Abstract
Background
The mesocorticolimbic system is particularly susceptible to the effects of chronic alcoholism. Disruption of this system has been linked to drug seeking and the development of Reward Deficiency Syndrome, a neurobiological framework for describing the development and relapsing patterns of addictions. In this study, we evaluated the association of alcoholism and sex with major connections of the medial forebrain bundle (MFB), a prominent mesocorticolimbic fiber pathway connecting the ventral tegmental area with the basal forebrain. Given sex differences in clinical consequences of alcohol consumption, we hypothesized that alcoholic men and women would differ in structural abnormalities of the MFB.
Methods
Diffusion magnetic resonance imaging (dMRI) data were acquired from 30 abstinent long-term alcoholic individuals (ALC; 9 men) and 25 non-alcoholic controls (NC; 8 men). Major connections of the MFB were extracted using multi-tensor tractography. We compared groups on MFB volume, fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD), with hemisphere and sex as independent variables. We also evaluated associations between abnormal structural measures and drinking measures.
Results
Analyses revealed significant group-by-sex interactions for FA and RD: while ALC men had lower FA and higher RD compared to NC men, ALC women had higher FA and lower RD compared to NC women. We also detected a significant negative association between FA and number of daily drinks in ALC women.
Conclusion
Alcoholism is associated with sexually dimorphic structural abnormalities in the MFB. The results expand upon other findings of differences in brain reward circuitry of alcoholic men and women.
Keywords: Alcohol, Reward system, Medial forebrain bundle, Diffusion MRI, Sex, Drinking history
Highlights
-
•
Alcoholism is linked to sexually dimorphic changes in the medial forebrain bundle.
-
•
Alcoholic men had lower FA and higher RD compared to normal men controls.
-
•
Alcoholic women had higher FA and lower RD compared to normal women controls.
-
•
Higher number of daily drinks was associated with greater brain damage in women.
-
•
Findings suggest sex-specific vulnerability to alcohol-related brain damage.
1. Introduction
Alcohol abuse has adverse consequences to brain structure (Oscar-Berman and Marinković, 2007; Sullivan et al., 2010). The mesocorticolimbic system is a focus of neurobiology of drug addiction, and understanding its structural connectivity is critical in drug reward research. The major fiber pathway of this system is the medial forebrain bundle (MFB), a long ascending and descending fiber tract between the telencephalon and brain stem, mediating most of the brain's monoamine systems (Koob, 2013). The MFB is a primary part of the reward system.
The reward system includes the amygdala, hippocampus, nucleus accumbens (NAc), and ventral diencephalon (basal forebrain, ventral tegmental area [VTA], and hypothalamus), as well as cortical areas having modulatory and oversight roles such as dorsolateral-prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), cingulate cortex, subcallosal cortex, temporal pole, parahippocampal gyri, and insula (Barbas, 2000; Heimer and Van Hoesen, 2006). These structures have been called the extended reward and oversight system (EROS) (Makris et al., 2008). Thus, the MFB interconnects EROS structures essential in the neurocircuitry of addiction (Koob, 2013; Koob and Volkow, 2009), in particular, the VTA, the NAc, the OFC, and DLPFC (Coenen et al., 2012). Traditionally, the anatomy of the MFB has been characterized in rodents (Nieuwenhuys et al., 1982), although the precise anatomical boundaries of the MFB in humans remain unclear (Coenen et al., 2012; Gálvez et al., 2015). The MFB has been visualized in a small number of diffusion tensor imaging (DTI) studies (Coenen et al., 2012, Coenen et al., 2016; Hana et al., 2015). For example, Coenen et al. (2012) depicted the MFB as a bipartite structure that originates in the brainstem and projects anterior and superior of the VTA towards the NAc and the septum. At the level of the VTA, the MFB splits into an inferomedial and a superolateral component. The inferomedial MFB follows the walls of the third ventricle anteriorly until reaching the lateral hypothalamus. The superolateral MFB transverses the thalamus and intermingles with the anterior limb of the internal capsule, fanning out medially and laterally towards the OFC and DLPFC (Coenen et al., 2012).
The mesocorticolimbic system is susceptible to damage associated with alcohol use and abuse (Bowirrat and Oscar-Berman, 2005; Makris et al., 2008; Oscar-Berman and Bowirrat, 2005; Oscar-Berman et al., 2014; Sawyer et al., 2017). Disruption of mesocorticolimbic circuitry has been linked to the neurobiology of addictions (Volkow et al., 2016) and the development of Reward Deficiency Syndrome (Blum et al., 2008; Bowirrat and Oscar-Berman, 2005), a neurobiological framework implicated in the impulsive, compulsive, and relapsing patterns of addictions. According to this framework, dopamine depletion in reward-related regions prompts an individual to consume psychoactive substances and/or engage in behaviors aimed at overcoming a hypo-dopaminergic state (Blum et al., 2008). In acute phases, this results in an increase of dopamine in reward centers, followed by dysregulation of the reward cascade, and the subsequent augmentation and perpetuation of craving behaviors. Prior studies looking at abnormalities in the reward system in alcoholism have focused primarily on grey matter areas (Makris et al., 2008; Sawyer et al., 2017) and the cingulum bundle (Harris et al., 2008; Segobin et al., 2015). However, little is known about the relationship between alcoholism and damage to white matter fibers of the mesocorticolimbic system (Oscar-Berman et al., 2014), and the integrity of the MFB in particular has not been examined.
Normal functioning of the brain-reward cascade involves interaction among neurotransmitters such as dopamine, opioid peptides, norepinephrine, and GABA (Bowirrat and Oscar-Berman, 2005; Volkow et al., 2016). The MFB is a neurochemically heterogeneous structure that contains projections from serotonin, norepinephrine, and dopamine systems (Coenen et al., 2012; Koob and Volkow, 2009). The MFB connects midbrain areas with subcortical and cortical structures of the reward system (Blum et al., 2008; Koob and Volkow, 2009; Volkow et al., 2016) that are hypothesized to play distinctive roles in each stage of the addiction cycle (Volkow et al., 2016). During the binge/intoxication stage, drugs may stimulate regions within the NAc and the striatum to engage stimulus-response habits. In the withdrawal stage, a negative emotional state resulting from dopamine depletion and lack of the rewarding stimuli involves the amygdala, which plays a role in negative reinforcement. The preoccupation or craving stage may involve structures that together, lead addicts to re-engage reward-seeking habits: the amygdala, hippocampus, and frontal lobes, particularly OFC and cingulate cortex, which are hypothesized to contain representations of outcomes and subjective values (Volkow et al., 2016). Given that each stage of addiction incorporates the interaction of several structures and neurotransmitters, it is critical to understand the reward system in alcoholism at a network level.
Alcoholism-related brain abnormalities in reward system regions include a reduction in volume of cortical and subcortical structures, particularly in the DLPFC, NAc, anterior insula, and amygdala, as well as white matter microstructural abnormalities in the cingulum bundle (Harris et al., 2008; Segobin et al., 2015). However, these studies included only men, or they did not evaluate sex effects between alcoholics and controls, even though the consequences of alcohol consumption differ between men and women (Ruiz and Oscar-Berman, 2013). That is, evidence from structural neuroimaging studies revealed prominent alcohol-related structural deficits in men (Oscar-Berman and Song, 2011; Sawyer et al., 2016; Seitz et al., 2016), while in women, recovery of brain structural indices could occur earlier (Ruiz et al., 2012). Furthermore, in an investigation of reward network volumes in alcoholism, Sawyer et al. (2017) found significant differences between men and women, particularly in the DLPFC and the ventral diencephalon.
Brain white matter is particularly susceptible to alcohol-related brain damage, as observed in post-mortem (Harper et al., 2003; de la Monte, 1988; Sutherland et al., 2013) and neuroimaging studies (Pfefferbaum and Sullivan, 2004; Ruiz et al., 2012; Seitz et al., 2016). Diffusion magnetic resonance imaging (dMRI) is sensitive to the rate and direction of water permeability (Basser and Pierpaoli, 2011), enabling the study of white matter structural alterations in vivo. Although the precise anatomical characterization of several brain fiber tracts such as the MFB with dMRI has been hampered by considerable fiber crossing and branching within these tracts (Coenen et al., 2012; Döbrössy et al., 2015), recent developments in dMRI tractography have significantly improved our ability to enhance spatial resolution and to obtain a more anatomically-accurate view of neural pathways (Alexander et al., 2007; Malcolm et al., 2010).
In the present study, using filtered multi-tensor tractography (Malcolm et al., 2010), we reconstructed major connections of the MFB in abstinent long-term chronic alcoholic men and women and healthy non-alcoholic volunteers. The aims of this study were: (1) to determine the impact of chronic alcoholism on the structure of the MFB; (2) to evaluate sex differences in the patterns of abnormal structural connectivity of the MFB; and (3) to investigate associations of structural abnormalities in the MFB with measures of drinking history. We hypothesized that alcoholism would be associated with abnormal structure in the MFB, which would be reflected by smaller volume of the tract, lower fractional anisotropy (FA) and axial diffusivity (AD), and higher radial diffusivity (RD). Additionally, we hypothesized that the degree of damage would be negatively associated with the severity of drinking histories.
2. Methods
2.1. Participants
This study included 30 abstinent long-term alcoholic individuals (ALC; 9 men), and 25 demographically equivalent non-alcoholic healthy controls (NC; 8 men) (Table 1). Participants were recruited from the Department of Veterans Affairs (VA) Healthcare System Boston Campus and Boston University Medical Center, as well as from local newspaper advertisements in the Boston metropolitan area, and website postings. The study was approved by our Investigational Review Boards. Participants gave their informed consent, and they were compensated for their time.
Table 1.
Characteristics of the research participants.
| Men and women |
Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NC (n = 25) |
ALC (n = 30) |
NC (n = 8) |
ALC (n = 9) |
NC (n=17) |
ALC (n = 21) |
|||||
| mean ± SD | mean ± SD | P* | mean ± SD | mean ± SD | P* | mean ± SD | mean ± SD | P* | P** | |
| Age (years) | 56.6 ± 15.8 | 54.0±10.6 | 0.72 | 50.7 ± 17.8 | 55.6 ± 10.1 | 0.44 | 59.1 ± 14.9 | 53.2 ± 11.0 | 0.34 | 0.53 |
| Education (years) | 15.2 ± 2.6 | 15.1 ± 2.7 | 0.62 | 15.1 ± 2.5 | 14.3 ± 3.3 | 0.64 | 15.2 ± 2.7 | 15.3 ± 2.3 | 0.78 | 0.35 |
| WAIS-III IQ | 110 ± 11.9 | 109.0 ± 16.2 | 0.47 | 112 ± 8.8 | 102.8 ± 15.5 | 0.13 | 109.2 ± 13.1 | 111.8 ± 16.1 | 0.93 | 0.17 |
| DD (ounces EtOH/day) | 0.34 ± 0.5 | 9.9 ± 9.1 | <0.001 | 0.6 ± 0.6 | 15.2 ± 11.8 | 0.003 | 0.2 ± 0.3 | 7.5 ± 6.8 | <0.001 | 0.02 |
| DHD (years) | 0.2 ±0.8 | 14.4 ± 7.9 | <0.001 | 0.5 ± 1.4 | 18.11 ± 10.4 | <0.001 | 0.1 ± 0.5 | 12.8 ± 6.2 | <0.001 | 0.08 |
| LOS (years) | 4.0 ± 9.0 | 7.1 ± 8.6 | 0.22 | 0.8 ± 2.0 | 2.0 ± 3.4 | 0.37 | 5.4 ± 10.5 | 9.4 ± 9.3 | 0.30 | 0.04 |
NC: non-alcoholic controls; ALC: abstinent alcoholics; WAIS-III IQ: Wechsler Adult Intelligence Scale Full Scale IQ scores (Wechsler, 1997); DD: Daily Drinks; EtOH: ethanol; DHD: Duration of Heavy Drinking; LOS: Length of Sobriety. P* values from an independent sample t-test between NC and AL. P** values from an independent sample t-test between ALC men and women.
Participants were evaluated with handedness and drug use questionnaires, medical history interviews, and the Diagnostic Interview Schedule (Robins et al., 1989), which provides psychiatric diagnosis according to the American Psychiatric Association (APA, 1994). Inclusion criteria for ALC individuals were: alcohol consumption of a minimum of 21 drinks per week for at least five years during their lives, and alcohol abstinence of at least four weeks prior to testing. Participants were excluded for any of the following: English was not among their first languages; left-handedness; Korsakoff's syndrome; cirrhosis; Human Immunodeficiency Virus; major head injury with loss of consciousness > 15 minutes; seizure disorder unrelated to alcoholism; any psychotic disorder; recurrent Major Depressive Disorder; Bipolar II disorder; score in the Hamilton Depression Scale > 15; or history of drug abuse once per week or more within the previous five years.
Drinking patterns were evaluated with the Alcohol Use Questionnaire (Cahalan et al., 1969), which includes length of sobriety (LOS; years), duration of heavy drinking (DHD; years), and the ounces of ethanol per day (approximately the number of daily drinks; DD). The LOS indicates the period between the MRI scan date and the last day participants recalled having an alcoholic drink. The DHD refers to the total number of years participants drank more than 21 drinks per week (1 drink: 355 ml beer, 148 ml wine, or 44 ml of hard liquor). The DD score indicates the average daily alcohol consumed during the last six months (for NC), or to the six months preceding the cessation of drinking (for ALC participants). Our sample of ALC participants had severe drinking histories: DD mean = 9.9 ounces of ethanol per day; DHD mean = 14.4 years with >21 drinks per week. The mean LOS was 7.1 years (Table 1).
2.2. Imaging analysis
2.2.1. Image acquisition and preprocessing
Images were obtained on a 3-Telsa whole body MRI Trio scanner (Siemens Medical Solutions USA, Inc., Malvern, PA) with an 8-channel head coil, at Massachusetts General Hospital, Boston, MA. Diffusion weighted images were acquired using an echo planar image sequence with the following parameters: TR = 9800 ms; TE = 94 ms; 60 gradient directions with b = 700 s/mm2; 10 images with b = 0 s/mm2. Each volume consisted of 64 axial slices of 2 mm thickness, and an acquisition matrix of 128 × 128 in a field of view of 256 mm, resulting in isotropic voxels of 2 mm in each orthogonal plane. The pre-processing steps included motion and eddy current correction using an affine registration algorithm in FSL (http://www.fmrib.ox.ac.uk/fsl). Diffusion tensor images were estimated from the Diffusion-Weighted Images (DWI) in Slicer Version 4 (http://www.slicer.org), based on the weighted-least-squares estimation. A T1 MRI acquisition was also performed (field of view of 256 mm, 1 mm3 isotropic voxels). For each DWI image, we obtained 2-tensor whole-brain tractography using the multi-fiber tracking method of Malcolm et al. (2010).
2.2.2. Extraction of major connections of the MFB
We extracted MFB connections between the brain stem and the following structures: nucleus accumbens (NAc), orbitofrontal cortex (OFC) (lateral and medial), anterior cingulate cortex (anterior caudal and rostral), hippocampus, and amygdala (Fig. 1).
Fig. 1.
The associations between major connections of the medial forebrain bundle (MFB) and structures within the Extended Reward and Oversight System (EROS). Image A shows a diagram representing several of the brain regions involved in EROS: The dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) connect with the thalamus and hypothalamus. Cortical areas also connect with the nucleus accumbens (NAc) in the ventral striatum, the midbrain ventral tegmental area (VTA), and other limbic structures (limbic brain stem, amygdala, and hippocampus). Limbic structures are interconnected with the NAc and the basal forebrain. The VTA projects to the NAc, thalamus, and hypothalamus, and to prefrontal cortex. The NAc projects to the thalamus, which projects to the prefrontal cortex (modified from Makris et al., 2008, with permission). Image B, C, and D show a 3D rendering of one participant’s major connections of the MFB on axial and sagittal views of a diffusion-weighted image, and its associations with the brain regions of the EROS system.
To extract the MFB, we overlaid a parcellation of cortical and subcortical structures on the white matter of DWI images. First, we processed the T1 images of each subject using FreeSurfer (Desikan et al., 2006; Fischl et al., 2002). This resulted in cortical and subcortical parcellations as described by Desikan et al. (2006) and Salat et al. (2009). Accuracy of this parcellation was visually inspected by a trained scientist (AMRG) and supervised by our expert neuroanatomist (NM). Next, the T1 images, with cortical parcellations, were registered to the DWI images using the advanced normalization tools (ANTS) (Avants et al., 2008). In order to separate the brain from surrounding tissue, brain masks were obtained using the Multi-Atlas Brain Segmentation Tool (del Re et al., 2015).
The connections between the brain stem and reward-related structures were extracted using the White Matter Query Language (WMQL) (Wassermann et al., 2016), a semi-automated fiber delineation method that allows extraction of fiber tracts based on FreeSurfer labels. WMQL allows for selecting grey-matter regions as building blocks of queries to form “sets”, which represent ROIs in the brain (e.g., Accumbens_area.right for the right NAc), and “operators”, which represent the relationships between tracts and these sets (e.g., endpoints_in Accumbens_area.right) or between query results (e.g., endpoints_in Accumbens_area.right AND endpoints_in (Brain_Stem NOT IN hemisphere.left)).
2.2.3. Brain measures
For each tract under investigation, we calculated measures of FA, RD, AD, and tract volume. FA has been linked previously to overall white matter abnormalities (Basser and Pierpaoli, 2011), while RD and AD have been linked with myelin and axonal pathology respectively (Song et al., 2003).
2.3. Statistical analyses
Statistical analyses were performed using the Statistical Package for Social Sciences Version 23, and with R version 3.3.2 (see Supplementary dataset, and Supplementary code). To investigate the impact of alcoholism on white matter volume and diffusion measures, we conducted four separate mixed models, one for each brain measure (tract volume, FA, RD, and AD). We chose a mixed model technique because, unlike repeated measures analyses of covariance, it does not have limitations on assumptions regarding sphericity and balanced sample sizes. As white matter volume can vary with age and head size, we used normalized volume values (i.e., tract volume of each participant divided by total intracranial volume), and controlled for the participant's age in all statistical analyses. In each model, the ‘brain-measure’ was entered as the dependent variable, and ‘group’, ‘sex,’ and ‘hemisphere’ as the independent factors, and ‘age’ was entered as a covariate (all fixed effects). In order to account for the correlated data for the multiple observations per subject, individual subject effects were modeled specified as random intercepts. Thus, the effect of ‘hemisphere’ was evaluated as a within-subjects measure. Interactions with age were examined to confirm homogeneity of regression slopes; these interactions were not significant and therefore not included in the final model. The interactions of group-by-sex-by-hemisphere, group-by-sex, group-by-hemisphere, and sex-by-hemisphere were investigated.
The second goal of our study was to evaluate sex differences for alcoholism abnormalities in structural connectivity of the MFB. To test this, following significant group-by-sex interactions, we evaluated men and women with post-hoc comparisons, using Tukey correction for multiple comparisons. Only those brain variables with significant effects in the initial mixed models were included in these analyses. Because the ALC men had more severe drinking histories and shorter duration of abstinence than the ALC women, a subset of the group of ALC women (n = 9) was selected according to the highest number of DHD and DD and lowest LOS.
To investigate how alcohol-drinking history (DD, DHD, and LOS) impacted structural connectivity and tract volume, we first averaged the left and right hemisphere values (because the models described above did not reveal significant hemisphere differences, after Bonferroni correction). We then used Spearman’s correlations between brain variables with significant group and sex effects in relation to drinking variables. Spearman rank-order correlations were used instead of Pearson correlations, given that the drinking measures were not normally distributed (as evaluated with the Shapiro-Wilk Test).
3. Results
Our principal findings were as follows: (1) a significant group-by-sex interaction in MFB FA, which was driven by lower FA values in ALC men, (2) a significant group-by-sex interaction in RD, driven by higher RD values in ALC men, and (3) significant association between DD and lower FA values in ALC women.
3.1. Alcoholism was associated with sex dimorphic changes in FA and RD
The main effect of group was not significant for any dMRI measures (Table S1, Supplementary tables). There was a significant group-by-hemisphere interaction for Tract Volume (F(1,50) = 4.50, P = 0.04, which did not survive Bonferroni correction for four tests; corrected threshold P<0.0125). Nonetheless, the interaction indicated that the NC group had 0.005 larger right hemisphere MFB (% of head volume) than left hemisphere MFB (t(50) = 2.50, P = 0.01), while ALC participants showed no significant laterality effect (t(50) = 0.42, P = 0.67). Subsequently, we explored sex differences through the interaction of group-by-sex in the mixed models, followed by post-hoc analyses. We conducted post-hoc comparisons for men and women separately to evaluate group differences in those variables that showed a significant group-by-sex difference in the initial mixed model.
We found significant group-by-sex interactions in FA, F(1,50) = 8.07, P = 0.01, and RD, F(1,50) = 5.96, P = 0.02. The interaction predicting FA remained significant after Bonferroni correction for four tests (corrected threshold P < 0.0125). Post-hoc comparisons indicated ALC men had 0.08 lower FA than NC men, t(50) = 2.39, P = 0.02, and 0.10 higher RD, t(50) = 2.15, P = 0.04 (Table 2, Fig. 2). In ALC women compared with NC women, FA was 0.04 higher, t(50) = 1.57, P = 0.12, and RD was 0.04 lower, t(50) = 1.21, P = 0.23, displaying a trend in the opposite directions from ALC men vs. NC men (Table 2, Fig. 2). Because the ALC men had more severe drinking histories and shorter duration of abstinence than the ALC women, we compared FA and RD differences in a subset of ALC women (n = 9) with drinking patterns comparable to the ALC men. The findings were similar to the larger group analyses: The group-by-sex interaction for FA (P = 0.02) and RD (P = 0.07) (Table S2, Supplementary tables), revealed a similar pattern, with ALC women showing comparable relative values compared to NC women (FA was 0.02 higher and RD was 0.03 lower, opposite to the direction observed in ALC men versus NC men).
Table 2.
Descriptive statistics showing the mean and standard deviations of dMRI measures.
| Men |
Women |
|||
|---|---|---|---|---|
| ALC | NC | ALC | NC | |
| Mean FA | 0.54 (0.11) | 0.62 (0.03) | 0.58 (0.06) | 0.55 (0.07) |
| Mean RD | 0.53 (0.16) | 0.44 (0.05) | 0.47 (0.07) | 0.50 (0.08) |
| Mean AD | 1.34 (0.08) | 1.33 (0.06) | 1.32 (0.08) | 1.30 (0.09) |
| Mean volume | 0.016 (0.013) | 0.023 (0.013) | 0.017 (0.007) | 0.019 (0.01) |
ALC: abstinent alcoholics; NC: non-alcoholic controls; FA: fractional anisotropy; AD: axial diffusivity; RD: radial diffusivity. Volumes are presented as proportion of head size.
Fig. 2.
Group differences in FA and RD in alcoholic men and women for measures with significant group-by-sex effects. The boxes and whiskers represent the interquartile range and 2.5-97.5 percentiles, respectively, and the circles represent individual observations beyond the whiskers. ALC-M: Alcoholic men; NC-M: Non-alcoholic men; ALC-W: Alcoholic women; NC-W: Non-alcoholic women.
3.2. Higher number of daily drinks was associated with lower FA in ALC women
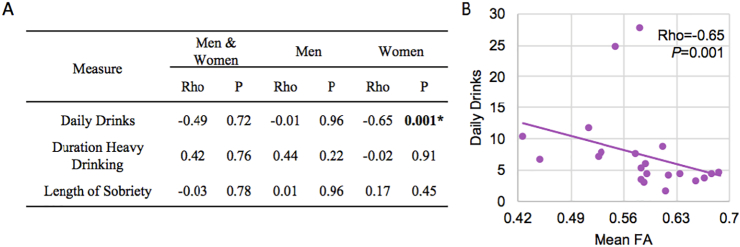
ALC men and women differed in DD (P = 0.02) and LOS (P = 0.04) (Table 1). To further evaluate the relationship between alcoholism and MFB structure, we correlated drinking variables (DHD, DD, and LOS) with FA in ALC men and women. There were no significant correlations in the ALC men. However, we found a significant negative correlation between the number of daily drinks and FA in ALC women, rho = −0.65, P = 0.001 (Fig. 3). This association remained significant after Bonferroni correction for nine tests (P = 0.005).
Fig. 3.
A) Correlations between fractional anisotropy (FA) and drinking measures. B) Significant correlation between mean FA and number of daily drinks in alcoholic women. *Indicates statistical significance of P<0.006 after Bonferroni correction for eight tests.
4. Discussion
The mesocorticolimbic system is critical in mediating the hedonic impact of alcohol consumption and in attributing incentive salience to reward alcohol-related stimuli (Berridge, 2007; Berridge et al., 2009; Blum et al., 2008; Davis et al., 2009; Robinson et al., 2005). In the present study, we used DTI tractography to delineate and extract principal connections of the MFB, a major reward-circuit pathway, in abstinent long-term ALC men and women. We tested the hypotheses that ALC individuals have abnormal structure in these pathways, and that the degree of abnormalities would be associated with measures of drinking histories. The group-by-sex interactions indicated that the FA and RD abnormalities were different for ALC men and women. That is, while ALC men had lower FA and higher RD compared to NC men, ALC women had a trend toward higher FA and lower RD compared to NC women. We also found that FA was negatively correlated with higher number of daily drinks in the group of ALC women.
4.1. Alcoholism and MFB Structure
Prior neuroimaging studies in alcoholism have demonstrated white matter abnormalities, specifically in cortical association fibers (Harris et al., 2008; Pfefferbaum et al., 2009; Seitz et al., 2016), the corpus callosum (Pfefferbaum et al., 2009; Ruiz et al., 2012), and mesencephalic fibers (Chanraud et al., 2009). The group differences in FA an RD in the MFB, are consistent with previous research showing structural abnormalities of the brain reward system in alcoholism. For example, Makris et al. showed that abstinent ALC subjects had grey matter volumetric deficits in the brain's extended reward and oversight system (EROS) (Makris et al., 2008). In a voxel-based analysis of DTI white matter, Harris at al. found alterations in the cingulum bundle and the white matter related to the OFC, which are also part of the EROS system (Harris et al., 2008). Furthermore, Segobin et al. reported white matter microstructural abnormalities in the cingulum bundle in a group of ALC individuals at risk for developing Korsakoff's syndrome (Segobin et al., 2015). More recently, Sawyer et al. not only confirmed the findings of Makris et al. (2008), but also showed that the volumetric abnormalities of the EROS network were different between men and women (Sawyer et al., 2017).
From a connectionist perspective, brain white matter fibers are important for the functional interaction of brain grey matter regions (Catani, 2005). Thus, disruption of brain structural connectivity may result in abnormal functioning, which would lead to the development and maintenance of maladaptive patterns of behavior. The FA and RD abnormalities observed in white matter pathways such as MFB, which interconnect critical portions of the brain reward network, support the concept of a Reward Deficiency Syndrome, i.e., that drugs of abuse cause a dysregulation in dopamine neurotransmission and a reduction of arousal and sensitivity to rewards (Blum et al., 2008; Bowirrat and Oscar-Berman, 2005). Specifically, we showed that white matter tracts interconnecting grey matter structures involved in the reward cascade and part of the EROS system (the brain stem, NAc, OFC, and cingulate cortex; see Fig. 1) are impacted in people with a history of alcoholism. These structures are rich in dopaminergic connections and are thought to mediate the hedonic impact of alcohol consumption and to attribute incentive salience to alcohol-related stimuli (Berridge, 2007; Berridge et al., 2009; Blum et al., 2008; Davis et al., 2009; Robinson et al., 2005).
4.2. Sexual dimorphism in MFB structure
The second goal of this study was to evaluate sex differences in the patterns of abnormal structural connectivity of the MFB. Congruent with prior studies of reward system structures, we found important group-by-sex interactions in FA and RD. ALC men had significantly decreased FA and increased RD relative to NC men, whereas ALC women showed reversed trends compared to NC women. This is consistent with other studies showing the existence of significant white matter structural abnormalities in alcoholic men but not in women in the middle longitudinal fascicle (Seitz et al., 2016), the cerebellum (Sawyer et al., 2016), and the corpus callosum (Ruiz et al., 2012). That is, while FA was lower in the group of ALC men compared to NC men, the opposite pattern was observed for ALC and NC women. Similarly, RD was elevated in the group of ALC men, but the pattern for ALC and NC women was reversed. This dimorphic pattern is congruent with the findings of Sawyer et al. (2017). They reported reduced total grey matter volume of the reward system and in the ventral diencephalon in the group of ALC men, but elevated values in ALC women, compared to NC groups.
Long-term alcohol abuse results in white matter damage, as reflected by a decrease in FA and an increase in RD, a decrease in white matter volume (Ruiz et al., 2012), as well as a reward volume reduction (Sawyer et al., 2017). Therefore, it is unlikely that long-term alcohol abuse results in increased white matter integrity (i.e., higher FA and lower RD). Also, despite the higher FA we observed in ALC women, the significant correlation between the number of daily drinks and lower FA found in this group is congruent with the notion that long-term alcohol use results in white matter damage of the reward system. Sawyer et al. (2017) suggested that women may have pre-existing larger reward volumes, reflected in our case as higher FA and lower RD, and that the observed dimorphism reflects sex-specific susceptibility to alcohol-related brain damage. This view is supported by preclinical models of addiction, which suggest that sex-specific differences in drug seeking behavior are due to differences in the stress response between female and male rats (Fox and Sinha, 2009). For example, female rats demonstrated greater hypothalamic-pituitary-adrenal (HPA) axis response to alcohol (Jury et al., 2017; Ogilvie and Rivier, 1996) and greater locomotor activity following cocaine exposure compared to male rats (Carroll et al., 2006). In humans, the patterns of stress response and drug seeking behavior vary according to the abused substance. In general, substance-abusing women show increased emotional sensitivity to stressful stimuli compared to men, which would translate into greater relapse vulnerability, the presence of comorbid affective disorders, and treatment outcome (Fox and Sinha, 2009).
Neuroimaging studies that examine sex differences in alcoholics' brain structure have yielded contradictory results, with some studies showing greater susceptibility in men (Ruiz et al., 2012; Sawyer et al., 2017, Sawyer et al., 2016; Seitz et al., 2016), other studies showing greater susceptibility in women (Hommer et al., 2001), and other studies not finding significant differences (Pfefferbaum et al., 2001). The results of the present study support the view that ALC men are either more vulnerable to alcohol-related brain damage or may have greater pre-morbid deficits compared with ALC women, congruent with Ruiz et al. (2012), Seitz et al. (2016) and Sawyer et al., 2017, Sawyer et al., 2016 Furthermore, a study of cortical thickness in adolescent binge drinkers reported similar ALC sex effects (Squeglia et al., 2012).
Another possible explanation of the sex differences observed in our sample is the difference in drinking behavior among ALC men and women. The number of daily drinks was significantly higher in the ALC men, suggesting higher rates of binge drinking and concomitant concentrations of blood alcohol levels, related to severe white matter deficits (McQueeny et al., 2009). Additionally, length of sobriety was shorter in the ALC men, thereby allowing for less brain structural improvement with abstinence (Ruiz et al., 2012).
It is important to note that studying the MFB in the context of alcoholism has implications for treatment approaches. For example, several nuclei within the trajectory of the MFB have been identified as targets for neuromodulation interventions (Döbrössy et al., 2015; Gálvez et al., 2015). Specifically, nonhuman animal studies on cocaine and nicotine addiction have showed that chronic deep brain stimulation of the NAc attenuates drug seeking behaviors (Liu et al., 2008; Pierce and Vassoler, 2013; Vassoler et al., 2013). Furthermore, remission of alcohol dependency symptoms has been observed in patients after stimulation of the NAc with deep brain stimulation (Heldmann et al., 2012; Kuhn et al., 2011, Kuhn et al., 2007; Müller et al., 2009).
4.3. Limitations
There are several limitations of this study. First, the small sample size, limited the capability for detecting significant associations between behavioral and diffusion measures in the group of ALC men. Thus, the small sample size may have reduced the power to detect a significant group-by-sex interaction for RD after Bonferroni correction. Second, ALC men had more severe drinking histories and shorter duration of abstinence than the ALC women. However, when we analyzed FA and RD differences in a subset of ALC women (n = 9) with drinking patterns comparable to the ALC men, the pattern of findings remained the same. Third, there is a strong comorbidity between cigarette smoking and alcohol abuse (Durazzo et al., 2013; Luhar et al., 2013), and cigarette smoking has been also associated with brain white matter abnormalities (Savjani et al., 2014; Zou et al., 2017). However, in this study, there were not sufficient data regarding smoking for additional analyses. The uneven numbers of men and women assigned to our subgroups were the result of selecting participants who had the same scanning parameters employed during our research at the time of the study. Importantly, the statistical method we employed was a mixed model technique, which does not have limitations regarding assumptions of sphericity and of balanced sample sizes. Another limitation is the large age range of the sample. While this may compromise the detection of significant effects, we were able to demonstrate the presence of significant differences in FA and RA in ALC men. We also believe that a heterogeneous sample is advantageous for external validity and generalizability of the findings. Finally, dMRI provides a means to study structural connectivity of the brain indirectly. This is because it is based on the diffusion pattern of water molecules, as restricted by cellular morphology, and thus is not reflective of a specific biological pathology. Therefore, the results of this study should be interpreted with caution. Nevertheless, the use of multi-tensor tractography in this study allowed us to trace tracts in regions where fibers cross one another, thus permitting a more anatomically accurate delineation of major connections of the MFB.
5. Conclusions
This study demonstrated that alcoholism is associated with structural abnormalities in major connections of the MFB, a primary pathway of the brain's reward circuitry. The findings also suggested sexual dimorphic abnormalities in the MFB. Specifically, ALC men had significantly lower FA and higher RD in MFB compared to healthy NC men, while the pattern for ALC and NC women was reversed. Furthermore, there was a significant association between drinking severity (number of DD) and lower FA in the ALC women. The significant group-by-sex interactions in FA and RD suggest sex-specific vulnerability to alcohol-related damage or gender dimorphic pre-morbid structure of the brain reward system. Whatever the nature and extent of sex differences in this system, it is important to note that studying the MFB in the context of alcoholism has important implications for treatment approaches and as targets for neuromodulation interventions (Döbrössy et al., 2015; Gálvez et al., 2015).
Acknowledgments
Acknowledgements
This study was supported by funds from: the National Institute on Alcohol Abuse and Alcoholism grants R01AA07112 (MOB), K05AA00219 (MOB); a U.S. Department of Veterans Affairs Clinical Science Research and Development grant I01-CX000326 (MOB); the National Institute on Aging grant R01AG042512 (MK and NM); the National Center for Complementary and Integrative Health grant R21AT008865 (NM and MK); the National Institute of Drug Abuse grant R21DA042271 (NM); the National Institute of Mental Health grants R01MH111917 (NM), R01MH102377 (MK); the National Institute of Neurological Disorders and Stroke grants R21NS077059 (NM), R21NS079905 (NM); and the Center for Functional Neuroimaging Technologies grant P41RR14075 from the National Center for Research Resources (now called National Center for Advancing Translational Sciences). We thank Diane Merritt, Trinity Urban, and Maria Valmas for assistance with recruitment, assessment, or neuroimaging of the research participants. The Boston University Clinical and Translational Science Institute provided consultation for statistical analyses (1UL1-TR001430). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, or the United States Government.
Financial disclosures
None of the authors report biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.03.025.
Appendix A. Supplementary data
Supplementary tables.
Supplementary dataset.
Supplementary code.
References
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th Ed. Washington; DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Avants B., Epstein C., Grossman M., Gee J. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res. Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Analysis of partial volume effects in diffusion-tensor MRI. J. Magn. Reson. 2011;213:560–570. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K., Chen A., Chen T.J., Braverman E.R., Reinking J., Blum S.H., Cassel K., Downs B.W., Waite R.L., Williams L., Prihoda T.J., Kerner M.M., Palomo T., Comings D.E., Tung H., Rhoades P., Oscar-Berman M. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theory Biol. Med. Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowirrat A., Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and reward deficiency syndrome. Am. J. Med. Genet. 2005;132B:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Cahalan D., Cisin I.H., Crossley H.M. 1969. American Drinking Practices: A National Study of Drinking Behavior and Attitudes (Monograph no. 6). Publications Division, Rutgers Center of Alcohol Studies. New Brunswick, NJ. [Google Scholar]
- Carroll M.E., Anderson M.M., Morgan A.D. Regulation of intravenous cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacology. 2006;190:331–341. doi: 10.1007/s00213-006-0600-3. [DOI] [PubMed] [Google Scholar]
- Catani M. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Chanraud S., Reynaud M., Wessa M., Penttilä J., Kostogianni N., Cachia A., Artiges E., Delain F., Perrin M., Aubin H.-J., Cointepas Y., Martelli C., Martinot J.-L. Diffusion tensor tractography in mesencephalic bundles: relation to mental flexibility in detoxified alcohol-dependent subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Panksepp J., Hurwitz T.A., Urbach H., Mädler B. Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. J. Neuropsychiatry Clin. Neurosci. 2012;24:223–236. doi: 10.1176/appi.neuropsych.11080180. [DOI] [PubMed] [Google Scholar]
- Coenen V.A., Schlaepfer T.E., Goll P., Reinacher P.C., Voderholzer U., Tebartz van Elst L., Urbach H., Freyer T. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 2016;22:282–289. doi: 10.1017/S1092852916000286. [DOI] [PubMed] [Google Scholar]
- Davis C.A., Levitan R.D., Reid C., Carter J.C., Kaplan A.S., Patte K.A., King N., Curtis C., Kennedy J.L. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity. 2009;91:432–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Döbrössy M.D., Furlanetti L.L., Coenen V.A. Electrical stimulation of the medial forebrain bundle in pre-clinical studies of psychiatric disorders. Neurosci. Biobehav. Rev. 2015;49:32–42. doi: 10.1016/j.neubiorev.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Durazzo T.C., Pennington D.L., Schmidt T.P., Mon A., Abé C., Meyerhoff D.J. Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcohol. Clin. Exp. Res. 2013;37:1794–1803. doi: 10.1111/acer.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fox H.C., Sinha R. Sex differences in drug-related stress-system changes: implications for treatment in substance-abusing women. Harvard Rev. Psychiatry. 2009;17:103–119. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez J.F., Keser Z., Mwangi B., Ghouse A.A., Fenoy A.J., Schulz P.E., Sanches M., Quevedo J., Selvaraj S., Gajwani P., Zunta-Soares G., Hasan K.M., Soares J.C. The medial forebrain bundle as a deep brain stimulation target for treatment resistant depression: a review of published data. Progr. Neuropsychopharmacol. Biol. Psychiatry. 2015;58:59–70. doi: 10.1016/j.pnpbp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Hana A., Hana A., Dooms G., Boecher-Schwarz H., Hertel F. Visualization of the medial forebrain bundle using diffusion tensor imaging. Front Neuroanat. 2015;9 doi: 10.3389/fnana.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C., Dixon G., Sheedy D., Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Progr. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Harris G.J., Jaffin S.K., Hodge S.M., Kennedy D., Caviness V.S., Marinković K., Papadimitriou G.M., Makris N., Oscar-Berman M. Frontal white matter and cingulum diffusion tensor imaging deficits in alcoholism. Alcohol. Clin. Exp. Res. 2008;32:1001–1013. doi: 10.1111/j.1530-0277.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L., Van Hoesen G.W. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci. Biobehav. Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Heldmann M., Berding G., Voges J., Bogerts B., Galazky I., Müller U., Baillot G., Heinze H.-J., Münte T.F. Deep brain stimulation of nucleus accumbens region in alcoholism affects reward processing. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0036572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D.W., Momenan R., Kaiser E., Rawlings R.R. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- Jury N.J., DiBerto J.F., Kash T.L., Holmes A. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol. 2017;58:53–60. doi: 10.1016/j.alcohol.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F. Addiction is a reward deficit and stress surfeit disorder. Front. Psychiatry. 2013;4:1–18. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J., Lenartz D., Huff W., Lee S., Koulousakis A., Klosterkoetter J., Sturm V. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J. Neurol. Neurosurg. Psychiatry. 2007;78:1152–1153. doi: 10.1136/jnnp.2006.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J., Gründler T.O.J., Bauer R., Huff W., Fischer A.G., Lenartz D., Maarouf M., Bührle C., Klosterkötter J., Ullsperger M., Sturm V. Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict. Biol. 2011;16:620–623. doi: 10.1111/j.1369-1600.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- Liu H.-Y., Jin J., Tang J.-S., Sun W.-X., Jia H., Yang X.-P., Cui J.-M., Wang C.-G. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict. Biol. 2008;13:40–46. doi: 10.1111/j.1369-1600.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- Luhar R.B., Sawyer K.S., Gravitz Z., Ruiz S.M., Oscar-Berman M. Brain volumes and neuropsychological performance are related to current smoking and alcoholism history. Neuropsychiatric Dis. Treat. 2013;9:1767–1784. doi: 10.2147/NDT.S52298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Oscar-Berman M., Jaffin S.K., Hodge S.M., Kennedy D.N., Caviness V.S., Marinković K., Breiter H.C., Gasic G.P., Harris G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm J.G., Shenton M.E., Rathi Y. Filtered multitensor tractography. IEEE Trans. Med. Imaging. 2010;29:1664–1675. doi: 10.1109/TMI.2010.2048121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T., Schweinsburg B.C., Schweinsburg A.D., Jacobus J., Bava S., Frank L.R., Tapert S.F. Altered white matter integrity in adolescent binge drinkers. Alcohol. Clin. Exp. Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S.M. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Müller U.J., Sturm V., Voges J., Heinze H.J., Galazky I., Heldmann M., Scheich H., Bogerts B. Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry. 2009;42:288–291. doi: 10.1055/s-0029-1233489. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R., Geeraedts L.M., Veening J.G. The medial forebrain bundle of the rat. I. General introduction. J. Comp. Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Ogilvie K.M., Rivier C. Gender difference in alcohol-evoked hypothalamic-pituitary-adrenal activity in the rat: ontogeny and role of neonatal steroids. Alcohol. Clin. Exp. Res. 1996;20:255–261. doi: 10.1111/j.1530-0277.1996.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M., Bowirrat A. Genetic influences in emotional dysfunction and alcoholism-related brain damage. Neuropsychiatric Dis. Treat. 2005;1:211–229. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M., Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol. Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M., Song J. Brain volumetric measures in alcoholics: a comparison of two segmentation methods. Neuropsychiatr. Dis. Treat. 2011;7:65–75. doi: 10.2147/NDT.S13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M., Valmas M.M., Sawyer K.S., Ruiz S.M., Luhar R.B., Gravitz Z.R. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. In: Pfefferbaum A., Sullivan E.V., editors. Alcohol and the Nervous System, Handbook of Clinical Neurology. Elsevier; 2014. pp. 183–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Sullivan E.V. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2004;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Deshmukh A., Sullivan E.V. Sex differences in the effects of alcohol on brain structure. Am. J. Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rosenbloom M., Rohlfing T., Sullivan E.V. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol. Psychiatry. 2009;65:680–690. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce R.C., Vassoler F.M. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology. 2013;229:487–491. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Re E.C., Gao Y., Eckbo R., Petryshen T.L., Blokland G.A.M., Seidman L.J., Konishi J., Goldstein J.M., McCarley R.W., Shenton M.E., Bouix S. A new MRI masking technique based on multi-atlas brain segmentation in controls and schizophrenia: a rapid and viable alternative to manual masking. J. Neuroimaging. 2015;26:28–36. doi: 10.1111/jon.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins L., Helzer J., Cottler L., Goldring E. 1989. NIMH Diagnostic Interview Schedule: Version III Revised (DIS-III-R). St. Louis, MO. [Google Scholar]
- Robinson S., Sandstrom S.M., Denenberg V.H., Palmiter R.D. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav. Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Ruiz S.M., Oscar-Berman M. Closing the gender gap: the case for gender-specific alcoholism research. J. Alcohol. Drug Depend. 2013;1 doi: 10.4172/2329-6488.1000e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz S.M., Oscar-Berman M., Sawyer K.S., Valmas M.M., Urban T., Harris G.J. Drinking history associations with regional white matter volumes in alcoholic men and women. Alcohol. Clin. Exp. Res. 2012;37:110–122. doi: 10.1111/j.1530-0277.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D., Greve D., Pacheco J., Quinn B., Helmer K., Buckner R., Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. NeuroImage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savjani R.R., Velasquez K.M., Thompson-Lake D.G.Y., Baldwin P.R., Eagleman D.M., Garza La, De R., II, Salas R. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend. 2014;145:134–142. doi: 10.1016/j.drugalcdep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Sawyer K.S., Oscar-Berman M., Mosher Ruiz S., Gálvez D.A., Makris N., Harris G.J., Valera E.M. Associations between cerebellar subregional morphometry and alcoholism history in men and women. Alcohol. Clin. Exp. Res. 2016;40:1262–1272. doi: 10.1111/acer.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer K.S., Oscar-Berman M., Barthelemy O.J., Papadimitriou G.M., Harris G.J., Makris N. Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Res. Neuroimaging. 2017;263:15–25. doi: 10.1016/j.pscychresns.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segobin S., Ritz L., Lannuzel C., Boudehent C., Vabret F., Eustache F., Beaunieux H., Pitel A.-L. Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Hum. Brain Mapp. 2015;36:2795–2808. doi: 10.1002/hbm.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz J., Sawyer K.S., Papadimitriou G., Oscar-Berman M., Ng I., Kubicki A., Mouradian P., Ruiz S.M., Kubicki M., Harris G.J., Makris N. Alcoholism and sexual dimorphism in the middle longitudinal fascicle: a pilot study. Brain Imaging Behav. 2016;35:1–12. doi: 10.1007/s11682-016-9579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ju W.-K., Lin S.-J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Squeglia L.M., Sorg S.F., Schweinsburg A.D., Wetherill R.R., Pulido C., Tapert S.F. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology. 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E.V., Harris R.A., Pfefferbaum A. Alcohol's effects on brain and behavior. Alcohol Res. Health. 2010;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Sutherland G.T., Sheedy D., Kril J.J. Using autopsy brain tissue to study alcohol-related brain damage in the genomic age. Alcohol. Clin. Exp. Res. 2013;38:1–8. doi: 10.1111/acer.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler F.M., White S.L., Hopkins T.J., Guercio L.A., Espallergues J., Berton O., Schmidt H.D., Pierce R.C. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J. Neurosci. 2013;33:14446–14454. doi: 10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Koob G.F., McLellan A.T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann D., Makris N., Rathi Y., Shenton M., Kikinis R., Kubicki M., Westin C.-F. The white matter query language: A novel approach for describing human white matter anatomy. Brain Struct. Funct. 2016;221:4705–4721. doi: 10.1007/s00429-015-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 3rd Ed. Psychological Corporation; San Antonio, TX: 1997. Wechsler Adult Intelligence Scale. [Google Scholar]
- Zou Y., Murray D.E., Durazzo T.C., Schmidt T.P., Murray T.A., Meyerhoff D.J. Effects of abstinence and chronic cigarette smoking on white matter microstructure in alcohol dependence: diffusion tensor imaging at 4T. Drug Alcohol Depend. 2017;175:42–50. doi: 10.1016/j.drugalcdep.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.
Supplementary dataset.
Supplementary code.