Abstract
Background
Huntington's disease (HD) is characterized by motor and behavioral symptoms, and cognitive decline. HD gene carriers and their caregivers report the behavioral and cognitive symptoms as the most burdensome. Apathy is the most common behavioral symptom of HD and is related to clinical measures of disease progression, like functional capacity. However, it is unknown whether apathy is directly related to the neurodegenerative processes in HD.
Objective
The aim is to investigate whether an association between atrophy of subcortical structures and apathy is present in HD, at baseline and after 2 years follow-up.
Method
Volumes of 7 subcortical structures were measured using structural T1 MRI in 171 HD gene carriers of the TRACK-HD study and apathy was assessed with the Problem Behaviors Assessment-Short, at baseline and follow-up visit. At baseline, logistic regression was used to evaluate whether volumes of subcortical brain structures were associated with the presence of apathy. Linear regression was used to assess whether subcortical atrophy was associated with the degree of apathy at baseline and with an increase in severity of apathy over time.
Results
At baseline, smaller volume of the thalamus showed a higher probability of the presence of apathy in HD gene carriers, but none of the subcortical structures was associated with the degree of apathy. Over time, no association between atrophy of any subcortical structures and change in degree of apathy was found.
Conclusion
The presence of apathy is associated with atrophy of the thalamus in HD, suggesting that apathy has an underlying neural cause and might explain the high incidence of apathy in HD. However, no association was found between atrophy of these subcortical structures and increase in severity of apathy over a 2-year time period.
Keywords: Apathy, Huntington's disease, Subcortical structures, Thalamus
Highlights
-
•
Apathy is associated with atrophy of the thalamus in HD.
-
•
Apathy is already present in the early stages of HD.
-
•
Severity of apathy does not drastically increase over a time period of 2 years.
1. Introduction
Huntington's disease (HD) is an autosomal dominant inherited, progressive neurodegenerative disorder, characterized by motor and behavioral symptoms, and cognitive decline (Roos, 2010). Despite motor symptoms being the most specific to HD, the highest burden reported by HD gene carriers and caregivers are the cognitive and behavioral symptoms (Hamilton et al., 2003). Behavioral symptoms are diverse and the degree of severity fluctuates for the majority of symptoms throughout disease progression (Thompson et al., 2012; van Duijn et al., 2007). The most common behavioral symptoms are depressive mood, irritability, and apathy with a prevalence varying between 33% to 76% for each symptom dependent on definition, measurement tools used, and disease stage (van Duijn et al., 2007). Of these symptoms, apathy is the only behavioral symptom that worsens as the disease progresses (Thompson et al., 2012; Martinez-Horta et al., 2016a; Tabrizi et al., 2013). In general, apathy has clinically been defined as “a disorder of diminished motivation, as manifested by reduced goal oriented behavior, emotions, and cognitions” (Starkstein and Leentjens, 2008) and has a strong influence on psychosocial functioning, including relationships with partners and caregivers, e.g. apathetic individuals need to be prompted into starting daily tasks such as getting dressed (Leroi et al., 2012; Aubeeluck et al., 2012).
In HD, apathy can develop early in the course of the disease (Thompson et al., 2012; Kingma et al., 2008) and can even be mildly present in pre-motormanifest gene carriers (Martinez-Horta et al., 2016a; Tabrizi et al., 2009). Over the course of the disease, apathy worsens and eventually apathy is severely present in almost all late stage gene carriers (Thompson et al., 2012). In addition, apathy itself is negatively related to functional capacity, cognitive performance and motor impairment in HD (Thompson et al., 2002). To better understand this behavioral symptom it is of interest to investigate the presence, severity and course of apathy in relation to the structural neurodegenerative processes that occur in HD.
Previous research has shown that apathy is caused by an interruption of the prefrontal cortex – basal ganglia circuit (Levy, 2012), specifically the anterior cingulate circuit in the brain (Haegelen et al., 2009; Tekin and Cummings, 2002). This circuit functionally connects the anterior cingulate cortex, nucleus accumbens, olfactory tubercle, and the ventromedial parts of the caudate nucleus and ventral putamen (Tekin and Cummings, 2002). In subcortical neurodegenerative diseases, such as Parkinson's disease and progressive supranuclear palsy, there is evidence that atrophy of the basal ganglia results in apathy (Haegelen et al., 2009; Cummings, 1993). One study showed that the nucleus accumbens, an important subcortical structure of the reward circuit (Riba et al., 2008), is associated with apathy in Parkinson's disease (Martinez-Horta et al., 2016b). In HD, it is not clear whether the same or other structures are related to apathy. Since degeneration of the basal ganglia is a key feature of HD, it is likely that these structures are associated with the occurrence of apathy in HD.
Dependent on disease stage, grey matter atrophy can be found in almost all grey matter structures in HD (Tabrizi et al., 2013; Aylward et al., 2011; Hobbs et al., 2010). The caudate nucleus is known to already show atrophy in pre-motor manifest HD gene carriers, far from estimated disease onset (Aylward et al., 2011; Douaud et al., 2006; Paulsen et al., 2006; Thieben et al., 2002) and also shows the highest rate of degeneration as the disease progresses (Tekin and Cummings, 2002; Bohanna et al., 2008; Georgiou-Karistianis et al., 2013; Montoya et al., 2006), followed by the putamen (Tabrizi et al., 2009; Aylward et al., 1996; Paulsen et al., 2008; Vonsattel et al., 1985). Volume loss of the nucleus accumbens is already present in the late pre-motormanifest stage (van den Bogaard et al., 2011). It is expected that volume loss of subcortical structures of the anterior cingulate circuit will be related to the development of apathy in HD patients.
Given the progressive nature of apathy and its close relationship with measures of disease progression such as a decrease of cognitive function (van Duijn et al., 2010), and general functioning (Thompson et al., 2012), it is possible that apathy is related to a neurodegenerative progress of subcortical grey matter in HD. Therefore, the aim of this study is to investigate the relationship between volume loss of subcortical structures and apathy in HD and whether there are changes over time.
2. Methods
2.1. Participants
TRACK-HD was a multicenter, longitudinal, observational study conducted at 4 different sites in the following cities: Vancouver (Canada), Paris (France), London (United Kingdom), and Leiden (the Netherlands). Of the 222 TRACK-HD participants, a total of 171 HD gene carriers (91 pre-motormanifest HD gene carriers and 80 motormanifest HD gene carriers) completed the baseline and follow-up visit after 24 months and were included in this study. HD gene carriers had a confirmed genetic testing, i.e. CAG ≥ 39. HD gene carriers with no substantial motor signs at baseline, as indicated with a total motor score (TMS) of ≤5 on the Unified Huntington's Disease Rating Scale (UHDRS), were defined as pre-motormanifest gene carriers. This pre-motormanifest group was further divided into ‘far from estimated disease onset’ (PreHD-A: >10.8 years) and ‘close to estimated disease onset’ (PreHD-B: <10.8 years), as calculated by the Langbehn formula (Langbehn et al., 2004). The group consisting of motormanifest HD gene carriers, as defined by a TMS of >5, was further divided into disease stage 1 and disease stage 2 based on the Total Functional Capacity (TFC) score (Shoulson and Fahn, 1979). All participating sites acquired ethical approval and all participants gave written informed consent prior study procedures. The study was conducted by trained professionals and all data was monitored, for a full description of the study, see Tabrizi et al. (Tabrizi et al., 2009).
2.2. Clinical measures
In addition to the collection of general sociodemographic and clinical characteristics, the short version of the Problem Behaviors Assessment (PBA-s) was administered. This is a semi-structured psychiatric interview designed for HD. The PBA-s consists of 11 items, each item measuring a different behavioral symptom such as apathy, depression and irritability. The PBA-s rates each behavioral symptom for both severity and frequency on a 5-point scale (Callaghan et al., 2015). Severity score ranges from absent (score 0) to severe (score 4) and frequency score ranges from absent (score 0) to every day/all day (score 4). In this study, both the product score of severity and frequency of the apathy item, and only the severity score of the apathy item were used.
In this study two concepts were evaluated: the degree of apathy and the presence of apathy (i.e. apathy is or is not present). To indicate the degree of apathy the product score of the apathy item is used. To indicate whether apathy is present a cut-off of ≥2 on only the severity apathy item was used.
2.3. MRI acquisition and processing
All participants underwent 3T MRI scanning at baseline and after 24 months follow-up on a Siemens or Philips whole body scanner depending on study site. 3D-T1-weighted image volumes were acquired with the following imaging parameters, as reported in the supplementary appendix in Tabrizi et al. (2009): TR = 2200 ms (Siemens)/7.7 ms (Philips), TE = 2.2 ms (Siemens)/3.5 ms (Philips), FA = 10° (Siemens)/8° (Philips), FOV = 28 cm (Siemens)/24 cm (Philips), matrix size 256 × 256 (Siemens)/224 × 224 (Philips), 208 (Siemens)/164 (Philips), sagittal slices to cover the entire brain with a slice thickness of 1.0 mm with no gap between slices.
Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) (Smith et al., 2004) was used for analyzing the structural T1-weighted images. Combined left and right volumes of the following seven subcortical brain regions were measured: nucleus accumbens, amygdala, caudate nucleus, hippocampus, pallidum, putamen, and thalamus, using FMRIB's Integrated Registration Segmentation Tool (FIRST) (Patenaude et al., 2011). All non-brain tissue was first removed from the T1-weighted image using a semi-automated brain extraction tool (BET), implemented in FSL (Smith, 2002). All images were registered to the Montreal Neurological Institute (MNI) 152-space standard image, using linear registration with 12° of freedom (Jenkinson et al., 2002). Then, segmentation of the seven subcortical regions was carried out and volumes for each region were calculated. Visual inspection was performed during the registration and segmentation steps. The volumes of these brain regions were corrected for estimated brain tissue volume, normalized for individual head size using SIENAX in FSL (Smith et al., 2002).
2.4. Statistics
To assess whether there were differences in the group characteristics at baseline an ANOVA or, when appropriate, a chi-square test was used.
The following groups of medication were identified to have a possible effect on the apathy scores: SSRIs, SNRIs, anti-psychotics, tricyclic antidepressants, buproprion, benzodiazepines, anti-epileptic, and tetrabenazine. One binary variable was created to indicate whether any of these medications were taken during the visit. We acknowledge that several different acting agents were treated as if they would have the same effect on apathy. Therefore, each model was run with and without the variable medication to identify the impact of medication on apathy.
A linear regression model between each subcortical brain structure and apathy product score was developed to investigate a possible association between volume of these structures and degree of apathy. As a next step a binary logistic regression between each subcortical brain structure and presence of apathy (i.e. apathetic versus not apathetic) was developed. Both regression models accounted for gender, medication use, group, age, study site and CAG length. As a last step depressive mood (severity ∗ frequency) was added as additional covariate.
To explore the relationship between apathy and volume loss over time, delta scores for apathy product score and delta scores for each brain structure were calculated to indicate change over time. For each subcortical brain structure, a linear regression model was designed to examine an association between delta score of apathy and delta score of the subcortical brain structures. Again, the model accounted for gender, medication use at baseline and follow-up, group, age, study site and CAG length. This model was run once for all participants and once only for participants with an increase in the degree of apathy over time.
IBM SPSS version 23 was used for the group characteristics analysis the significance threshold was set to 0.05. Baseline and follow-up models were corrected for multiple comparisons; i.e. p < 0.007 (significant threshold of 0.05 divided by the number of executed tests).
3. Results
Group characteristics are described in Table 1. The four groups differed significantly in age, CAG length, medication use, and apathy scores. On average, all participants were seen 23 months (SD: 1 month) after baseline visit.
Table 1.
Group characteristics.
| PreA N = 52 |
PreB N = 39 |
HD1 N = 50 |
HD2 N = 30 |
p-Value | |
|---|---|---|---|---|---|
| Gender: m/fa | 25/27 | 18/21 | 19/31 | 17/13 | p = 0.43 |
| Age in years (SD) at baselineb | 46 (9) | 46 (9) | 51 (10) | 56 (8) | p < 0.001 |
| CAG lengthb | 42 (2) | 44 (2) | 44 (4) | 43 (2) | p = 0.001 |
| Medication use at baseline (%)a | 9 (17%) | 9 (23%) | 18 (36%) | 26 (87%) | p < 0.001 |
| Medication use at baseline and FU (%)a | 8 (15%) | 9 (23%) | 18 (36%) | 25 (83%) | p < 0.001 |
| Apathy at baseline (%)a | 6 (12%) | 6 (15%) | 12 (24%) | 15 (50%) | p = 0.001 |
| Apathy at FU (%)a | 5 (9%) | 10 (25%) | 18 (36%) | 21 (70%) | p < 0.001 |
| Months between visitsb | 23 (1) | 23 (1) | 24 (1) | 24 (1) | p = 0.33 |
Subgroups are created on baseline characteristics: PreA: pre-motormanifest A; PreB: pre-motormanifest B; HD1: motormanifest stage 1; HD2: motormanifest stage 2; FU: follow-up visit; p-value for main comparison, no post-hoc results are displayed.
Total number.
Mean (standard deviation).
3.1. Baseline visit
Throughout the consecutive disease stages the percentage of participants with apathy steadily increased: at baseline 12% in the PreHD-A groups to 50% in the stage 2 HD group, see Table 1.
The linear regression model did not reveal any association between volume of the separate subcortical brain structures and the apathy product score. The results did not change by adding the covariate depressive mood or by excluding the covariate medication use in the original model.
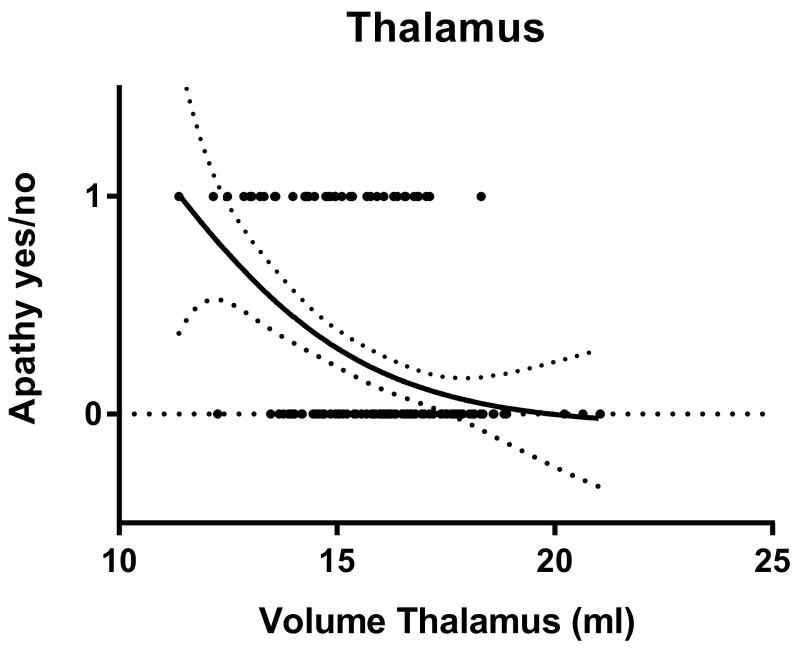
The logistic model showed that only a smaller volume of the thalamus (OR = 0.57; 95% CI: 0.38–0.84; p = 0.004) was associated with the presence of apathy; i.e. smaller thalamus indicates a higher probability of presence of apathy, see Fig. 1. No other associations were found, and the results did not change when the covariate depressive mood was added. If medication use was excluded as a covariate in our original model, again, only the thalamus was associated with the presence of apathy (OR = 0.56; 95% CI: 0.38–0.82; p = 0.003). Volumes of the nucleus accumbens, amygdala, caudate nucleus, hippocampus, pallidum and putamen were not associated with the apathy product score.
Fig. 1.
Probability of being apathetic based on volume of the thalamus.
3.2. Follow-up
Overall, the percentage of apathetic participants increased over a time period of 2 years, see Table 1. When comparing the apathy product score at baseline with the apathy product score at follow-up for 16% of the participants apathy product score decreased by at least one point. Of 53% of the participants the apathy severity score stayed exactly the same and for 31% of the participants the apathy severity score increased by at least one point.
For the linear regression model over time no significant associations were found. None of the volumes of the subcortical brain structures were associated with change in the apathy product score between the two assessments; removing the covariate medication use did not make any difference. Additional analysis with only participants with increase of the apathy product score included in the analysis did not show other results, data not shown.
4. Discussion
This study investigated the relationship between atrophy of subcortical brain structures and apathy in HD gene carriers at baseline and after 2 years follow-up. Cross-sectional analyses at baseline revealed that only atrophy of the thalamus was associated with the presence of apathy in HD, but no association between atrophy of the subcortical brain structures and the degree of apathy at baseline or over time were found. The former finding supports the notion that the prefrontal cortex – basal ganglia circuit is involved in occurrence of apathy in HD, i.e. disruption of the circuit at the level of the thalamus is related to apathy. However, solely association with the thalamus is not specific to any of the circuits as the thalamus connects the subcortical brain structures and the cortex in all prefrontal cortex – basal ganglia circuits. Since disruption of the anterior cingulate circuit was associated with apathy in other neurodegenerative diseases (Levy, 2012; Haegelen et al., 2009; Cummings, 1993; O'Callaghan et al., 2013), it is most likely that this circuit is also involved in the occurrence of apathy in HD. However, with only one structure being associated with apathy in our study, there is no conclusive evidence that the presence of apathy is associated with this specific circuit in HD. We only evaluated possible associations between apathy and atrophy of subcortical structures in HD. This is in accordance with findings that subcortical structures are associated with apathy in other neurodegenerative disease (Haegelen et al., 2009; Cummings, 1993; Martinez-Horta et al., 2016b) and that degeneration of subcortical structures is prominently present in HD (Aylward et al., 2011; Paulsen et al., 2006; van den Bogaard et al., 2011; Tabrizi et al., 2012). However, we have neglected a possible association with parts of the prefrontal cortex which might also differentiate between the prefrontal cortex – basal ganglia circuits. It is known that HD is a whole brain disease and the cortex degenerates in the early HD stages (Tabrizi et al., 2009; Tabrizi et al., 2011). In these early stages apathy also drastically increases (Thompson et al., 2012) which is also supported by our results. This leads us to speculate that the underlying neural cause of apathy might not only be ascribed to atrophy of subcortical brain structures, but might also be associated with atrophy of the cortex. For future research, we suggest to evaluate a possible association between the cortex and apathy in HD. In addition, it might be useful to explore other measurement tools for neural dysfunction, such as structural integrity or dopamine binding rather than volume reduction, to assess the relationship between apathy and neurodegenerative process in HD.
In the cross-sectional analysis we only found an association between the presence of apathy and volume reduction of the thalamus but not between the degree of apathy and volume reduction of any subcortical brain structure. To measure the degree of apathy in HD, we used the product score of severity and frequency of the apathy PBA-s item, as was done previously (Tabrizi et al., 2009). However, the product score bares some degree of uncertainty in what exactly is measured, it is unknown whether a high product score is a result of a high severity score, a high frequency score or a mix of both. This means that different clinical presentations may have the same product score, e.g. someone with chronically mild apathy may have the same score as someone with occasionally severe apathy. In our opinion, these cases are not equal; hypothesis is that it is more likely that severity – rather than frequency – of apathy is related to the neurodegenerative process in HD. McNally et al. (McNally et al., 2015) have also pointed out in their re-evaluation of the PBA-s that using the product score might statistically not be appropriate as it is not a ratio scale. For future research, it would be of interest to further evaluate the use of the different PBA-s scores.
Over a time period of 2 years follow-up, we did not find any association between atrophy of the subcortical brain structures and change in the severity of apathy. In our cohort, apathy was already present in the early stages and the number of apathetic HD gene carriers increased. Over a time period of 2 years, in 31% of the HD gene carriers apathy scores worsened, while in 16% of the HD gene carriers apathy scores improved. The last finding was rather unexpected, as previous studies have shown that apathy worsens over time in HD (Hamilton et al., 2003; Thompson et al., 2012), to our knowledge only one other study found that over a time period of 2 years some apathetic individuals improved (Reedeker et al., 2011). A possible explanation might be that apathy itself is related to depression and the use of psychotropic medication; successful treatment of depression and/or use of other medication can affect apathy (van Duijn et al., 2010). As medication use has such an influence on apathy, our statistical model was adjusted for medication use. We acknowledge that by creating a binary variable (i.e. use or no use of certain medication), the different acting agents were treated as if they all have the same effect on apathy. However, more research is needed to investigate whether medication itself triggers apathy or whether apathetic HD gene carriers are more likely to use certain medication, which is important for the prescription of effective individualized medication in HD. From our longitudinal results, we can only conclude that the severity in apathy does not drastically increase over a time period of 2 years in the pre-motormanifest and early stage of the disease, for the majority of individuals the apathy score stayed the same. The time period of 2 years might be too short to find a significant increase in apathy in a pre-motor manifest and early HD population considering that disease duration is 17–20 years (Roos, 2010). This is supported by Thompson et al.'s study (Thompson et al., 2012) in which more increase in apathy was found over a longer time period of on average 5 years and more advanced HD gene carriers.
In conclusion, apathy is present in early stages of HD and is associated with atrophy of the thalamus in HD gene carriers, suggesting that occurrence of apathy has an underlying neural cause. Further research is necessary to evaluate apathy over a longer time period in more advanced stages and to evaluate the possible association between apathy and cortical atrophy in HD.
Acknowledgments
Acknowledgments
TRACK-HD was supported by the CHDI Foundation, Inc., a not for profit organization dedicated to finding treatments for Huntington's disease. The authors wish to extend their gratitude to the TRACK-HD study participants and their families. Some of this work was undertaken at UCLH/UCL and the University of Manchester who acknowledge support from the respective Department of Health's NIHR Biomedical Research Centres.
TRACK-HD investigators
| Last name | First name | Location | |
| 1 | Acharya | T | Iowa |
| 2 | Arran | Natalie | Manchester |
| 3 | Axelson | Eric | Iowa |
| 4 | Bechtel | Natalie | Muenster |
| 5 | Berna | Claire | UCL |
| 6 | Borowsky | Beth | CHDI |
| 7 | Bohlen | Stefan | Muenster |
| 8 | Callaghan | Jenny | Manchester |
| 9 | Campbell | Colin | Indiana/Monash |
| 10 | Campbell | Melissa | Monash |
| 11 | Cash | David M. | IXICO |
| 12 | Coleman | Allison | UBC, Vancouver |
| 13 | Crawford | Helen | UCL |
| 14 | Dar Santos | Rachelle | UBC, Vancouver |
| 15 | Decolongon | Joji | UBC, Vancouver |
| 16 | Fox | Nick C | UCL |
| 17 | Frost | Chris | LSHTM |
| 18 | Gibbard | Claire | UCL |
| 19 | van der Grond | Jeroen | LUMC, Leiden |
| 20 | 't Hart | Ellen P. | LUMC, Leiden |
| 21 | Hicks | Stephen | Oxford |
| 22 | Hobbs | Nicola Z | UCL |
| 23 | Jauffret | Celine | Paris |
| 24 | Jones | Rebecca | LSHTM |
| 25 | Justo | Damian | Paris |
| 26 | Kennard | Chris | Oxford |
| 27 | Labushchagne | Izelle | Monash |
| 28 | Lahiri | Nayana | UCL |
| 29 | Landwehrmeyer | Bernhard | Ulm |
| 30 | Langbehn | Douglas | Iowa |
| 31 | Lehericy | Stéphane | Paris |
| 32 | Malone | Ian | UCL |
| 33 | Marelli | Cecilia | Paris |
| 34 | Milchman | Cassie | Monash |
| 35 | Nigaud | Kevin | Paris |
| 36 | Owen | Gail | UCL |
| 37 | Pepple | Tracey | UCL |
| 38 | Queller | Sarah | Indiana |
| 39 | Read | Joy | UCL |
| 40 | Reilmann | Ralf | UCL |
| 41 | Rosas | H Diana | MGH |
| 42 | Say | Miranda J | UCL |
| 43 | Stopford | Cheryl | Manchester |
| 44 | Stout | Julie C | Monash |
| 45 | Sturrock | Aaron | UBC, Vancouver |
| 46 | Valabrègue | Romain | Paris |
| 47 | Whitehead | Daisy | UCL |
| 48 | Wild | Edward | UCL |
References
- Aubeeluck A.V., Buchanan H., Stupple E.J. All the burden on all the carers': exploring quality of life with family caregivers of Huntington's disease patients. Qual. Life Res. 2012;21(8):1425–1435. doi: 10.1007/s11136-011-0062-x. [DOI] [PubMed] [Google Scholar]
- Aylward E.H. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch. Neurol. 1996;53(12):1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- Aylward E.H. Longitudinal change in regional brain volumes in prodromal Huntington disease. J. Neurol. Neurosurg. Psychiatry. 2011;82(4):405–410. doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohanna I. Magnetic resonance imaging as an approach towards identifying neuropathological biomarkers for Huntington's disease. Brain Res. Rev. 2008;58(1):209–225. doi: 10.1016/j.brainresrev.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Callaghan J. Reliability and factor structure of the short problem behaviors assessment for Huntington's disease (PBA-s) in the TRACK-HD and REGISTRY studies. J. Neuropsychiatr. Clin. Neurosci. 2015;27(1):59–64. doi: 10.1176/appi.neuropsych.13070169. [DOI] [PubMed] [Google Scholar]
- Cummings J.L. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993;50(8):873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Douaud G. Distribution of grey matter atrophy in Huntington's disease patients: a combined ROI-based and voxel-based morphometric study. NeuroImage. 2006;32(4):1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Georgiou-Karistianis N. Structural MRI in Huntington's disease and recommendations for its potential use in clinical trials. Neurosci. Biobehav. Rev. 2013;37(3):480–490. doi: 10.1016/j.neubiorev.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Haegelen C. The subthalamic nucleus is a key-structure of limbic basal ganglia functions. Med. Hypotheses. 2009;72(4):421–426. doi: 10.1016/j.mehy.2008.07.065. [DOI] [PubMed] [Google Scholar]
- Hamilton J.M. Behavioural abnormalities contribute to functional decline in Huntington's disease. J. Neurol. Neurosurg. Psychiatry. 2003;74(1):120–122. doi: 10.1136/jnnp.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs N.Z. The progression of regional atrophy in premanifest and early Huntington's disease: a longitudinal voxel-based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2010;81(7):756–763. doi: 10.1136/jnnp.2009.190702. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kingma E.M. Behavioural problems in Huntington's disease using the problem Behaviours assessment. Gen. Hosp. Psychiatry. 2008;30(2):155–161. doi: 10.1016/j.genhosppsych.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Langbehn D.R. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin. Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Leroi I. Carer burden in apathy and impulse control disorders in Parkinson's disease. Int. J. Geriatr. Psychiatry. 2012;27(2):160–166. doi: 10.1002/gps.2704. [DOI] [PubMed] [Google Scholar]
- Levy R. Apathy: a pathology of goal-directed behaviour: a new concept of the clinic and pathophysiology of apathy. Rev. Neurol. (Paris) 2012;168(8–9):585–597. doi: 10.1016/j.neurol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington's disease. Parkinsonism Relat. Disord. 2016;25:58–64. doi: 10.1016/j.parkreldis.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Martinez-Horta S. Non-demented Parkinson's disease patients with apathy show decreased grey matter volume in key executive and reward-related nodes. Brain Imaging Behav. 2016;11(5):1334–1342. doi: 10.1007/s11682-016-9607-5. [DOI] [PubMed] [Google Scholar]
- McNally G. Exploring the validity of the short version of the Problem Behaviours Assessment (PBA-s) for Huntington's disease: a rasch analysis. J. Huntingtons Dis. 2015;4(4):347–369. doi: 10.3233/JHD-150164. [DOI] [PubMed] [Google Scholar]
- Montoya A. Brain imaging and cognitive dysfunctions in Huntington's disease. J. Psychiatry Neurosci. 2006;31(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C., Bertoux M., Hornberger M. Beyond and below the cortex: the contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J. Neurol. Neurosurg. Psychiatry. 2013;85(4):371–378. doi: 10.1136/jnnp-2012-304558. [DOI] [PubMed] [Google Scholar]
- Patenaude B. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen J.S. Brain structure in preclinical Huntington's disease. Biol. Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Paulsen J.S. Detection of Huntington's disease decades before diagnosis: the predict-HD study. J. Neurol. Neurosurg. Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedeker N. Incidence, course, and predictors of apathy in Huntington's disease: a two-year prospective study. J. Neuropsychiatr. Clin. Neurosci. 2011;23(4):434–441. doi: 10.1176/jnp.23.4.jnp434. [DOI] [PubMed] [Google Scholar]
- Riba J. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3(6) doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R.A. Huntington's disease: a clinical review. Orphanet. J. Rare. Dis. 2010;5(1):40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulson I., Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. 2002;17(1):479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Starkstein S.E., Leentjens A.F. The nosological position of apathy in clinical practice. J. Neurol. Neurosurg. Psychiatry. 2008;79(10):1088–1092. doi: 10.1136/jnnp.2007.136895. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi S.J. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J. Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11(1):42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- Tabrizi S.J. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12(7):637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- Tekin S., Cummings J.L. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J. Psychosom. Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thieben M.J. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125(Pt 8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Thompson J.C. Behavior in Huntington's disease: dissociating cognition-based and mood-based changes. J. Neuropsychiatr. Clin. Neurosci. 2002;14(1):37–43. doi: 10.1176/jnp.14.1.37. [DOI] [PubMed] [Google Scholar]
- Thompson J.C. Longitudinal evaluation of neuropsychiatric symptoms in Huntington's disease. J. Neuropsychiatr. Clin. Neurosci. 2012;24(1):53–60. doi: 10.1176/appi.neuropsych.11030057. [DOI] [PubMed] [Google Scholar]
- van den Bogaard S.J. Early atrophy of pallidum and accumbens nucleus in Huntington's disease. J. Neurol. 2011;258(3):412–420. doi: 10.1007/s00415-010-5768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn E., Kingma E.M., van der Mast R.C. Psychopathology in verified Huntington's disease gene carriers. J. Neuropsychiatr. Clin. Neurosci. 2007;19(4):441–448. doi: 10.1176/jnp.2007.19.4.441. [DOI] [PubMed] [Google Scholar]
- van Duijn E. Correlates of apathy in Huntington's disease. J. Neuropsychiatr. Clin. Neurosci. 2010;22(3):287–294. doi: 10.1176/jnp.2010.22.3.287. [DOI] [PubMed] [Google Scholar]
- Vonsattel J.P. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 1985;44(6):559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]