Abstract
Complications of insertable loop recorder (ILR) are rare. The ILR is a cardiac monitoring device placed subcutaneously in the left parasternal region. It is commonly used for continuous monitoring in patients with unexplained and recurrent episodes of palpitations and syncope. We report a rare complication of subacute breast implant rupture in a patient after ILR placement. To the best of our knowledge, this is the first such reported case in the literature.
Keywords: insertable loop recorder, implantable loop recorder, breast implant rupture
INTRODUCTION
The insertable loop recorder (ILR) is a device commonly used for continuous electrocardiographic monitoring in patients with unexplained syncope and suspected cardiac arrhythmias.1 Another indication is the detection of a possible underlying cause of cryptogenic strokes. With a battery life of about 3 years, the ILR stays subcutaneously under the chest wall throughout the monitoring period and helps detect infrequent paroxysmal cardiac events. It is a small device implanted in the left prepectoral and parasternal position under local anesthesia. Except for a few minor complications such as pain and infection at the incision site, short-term chest discomfort, and breast tissue sensitivity (in females), the ILR is a relatively safe device. It rarely causes any major complications during the intra- or post-procedure period. We hereby report a very rare complication of subacute breast implant rupture in a middle-aged woman. To the best of our knowledge, this is the first such case to be reported in the literature.
METHODS
We searched PubMed/Medline and Google Scholar for articles and case reports of breast implant rupture in patients with ILR implantation. The keywords used to conduct the relevant literature search, alone and/or in combination, were “insertable loop recorder,” “implantable loop recorder,” “breast implant rupture,” “safety,” and “complications.” According to our search, no similar cases have been reported.
CASE PRESENTATION
A 44-year-old woman with a history of smoking, atypical chest pain syndrome, and minimal nonobstructive coronary artery disease presented with symptoms of palpitations and recurrent unexplained syncope and presyncope. The other pertinent history was remarkable for bilateral surgical implantation of silicone breast implants for breast augmentation 2 years before her current presentation. After a 30-day event monitor failed to reveal any particular diagnosis, she underwent implantation of a Reveal LINQ (Medtronic) ILR that was placed subcutaneously in the left parasternal location under local anesthesia. Immediately after implantation, the patient underwent routine investigation to check the voltage signals. As a result, the device was readjusted to obtain the optimal signal intensity. After the procedure, the patient complained of mild incision and left breast discomfort and sensitivity for several days, which eventually resolved.
About 10 weeks later, the patient presented to the outpatient clinic with left breast discomfort and mild sensitivity associated with a slow, progressive flattening of the left breast contour over the last few weeks. Physical examination of the left breast was suspicious for a breast implant rupture presenting subacutely.
To confirm this suspected diagnosis, a computed tomography (CT) scan of the chest was ordered (Figure 1). It showed the abnormal and flattened contour of the left breast implant compared to the right, confirming the diagnosis of left breast implant rupture. The axial view of the chest also showed the position of the ILR in close contact with the left breast implant rupture site.
Figure 1.
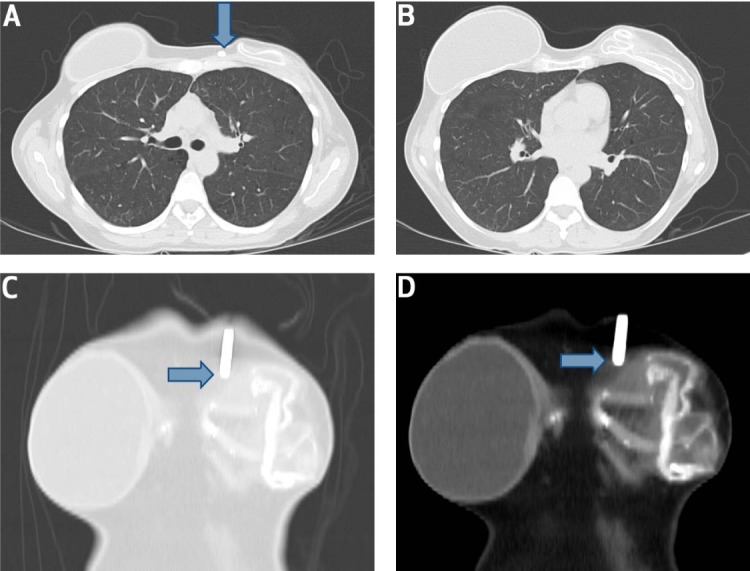
Computed tomography scan of the chest (axial view) in a patient with subacute left breast implant rupture. (A) An insertable loop recorder (ILR) adjoins the site of left breast implant rupture (blue arrow). (B) Axial view of chest shows a clear difference in the morphology of the two breast implants. (C, D) Frontal views reveal close contact of the IRL (blue arrows) with the ruptured left breast implant contour.
The patient was subsequently referred to see her breast surgeon, and reimplantation of a new breast implant was recommended.
DISCUSSION
The ILR is a small leadless box one-third the size of an AAA battery (~1 cc) that has two self-contained electrodes and is capable of generating single-lead electrocardiograms (ECGs). It functions by continuously recording and deleting the patient's ECG (hence, “loop”), with the ability for a particular segment to be “frozen” and stored through manual activation (with a hand-held activator) or automatic activation (when heart rate exceeds or falls below preprogrammed parameters for tachy- or bradycardia).1 It is placed in the left parasternal region in a small subcutaneous pocket created using local anesthesia. One of the two recommended locations positions the device 45° relative to the sternum over the fourth intercostal place, with the superior end of the device placed 2 cm lateral to the sternal border. The other position involves placing the device 2 cm lateral and parallel to the sternum over the fourth intercostal space. If these are not suitable, the ILR can also be inserted in the fifth intercostal space at 90° relative to the sternum, using preinsertion surface mapping for optimal insertion.
The ILR is particularly useful when the symptoms are infrequent or when aggregate long-term data (burden of AF) is required.2 It is used for diagnosing recurrent, unexplained palpitations; for long-term monitoring in patients with cryptogenic stroke or to monitor for arrhythmia recurrence (e.g., in patients with atrial fibrillation (AF) treated with rhythm control strategy); and for post–myocardial-infarction risk stratification in patients with certain genetic disorders.2 ILR use has been observed to be reasonably safe in patients.3 Apart from minor complications at the implantation site—such as bleeding or bruising, pain, infection at incision site, local tissue reaction, device migration, sensitivity of breast tissue (in females) and, rarely, poor R-wave sensing that necessitates device relocation—no major complications during the implantation procedure or follow-up have been reported.3,4
Our patient presented to the clinic reporting of symptoms consistent with a left breast implant rupture. Her presentation was subacute, and she described gradual development of pain/sensitivity and progressive flattening of the left breast contour that started a few weeks after the ILR implantation. Although most breast implant ruptures are spontaneous, trauma and a leak in the valve area or the implant base are some of the common causes.5 Risk factors for rupture include iatrogenic sources (e.g., damage by surgical instrument or excessive force to the chest—for example, during closed capsulotomy and compression during mammography), increasing implant age, seat belt contusion injury, blunt trauma, or severe capsular contracture.6 In this unusual case, iatrogenic rupture occurred when a central venous catheter was inadvertently placed into an implant, rupturing it after a unit of blood had been transfused into the prosthesis.7 Physical examination is considered inadequate for correctly evaluating implant rupture as it can detect only 30% of ruptures in asymptomatic patients. Between mammography, ultrasonography, CT, and magnetic resonance imaging (MRI), MRI is the most effective tool to diagnose a silicone breast implant rupture.8
The ILR is a small device implanted in a 1-day outpatient/inpatient procedure and has no associated major adverse events, which made it challenging to diagnose breast implant rupture. Because the patient probably had a rupture of the left breast implant, the possibility of routine life mechanical factors such as physical pressure comes into play. This mechanical stress can also play a critical role in exacerbating the repeated contact between the breast implant and the ILR, thus increasing the chances of rupture.
CONCLUSION
ILR implantation may be associated with a rare potential complication of artificial breast implant rupture. Fluoroscopic guidance during implantation may be helpful and should be considered to avoid this potential complication.
Footnotes
Conflict of Interest Disclosure:
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1.Chopra HK, Wander GS, Chandra P., Kumar V. Atrial Fibrillation Update: A Textbook of Cardiology. London: JP Medical Ltd; 2017. [Google Scholar]
- 2.Mittal M., Steinberg JS. Patient Remote Monitoring in Cardiology. New York: Demos Medical Publishing; 2012. [Google Scholar]
- 3.Solano A., Menozzi C., Maggi R. et al. Incidence, diagnostic yield and safety of the implantable loop-recorder to detect the mechanism of syncope in patients with and without structural heart disease. Eur Heart J. 2004 Jul;25(13):1116–9. doi: 10.1016/j.ehj.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Silveira I., Sousa MJ, Antunes N. et al. Efficacy and Safety of Implantable Loop Recorder: Experience of a Center. J Atr Fibrillation. 2016 Aug 31;9(2):1425. doi: 10.4022/jafib.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tark KC, Jeong HS, Roh TS, Choi JW. Analysis of 30 Breast Implant Rupture Cases. Aesthetic Plast Surg. 2005 Nov-Dec;29(6):460–9. doi: 10.1007/s00266-004-0146-x. [DOI] [PubMed] [Google Scholar]
- 6.Handel N., Garcia E., Wixtrom R. Breast Implant Rupture: Causes, Incidence, Clinical Impact, and Management. Plastic and Reconstructive Surgery. 2013 Nov;132(5):1128–37. doi: 10.1097/PRS.0b013e3182a4c243. [DOI] [PubMed] [Google Scholar]
- 7.Shoaib BO, Patten BM, Calkins DS. Adjuvant breast disease: an evaluation of 100 symptomatic women with breast implants or silicone fluid injections. Keio J Med. 1994 Jun;43(2):79–87. doi: 10.2302/kjm.43.79. [DOI] [PubMed] [Google Scholar]
- 8.Brown SL, Silverman BG, Berg WA. Rupture of silicone-gel breast implants: causes, sequelae, and diagnosis. Lancet. 1997 Nov 22;350(9090):1531–7. doi: 10.1016/S0140-6736(97)03164-4. [DOI] [PubMed] [Google Scholar]


