ABSTRACT
Cytokine receptors often act via the Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway to form a signalling cascade that is essential for processes such as haematopoiesis, immune responses and tissue homeostasis. In order to transduce ligand activation, cytokine receptors must dimerise. However, mechanisms regulating their dimerisation are poorly understood. In order to better understand the processes regulating cytokine receptor levels, and their activity and dimerisation, we analysed the highly conserved JAK/STAT pathway in Drosophila, which acts via a single receptor, known as Domeless. We performed a genome-wide RNAi screen in Drosophila cells, identifying MASK as a positive regulator of Domeless dimerisation and protein levels. We show that MASK is able to regulate receptor levels and JAK/STAT signalling both in vitro and in vivo. We also show that its human homologue, ANKHD1, is also able to regulate JAK/STAT signalling and the levels of a subset of pathway receptors in human cells. Taken together, our results identify MASK as a novel regulator of cytokine receptor levels, and suggest functional conservation, which may have implications for human health.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Cytokine receptor, ANKYRIN domain, Dimerisation, RNAi, JAK/STAT
Summary: A genome-wide RNAi screen in Drosophila identifies MASK as a positive regulator of the JAK/STAT signalling via stabilisation of the pathway receptor. This function is conserved in human cells.
INTRODUCTION
The ability to bind to extracellular ligands and transduce the resulting interaction across the plasma membrane represents the central biological function of cytokine receptors. Such receptors include the single-pass transmembrane proteins that ultimately stimulate the Janus kinase and signal transducer and activator of transcription (JAK/STAT) pathway (Arbouzova and Zeidler, 2006; Vainchenker and Constantinescu, 2013). This group of receptors are typically homo- or hetero-dimerised with an extracellular structure consisting of multiple fibronectin type III repeats in which two of the distal repeats form a cytokine-binding motif (Tanaka et al., 2014) (CBM; Fig. 1A). On the C-terminal intracellular side, long, β-type cytokine receptors, such as glycoprotein 130 (GP130; also known as IL6ST), oncostatin M receptor B (OSMRB), thromobopoietin receptor (TPOR, also known as MPL) and the Drosophila receptor Domeless (Dome), contain juxta-membrane domains via which cytosolic JAK tyrosine kinases associate. By contrast, shorter α-type receptors such as Interleukin (IL)-6Rα, participate in the formation of ligand-binding complexes but lack the intracellular domains bound by downstream pathway components (Heinrich et al., 2003).
Fig. 1.
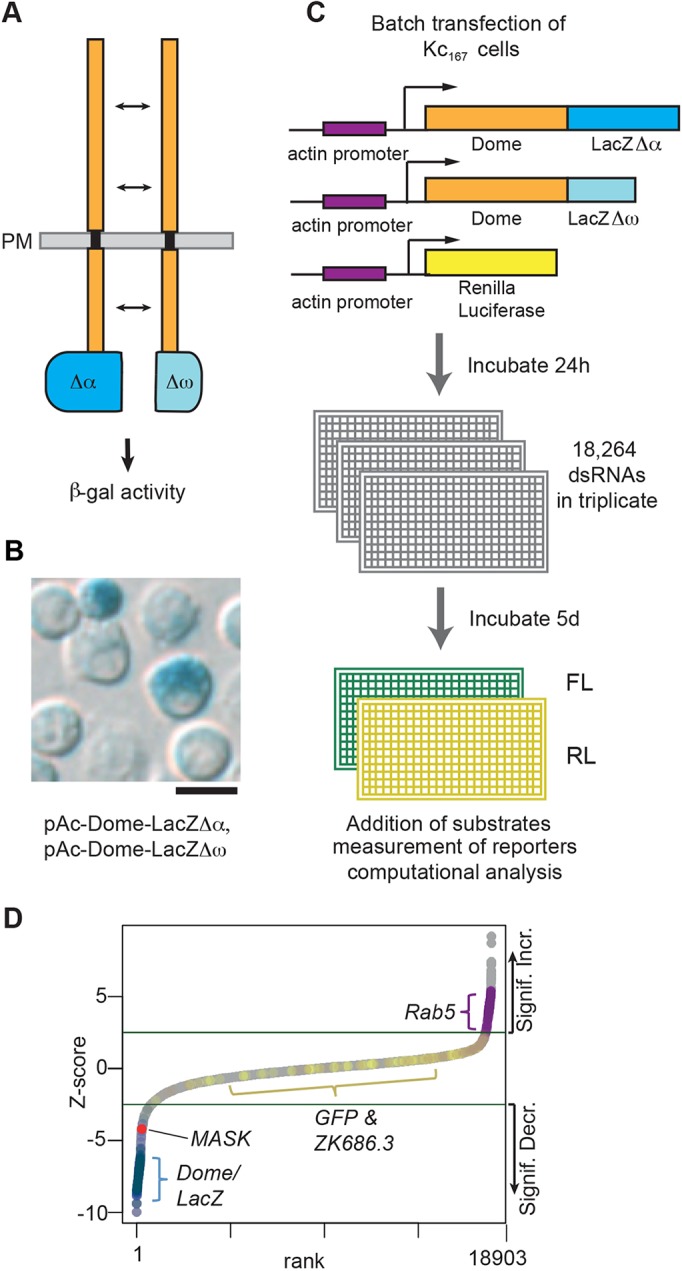
A split β-galactosidase genome-wide RNAi screen to identify modulators of Dome dimerisation and levels. (A) Schematic representation of the Dome–β-galΔα and Dome–β-galΔω complementation assay. PM, plasma membrane. (B) Drosophila Kc167 cells transiently transfected with plasmids expressing the proteins shown in A show β-galactosidase activity as determined by X-gal staining. Scale bar: 10 μm. (C) Workflow of the genome-wide RNAi screen for modulators of Dome dimerisation and levels as undertaken in Drosophila Kc167 cells. FL, firefly luciferase; RL, Renilla luciferase. (D) Ranked Z-scores from the genome-wide RNAi screen. Green lines illustrate Z-score cut-offs of significant increase or significant decrease. Controls are shown (Dome, LacZ, GFP, ZK686.3, Rab5) with MASK highlighted in red.
Cytokine binding to the extracellular domains of a receptor complex induces a conformational change, which either reorients a preformed dimer (Brown et al., 2005; Remy et al., 1999) or induces receptor dimerisation/oligomerisation (Thomas et al., 2011). In the case of erythropoietin (EPO), ligand binding has been shown to be sufficient to bring about receptor dimerisation (Boger and Goldberg, 2001) while the related receptor in Drosophila, Domeless (Dome), is dimerised in vivo via a ligand and JAK/STAT pathway-independent mechanism (Brown et al., 2003).
In canonical JAK/STAT pathway signalling, ligand activation results in JAK auto-phosphorylation, followed by trans-phosphorylation of intracellular tyrosine residues in the receptor tail and recruitment of latent STAT molecules (Vainchenker and Constantinescu, 2013). These STATs are then themselves tyrosine phosphorylated, dimerise and translocate to the nucleus where they bind to palindromic DNA sequences in the promoters of pathway target genes and thus regulate gene expression.
In humans, JAK/STAT pathway signalling is mediated by four JAK and six STAT proteins, playing key roles both during embryonic development, adult homeostasis and multiple diseases (Arbouzova and Zeidler, 2006; Vainchenker and Constantinescu, 2013). These core components are themselves downstream of multiple receptors and ligands with cell-specific differences, redundancy and crosstalk between pathway components, making the dissection of signalling processes particularly challenging. For example, signalling by the pro-inflammatory cytokine IL-6, occurs via receptor heterodimers made up of the long β-type GP130 receptor and the shorter α-type IL-6R, with both membrane-bound and soluble forms of IL-6R able to form signalling competent complexes with GP130 to stimulate the downstream pathway (Tanaka et al., 2014). By contrast, the production of erythrocytes and platelets is dependent on homodimerised EPO receptor (EPOR) and TPOR, respectively, receptors which function upstream of JAK2 and STAT5 (Seubert et al., 2003).
Although the core JAK and STAT pathway components have been extensively studied, the regulatory processes controlling upstream pathway receptors are less well understood. One key mechanism regulating receptor levels at the plasma membrane is endocytosis. Originally considered as a mechanism to attenuate pathway signalling following activation (Liu and Shapiro, 2003), it is now clear that the endocytosis and trafficking of ligand–receptor complexes into endosomes, and continued pathway signalling from this internalised compartment, not only occurs, but is also frequently qualitatively changed (reviewed in Cendrowski et al., 2016). Although uncertainty remains, changes in the micro-environment within a maturing endosome, such as reduced pH, trapping of the ligand, alterations in the receptor complex and changes to ligand–receptor affinities are all likely to occur (Kurgonaite et al., 2015). Indeed, there is compelling evidence that even closely related receptors, interferon (IFN) type I and type II, are regulated through varying mechanisms (de Weerd and Nguyen, 2012). Ultimately, receptor recycling to the plasma membrane or destruction of the complex within the lysosome also changes the levels of functional receptors (Chmiest et al., 2016; Gesbert et al., 2004).
In order to better understand the processes regulating cytokine receptor levels, activity and dimerisation, we set out to exploit the lower complexity of the Drosophila JAK/STAT signalling pathway which consists of a single JAK and STAT-like molecule together with a single full-length receptor, Dome, and a single short antagonistic receptor, termed Latran (Lat; also known as Eye Transformer) (reviewed in Zeidler and Bausek, 2013). Using this system, we undertook an RNA-interference (RNAi)-based screen for regulators of the Dome receptor. A previous report indicated that JAK/STAT pathway activation downstream of the Dome receptor requires homodimerisation of the receptor (Brown et al., 2003). Furthermore, the authors showed that dimerisation is developmentally regulated by an as-yet-unidentified ligand- and signalling-independent mechanism in vivo. In this study, we employed a molecular complementation assay utilising two truncated forms of the β-galactosidase (β-gal) enzyme, termed Δα and Δω (Rossi et al., 1997), and fused these to the cytosolic C-terminal ends of the Dome receptor. Although enzymatically inactive in isolation, dimerisation of two Dome molecules brings together both a Δα and a Δω truncation, allowing molecular complementation and the reconstitution of β-gal activity (Fig. 1A).
Here, we present our use of the above molecular complementation assay to undertake RNAi screens for factors able to modulate Dome levels and/or dimerisation. We present our genome-wide analysis of this screen, and go on to follow up by analysing the conserved Multiple Ankyrin repeats and KH domain-containing protein, MASK. By using both biochemical and genetic approaches, we show that MASK promotes Dome dimerisation and stability and demonstrate that JAK/STAT pathway activity is reduced following MASK knockdown. We go on to demonstrate that MASK binds directly to the Dome receptor via its medial A2 cluster of ankyrin repeats to stabilise the resulting complex. We show that the conserved human homologue ANKHD1 is also able to regulate both JAK/STAT pathway activity and the stability of a subset of human cytokine receptors. This study therefore identifies a novel regulator of cytokine receptor levels, providing insights into the regulation of this disease-relevant signalling pathway.
RESULTS
A split β-galactosidase assay for monitoring receptor dimerisation
Genome-scale RNAi screening has previously identified multiple regulators of JAK/STAT transcriptional activity (Kallio et al., 2010; Müller et al., 2005). However, changes in gene expression do not provide insights into the molecular mechanisms via which regulators of the pathway act. We therefore modified an assay for Dome dimerisation that used a split β-gal complementation system (Brown et al., 2003), in which the coding region for the β-gal enzyme containing one of two inactivating deletions (termed Δα and Δω) was attached to the intracellular terminus of the Dome receptor (Fig. 1A). The Δα and Δω fragments are themselves unable to complement unless they are brought into close proximity by fusing them to proteins that physically interact (Rossi et al., 1997). As previously demonstrated in vivo (Brown et al., 2003), each individual β-gal fusion protein is inactive in isolation and shows enzymatic activity only when co-expressed in the same cells (Fig. S1A). We note that, although all combinations of tagged Dome receptor can dimerise (e.g. DomeΔα–DomeΔα), homodimers will not be detected as having β-gal activity. However, since all dimer combinations are assumed to form with equal probability, the detectable population of heterodimers is representative of the overall population of all dimerised molecules. We adapted this technique for use in cultured Drosophila cells (Fig. 1B) and optimised a luminescent readout for β-gal enzymatic activity (Fig. S1A; Materials and Methods). Although designed to detect receptor dimers, our assay was also inherently sensitive to the absolute level of these dimers, since any changes in the amount of protein would also result in changes in β-gal activity.
A genome-wide RNAi screen identifies modulators of Dome dimerisation and stability
We performed a genome-wide RNAi screen using a second-generation, in silico optimised double-stranded RNA (dsRNA) library targeting 97.9% of the Drosophila genome (Fig. 1C; Fig. S1B), and analysed the resulting >110,000 data points using best-practice analysis techniques (Fisher et al., 2012). To avoid variation in transfection efficiency, which could affect results in the assay, we transfected a single batch of pooled cells that was aliquoted across a whole-genome replicate. As expected, negative controls (targeting GFP or the C. elegans gene zk686.3) did not significantly affect our assay, while knockdown of the endocytic trafficking component Rab5 increased levels of dimerised Dome, consistent with previous reports (Stec et al., 2013; Vidal et al., 2010). Conversely, knockdown of either dome itself or lacZ strongly decreased β-gal activity (Fig. 1D; Fig. S1B). Using techniques previously developed for similar genome-scale screens (Fisher et al., 2012; Müller et al., 2005), we first analysed three replicates of initial screening [available via GenomeRNAi (http://www.genomernai.org)] and then identified potential hits, which we subsequently retested in secondary re-screens (Table S1). Based on both primary and secondary screening, 43 candidates with consistent and robust Z-scores were selected for further analysis (Table S2; see Materials and Methods for precise selection criteria). Previous work undertaken in vivo suggested that ligand expression is not sufficient for Dome dimerisation (Brown et al., 2003). To test this finding in the context of our 43 candidates, we repeated the original Dome dimerisation assay in the presence of co-expressed pathway ligand and found that most (79%, 34/43) of the original hits had the same effect as in the absence of ligand (Table S2). In addition, it has also been shown that Dome can form heterodimers with the short, negatively acting, pathway receptor Lat, and that Lat can also form homodimers with itself (Fisher et al., 2016; Makki et al., 2010). We therefore set up cell based assays to test for Dome–Lat heterodimer and Lat–Lat homodimer formation and used this to test the 43 candidate genes. We found that 90% (36/40) of candidates affect Dome–Lat and Lat–Lat dimers, with 31 of these common to both (Table S2).
Although our molecular complementation assay requires receptor dimerisation to produce β-gal enzymatic activity, changes in signal can also be a consequence of changes in overall receptor levels due to alterations in gene expression level, mRNA stability or protein stability/turnover. In order to distinguish between those hits that promote or inhibit dimerisation and those that simply change protein levels, we next sought to quantify total protein levels using an independent technical approach. We therefore used quantified western blotting undertaken in triplicate (see Materials and Methods for assay design) to examine the effects of the 43 genes on overall Dome protein levels. Of the candidates tested, 31 altered Dome protein levels by at least 25% (Table S2, also see Fig. S1C,D for examples) while the remaining 13 appeared to change dimerisation without affecting overall protein levels. It should be noted that this secondary assay used a different form of tagged Dome, and so protein concentration cannot be directly compared to quantitative changes in the enzymatic activity of β-gal measured in the original screen (i.e. a 25% change in protein levels would not necessarily relate to a 25% change in luciferase values).
Given the changes in Dome dimerisation and protein levels, we also assessed the effect of our hits on JAK/STAT-dependent transcription using the 6x2xDrafLuc reporter (Müller et al., 2005). Surprisingly, while the gene knockdown mediated by some dsRNAs clearly affected JAK/STAT transcriptional activity, a large proportion had little or no effect on the 6x2xDrafLuc reporter (Table S2). This rather unexpected result suggests that either the levels of dimerised receptor are not rate limiting in this system, or that alternative regulatory pathways are able to compensate for changes in Dome dimer activity.
We next undertook an analysis of our 43 candidates to identify gene ontological (GO) terms disproportionately enriched or depleted relative to the whole Drosophila genome (Mi et al., 2017). This identified strong overrepresentation of genes involved in endocytosis (GO:0006897), actin cytoskeleton (GO:0015629) and cellular component morphogenesis (GO:0032989).
One strikingly overrepresented GO group identified was that of actin-related proteins. These were initially identified as strong hits that resulted in significant upregulation of Dome protein levels (Table S2; Fig. S1E). Upon further examination by quantitative real-time PCR (qPCR), we found that RNAi-mediated knockdown of Act42A resulted in a significant increase in the transcription of Dome construct transfected into our cells and expressed by an actin5c-derived promoter (Fig. S1F). Given that this result indicated the existence of a feedback loop regulating the actin promoter used in this construct, actin-related genes were classified as non-specific in this assay.
MASK regulates levels of dimerised Dome
Throughout the multiple rounds of screening and secondary assays undertaken, RNAi targeting MASK consistently generated strong effects in the dimerisation assay, on receptor levels and on JAK/STAT pathway transcriptional activity (bold values in Table S2). We, therefore, set out to better investigate the mechanisms underlying this activity.
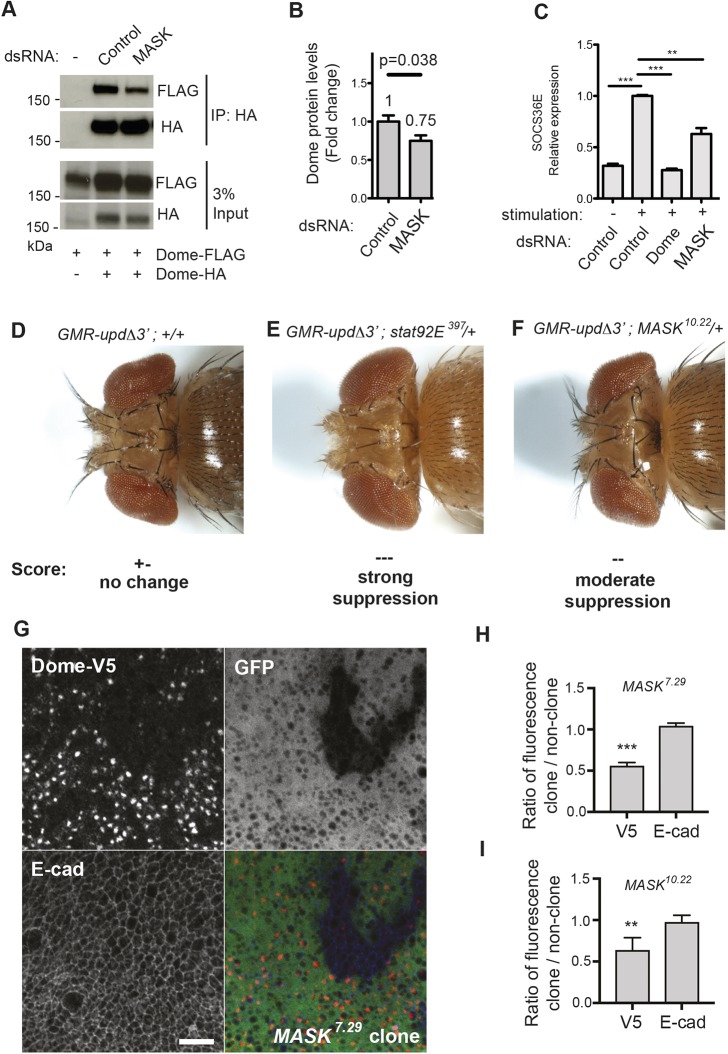
In order to confirm the screen-based identification of MASK, we retested its effect using an alternative dimerisation assay. Using co-immunoprecipitation of differentially epitope-tagged Dome molecules followed by quantification of western blots, we found that knockdown of MASK was sufficient to reduce Dome dimerisation by 50% (±10%, P<0.013, n=3) (Fig. 2A). Furthermore, given the nature of immunoprecipitation experiments, this approach is independent of potential protein level changes and therefore represents a specific assay for Dome–Dome dimerisation. In order to assess whether MASK knockdown also altered Dome–FLAG protein levels, we returned to our semi-quantitative western blotting secondary assay. This allowed us to confirm that a change in Dome dimerisation, due to MASK knockdown, was also accompanied by an ∼25% decrease in the steady-state level of Dome protein detected (Fig. 2B). As such, knockdown of MASK resulted in both the destabilisation of Dome–Dome dimers and also a reduction in the absolute levels of Dome itself.
Fig. 2.
MASK regulates pathway activity and receptor levels in vivo. (A) Co-expression of Dome–HA and Dome–FLAG followed by Dome–HA immunoprecipitation (IP) in Drosophila Kc167 cells. Levels of co-precipitated Dome–FLAG are modulated by treatment with the MASK dsRNA. The input is shown in the lower panels. (B) Quantification of the steady-state Dome–FLAG protein levels expressed by Kc167 cells after knockdown of MASK. Results represent the mean±s.d. fold change. P-value is determined by a Student's t-test (n=3). (C) Expression of the JAK/STAT pathway target gene SOCS36E following Upd2 ligand stimulation and treatment with the indicated dsRNAs. Results are the mean±s.d. (n=3). **P<0.01, ***P<0.001 (one-way ANOVA with Dunnett's post-hoc test). (D–F) Dorsal view of eye overgrowth phenotypes caused by ectopic Upd ligand expression driven by GMR-UpdΔ3′. Loss of one copy of STAT92E or MASK suppresses overgrowth. (G) Mitotic clones of MASK7.29 cause a reduction in tubulin-GAL4-driven UAS-Dome-V5 (red) fluorescence, whereas E-cadherin (blue) levels are unaffected. Clones were identified through loss of native GFP (green). Scale bar: 10 μm. (H,I) Quantification of Dome–V5 and E-cad levels in MASK7.29 (H) or MASK10.22 (I) mutant clones. Ratios of fluorescence intensity inside clones and in nearby twin-spots were taken to control for variations across discs. Measurements were averaged (mean±s.d.) over four or more discs with at least two clones per disc. **P<0.01, ***P<0.001 (one-sample t-test with expected mean of 1).
Consistent with the decrease in levels and dimerisation of receptor, we also found that Unpaired (Upd)-induced JAK/STAT transcriptional activation was reduced following knockdown of MASK, both at the level of a luciferase-based JAK/STAT-sensitive reporter (Table S2) and also via the reduction in transcription of the STAT92E target gene, SOCS36E (Fig. 2C). This result was confirmed by using two independent dsRNAs, each of which reduced both MASK mRNA by >70% (Fig. S2A), and pathway-induced transcription (Fig. S2B). The requirement for MASK in JAK/STAT signalling was further demonstrated using two additional independent STAT92E reporter assays, each of which showed strongly and significantly reduced activation following MASK knockdown (Fig. S2C). Taken together, these findings confirm that MASK functions as a positive regulator of the Drosophila JAK/STAT pathway.
Previous reports have identified MASK as a regulator of the Ras/Raf and Hippo/Warts pathways (Sansores-Garcia et al., 2013; Sidor et al., 2013; Smith et al., 2002), we examined the effects of silencing known components of the Ras (csw, ras85D, ras64B and raf) and Hippo (hpo, wts and yki) pathways in order to identify potential pathway crosstalk with our JAK/STAT pathway assays. Analysed as Z-scores relative to the original genome-wide screen dataset, neither Dome dimerisation, stability (Fig. S2D) nor STAT92E transcriptional activity (Fig. S2E) were significantly affected by knockdown of any of the Ras or Hippo pathway components tested. This suggests that MASK is acting directly on the JAK/STAT pathway.
MASK regulates JAK/STAT signalling in vivo
In order to support the cell and RNAi-based data, we also undertook in vivo JAK/STAT pathway assays using previously characterised loss-of-function MASK alleles (Smith et al., 2002). Ectopic JAK/STAT pathway activation is sufficient to drive over-proliferation within the developing eye imaginal disc, a process that is sensitive to downstream JAK/STAT pathway activity (Fig. 2D,E) (Bach et al., 2003; Mukherjee et al., 2006). By using this test, we found that JAK/STAT pathway-induced eye overgrowth was markedly reduced in genetic backgrounds heterozygous for independent MASK loss-of-function alleles (Fig. 2F; Fig. S2F–H).
We next explored whether MASK was required to maintain Dome protein levels in vivo. Since existing MASK mutant alleles are embryonic lethal, we induced mitotic clones of either the hypomorphic allele MASK7.29 or the null MASK10.22 allele in developing wing discs (Fig. 2G,H). In the absence of reliable antibodies against Dome, we ubiquitously expressed epitope-tagged Dome–V5 throughout the wing disc using tubulin-GAL4 (Fig. 2G). As observed previously (Makki et al., 2010), Dome was found to accumulate in intracellular vesicles, with weak staining observed at the plasma membrane. Although MASK mutant clones proliferate poorly and are therefore relatively small, the levels of Dome detected in mutant areas were significantly lower than those in surrounding wild-type tissue (Fig. 2G–I). When Dome levels inside clones (which are identified by their lack of GFP marker expression) were quantified relative to equivalent neighbouring wild-type regions, a significant reduction in Dome–V5 levels was observed for both MASK alleles (Fig. 2H,I). By contrast, another single-pass transmembrane protein E-cadherin (E-cad), which is not a JAK/STAT pathway receptor, was unaffected by loss of MASK (Fig. 2H,I). Given that transcription of Dome in this experiment is driven via a uniformly expressed heterologous tubulin promoter, we conclude that changes in Dome are a function of reduced protein levels rather than a change in gene expression. These results suggest that MASK acts as a positive regulator of the JAK/STAT pathway in vivo in Drosophila and is able to modulate pathway receptor levels.
MASK can physically associate with Dome
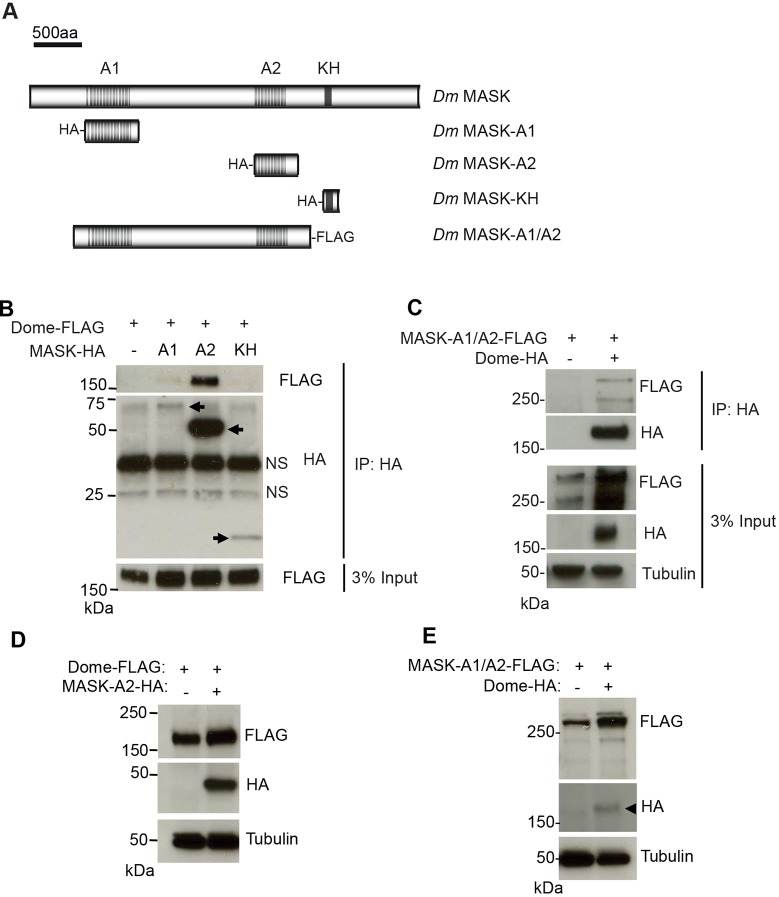
Given the ability of ankyrin repeats to mediate protein–protein interactions (Bennett and Chen, 2001; Michaely et al., 1999) and the ability of Drosophila MASK to modulate Dome receptor levels, we reasoned that MASK proteins might directly bind to cytokine receptors. We therefore expressed constructs encoding each of the ankyrin repeat domains and the KH domain of MASK (Sansores-Garcia et al., 2013) in Drosophila Kc167 cells (Fig. 3A) and tested the encoded proteins for binding to Dome. We found that the MASK-A2 and, more weakly, the MASK-A1 ankyrin repeat domains were able to co-precipitate Dome–FLAG (Fig. 3B). Although no interaction was detected with the MASK-KH region, we are unable to rule out binding due to much lower expression levels of the MASK-KH fragment (Fig. 3B). The interaction with MASK ankyrin repeat domains was found to be reciprocal, with a FLAG-tagged construct containing both ankyrin repeat domains (MASK-A1A2; Fig. 3A) being immunoprecipitated with Dome–HA (Fig. 3C).
Fig. 3.
MASK physically associates with Dome. (A) Schematic representation of Drosophila MASK protein and constructs used in this study. (B) Immunoprecipitation (IP) of the indicated HA–MASK constructs from Kc167 cells also expressing Dome–FLAG (arrows). Dome–FLAG is co-immunoprecipitated with HA–MASK-A1 and HA–MASK-A2. Levels of Dome–FLAG present in the input lysate are shown in the lower panel. NS, non-specific band. (C) Co-precipitation of MASK-A1/A2-FLAG following immunoprecipitation of Dome–HA. Levels of MASK-A1/A2-FLAG, Dome-HA and α-Tubulin present in the total Kc167 cell lysates are shown in the lower panels. (D) Steady-state levels of Dome–FLAG expressed in Drosophila Kc167 cells are increased following the co-expression of HA–MASK-A2. Levels of α-Tubulin indicate loading parity. (E) Steady-state levels of MASK-A1/A2–FLAG expressed in Drosophila Kc167 cells are increased following the co-expression of Dome–HA (arrowhead). Levels of α-Tubulin indicate loading parity.
These results suggest that MASK forms a physical complex with Dome and suggests that this interaction occurs via its ankyrin repeat domains.
Increased MASK levels leads to raised receptor levels
Given the negative effect of RNAi-mediated MASK knockdown on receptor stability, we tested whether increased levels of MASK fragments might have the opposite effect. In contrast to what was seen in loss-of-function experiments, we found that expression of the second MASK ankyrin repeat cluster (MASK-A2; Fig. 3A) (Sansores-Garcia et al., 2013) was sufficient to increase the steady-state levels of Dome in Kc167 cells (Fig. 3D), while overexpression of exogenous Dome was also sufficient to reciprocally stabilise MASK-A1A2 (Fig. 3E). These results support the initial finding that MASK is a positive regulator of Dome stability and, when taken together with the physical interactions between Dome and MASK, suggest that Dome and MASK associate to form a stable protein complex.
Conservation of MASK function in human cells
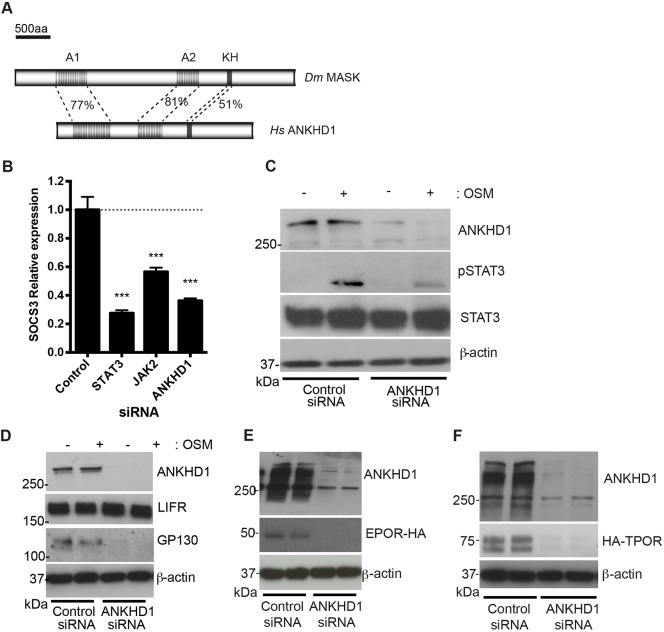
Since MASK is evolutionarily conserved between humans and Drosophila at the primary sequence level (Fig. 4A), we tested whether its function in modulating JAK/STAT signalling is also conserved. To test the effects of knocking down the closely related ANKHD1 on the human JAK/STAT pathway, we used qPCR in HeLa cells to detect the mRNA of the pathway target gene SOCS3 (Murray, 2007). As expected, mRNA levels of SOCS3 were strongly decreased following silencing of JAK2 and STAT3, while siRNA-mediated silencing of ANKHD1 (whose efficiency is shown in Fig. S3A) was also sufficient to significantly reduce expression (Fig. 4B). Consistent with this, ANKHD1 knockdown was also sufficient to significantly reduce the oncostatin M (OSM)-stimulated phospho-STAT1 and phospho-STAT3 levels, a hallmark of pathway activation (Fig. 4C; Fig. S3B).
Fig. 4.
MASK function is conserved in human cells. (A) Schematic representation of protein structure of Drosophila (Dm) MASK and human (Hs) ANKHD1 with percentage identity between sequences indicated for the highlighted regions. (B) SOCS3 mRNA expression in HeLa cells transfected with indicated siRNA and following OSM stimulation as indicated. ***P<0.001 (one-way ANOVA with Dunnett's posthoc test). Results are mean±s.d. (n=3). (C) HeLa cell extracts treated with siRNA targeting ANKHD1 have reduced levels of phospho-STAT3 upon OSM stimulation, whereas total STAT3 levels are unaffected. Blots confirm knockdown of ANKHD1 levels. (D) A representative blot of HeLa cells treated with control siRNA or siRNA targeting ANKHD1 in (n=3). Silencing of ANKHD1 leads to a loss of both ANKHD1 protein and endogenous GP130 protein. By contrast, levels of LIFR are not changed. β-actin levels are unaffected. (E,F) HeLa cell extracts treated with siRNA targeting ANKHD1 have reduced HA–EpoR (G) and TpoR–HA (H) compared to controls. Blots confirm knockdown of ANKHD1 levels.
Given that knockdown of Drosophila MASK led to a reduction in Dome protein levels (Fig. 2B,G), we tested cytokine receptor levels in human cells. Strikingly, while knockdown of ANKHD1 had no detectable effect on leukaemia inducible factor receptor (LIFR), it led to the almost complete loss of the endogenous GP130, the long cytokine receptor central to IL6-class cytokine receptor complexes (Fig. 4D) (Heinrich et al., 2003). Given that the change in GP130 protein level could be the consequence of changes in protein stability, mRNA stability or transcriptional regulation, we tested the ability of ANKHD1 to alter the levels of HA-tagged TPOR and EPOR expressed from a CMV promoter in HeLa cells in the presence or absence of ANKHD1. The amount of both receptors were greatly reduced following treatment with ANKHD1 siRNA (Fig. 4E,F). Since these receptors were expressed from a constitutive promoter, they are unlikely to be affected by changes in transcriptional control, further supporting a model in which ANKHD1 functions at a post-transcriptional level.
Taken together, these results suggest that the human homologue of MASK, ANKHD1, also acts as a positive regulator of JAK/STAT signalling and modulates the levels of a subset of human cytokine receptors.
DISCUSSION
The development and maintenance of multicellular life is absolutely dependent on the ability of cells to communicate with one another – a process that requires transmembrane receptor molecules. In this paper, we describe a screen to identify the factors involved in the dimerisation and stability of the Drosophila receptor associated with JAK/STAT pathway activation. This single-pass transmembrane receptor, termed Dome, forms homodimers in a spatially and temporally restricted manner during embryonic development. This dimerisation is required for downstream signalling, but is unaffected by the presence of the pathway ligand Upd (Brown et al., 2003). More recently, a related, but shorter receptor, Lat, was identified, which acts negatively to downregulate JAK/STAT pathway signalling (Kallio et al., 2010; Makki et al., 2010). Strikingly, Lat has also been shown to be able to form both homodimers and heterodimers with Dome (Fisher et al., 2016; Makki et al., 2010), with the formation of signalling-incompetent Dome–Lat heterodimers thought to represent the mechanism of negative regulation (Fisher et al., 2016). However, while the receptors themselves have been characterised, the mechanisms mediating receptor dimerisation required to generate a signalling competent complex are unknown. As such, the data reported here represents the first comprehensive description of the components required for this process.
One caveat of the screen design presented is the fact that the β-gal activity measured is influenced by both the efficiency of dimer formation and the stability/levels of Dome protein – although we are able to rule out effects on transcriptional regulation owing to the use of a ubiquitous actin promoter. In order to differentiate between these two influences, we undertook secondary screens using semi-quantitative western blotting to assess protein levels. In this way, we differentiated between those genes modulating dimerisation, those modulating protein levels and those that regulate both aspects. Based on this insight, the identification of hits that change β-gal activity but not protein levels (e.g. sec61β and CG6106) suggest that failure to dimerise does not inherently affect protein stability. By contrast, hits such as MASK that change both dimerisation and protein levels may be affecting both processes, although it is also possible that the loss of receptor stability following MASK knockdown may result in the breakdown of existing dimers as a prelude to protein destruction.
While transmembrane proteins destined for insertion into the plasma membrane are processed via conserved endoplasmic reticulum (ER) and Golgi pathways, it is clear that knockdown of MASK does not globally affect the production and/or trafficking of all membrane-spanning proteins. Rather, the requirement for MASK proteins is specific to a subset of transmembrane proteins, affecting Dome but not E-cadherin in Drosophila (Fig. 2G–I), and GP130, EPOR and TPOR, but not LIFR, in human HeLa cells (Fig. 4D–F). This is particularly interesting in the context of LIFR, since this protein is known to form a signalling complex with GP130 (Gearing et al., 1991, 1992). However, it has been shown that addition of ligand is a key factor in inducing LIFR–GP30 heterodimerisation, suggesting that GP130 may be trafficked independently of LIFR in unstimulated cells (Giese et al., 2005). Indeed, evidence suggests that LIFR and GP130 can be internalised and degraded via different mechanisms (Blanchard et al., 2000).
In order to obtain a mechanistic insight into the function of MASK, we also undertook a structure–function analysis of MASK itself. This showed that MASK and Dome form stable physical interactions, as shown by reciprocal co-immunoprecipitation, with this interaction being primarily mediated by the second, central A2 group of ankyrin domains present in MASK (Fig. 3B). Furthermore, we also show that the overexpression of the MASK-A1/A2 region is able to stabilise Dome levels (Fig. 3E), suggesting that MASK–Dome complexes (and possibly MASK–Dome–Dome complexes) may be inherently more stable than Dome alone. Although largely speculative, it is possible that the interactions seen between Dome and both the A1 and A2 regions of MASK (Fig. 3B) may point to a model in which one Dome receptor may bind to each ankyrin domain so promoting the dimerisation and stabilisation of Dome dimers. Although it is formally possible that MASK alters dome mRNA stability, this physical association with Dome suggests regulation at the protein level. We have previously demonstrated that Dome is constitutively internalised and degraded via the lysosome, but not recycled to a significant degree (Fisher et al., 2016; Stec et al., 2013). One could therefore speculate that association with MASK stabilises Dome, slowing the degradation process.
In humans, ANKHD1 has a paralogue on chromosome 4, named ANKRD17 (Sansores-Garcia et al., 2013; Sidor et al., 2013) with the two proteins sharing 71% identity, with greater sequence similarity in the regions of ankyrin repeats and the KH domain (Poulin et al., 2003). Strikingly, ANKRD17 has been demonstrated to physically interact with receptors involved in the innate immune response, and plays a role in the release of cytokines (Menning and Kufer, 2013) and interferons (Wang et al., 2012). These findings serve to support our own data, and suggest that ANKHD1 and ANKRD17 may also be acting to regulate receptor stability and dimerisation in humans.
Taken together, we present the first systematic screen, of which we are aware, to identify the factors responsible for the dimerisation of a JAK/STAT pathway receptor. We characterise one of these hits, MASK, and show that it regulates JAK/STAT pathway activity and forms a complex with the pathway receptor. We show that MASK is required to maintain the stability of Dome protein both in vivo and in cells, and may well also play a role in receptor dimerisation. Finally, we demonstrate the evolutionary conservation of the MASK homologue ANKHD1 at the sequence and functional levels. As such, this work provides a valuable insight into this aspect of JAK/STAT pathway and highlights a novel level of regulation of this important and disease-relevant pathway.
MATERIALS AND METHODS
Cell culture and biochemistry
Drosophila Kc167 cells were obtained from the Drosophila Genomics Resource Center (DGRC) and maintained according to standard procedures (Fisher et al., 2012). HeLa cells were maintained in DMEM supplemented with 10% serum. All cells are regularly screened for contamination. Plasmid transfections were carried out using Effectene (Qiagen) according to manufacturer's instructions. Reverse transfections with siRNA were carried out using Lipofectamine RNAiMAX (Invitrogen) using 10 nM final concentrations of single siRNAs (Dharmacon), targeting ANKHD1 (D-014405-01 or D-014405-02) where comparable results in terms of knockdown efficiency and reduction in JAK/STAT pathway activity were seen for both, or non-targeting siRNA (D-001210-01) as a control. Stimulation of mammalian JAK/STAT pathway was carried out using human recombinant oncostatin M (295-OM-010, R&D systems) at a final concentration of 10 ng/ml for 20 min. Immunoprecipitation experiments were carried out as previously described (Stec et al., 2013). Proteins were separated on 4–15% TGX SDS-PAGE precast gels (Bio-Rad) and transferred onto nitrocellulose membranes.
Drosophila RNAi screen hits were assessed for their effects on Dome protein levels – although it should be noted that this assay could not distinguish between modulation of mRNA stability or protein turnover. Kc167 cells were batch-transfected with Dome–FLAG, incubated for 24 h, then split into 24-well plates with 4 µg dsRNA. After 5 days RNAi treatment, cells were lysed as described (Stec et al., 2013). Lysates were boiled in 2× Laemmli sample buffer and analysed by western blotting. FLAG/tubulin fold-changes were calculated for each RNAi condition in comparison to the average of three negative controls per gel. Each screen hit was analysed in duplicate by a researcher who was blind to the treatment.
Genome-wide RNAi screening
The genome-wide SRSFv1 library, in 384-well format, was used as previously described (Fisher et al., 2012). Controls were manually added into empty wells (250 ng dsRNA in 5 µl water): GFP and the C. elegans gene bearing no sequence homology in Drosophila, zk686.3, were used as baseline controls; technical controls targeting transfected plasmids were dome, LacZ and RLuc; and Rab5 was used as a positive control. Genome-wide screening was carried out in biologically independent triplicates. Kc167 cells were batch-transfected in T75 flasks with 4 µg pAc-Dome-LacZ-Δα, pAc-Dome-LacZ-Δω and pAc-RLuc and incubated for 24 h. Cells were pooled in serum-free medium, and 15,000 cells seeded in every 384 well. After 1 h, medium was added to give a final serum concentration of 10%. After 5 days cells were assayed for β-gal activity using the β-glo assay system (Promega), which involves firefly luciferase reactions (FL), followed by measurement of Renilla luciferase (RL) activity as a viability control. Luciferase activities were measured on a Varioskan plate reader (Thermo). Raw data can be supplied by the corresponding author upon request.
Data analysis
Firefly and Renilla luciferase values for each well were processed using the CellHTS2 Bioconductor package (Boutros et al., 2006). Values were median-centred to normalise for plate-to-plate variation. Ratios of luciferase (FL:RL) were used to calculate the robust Z-scores, which were considered significant at ≥2.5 or ≤−2.5. Individual FL and RL values were also assessed, since they were not always linear with respect to one another. Secondary analyses were carried out with newly synthesised dsRNAs, and hits were considered significant at the less stringent ≥+2 or ≤−2 level. 43 robust hits were selected at this stage and sequenced to confirm correct target genes.
Drosophila genotypes
The full genotypes of Drosophila in Fig. 2 were: Fig. 2D, w, GMR-updΔ3′ / w1118; Fig. 2E, w, GMR-updΔ3′ /+;; stat92E397/+; Fig. 2F, w, GMR-updΔ3′ /+;; MASK10.22/+; Fig. 2G,H, w UbxFLP ;;UAS-Dome-V5 FRT82 MASK7.29 / tub-GAL4 FRT82 Ubq-GFP; and Fig. 2I, w UbxFLP ;;UAS-Dome-V5 FRT82 MASK10.22 / tub-GAL4 FRT82 Ubq-GFP. MASK alleles were a gift of Michael Simon (Department of Biology, Stanford University, USA) (Smith et al., 2002).
Drosophila phenotypes
Eye overgrowth assays were double-blind scored alongside stat92E and w1118 out-crosses (n>20 flies per genotype with >2 repeats). Adult flies were photographed using a Nikon SMZ1500 stereo-microscope and the Nikon Elements extended depth of focus software package.
Wing discs were dissected from wandering third-instar larvae raised at 25°C. Inverted carcasses were fixed in 4% paraformaldehyde in PBS for 20 min, blocked and incubated in primary antibodies overnight at 4°C. Tissues were washed in PBS containing 0.2% Triton X-100 (PBST) and incubated in secondary antibodies overnight at 4°C. After washing, discs were mounted in mounting medium and imaged on Nikon A1R GaAsP confocal microscope using a 60× NA1.4 apochromatic lens, with a pixel size of 70 nm, and the pinhole was set to 1.2 AU.
Antibodies
For western blotting, antibodies used were against ANKHD1 (Sigma, HPA008718), β-actin (Abcam, ab8226), GP130 (Cell Signaling Technologies, 3732), pSTAT1 (Cell Signaling Technologies, 9167), STAT3 (Cell Signaling Technologies, 12640), pSTAT3 (Cell Signaling Technologies, 9145), FLAG (M2, Sigma), HA (3F10, Roche), Drosophila α-tubulin (DM1A, Sigma), and were all used at 1:1000. For immunohistochemistry, the primary antibodies were against E-cadherin (dCAD2, DSHB, 1:20) and V5 (E10/V4RR, Invitrogen, 1:500).
Cloning of expression constructs
Dome-LacZ-Δα and -Δω fragments were cut from pUAST vectors (Brown et al., 2003) and ligated into pAc5.1 vector (Invitrogen) using the KpnI and XbaI restriction sites (a partial digestion of KpnI sites was used for Δω). pAc-Dome-FLAG and pAc-Dome-HA were described in Stec et al. (2013). Drosophila MASK-A1/A2 was PCR amplified from cDNA clone LD31446 (DGRC). Gateway cloning of PCR product was carried out using the pENTR/D-TOPO Cloning Kit (Invitrogen) and introduced into the pAWF vector (Drosophila Gateway Vector Collection) using Gateway LR Clonase II Enzyme Mix (Invitrogen), according to the manufacturer's instructions. HA–MASK constructs were a gift from Georg Halder (VIB-KU Leuven Center for Cancer Biology, Belgium) (Sansores-Garcia et al., 2013). HA-tagged EPOR and TPOR constructs were a gift from Stefan Constantinescu (Ludwig Institute for Cancer Research, Brussels, Belgium). TotM-luc STAT92E reporter construct was a gift from Mika Ramet (BMT/Experimental Immunology, University of Tampere, Finland) (Kallio et al., 2010) while the 10xSTAT-luc construct was a gift from Norbert Perrimon (Department of Genetics, Harvard Medical School, USA) (Baeg et al., 2005).
Quantitative real-time PCR
Total RNA was extracted from cells using TRIZOL Reagent (Invitrogen) following the manufacturer's instructions. Synthesis of cDNA was carried out using the High Capacity RNA-to-cDNA kit (Applied Biosystems) from 2 µg total RNA. To confirm gene knockdown by RNAi or to measure levels of target gene expression, qPCR was carried out using SsoAdvanced SYBRGreen Supermix (BioRad) on a CFX-96 Touch new generation Real-Time PCR Detection System (BioRad). Change in expression levels between experimental conditions was calculated compared to housekeeping gene expression (either Drosophila RpL32 or human β-actin) using the ΔΔCT method (Bina et al., 2010). Statistical analysis was carried out using one-way ANOVA tests in Prism (Graphpad). Primers are listed in Table S3. TAQMAN qPCR probes were designed for multiplexing (IDT oligo).
Supplementary Material
Acknowledgements
The authors would like to thank Lauren Francis, Kirsty Johnstone, Amy Taylor and Lucie N'Koy for technical support. Stefan Constantinescu, Mike Simon, Georg Halder, Mika Ramet and Norbert Perrimon kindly provided reagents.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.H.F., M.P.Z.; Methodology: K.H.F., M.F., S.J.T.; Software: S.B.; Validation: K.H.F., M.F., M.P.Z.; Formal analysis: K.H.F., S.B., M.P.Z.; Investigation: K.H.F., M.F., D.P., N.B., M.A.A., S.J.T., S.B.; Data curation: K.H.F.; Writing - original draft: K.H.F., M.P.Z.; Writing - review & editing: K.H.F., M.P.Z.; Visualization: K.H.F., M.F., S.B., M.P.Z.; Supervision: S.B., M.P.Z.; Project administration: M.P.Z.; Funding acquisition: M.P.Z.
Funding
Funding was provided by a Cancer Research UK Senior Cancer Fellowship (C24089/A8301), the EU Seventh Framework Programme (FP7) Project ‘Cancer Pathways’ (Project no. 201666) and a Wellcome Trust equipment grant (ref. 084757) to M.P.Z. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.209551.supplemental
References
- Arbouzova N. I. and Zeidler M. P. (2006). JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133, 2605-2616. 10.1242/dev.02411 [DOI] [PubMed] [Google Scholar]
- Bach E. A., Vincent S., Zeidler M. P. and Perrimon N. (2003). A sensitized genetic screen to identify novel regulators and components of the Drosophila janus kinase/signal transducer and activator of transcription pathway. Genetics 165, 1149-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg G.H., Zhou R. and Perrimon N. (2005). Genome-wide RNAi analysis of JAK/STAT signaling components in Drosophila. Genes Dev. 19, 1861-1870. 10.1101/gad.1320705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. and Chen L. (2001). Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr. Opin. Cell Biol. 13, 61-67. 10.1016/S0955-0674(00)00175-7 [DOI] [PubMed] [Google Scholar]
- Bina S., Wright V. M., Fisher K. H., Milo M. and Zeidler M. P. (2010). Transcriptional targets of Drosophila JAK/STAT pathway signalling as effectors of haematopoietic tumour formation. EMBO Rep. 11, 201-207. 10.1038/embor.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard F., Duplomb L., Wang Y., Robledo O., Kinzie E., Pitard V., Godard A., Jacques Y. and Baumann H. (2000). Stimulation of leukemia inhibitory factor receptor degradation by extracellular signal-regulated kinase. J. Biol. Chem. 275, 28793-28801. 10.1074/jbc.M003986200 [DOI] [PubMed] [Google Scholar]
- Boger D. L. and Goldberg J. (2001). Cytokine receptor dimerization and activation: prospects for small molecule agonists. Bioorg. Med. Chem. 9, 557-562. 10.1016/S0968-0896(00)00276-5 [DOI] [PubMed] [Google Scholar]
- Boutros M., Brás L. P. and Huber W. (2006). Analysis of cell-based RNAi screens. Genome Biol. 7, R66 10.1186/gb-2006-7-7-r66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Hu N. and Hombría J. C. (2003). Novel level of signalling control in the JAK/STAT pathway revealed by in situ visualisation of protein-protein interaction during Drosophila development. Development 130, 3077-3084. 10.1242/dev.00535 [DOI] [PubMed] [Google Scholar]
- Brown R. J., Adams J. J., Pelekanos R. A., Wan Y., McKinstry W. J., Palethorpe K., Seeber R. M., Monks T. A., Eidne K. A., Parker M. W. et al. (2005). Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat. Struct. Mol. Biol. 12, 814-821. 10.1038/nsmb977 [DOI] [PubMed] [Google Scholar]
- Cendrowski J., Mamińska A. and Miaczynska M. (2016). Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor. Rev. 32, 63-73. 10.1016/j.cytogfr.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Chmiest D., Sharma N., Zanin N., Viaris de Lesegno C., Shafaq-Zadah M., Sibut V., Dingli F., Hupé P., Wilmes S., Piehler J. et al. (2016). Spatiotemporal control of interferon-induced JAK/STAT signalling and gene transcription by the retromer complex. Nat. Commun. 7, 13476 10.1038/ncomms13476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd N. A. and Nguyen T. (2012). The interferons and their receptors–distribution and regulation. Immunol. Cell Biol. 90, 483-491. 10.1038/icb.2012.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. H., Wright V. M., Taylor A., Zeidler M. P. and Brown S. (2012). Advances in genome-wide RNAi cellular screens: a case study using the Drosophila JAK/STAT pathway. BMC Genomics 13, 506 10.1186/1471-2164-13-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. H., Stec W., Brown S. and Zeidler M. P. (2016). Mechanisms of JAK/STAT pathway negative regulation by the short coreceptor Eye Transformer/Latran. Mol. Biol. Cell 27, 434-441. 10.1091/mbc.e15-07-0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Thut C. J., VandeBos T., Gimpel S. D., Delaney P. B., King J., Price V., Cosman D. and Beckmann M. P. (1991). Leukemia inhibitory factor receptor is structurally related to the IL-6 signal transducer, gp130. EMBO J. 10, 2839-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Comeau M. R., Friend D. J., Gimpel S. D., Thut C. J., McGourty J., Brasher K. K., King J. A., Gillis S. and Mosley B. (1992). The IL-6 signal transducer, gp130: an oncostatin M receptor and affinity converter for the LIF receptor. Science 255, 1434-1437. 10.1126/science.1542794 [DOI] [PubMed] [Google Scholar]
- Gesbert F., Sauvonnet N. and Dautry-Varsat A. (2004). Clathrin-lndependent endocytosis and signalling of interleukin 2 receptors IL-2R endocytosis and signalling. Curr. Top. Microbiol. Immunol. 286, 119-148. [PubMed] [Google Scholar]
- Giese B., Roderburg C., Sommerauer M., Wortmann S. B., Metz S., Heinrich P. C. and Müller-Newen G. (2005). Dimerization of the cytokine receptors gp130 and LIFR analysed in single cells. J. Cell Sci. 118, 5129-5140. 10.1242/jcs.02628 [DOI] [PubMed] [Google Scholar]
- Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G. and Schaper F. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1-20. 10.1042/bj20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio J., Myllymäki H., Grönholm J., Armstrong M., Vanha-aho L.-M., Mäkinen L., Silvennoinen O., Valanne S. and Rämet M. (2010). Eye transformer is a negative regulator of Drosophila JAK/STAT signaling. FASEB J. 24, 4467-4479. 10.1096/fj.10-162784 [DOI] [PubMed] [Google Scholar]
- Kurgonaite K., Gandhi H., Kurth T., Pautot S., Schwille P., Weidemann T. and Bökel C. (2015). Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling. J. Cell Sci. 128, 3781-3795. 10.1242/jcs.170969 [DOI] [PubMed] [Google Scholar]
- Liu J. and Shapiro J. I. (2003). Endocytosis and signal transduction: basic science update. Biol. Res. Nurs. 5, 117-128. 10.1177/1099800403256860 [DOI] [PubMed] [Google Scholar]
- Makki R., Meister M., Pennetier D., Ubeda J. M., Braun A., Daburon V., Krzemień J., Bourbon H. M., Zhou R., Vincent A. et al. (2010). A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 8, e1000441 10.1371/journal.pbio.1000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menning M. and Kufer T. A. (2013). A role for the Ankyrin repeat containing protein Ankrd17 in Nod1- and Nod2-mediated inflammatory responses. FEBS Lett. 587, 2137-2142. 10.1016/j.febslet.2013.05.037 [DOI] [PubMed] [Google Scholar]
- Mi H., Huang X., Muruganujan A., Tang H., Mills C., Kang D. and Thomas P. D. (2017). PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45, D183-D189. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaely P., Kamal A., Anderson R. G. and Bennett V. (1999). A requirement for ankyrin binding to clathrin during coated pit budding. J. Biol. Chem. 274, 35908-35913. 10.1074/jbc.274.50.35908 [DOI] [PubMed] [Google Scholar]
- Mukherjee T., Schäfer U. and Zeidler M. P. (2006). Identification of Drosophila genes modulating Janus kinase/signal transducer and activator of transcription signal transduction. Genetics 172, 1683-1697. 10.1534/genetics.105.046904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Kuttenkeuler D., Gesellchen V., Zeidler M. P. and Boutros M. (2005). Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 436, 871-875. 10.1038/nature03869 [DOI] [PubMed] [Google Scholar]
- Murray P. J. (2007). The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178, 2623-2629. 10.4049/jimmunol.178.5.2623 [DOI] [PubMed] [Google Scholar]
- Poulin F., Brueschke A. and Sonenberg N. (2003). Gene fusion and overlapping reading frames in the mammalian genes for 4E-BP3 and MASK. J. Biol. Chem. 278, 52290-52297. 10.1074/jbc.M310761200 [DOI] [PubMed] [Google Scholar]
- Remy I., Wilson I. A. and Michnick S. W. (1999). Erythropoietin receptor activation by a ligand-induced conformation change. Science 283, 990-993. 10.1126/science.283.5404.990 [DOI] [PubMed] [Google Scholar]
- Rossi F., Charlton C. A. and Blau H. M. (1997). Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation. Proc. Natl. Acad. Sci. USA 94, 8405-8410. 10.1073/pnas.94.16.8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansores-Garcia L., Atkins M., Moya I. M., Shahmoradgoli M., Tao C., Mills G. B. and Halder G. (2013). Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr. Biol. 23, 229-235. 10.1016/j.cub.2012.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert N., Royer Y., Staerk J., Kubatzky K. F., Moucadel V., Krishnakumar S., Smith S. O. and Constantinescu S. N. (2003). Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol. Cell 12, 1239-1250. 10.1016/S1097-2765(03)00389-7 [DOI] [PubMed] [Google Scholar]
- Sidor C. M., Brain R. and Thompson B. J. (2013). Mask proteins are cofactors of Yorkie/YAP in the Hippo pathway. Curr. Biol. 23, 223-228. 10.1016/j.cub.2012.11.061 [DOI] [PubMed] [Google Scholar]
- Smith R. K., Carroll P. M., Allard J. D. and Simon M. A. (2002). MASK, a large ankyrin repeat and KH domain-containing protein involved in Drosophila receptor tyrosine kinase signaling. Development 129, 71-82. [DOI] [PubMed] [Google Scholar]
- Stec W., Vidal O. and Zeidler M. P. (2013). Drosophila SOCS36E negatively regulates JAK/STAT pathway signaling via two separable mechanisms. Mol. Biol. Cell 24, 3000-3009. 10.1091/mbc.e13-05-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M. and Kishimoto T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect Biol. 6, a016295 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Moraga I., Levin D., Krutzik P. O., Podoplelova Y., Trejo A., Lee C., Yarden G., Vleck S. E., Glenn J. S. et al. (2011). Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 146, 621-632. 10.1016/j.cell.2011.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W. and Constantinescu S. N. (2013). JAK/STAT signaling in hematological malignancies. Oncogene 32, 2601-2613. 10.1038/onc.2012.347 [DOI] [PubMed] [Google Scholar]
- Vidal O. M., Stec W., Bausek N., Smythe E. and Zeidler M. P. (2010). Negative regulation of Drosophila JAK-STAT signalling by endocytic trafficking. J. Cell Sci. 123, 3457-3466. 10.1242/jcs.066902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Tong X., Li G., Li J., Deng M. and Ye X. (2012). Ankrd17 positively regulates RIG-I-like receptor (RLR)-mediated immune signaling. Eur. J. Immunol. 42, 1304-1315. 10.1002/eji.201142125 [DOI] [PubMed] [Google Scholar]
- Zeidler M. P. and Bausek N. (2013). The Drosophila JAK-STAT pathway. JAKSTAT 2, e25353 10.4161/jkst.25353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.