tukey post hoc, p < .001). There was a significant effect of group on 'immobile' time (one-way ANOVA), F(2, 19) = 24.01, p < .0001, where 10-hit mice spent less time ‘immobile' than both sham and 1-hit groups (Tukey post hoc, p < .0001). A significant effect of group on ‘mobile' time (one-way ANOVA), F(2, 19) = 19.76, p < .0001, where 10-hit mice spent more time ‘mobile' than both sham and 1 hit groups (Tukey post hoc, p < .0001) was also observed. Finally, there remained a significant effect of group on ‘high mobility' time (one-way ANOVA), F(2, 17) = 15.21, p = .0002; Figure 7(d), at 6 MPI, where 10-hit mice spent more time ‘high mobility' than either sham or 1-hit groups (Tukey post hoc, p < .01 and p < .001, respectively).
Mice were tested on the MWM task at 6 MPI to assess long-term effects of injury on learning and memory. There was a significant interaction between group and latency to find the platform over 5 days of testing (two-way repeated measures ANOVA), F(8, 76) = 2.858, p = .0078; Figure 7(e). There was a main effect of time on time to reach platform, F(4, 76) = 25.27, p < .0001, as well as a main effect of group, F(2, 19) = 5.264, p = .0152. Mice receiving 10 hits had significantly longer latency to reach platform on testing Day 3 compared with sham mice (Tukey post hoc, p < .01), on testing Day 4 compared with sham (p < .05) and 1-hit (p < .01) mice, and on Day 5 compared with both sham and 1-hit mice (p < .01). During the probe trial, mice with 10 hits spent less time in the target quadrant than both sham and 1-hit animals (one-way ANOVA), F(2, 18) = 8.085, p < .0031, Tukey post hoc, p < .01.
Are Pathological Changes Persistent Out to 6 MPI?
To assess white matter integrity, volumetric analysis was performed using a Cavalieri probe. There was a significant effect of injury condition on corpus callosum volume (one-way ANOVA), F(2, 15) = 9.617, p = .0021; Figure 7(f), at 6 MPI. Mice that received 10 hits had significantly smaller corpora callosa compared with both sham and 1-hit mice (Tukey post hoc, p < .01, p <.05); the average corpora callosa volume was reduced 35% compared with shams. Corpus callosum volume negatively correlated with latency to time in open arms on the EPM at 6 MPI (r2 = .6083, p = .0001, Figure 7(g)). Corpus callosum volume also correlated with MWM Day 5 latency to find the platform at 6 MPI (r2 = .4540, p = .0022, Figure 7(h)).
Stereological quantification of NG2+ oligodendrocyte precursors in the corpus callosum of injured and uninjured mice was also performed. There was no significant effect of absolute number of NG2+ cells between groups (one-way ANOVA), F(2, 15)=1.012, p = .3871; however, there was a strong correlation between NG2+ cells and corpus callosum volume at 6 MPI (r2 = .5781, p = .0002), whereby there were more NG2+ cells in larger corpora collosa. CE values for sham, 1-hit, and 10-hit groups averaged 0.13, 0.17, and 0.16, respectively.
Following rmCHI, and as reported by numerous groups, extensive glial activation and inflammation were observed at both 2 MPI (from the injury severity cohorts) and 6 MPI (from the long-term cohorts). Specifically, hypertrophic GFAP+ astrocytes (Figure 8) and activated Iba1+ microglia (Figure 9) were observed in 10-hit animals, particularly localized to corpus callosum tracts in comparison with sham and 1-hit animals.
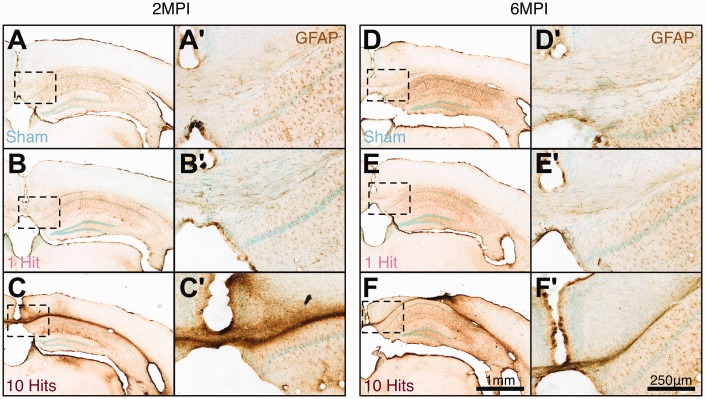
Figure 8.
rmCHI induces chronic astrogliosis at 2 MPI and 6 MPI. (a, d) Baseline levels of GFAP immunoreactivity in C57Bl/6J mice. 1-hit mice have similar GFAP immunoreactivity at 2 MPI (b, b') and 6 MPI (e, e') to sham mice at 2 MPI (a, a') and 6 MPI (d, d'). Mice receiving 10 hits have prominent GFAP immunoreactivity at 2 MPI (c, c') and at 6 MPI (f, f'), particularly in the corpus callosum, cortex below impact location, and hippocampus. Low magnification scale bar = 1 mm; high magnification scale bar = 250 μm.
rmCHI = repeated mild closed head injury; MPI = months postinjury; GFAP = glial fibrillary acidic protein.
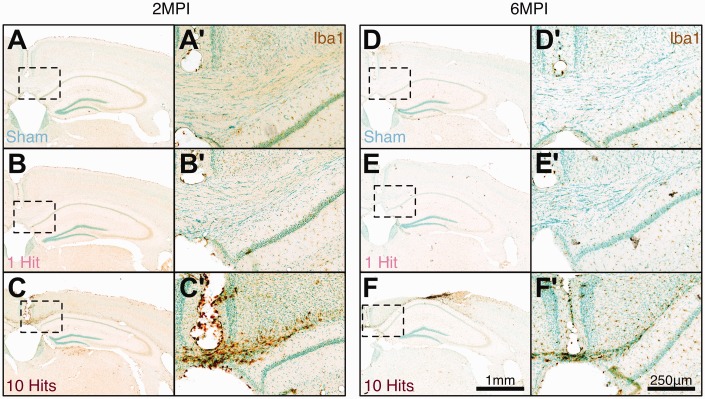
Figure 9.
rmCHI induces chronic microglial inflammation at 2 MPI and 6 MPI in white matter tracts. (a, d) Baseline levels of Iba1 immunoreactivity in sham C57Bl/6J mice. 1-hit mice have similar Iba1 immunoreactivity at 2 MPI (b, b') and 6 MPI (e, e') to sham mice at 2 MPI (a, a') and at (d, d') 6 MPI. (c, c') Mice receiving 10 hits have prominent Iba1 immunoreactivity at 2 MPI and (f, f') at 6 MPI, particularly in the corpus callosum. Low magnification scale bar = 1 mm; high magnification scale bar = 250 μm.
rmCHI = repeated mild closed head injury; MPI = months postinjury.
Differences Between NSG and C57Bl/6J Mice
We tested the effect of rmCHI on a newly developed mouse strain from Jackson Laboratories, NSG (catalog #005557), to lay the groundwork for future testing of cellular therapies. Surprisingly, NSG mice exhibited no significant changes as a result of any injury parameter tested on any behavioral or histological measure assessed in comparison with NSG sham controls. NSG mice were tested on the EPM at 1 MPI. There were no differences between groups. Specifically, there was no effect of group on time spent in open arms (Figure 10(a), one-way ANOVA), F(3, 43)=1.302, p = .2865, on distance traveled (Figure 10(b), one-way ANOVA), F(3, 43) = 0.5475, p = .6525, or open arm entries (one-way ANOVA), F(3, 43) = .9388, p = .4302, at 1 MPI. Mice were also tested for learning performance at 2 MPI on the MWM. There were no differences detected in performance between groups (Figure 10(c), two-way repeated measures ANOVA), F(12, 172)=0.4904, p = .9184. Moreover, the white matter atrophy we observed in C57Bl/6J mice was not observed in NSG mice at 6 MPI at any injury level (Figure 10(d), one-way ANOVA), F(3, 19) = 0.5767, p = .6573. A summary of the effects of rmCHI on a range of behavioral assessments at different time points is shown in Table 1 for both C57Bl/6J and NSG mice. A summary of correlations between corpus callosum volume and NeuN number versus several behavioral endpoints is provided in Table 2.
Figure 10.
Nod-scid gamma (NSG) mice do not exhibit changes in behavioral function nor white matter atrophy following rmCHI. (a) No differences were observed at 1 MPI on time in open arm, or (b) total distance traveled in NSG mice receiving 1 or 5 hits at 5 m/s. (c) No differences were observed in the latency to find a hidden platform during the Morris water maze testing at 2 MPI. (d) Corpus callosum atrophy was not observed at 6 MPI in NSG mice receiving any level of injury. In contrast to the gliosis and microglial inflammation observed in C57Bl/6J mice, there appeared to be reduced staining for GFAP (d) and Iba1 (e) in NSG mice following rmCHI. NSG mice were treated and stained identical to those shown in Figures 8 and 9, although not in parallel with the staining of C57Bl/6J mice.
rmCHI = repeated mild closed head injury; DPI = days postinjury; MPI = months postinjury; GFAP = glial fibrillary acidic protein.
Table 1.
Summary of Deficits Observed on Behavioral Tasks Assessed Following Repeated Mild Closed Head Injury in C57Bl/6J and NSG Mice.
| Task | Time point | Strain | Sham (n) | 1 Hit at 5.0 m/s | 5 Hits at 2.5 m/s | 5 Hits at 5.0 m/s | 10 Hits at 5.0 m/s | Significant deficit | Summary |
|---|---|---|---|---|---|---|---|---|---|
| Rotarod | 1, 4, 10, 21, 35 DPI | C57BL/6 mice | 14 | 11 | – | 9 | 13 | No | Deficits at 1 DPI for 10-hit group: 10 vs. sham |
| Horizontal ladder beam | 1 MPI | C57BL/6 mice | 14 | 11 | – | 9 | 13 | No | N.S. |
| Tail suspension test | 1 MPI | C57BL/6 mice | 14 | 11 | – | 9 | 13 | No | N.S. |
| Elevated plus maze (bright room) | 1 MPI | C57BL/6 mice | 14 | 11 | – | 9 | 13 | Yes | 10 Hits and 5 hits vs. 1 and sham: more time in open arm, more distance traveled, more open arm entries; closed arm entries (N.S.) |
| Elevated plus maze (dark room) | 1 MPI | C57BL/6 mice | 8 | 11 | – | – | 9 | Yes | 10 Hits vs. 1 and sham: more time in open arm, trend for more distance traveled (N.S.) |
| Elevated plus maze (dark room) | 1 MPI | C57BL/6 mice | 12 | – | 12 | 12 | – | Yes | Time in open arms: 5 hits at 5.0 m/s > 3 hits at 5.0 m/sTime in open arms: 5 hits at 5.0 m/s > 5 hits at 2.5 m/sTime in open arms: 5 hits at 5.0 m/s > shamTime in open arms: 3 hits at 5.0 m/s > sham |
| Morris water maze | 2 MPI | C57BL/6 mice | 14 | 11 | – | 9 | 13 | Yes | 10 Hits and 5 hits vs. 1 and sham: deficits in learning platform location (latency to reach platform), decreased distance traveled in probe, decreased platform crossings in probe, decreased time in target quadrant in probe |
| Morris water maze | 2 MPI | C57BL/6 mice | 12 | – | 12 | 12 | – | Yes | Learning deficits at D4 and D5 trainingSham ≤ 5 hits at 2.5 m/s < 3 hits at 5.0 m/s ≤ 5 hits at 5.0 m/s |
| Forced swim test | 2 MPI | C57BL/6 mice | 8 | 11 | – | – | 9 | Yes | 10 Hits vs. 1 and sham: less time “immobile,” more time “high mobility” |
| Elevated plus maze (dark room) | 6 MPI | C57BL/6 mice | 6 | 9 | – | – | 7 | Yes | 10 Hits vs. 1 and sham: more time in open arm, more distance traveled |
| Forced swim test | 6 MPI | C57BL/6 mice | 6 | 9 | – | – | 7 | Yes | 10 Hits vs. 1 and sham: less time “immobile,” more time “high mobility” |
| Morris water maze | 6 MPI | C57BL/6 mice | 6 | 9 | – | – | 7 | Yes | 10 Hits vs. 1 and sham: deficits in learning platform location (latency to reach platform); |
| Elevated plus maze (dark room) | 1 MPI | NOD-scid gamma | 12 | 12 | 12 | 11 | – | No | N.S. |
| Morris water maze | 1 MPI | NOD-scid gamma | 12 | 12 | 12 | 11 | – | No | N.S. |
| Elevated plus maze (dark room) | 6 MPI | NOD-scid gamma | 6 | 6 | 6 | 6 | – | No | N.S. |
| Forced swim test | 6 MPI | NOD-scid gamma | 6 | 6 | 6 | 6 | – | No | N.S. |
| Novel object recognition | 6 MPI | NOD-scid gamma | 6 | 6 | 6 | 6 | – | No | N.S. |
| Novel place recognition | 6 MPI | NOD-scid gamma | 6 | 6 | 6 | 6 | – | No | N.S. |
Note. NSG = NOD-scid gamma mice; DPI = days postinjury; MPI = months postinjury.
Table 2.
Correlations between corpus callosum volume, NeuN neuron counts, and behavior.
| Time point | Pearson’s r | Pearson’s r2 | One-tailed p value | |
|---|---|---|---|---|
| Correlations to corpus callosum volume | ||||
| EPM. Time spent in open arms | 2 MPI | −.633 | .400 | .0005 |
| EPM. Distance traveled | 2 MPI | −.637 | .406 | .0004 |
| EPM. Open arm entries | 2 MPI | −.553 | .306 | .0025 |
| EPM. Time spent in open arms | 6 MPI | −.780 | .608 | .0001 |
| Ladder beam errors | 2 MPI | −.315 | .099 | .0715 |
| MWM. Day 5 latency to platform | 2 MPI | −.438 | .192 | .0161 |
| MWM. Probe trial time in target quadrant | 2 MPI | .363 | .131 | .0446 |
| MWM. Probe platform crossings | 2 MPI | .430 | .185 | .0018 |
| MWM. Day 5 latency to platform | 6 MPI | −.674 | .454 | .0011 |
| NG2+ cells in corpus callosum | 6 MPI | .760 | .578 | .0001 |
| Correlations to NeuN neuron number | ||||
| EPM. Time spent in open arms | 2 MPI | −.527 | .278 | .0041 |
| EPM. Distance traveled | 2 MPI | −.436 | .190 | .0166 |
| MWM. Day 5 latency to platform | 2 MPI | −.509 | .260 | .0055 |
| MWM. Probe trial time in target quadrant | 2 MPI | .250 | .062 | .1197 |
Note. EPM = elevated plus maze; MPI = months postinjury; NG2 = neural/glial antigen 2; MWM = Morris water maze.
Exacerbation of Tau Phosphorylation in hTau Mice Following rmCHI
We were unable to assess hTau mice for behavioral changes as the group sizes were too small and hTau mice are difficult to test accurately due to hyperactivity. Instead, we compared the level of pTau (CP13 and PHF-1) in the hippocampus of sham-treated and rmCHI-challenged hTau mice in response to the 5-hit model using immunocytochemistry. We observed that 10-month-old female hTau mice exhibit increased staining of pTau (CP13) 6 months post-rmCHI (Figure 11(a) and (b)); PHF-1 staining was also present (data not shown). Increases in hyperphosphorylated tau were most robust within the granule cell layer and apical dendrites of the dentate gyrus. We observed similar increases in CP13 immunostaining in male hTau mice in response to the 5-hit rmCHI injuries. Conversely, no CP13 or PHF-1 staining was seen in WT mice regardless of injury status or gender (data not shown).
On Western blots (Figure 11(c) and (d)), total tau levels (HT7) were unchanged in WT mice after rmCHI exposure (1.45 ± 0.075 in sham-treated vs. 1.48 ± 0.045 in rmCHI animals). In hTau mice, total tau levels (HT7) were slightly increased, though not significantly (2.20 ± 0.194 vs. 2.41 ± 0.17) after rmCHI. Levels of tau phosphorylated at Thr231/Ser235 tau (AT8) were slightly increased both in WT (1.06 ± 0.054 vs. 1.13 ± 0.068) and in hTau mice (1.75 ± 0.0116 vs. 1.94 ± 0.108) after rmCHI. There were slight conformational changes in tau toward tangle formation (via MC1) in both WT (0.888 ± 0.114 vs. 0.947 ± 0.104) and in hTau mice (2.40 ± 0.105 vs. 2.55 ± 0.14) 3 months after rmCHI. pTau at Ser202 (CP13) was detected only in hTau mice and was increased after rmCHI (0.626 ± 0.144 vs. 0.833 ± 0.076). Hyperphosphorylated tau at Ser396/Ser404 (PHF-1) was also detected only in hTau mice and was significantly increased after rmCHI in comparison with shams (0.789 ± 0.0242 vs. 0.915 ± 0.027, p < .0002). These data suggest that hTau mice with rmCHI may be suitable to model CTE.
Discussion
rmTBI Is Not ‘One' Syndrome
TBI is often used to describe head injuries encompassing a wide range of precipitating events, followed by a wide range of cognitive and pathological consequences. To better study this variety, many experimental animal models have been developed to recapitulate specific consequences of head injuries, depending on the model chosen. While it may seem ideal to have one universal rodent model to facilitate comparisons across research groups, it is impossible to account for every injury type or every injury sequelae due to the variability of injuries, so standardizing to one model would be severely restricting. It is therefore useful to have multiple models of TBI and rmCHI that have their own characteristics relevant to a specific clinical injury phenotype—provided those models have been well characterized and recapitulate specific features of the human condition. We chose to combine a well-controlled cortical impact (CCI) model with a freely moving head so that rotational/axon shearing forces were combined with CCI. Our goal was to replicate chronic symptoms and pathologies clinically similar to that of patients with rmCHI and to look for correlations between behavioral function and measures of pathology as a result of rmCHI. Our model induces long-term cognitive deficits, white matter atrophy, cortical neuronal loss, demyelination, and chronic neuroinflammation, particularly within the corpus callosum. This model uses commercially available equipment allowing for replication across laboratories. Avoiding use of a fixed head stereotaxic position allows for rotational forces in the anterior–posterior axis and coup–contrecoup intracranial impacts. Another benefit is the reduced time under anesthesia (7 min), minimizing confounding neuroprotective effects of isoflurane (Statler et al., 2000, 2006). This short procedure allows for high-throughput and large animal cohorts, useful when assessing behavior. An experienced solo experimenter could run this procedure on 10 mice per hour. In addition, there is low variability within groups and high reproducibility across users.
Long-Term Cognitive and Behavioral Deficits Following rmCHI
Clinically, head injuries can result in a range of symptoms. Patients with a history of TBI frequently develop depression (33%) with comorbidities including anxiety (77%) and aggressive behavior (57%; Jorge et al., 2004), as well as deficits in working memory (Collins et al., 1999a, 1999b; Iverson et al., 2006). In the rmCHI model presented here, we recapitulate these behavioral deficits following repeated injury; mice receiving a single impact at the standardized injury parameters (5 m/s and 1 mm depth) do not display detectable behavioral changes, whereas 3- and 5-hit mice do, supporting the argument that repeated injuries of a mild severity can lead to impairments as the result of cumulative effects. When tested on the MWM, mice receiving either 5 or 10 hits took longer to find a hidden platform over 5 days of testing at both 2 and 6 MPI, whereas mice receiving a single hit were able to learn the task. Learning the water maze task is a dorsal hippocampus-mediated task (Moser et al., 1993). Although no gross hippocampal changes were observed in C57Bl/6J mice, hippocampal neuron counts or synapse quantification could provide more insight for the role of these learning deficits following rmCHI. Supporting the possibility of discrete hippocampal changes, we observed focal hippocampal increases in hyperphosphorylated tau 3 MPI in hTau mice, suggesting this model can induce hippocampal changes that may affect cognition.
Deficits in learning at chronic time points post-rmCHI have been reported for the MWM (Meehan et al., 2012; Mannix et al., 2013). Using a weight drop model of rmCHI, mice with 5 daily or 5 weekly, but not 5 monthly, head impacts had deficits in the MWM (Mannix et al., 2013). Moreover, this study also observed deficits in the task persisting up to 1 year post-injury. In a modified CCI mCHI injury, where animals received 2 impacts over 2 days, deficits in learning were observed at 7 weeks post-injury (Shitaka et al., 2011). However, neither of these studies correlated water maze performance with associated neuropathologies. We observed that both corpus callosum atrophy and loss of cortical NeuN+ neurons correlated with performance on the MWM. Models of rmCHI with long-lasting deficits are critical for future intervention studies and for understanding CTE. Importantly, we did not see an exacerbation of deficits (behavioral or pathological) in the 10-hit animals compared with 5-hit animals, nor significant differences between animals that received 5 hits over 5 days versus 5 hits over 10 days, suggesting a threshold or ceiling for cumulative effects at one injury every other day. Reducing the injuries to 3 hits over 6 days in comparison with 5 hits does not change the distance traveled in the EPM but results in an intermediate effect on the time in open arms. Both 3-hit and 5-hit mice have similar deficits in learning on the MWM. In a fixed skull open scalp rmTBI model, Bolton Hall et al. (2016) reported that mice receiving 5 hits spaced 48 hr apart did not differ significantly from mice receiving 5 hits 24 hr apart, supporting the timing of a ceiling effect.
An animal spending more time in closed arms on the EPM is typically considered to have elevated anxiety (Lister, 1987), whereas the extent of movement versus immobility on the FST is considered a surrogate for depression (Petit-Demouliere et al., 2005). However, more time spent in open arms can also be indicative of increased risk-taking behavior. At both 1 MPI and 6 MPI, mice receiving rmCHI displayed significant changes in both anxiety and depression-linked behavioral tasks; in contrast, mice with a signal hit exhibited no detectable deficits. On the EPM, mice with rmCHI were hyperactive, spent more time in open arms, and had more open arm entries. rmCHI mice also traveled greater distances in the EPM compared with shams. Five-hit and 10-hit mice frequently extended their bodies out into open space beyond the sides of arms, whereas shams and 1-hit mice rarely, if ever, extended their bodies out over the sides of the maze. This suggest an overall hyperactive state as well as a varied or greater risk-taking profile (e.g., based on risk assessment, decision-making, exploration, and vertical activity; Rodgers and Johnson, 1995). Common long-term sequelae of people with TBI are disinhibition and aggression (Arciniegas and Wortzel, 2014). In support of this possibility, we also observed a hyperactive state in the FST at 2 and 6 MPI (Figure 7). Opposite to the predicted depressive-like behavior of more immobile time, rmCHI mice displayed more moving time as well more ‘highly mobile' time. These mice responded more aggressively to the task and tried to escape the test more than uninjured or single hit animals. While counterintuitive, viewed from the perspective of emotional ‘dyscontrol' common in individuals with TBI, the phenotype we observed in our model has external validity and argues for more thorough testing of emotional and behavioral changes in mice post-rmCHI in addition to standard learning and memory tasks.
Similar changes in anxiety-like behavior on the EPM has also been reported at chronic time points of 1 and 6 months following rmCHI (Petraglia et al., 2014) where animals spent significantly more time in open arms (as observed here); however, to our knowledge, no data on hyperactivity following rmCHI on this test have been previously reported. In our model, rmCHI mice were indistinguishable from shams on the tail suspension task, whereas Bajwa et al. (2016) reported that rmCHI mice and CCI injured mice become immobile more quickly than sham animals; we did not specifically measure the 'time to immobile' to be able to make a clearer comparison. Chronic time points assessed at greater than 1 MPI are vital to investigating disease progression and detecting sustained or increased deficits over time (Gold et al., 2013). Interestingly, a slight progression of behavioral deficits was observed in the retest of the FST at 6 MPI versus 2 MPI, whereas deficits in performance on the EPM and MWM remained constant to 6 MPI. Discovery of behavioral measures that show disease progression over time could be valuable in linking cognitive changes and with neuropathological progression.
Long-Term Neuropathological Findings Post-rmCHI
Animals receiving rmCHI exhibited significant white matter atrophy of the corpus callosum. Atrophy was observed as early as 2 month post-injury (the earliest timepoint measured) and remained 6 months following injury. White matter volume loss was associated with demyelination as well as chronic astroglial neuroinflammation and reactive microglia. In addition, we observed significant cortical neuronal loss following rmCHI. Loss of neurons was associated with cortical volume loss, while no changes in neuronal density were observed. Chronic neuroinflammation at both 6 and 12 MPI has been previously reported in other experimental models of rmCHI (Shitaka et al., 2011; Mouzon et al., 2012). One might hypothesize that cortical neurons projecting through the corpus callosum have become demyelinated, or the neurons and axonal projections through the corpus callosum have died. Importantly, we observed cortical neuronal loss in areas sending contralateral projections. Demyelination can occur due to several injury mechanisms. Upon oligodendrocyte death, oligodendrocyte progenitor cell (OPC) populations migrate to the site, divide, and differentiate into newborn oligodendrocytes capable of repopulating lesions and subsequently remyelinating axons (Kirby et al., 2006; Franklin and Ffrench-Constant, 2008). Mature, myelinating oligodendrocytes could be selectively more susceptible to physical trauma or fail to remyelinate disrupted or demyelinated axons. Another possibility is a dysfunction of OPC populations. OPCs are evenly distributed throughout the brain, including the corpus callosum. rmCHI could lead to failure of OPC migration to injury sites, or impairment in cell division and differentiation. NG2+ oligodendrocytes have been shown to proliferate up to 7 days post-TBI in an experimental rodent model, but proliferation subsides by 21 DPI (Flygt et al., 2017). Seeing as much of the observed inflammation was occurring within the white matter tracts in the current model, we attempted to clarify the interplay between white matter degeneration and neuronal loss. We quantified the number of NG2+ OPCs in the corpus callosum, as these cells have been shown to proliferate between 7 and 21 days post-TBI, but no quantification has been performed at more chronic time points. At 6 MPI, we found that the overall density of NG2+ cells relative to volume remains constant, suggesting that the damage occurring in white matter following this experimental model of rmCHI is not due to a lack of oligodendrocyte progenitors and remyelination potential. To further elucidate the targets of the observed neuroinflammation, future studies should focus on quantification and assessment of a variety of cell subpopulations, including both immature and mature oligodendrocytes, as well as cortical layer segmented analysis.
Linking Neuropathology to Behavioral Changes
The corpus callosum is the major thoroughfare for cortical neuronal commiserate fibers between hemispheres. While it has been demonstrated that even a single TBI can lead to a 25% reduction in white matter in humans (Johnson et al., 2013a), the link between white matter disruption and cognitive/emotional impairments has yet to be clearly elucidated. This relationship has been investigated in clinical studies focusing on associations between diffusion-tensor imaging, particularly in white matter tracts, with emotional and cognitive changes following TBI (Kraus et al., 2007; Cubon et al., 2011; Shenton et al., 2012), although a clear relationship has yet to be illuminated. In addition, inflammation is a consistent feature of neurotrauma (Mckee and Lukens, 2016). In Rag1(−/−) mice, which lack mature T- and B-cells, there were no observed differences in injury response or neurological score in the acute phase of injury (≤ 7 DPI; Weckbach et al., 2012). Further, Weckback noted that C3a levels were significantly reduced in Rag1(−/−) mice after CHI in comparison with WT controls. However, these were Rag1(−/−) mice, not the Rag1(−/−) gamma (interleukin 2 [IL2] receptor common γ chain null) NSG mice used in the present study. In the present study, C57Bl/6J mice receiving rmCHI exhibited long-term microgliosis in the hippocampus and corpus callosum. Conversely, NSG exhibited no behavioral deficits nor atrophy or gliosis within the corpus callosum. Chronic Iba1+ microglia activation has also been reported in white matter tracts including corpus callosum, olfactory nerve layer, optic tract, and the brachium of superior colliculus in the CHIMERA model (Namjoshi et al., 2014) using C57Bl/6J mice. Chronic microgliosis has also been observed in humans post-TBI, especially in white matter tracts (Smith et al., 2013).
The contribution of microgliosis to axonal injury has yet to be fully investigated. Several groups have observed white matter gliosis following trauma. Chronic Iba1+ reactive microglia have been observed post-TBI in rodents, especially in white matter tracts following two mild closed head TBIs (Shitaka et al., 2011). However, in a mouse model of rmCHI, acute administration of valganciclovir to CD11b-thymidine kinase transgenic mice depleting macrophages had no effect on chronic axonal injury (Bennett and Brody, 2014). Low-dose valganciclovir reduced microglia in the corpus callosum and external capsule by 35% after rmCHI; however, axonal injury was unaltered; higher doses of valganciclovir or longer exposure were found to be toxic. Dr. Kim Green’s laboratory previously showed that PLX5622 at 1,200 mg/kg in chow can reduce the number of microglia in the central nervous system by 80% to 95%, depending on the duration of drug treatment (Dagher et al., 2015). Moreover, the brains of mice treated for 14 days at the 1,200 mg/kg dose of PLX5622, when exposed to lipopolysaccharide (0.5 mg/kg) via IP injection, expressed significantly less mRNA for IL-1ß (60% less) and tumor necrosis factor-α (70% less) than mice with a normal population of microglia. The 300 mg/kg PLX5622-treated animals, which reduced microglia only by 35%, did not attenuate the IL-1β levels in lipopolysaccharide-treated mice. This may explain why Bennett and Brody (2014), where they reduced microglia by only 35%, did not see any protection from 2 hits.
Immunodeficient Models and Stem Cell Transplants
Stem cell transplants may be an option for treating the effects of repeated mTBI if the deficits are severe enough, chronic, and suitable targets for cellular engraftment result from the injury. One strategy to alleviate these symptoms is to introduce multipotent neural stem cells that are capable of differentiating into neurons, oligodendrocytes, or astrocytes. One strategy might be to introduce neural stem cells to repopulate neuronal loss in the cortex, increase oligodendrocyte progenitor pools within corpus callosum, or increase oligodendrocytes and myelination for commissural fibers within the corpus callosum. Previous studies have shown efficacy of neural stem cell transplantation in acute models of TBI (Shear et al., 2004; Skardelly et al., 2011; Ma et al., 2012; Wang et al., 2012; Beretta et al., 2017; Haus et al., 2016), but this approach has not been investigated in a rodent model of rmCHI.
It is imperative to test clinically relevant cell populations, that is, human as opposed to murine cells. However, testing human stem cells in a rodent requires either multiple immunosuppressive agents or the use of immunodeficient animals (Anderson et al., 2011) to prevent rejection. Without the knowledge that white matter deficits are significant and sustained following rmCHI, targeting this syndrome with a cell therapy would seem premature. We chose a newly developed mouse from Jackson laboratories, NSG mice (catalog # 005557), which are similar to the classic immunocompromised mouse line, NOD-scids; both lack T and B cells. In addition, NSG mice carry a null allele of the IL2 receptor gamma, resulting in a deficiency of natural killer cells, antigen presenting cells, complement, and IL2 receptor gamma chain, while also having a longer life span than NOD-scids, making them even more attractive for long-term survival studies following cell transplantation. However, the finding that NSG mice exhibited neither behavioral nor frank pathological consequences following rmCHI, while suggesting that the inflammatory response to rmCHI is a significant component, indicates that testing a cell therapy in NSG mice at this time would be ill-advised.
Summary
There are shortcomings of any model and experiment, the present study included. For example, we do not know what the minimum delay between injuries is such that pathological or cognitive deficits are observed after 3, 5, or 10 hits, but not after 1 hit. In addition to different timing, different velocities and depth of impact should also be compared. Furthermore, the observation that 5 hits delivered every other day versus 5 days in a row both result in similar findings suggests that there is a ceiling effect of the injury paradigm. Finally, a more precise threshold for impactor velocity, depth of injury, and timing between injuries of injury severity would better classify the injury as mild, moderate, or severe and allow others to calibrate the level of injury to different paradigms. We would argue that any combination of parameters that results in undetectable functional or pathological consequences is by definition mild. In the present study, 5 and 10 impacts at 5.0 m/s speed and 1 mm depth of impact were sufficient to produce both behavioral and pathological deficits that were correlated with each other.
In humans, acute trauma results in one set of repercussions and secondary degeneration. But we are now learning that the consequences of concussion, and particularly repeated or multiple concussions, such as those sustained by athletes in a wide range of sports, or individuals in the military who are exposed to improvised explosive devices, concussive forces from shoulder fired missiles, grenades, or mortars, have their own sequelae of repercussions, leading to increased risk of developing CTE. Animal models that can mirror more facets of CTE, such as the use of hTau mice reported here, will enable us to explore the potential to block degenerative pathways or enhance regeneration post-concussion. This study lays the groundwork for further exploring the link between rmCHI, neuroinflammation, and the association of discrete neuropathological features with cognitive and emotional alterations.
Summary Statement
Mice with repeated mild concussions, but not a single concussion, exhibit a variety of functional deficits 2 and 6 MPI. These deficits are associated with thinning of the white matter and loss of cortical neurons.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The preceding studies were supported by a grant from the California Institute for Regenerative Medicine (CIRM): Early Translational Award TR2-01767 awarded to B. J. C., a grant from the Department of Defense, W81XWH-15-1-0435 (MR 141282), to Cathy Cahill and B. J. C., and Predoctoral Fellowships from the National Institutes of Health for “Training in the Neurobiology of Aging” (T32 AG00096-30) and for “Training Program in Stem Cell Translational Medicine for Neurological Disorders” (T32 NS082174-01) awarded to E. M. G.
References
- Abisambra J. F., Scheff S. (2014). Brain injury in the context of tauopathies. J Alzheimers Dis, 40, 495–518. [DOI] [PubMed] [Google Scholar]
- Acabchuk R., Briggs D. I., Angoa-Perez M., Powers M.Wolferz R. Jr. Soloway M., Stern M., Talbot L. R., Kuhn D. M., Conover J. C. (2016). Repeated mild traumatic brain injury causes focal response in lateral septum and hippocampus. Concussion, 1, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. J., Haus D. L., Hooshmand M. J., Perez H., Sontag C. J., Cummings B. J. (2011). Achieving stable human stem cell engraftment and survival in the CNS: Is the future of regenerative medicine immunodeficient? Regen Med, 6, 367–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C., Kress Y., Espinoza M., De Silva R., Tucker K. L., Barde Y. A., Duff K., Davies P. (2003). Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem, 86, 582–590. [DOI] [PubMed] [Google Scholar]
- Arciniegas D. B., Wortzel H. S. (2014). Emotional and behavioral dyscontrol after traumatic brain injury. Psychiatr Clin North Am, 37, 31–53. [DOI] [PubMed] [Google Scholar]
- Bajwa N. M., Halavi S., Hamer M., Semple B. D., Noble-Haeusslein L. J., Baghchechi M., Hiroto A., Hartman R. E., Obenaus A. (2016). Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PLoS One, 11, e0146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaya M. K., Rao A. M., Dogan A., Donaldson D., Dempsey R. J. (1997). The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett, 226, 33–36. [DOI] [PubMed] [Google Scholar]
- Bazarian J. J., Mcclung J., Shah M. N., Cheng Y. T., Flesher W., Kraus J. (2005). Mild traumatic brain injury in the United States, 1998–2000. Brain Inj, 19, 85–91. [DOI] [PubMed] [Google Scholar]
- Bennett R. E., Brody D. L. (2014). Acute reduction of microglia does not alter axonal injury in a mouse model of repetitive concussive traumatic brain injury. J Neurotrauma, 17, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta S., Cunningham K. M., Haus D. L., Gold E. M., Perez H., Lopez-Velazquez L., Cummings B. J. (2017). Effects of human ES-derived neural stem cell transplantation and kindling in a rat model of traumatic brain injury. Cell Transplant, 26(7), 1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Hall A. N., Joseph B., Brelsfoard J. M., Saatman K. E. (2016). Repeated closed head injury in mice results in sustained motor and memory deficits and chronic cellular changes. PLoS One, 11, e0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody D. L., Benetatos J., Bennett R. E., Klemenhagen K. C., Mac Donald C. L. (2015). The pathophysiology of repetitive concussive traumatic brain injury in experimental models; new developments and open questions. Mol Cell Neurosci, 66, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R. A., O'donnell M. L., Creamer M., Mcfarlane A. C., Clark C. R., Silove D. (2010). The psychiatric sequelae of traumatic injury. Am J Psychiatry, 167, 312–320. [DOI] [PubMed] [Google Scholar]
- Chabrier M. A., Cheng D., Castello N. A., Green K. N., Laferla F. M. (2014). Synergistic effects of amyloid-beta and wild-type human tau on dendritic spine loss in a floxed double transgenic model of Alzheimer’s disease. Neurobiol Dis, 64, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. W., Grindel S. H., Lovell M. R., Dede D. E., Moser D. J., Phalin B. R., Nogle S., Wasik M., Cordry D., Daugherty K. M., Sears S. F., Nicolette G., Indelicato P., Mckeag D. B. (1999. a). Relationship between concussion and neuropsychological performance in college football players. JAMA, 282, 964–970. [DOI] [PubMed] [Google Scholar]
- Collins M. W., Lovell M. R., Mckeag D. B. (1999. b). Current issues in managing sports-related concussion. JAMA, 282, 2283–2285. [DOI] [PubMed] [Google Scholar]
- Coronado V. G., Mcguire L. C., Sarmiento K., Bell J., Lionbarger M. R., Jones C. D., Geller A. I., Khoury N., Xu L. (2012). Trends in traumatic brain injury in the U.S. and the public health response: 1995-2009. J Safety Res, 43, 299–307. [DOI] [PubMed] [Google Scholar]
- Cubon V. A., Putukian M., Boyer C., Dettwiler A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma, 28, 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings B. J., Engesser-Cesar C., Cadena G., Anderson A. J. (2007). Adaptation of a ladder beam walking task to assess locomotor recovery in mice following spinal cord injury. Behav Brain Res, 177, 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher N. N., Najafi A. R., Kayala K. M., Elmore M. R., White T. E., Medeiros R., West B. L., Green K. N. (2015). Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-Ad mice. J Neuroinflammation, 12, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M., Xu L., Wald M. M., Coronado V. G. (2010). Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths 2002-2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Google Scholar]
- Flygt J., Clausen F., Marklund N. (2017). Diffuse traumatic brain injury in the mouse induces a transient proliferation of oligodendrocyte progenitor cells in injured white matter tracts. Restor Neurol Neurosci, 35, 251–263. [DOI] [PubMed] [Google Scholar]
- Franklin R. J., Ffrench-Constant C. (2008). Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci, 9, 839–855. [DOI] [PubMed] [Google Scholar]
- Gerberding J. L., Binder S. (2003). Report to congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Gold E. M., Su D., Lopez-Velazquez L., Haus D. L., Perez H., Lacuesta G. A., Anderson A. J., Cummings B. J. (2013). Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med, 8, 483–516. [DOI] [PubMed] [Google Scholar]
- Greve M. W., Zink B. J. (2009). Pathophysiology of traumatic brain injury. Mt Sinai J Med, 76, 97–104. [DOI] [PubMed] [Google Scholar]
- Haus D. L., Lopez-Velazquez L., Gold E. M., Cunningham K. M., Perez H., Anderson A. J., Cummings B. J. (2016). Transplantation of human neural stem cells restores cognition in an immunodeficient rodent model of traumatic brain injury. Exp Neurol, 281, 1–16. [DOI] [PubMed] [Google Scholar]
- Henderson V. C., Kimmelman J., Fergusson D., Grimshaw J. M., Hackam D. G. (2013). Threats to validity in the design and conduct of preclinical efficacy studies: A systematic review of guidelines for in vivo animal experiments. PLoS Med, 10, e1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring S. A., Cantu R. C., Guskiewicz K. M., Putukian M., Kibler W. B., Bergfeld J. A., Boyajian-O'neill L. A., Franks R. R., Indelicato P. A., & American College of Sports Medicine. (2011). Concussion (mild traumatic brain injury) and the team physician: A consensus statement–2011 update. Med Sci Sports Exerc, 43, 2412–2422. [DOI] [PubMed] [Google Scholar]
- Hicks R. R., Smith D. H., Lowenstein D. H., Saint Marie R., Mcintosh T. K. (1993). Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma, 10, 405–414. [DOI] [PubMed] [Google Scholar]
- Huang L., Coats J. S., Mohd-Yusof A., Yin Y., Assaad S., Muellner M. J., Kamper J. E., Hartman R. E., Dulcich M., Donovan V. M., Oyoyo U., Obenaus A. (2013). Tissue vulnerability is increased following repetitive mild traumatic brain injury in the rat. Brain Res, 1499, 109–120. [DOI] [PubMed] [Google Scholar]
- Iliff J. J., Chen M. J., Plog B. A., Zeppenfeld D. M., Soltero M., Yang L., Singh I., Deane R., Nedergaard M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci, 34, 16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson G. L., Brooks B. L., Collins M. W., Lovell M. R. (2006). Tracking neuropsychological recovery following concussion in sport. Brain Inj, 20, 245–252. [DOI] [PubMed] [Google Scholar]
- Johnson V. E., Meaney D. F., Cullen D. K., Smith D. H. (2015). Animal models of traumatic brain injury. Handb Clin Neurol, 127, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. E., Stewart J. E., Begbie F. D., Trojanowski J. Q., Smith D. H., Stewart W. (2013. a). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain, 136, 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. E., Stewart W., Smith D. H. (2013. b). Axonal pathology in traumatic brain injury. Exp Neurol, 246, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R. E., Robinson R. G., Moser D., Tateno A., Crespo-Facorro B., Arndt S. (2004). Major depression following traumatic brain injury. Arch Gen Psychiatry, 61, 42–50. [DOI] [PubMed] [Google Scholar]
- Kane M. J., Angoa-Pérez M., Briggs D. I., Viano D. C., Kreipke C. W., Kuhn D. M. (2012). A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods, 203, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B. B., Takada N., Latimer A. J., Shin J., Carney T. J., Kelsh R. N., Appel B. (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci, 9, 1506–1511. [DOI] [PubMed] [Google Scholar]
- Kraus M. F., Susmaras T., Caughlin B. P., Walker C. J., Sweeney J. A., Little D. M. (2007). White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain, 130, 2508–2519. [DOI] [PubMed] [Google Scholar]
- Langlois J. A., Sattin R. W. (2005). Traumatic brain injury in the United States: Research and programs of the centers for disease control and prevention (CDC). J Head Trauma Rehabil, 20, 187–188. [DOI] [PubMed] [Google Scholar]
- Laskowski R. A., Creed J. A., Raghupathi R. (2015). Pathophysiology of mild TBI: Implications for altered signaling pathways. In F. H. Kobeissy (Ed.), Brain neurotrauma: Molecular, neuropsychological, and rehabilitation aspects (pp. 35–42). Boca Raton, FL.
- Laurer H. L., Bareyre F. M., Lee V. M., Trojanowski J. Q., Longhi L., Hoover R., Saatman K. E., Raghupathi R., Hoshino S., Grady M. S., Mcintosh T. K. (2001). Mild head injury increasing the brain’s vulnerability to a second concussive impact. J Neurosurg, 95, 859–870. [DOI] [PubMed] [Google Scholar]
- Lister R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl), 92, 180–185. [DOI] [PubMed] [Google Scholar]
- Longhi L., Saatman K. E., Fujimoto S., Raghupathi R., Meaney D. F., Davis J., Mcmillan B. S. A., Conte V., Laurer H. L., Stein S., Stocchetti N., Mcintosh T. K. (2005). Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery, 56, 364–374; Discussion 364–374. [DOI] [PubMed] [Google Scholar]
- Ma H., Yu B., Kong L., Zhang Y., Shi Y. (2012). Neural stem cells over-expressing brain-derived neurotrophic factor (BDNF) stimulate synaptic protein expression and promote functional recovery following transplantation in rat model of traumatic brain injury. Neurochem Res, 37, 69–83. [DOI] [PubMed] [Google Scholar]
- Main B. S., Sloley S. S., Villapol S., Zapple D. N., Burns M. P. (2017). A mouse model of single and repetitive mild traumatic brain injury. J Vis Exp, 124. [DOI] [PMC free article] [PubMed]
- Malkesman O., Tucker L. B., Ozl J., Mccabe J. T. (2013). Traumatic brain injury – Modeling neuropsychiatric symptoms in rodents. Front Neurol, 4, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R., Meehan W. P., Mandeville J., Grant P. E., Gray T., Berglass J., Zhang J., Bryant J., Rezaie S., Chung J. Y., Peters N. V., Lee C., Tien L. W., Kaplan D. L., Feany M., Whalen M. (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol, 74, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee A. C., Cantu R. C., Nowinski C. J., Hedley-Whyte E. T., Gavett B. E., Budson A. E., Santini V. E., Lee H. S., Kubilus C. A., Stern R. A. (2009). Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol, 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckee C. A., Lukens J. R. (2016). Emerging roles for the immune system in traumatic brain injury. Front Immunol, 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan W. P., Zhang J., Mannix R., Whalen M. J. (2012). Increasing recovery time between injuries improves cognitive outcome after repetitive mild concussive brain injuries in mice. Neurosurgery, 71, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E., Moser M. B., Andersen P. (1993). Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci, 13, 3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., Crawford F. (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma, 29, 2761–2773. [DOI] [PubMed] [Google Scholar]
- Mouzon B. C., Bachmeier C., Ferro A., Ojo J. O., Crynen G., Acker C. M., Davies P., Mullan M., Stewart W., Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol, 75, 241–254. [DOI] [PubMed] [Google Scholar]
- Mul J. D., Zheng J., Goodyear L. J. (2016). Validity assessment of 5 day repeated forced-swim stress to model human depression in young-adult C57bl/6j and Balb/Cj mice. eNeuro, 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani N., Goswami R., Colella B., Khodadadi M., Ebraheem A., Davis K. D., Tator C. H., Wennberg R., Mikulis D. J., Ezerins L., Tartaglia M. C. (2016). The association between white-matter tract abnormalities, and neuropsychiatric and cognitive symptoms in retired professional football players with multiple concussions. J Neurol, 263(7), 1332–1341. [DOI] [PubMed] [Google Scholar]
- Namjoshi D. R., Cheng W. H., Mcinnes K. A., Martens K. M., Carr M., Wilkinson A., Fan J., Robert J., Hayat A., Cripton P. A., Wellington C. L. (2014). Merging pathology with biomechanics using chimera (closed-head impact model of engineered rotational acceleration): A novel, surgery-free model of traumatic brain injury. Mol Neurodegener, 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J. N., Deshane A. S., Niedzielko T. L., Smith C. D., Floyd C. L. (2016). Greater neurobehavioral deficits occur in adult mice after repeated, as compared to single, mild traumatic brain injury (mTBI). Behav Brain Res, 298, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo J. O., Mouzon B. C., Crawford F. (2016). Repetitive head trauma, chronic traumatic encephalopathy and tau: Challenges in translating from mice to men. Exp Neurol, 275(Pt 3), 389–404. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. (2005). Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology (Berl), 177, 245–255. [DOI] [PubMed] [Google Scholar]
- Petraglia A. L., Plog B. A., Dayawansa S., Chen M., Dashnaw M. L., Czerniecka K., Walker C. T., Viterise T., Hyrien O., Iliff J. J., Deane R., Nedergaard M., Huang J. H. (2014). The spectrum of neurobehavioral sequelae after repetitive mild traumatic brain injury: A novel mouse model of chronic traumatic encephalopathy. J Neurotrauma, 31, 1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M., Greco T., Alexander D., Giza C. C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis Model Mech, 6, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins M. L., Hales A., Reger M., Giza C. C., Hovda D. A. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci, 32, 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. (2004). Cell death mechanisms following traumatic brain injury. Brain Pathol, 14, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlackhansingh A. F., Brooks D. J., Greenwood R. J., Bose S. K., Turkheimer F. E., Kinnunen K. M., Gentleman S., Heckemann R. A., Gunanayagam K., Gelosa G., Sharp D. J. (2011). Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol, 70, 374–383. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Johnson N. J. (1995). Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav, 52, 297–303. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W., Langlois J. A., Thomas K. E., Xi Y. L. (2006). Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil, 21, 544–548. [DOI] [PubMed] [Google Scholar]
- Shear D. A., Tate M. C., Archer D. R., Hoffman S. W., Hulce V. D., Laplaca M. C., Stein D. G. (2004). Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res, 1026, 11–22. [DOI] [PubMed] [Google Scholar]
- Shenton M. E., Hamoda H. M., Schneiderman J. S., Bouix S., Pasternak O., Rathi Y., Vu M. A., Purohit M. P., Helmer K., Koerte I., Lin A. P., Westin C. F., Kikinis R., Kubicki M., Stern R. A., Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav, 6, 137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitaka Y., Tran H. T., Bennett R. E., Sanchez L., Levy M. A., Dikranian K., Brody D. L. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol, 70, 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardelly M., Gaber K., Burdack S., Scheidt F., Hilbig H., Boltze J., Forschler A., Schwarz S., Schwarz J., Meixensberger J., Schuhmann M. U. (2011). Long-term benefit of human fetal neuronal progenitor cell transplantation in a clinically adapted model after traumatic brain injury. J Neurotrauma, 28, 401–414. [DOI] [PubMed] [Google Scholar]
- Smith C., Gentleman S. M., Leclercq P. D., Murray L. S., Griffin W. S. T., Graham D. I., Nicoll, J. A. R. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol Appl Neurobiol, 39, 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler K. D., Alexander H., Vagni V., Holubkov R., Dixon C. E., Clark R. S., Jenkins L., Kochanek P. M. (2006). Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res, 1076, 216–224. [DOI] [PubMed] [Google Scholar]
- Statler K. D., Kochanek P. M., Dixon C. E., Alexander H. L., Warner D. S., Clark R. S., Wisniewski S. R., Graham S. H., Jenkins L. W., Marion D. W., Safar P. J. (2000). Isoflurane improves long-term neurologic outcome versus fentanyl after traumatic brain injury in rats. J Neurotrauma, 17, 1179–1189. [DOI] [PubMed] [Google Scholar]
- Tremblay S., Henry L. C., Bedetti C., Larson-Dupuis C., Gagnon J. F., Evans A. C., Theoret H., Lassonde M., De Beaumont L. (2014). Diffuse white matter tract abnormalities in clinically normal ageing retired athletes with a history of sports-related concussions. Brain, 137, 2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K., Laurer H., Mcintosh T., Praticò D., Martinez D., Leight S., Lee V. M. Y., Trojanowski J. Q. (2002). Repetitive mild brain trauma accelerates Aβ deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci, 22, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Gao J., Yang Q., Parsley M. O., Dunn T. J., Zhang L., Dewitt D. S., Denner L., Prough D. S., Wu P. (2012). Molecular mechanisms underlying effects of neural stem cells against traumatic axonal injury. J Neurotrauma, 29, 295–312. [DOI] [PubMed] [Google Scholar]
- Weckbach S., Neher M., Losacco J. T., Bolden A. L., Kulik L., Flierl M. A., Bell S. E., Holers V. M., Stahel P. F. (2012). Challenging the role of adaptive immunity in neurotrauma: Rag1(−/−) mice lacking mature B and T cells do not show neuroprotection after closed head injury. J Neurotrauma, 29, 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston C. N., Noel A., Neustadtl A., Parsadanian M., Barton D. J., Chellappa D., Wilkins T. E., Alikhani A. D., Zapple D. N., Villapol S., Planel E., Burns M. P. (2016). Dendritic spine loss and chronic white matter inflammation in a mouse model of highly repetitive head trauma. Am J Pathol, 186, 552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowicz M. W., Fisher A. M., Minaeva O., Goldstein L. E. (2017). Considerations for experimental animal models of concussion, traumatic brain injury, and chronic traumatic encephalopathy – These matters matter. Front Neurol, 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.