Abstract
Background
The concentration and size of lipoprotein particles are associated with race, inflammation and disease. When triglycerides are high, as in pregnancy, lipoprotein particle size may have physiologic importance beyond conventional lipid measurements. We considered that lipoprotein particles may be related to preterm birth (PTB), and explored race differences.
Methods
Samples were collected at 9 weeks gestation (22 PTB [<37 weeks]; 42 term births [≥37 weeks]). Lipids were assayed using standard techniques. Concentrations of high-density lipoprotein, low-density lipoprotein, very low-density lipoprotein particles (HDL-P, LDL-P, and VLDL-P) and markers of systemic inflammation were quantified using NMR spectroscopy and related to PTB.
Results
Women with PTB had lower VLDL-P (−10.66 nmol/L, p=0.03) and higher systemic inflammation (+19.2 μmol/L, p=0.02) compared to women with term births, independent of race, pre-pregnancy BMI and smoking. Black vs. White women had lower VLDL-P and higher HDL-cholesterol (both p<0.05). Race-specific results indicated that large HDL-P and inflammation (GlycB) were higher with PTB vs. term birth among black women only.
Conclusion
Women with PTB had lower VLDL-P early in pregnancy, which may represent impaired lipid response. Black-White differences in the lipoprotein profile are similar to non-pregnant adults, but race-specific lipoprotein and inflammation associations with PTB warrant further study.
Keywords: Inflammation, Lipids, Lipoproteins, Pregnancy, Prematurity
Preterm birth (PTB, <37 weeks) is the most common pregnancy complication, affecting 10% of U.S. births and is the leading cause of perinatal morbidity and mortality.1 With the exception of the past few years, rates of singleton PTBs have risen for two decades,2 and Black compared to White women are disproportionately affected. Rates of obesity among women of reproductive age have increased 70% in the past decade, 3 increasing the likelihood that the vascular and metabolic health of women entering pregnancy has worsened. Dysregulated lipid metabolism and inflammation are two metabolic aberrations associated with adiposity, and these may converge in women with PTB. 4 Lipid metabolism and inflammatory markers also differ by race, and may contribute to race disparities in PTB.5,6
Normal pregnancy is associated with profound changes in maternal lipoprotein metabolism to support placental steroid hormone synthesis and the nutritional needs of the fetus. However, emerging evidence indicates that elevated lipids before or during pregnancy may be associated with PTB risk. 7–11 During gestation, low-density lipoprotein (LDL) particles tend to shift from large to smaller, denser and more atherogenic and pro-inflammatory particles. 12 Small LDL particles are higher in preeclampsia and in obese women, 13,14 and both preeclampsia and obesity are leading contributors to indicated PTB.
In cardiovascular research, concentrations of lipoprotein particles (e.g., total and small LDL and HDL) provide insight into risk for disease beyond traditional measurements of total lipid concentrations, particularly when triglycerides and inflammation levels are high. 15,16 In the setting of high triglycerides and excess inflammation HDL and LDL particles become smaller and cholesterol-depleted, causing the concentration of HDL or LDL particles (HDL-P or LDL-P) to be discordant from the concentration of cholesterol (LDL-C or HDL-C.)16 Pregnancy is a potentially susceptible time for this discordance given the rapid elevations in triglycerides and excess inflammation seen in normal gestation. Therefore, we considered that higher lipoprotein particle concentrations and smaller lipoprotein particle size assessed in maternal plasma collected before 18 weeks’ gestation would be related to PTB. We also evaluated two novel markers of systemic inflammation, GlycA and GlycB that quantify enzymatically glycosylated acute phase proteins. In addition, a priori we hypothesized that associations may vary by maternal race given the race differences in PTB and lipid profiles
Materials and Methods
Women in the current study were enrolled in a larger prospective cohort designed to investigate the role of maternal nutritional influences on PTB (2004–2011, Pittsburgh, PA). Women aged 14–50 years who were free of chronic hypertension, diabetes, renal disease, and rheumatologic disorders carrying singleton infants were eligible. Women were enrolled before 18 weeks’ gestation and non-fasting maternal serum and plasma were collected. The Institutional Review Board of the University of Pittsburgh approved the protocol and all participants provided informed consent.
Within the larger cohort (n=674) we identified all women enrolled after 2007 who had serum lipids measured and had plasma available for quantification of lipoproteins (n=225; 33.4%). Of these, we selected all the cases of PTB (n=22) and 2:1 randomly selected controls (n=44). Sample was insufficient to quantify lipoproteins in 2 women, thus final sample include 22 cases of PTB and 42 controls (n=64). When compared to women in the larger cohort, the women included in this analysis were not different according to age, race/ethnicity, pre-pregnancy body mass index (BMI), and education (all p>0.10).
Plasma lipoproteins were quantified using NMR spectroscopy by LipoScience (Raleigh, NC). 17 Using spectral analysis, the composite lipoprotein signal is decomposed to give the signal amplitudes (and particle concentrations) of several VLDL, LDL, and HDL subclasses. Interassay CVs for HDL and LDL particles were <4%. We also evaluated the lipoprotein insulin resistance score (LP-IR) which is a weighted composite of the 6 parameters that are particularly sensitive to the metabolic changes associated with insulin resistance. 18,19 Two biomarkers of systemic inflammation, GlycA and GlycB that quantify global glycosylated protein burden were measured by NMR spectroscopy. They represent levels of O- and N-linked glycoproteins; GlycA includes β-D-N-Acetylglucosamine (GLcNAc) and β-D-N-Acetylgalactosamine (GalNAc) and GlycB (α-N-Acetylneuraminic acid [NeuNAc, aka sialic acid]). Between-run precision of GlycA and GlycB levels was <4%.
Serum total cholesterol, HDL cholesterol, LDL cholesterol and triglyceride concentrations were determined enzymatically (Pointe Scientific, Canton, MI). The inter- and intra-assay variability was < 10% and < 5%, respectively.
Gestational age was based on the best obstetrical estimate using last menstrual period and ultrasounds early in pregnancy. PTB was defined as delivery before 37 completed weeks’ gestation. Cases were further classified as spontaneous (those following spontaneous onset of labor or preterm premature rupture of membranes) and medically indicated. Women completed a structured interview and reported race, age, smoking status, and parity. Race was self-reported as Non-Hispanic Black, Non-Hispanic White, or Asian (Chinese or Japanese). The 13 women who reported their race/ethnicity as Chinese or Japanese were excluded for the race-specific comparisons due to small numbers. Body mass index (BMI, kg/m2) was calculated using reported pre-pregnancy weight and measured height.
Lipoprotein particle concentrations and mean size were compared according to PTB status, and according to maternal race using t-tests or Wilcoxon rank sum test when not normally distributed. These were adjusted for pre-pregnancy BMI, race and smoking using linear regression. We replicated analyses in women with triglycerides above the sample median (>78 mg/dl) to explore the possibility that lipoprotein differences (discordance) from lipid levels would be exacerbated in the setting of high triglycerides. We calculated Spearman correlation coefficients between all lipoprotein variables, pre-pregnancy BMI and gestational week of sample collection to characterize early gestational changes. Lipoproteins were also evaluated according to race-PTB groups using linear regression. We considered a two-sided p<0.05 as significant, and did not account for multiple comparisons given the exploratory nature of our study and the modest sample size.
Results
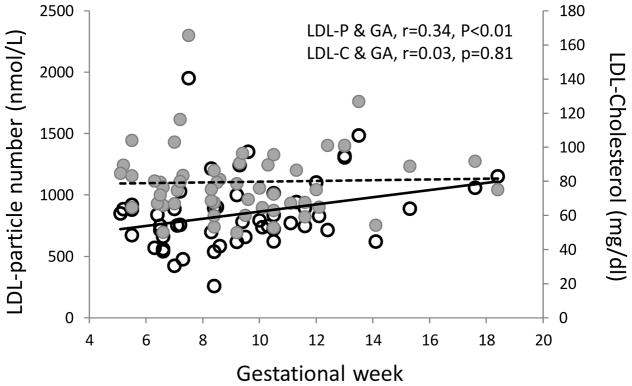
In plasma collected on average at 9 weeks’ gestation (SD 3.0; range 5–18), the concentration of total LDL-P was higher with later gestational week of sampling (r=0.34, p<0.01; Figure 1). In contrast, LDL-cholesterol was not associated with gestational week in these samples (r=0.03, p=0.81). The only other marker correlated with gestational week was triglycerides (r=0.43, p<0.01).
Figure 1.
LDL-cholesterol (closed circles, dotted line) and LDL-particle (open circles, solid line) concentrations according to gestational week of sample collection
Abbreviations: LDL, Low-density Lipoprotein; LDL-P, Low-density Lipoprotein Particles; LDL-C, Low-density Lipoprotein Cholesterol; GA, Gestational Age in Weeks.
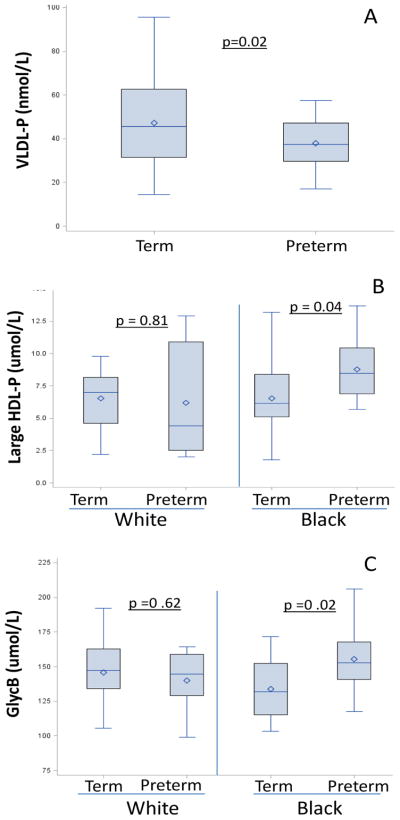
Among traditional cholesterol-content measures, triglycerides tended to be lower among women with preterm compared to term births, but total, LDL and HDL cholesterol levels did not differ by PTB status (Table 1). Women with subsequent PTB had lower concentrations of total VLDL-P in the first trimester (35.2 vs. 45.7 nmol/L, difference −10.5; p=0.02; Figure 2 panel A), due to significantly fewer small VLDL-P which made up over 80% of total VLDL-P. The difference in total VLDL-P among women with preterm v term births was 10.66 nmol/L (p=0.03) after adjustment for pre-pregnancy BMI, race, and smoking. Of note, this adjusted estimate was similar to the unadjusted difference. No other lipoprotein subclass particle counts or size differed according to PTB status.
Table 1.
Maternal characteristics, lipoprotein subclasses and size and traditional lipids in pregnant women, according to preterm birth status
| Term (n=42) | PTB (n=22) | p | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age (years) | 23.4 (4.4) | 23.5 (3.2) | 0.93 |
| Pre-pregnant BMI (kg/m2) | 27.1 (7.7) | 28.8 (6.7) | 0.42 |
| Smoking, n (%) | 12 (30.0) | 6 (27.3) | 0.82 |
| Race/ethnicity, n (%) | 0.22 | ||
| White | 17 (41.4) | 6 (28.6) | |
| Black | 14 (34.2) | 12 (57.14) | |
| Other | 10 (24.4) | 3 (14.3) | |
| Lipoproteins | |||
| VLDL particles (nmol/L) | |||
| Total VLDL and chylomicrons | 45.7 (31.5, 62.7) | 35.2 (29.2, 47.1) | 0.02 |
| Large LVDL and chylomicrons | 1.30 (0.8, 1.95) | 1.30 (0.6, 1.6) | 0.13 |
| Medium VLDL | 5.60 (2.95, 8.40) | 5.0 (3.2, 6.2) | 0.28 |
| Small VLDL | 36.2 (26.7, 53.4) | 30.8 (21.2, 40.8) | 0.03 |
| IDL particles (nmol/L) | 130.7 (80.2) | 122.4 (78.2) | 0.69 |
| LDL particles (nmol/L) | |||
| Total | 817.9 (315.9) | 826.6 (255.5) | 0.91 |
| Large | 320.2 (222.6) | 348.1 (283.7) | 0.67 |
| Small | 414.5 (196.8) | 402.5 (215.4) | 0.83 |
| HDL particles (μmol/L) | |||
| Total | 27.4 (5.6) | 29.2 (6.6) | 0.26 |
| Large | 6.7 (2.8) | 7.4 (3.4) | 0.39 |
| Medium | 7.5 (3.5) | 7.2 (3.1) | 0.76 |
| Small | 13.3 (2.8) | 14.7 (4.6) | 0.21 |
| VLDL size (nm) | 43.1 (4.2) | 43.7 (5.0) | 0.63 |
| LDL size (nm) | 20.8 (0.5) | 20.8 (0.4) | 0.80 |
| HDL size (nm) | 9.7 (0.4) | 9.6 (0.5) | 0.50 |
| Lipoprotein Insulin Resistance index | 29.5 (19.0, 37.0) | 31.5 (14.0, 38.0) | 0.96 |
| GlycA (μmol/L) | 368.4 (50.8) | 389 (59.2) | 0.16 |
| GlycB (μmol/L) | 139.4 (22.0) | 157.3 (37.5) | 0.02 |
| Cholesterol contents | |||
| Total cholesterol (mg/dl) | 157.9 (27.8) | 158.4 (35.7) | 0.95 |
| HDL-cholesterol (mg/dl) | 47.2 (11.7) | 50.4 (15.1) | 0.36 |
| LDL-cholesterol (mg/dl) | 81.4 (21.9) | 76.8 (68.5) | 0.42 |
| Triglycerides (mg/dl) | 84.9 (63.2, 114.0) | 74.0 (56.0, 87.6) | 0.08 |
Values are mean (SD) unless noted with * as median (IQR)
Abbreviations: PTB, Preterm Birth; BMI, Body Mass Index; VLDL, Very Low-density Lipoprotein; IDL, Intermediate-density Lipoprotein; LDL, Low-density Lipoprotein; HDL, High-density Lipoprotein; GlycA, Glycoprotein A; GlycB, Glycoprotein B.
Figure 2.
VLDL-P (panel A) according to preterm birth status; Large HDL-P (panel B) and Glyc B (panel C) according to race-preterm birth strata
Abbreviations: VLDL-P, Very Low-density Lipoprotein Particles; HDL-P, High-density Lipoprotein Particles.
GlycB, a measure of sialic acid and a marker of systemic inflammation, was higher in women with subsequent PTB compared to those with term births (157.3 vs. 139.4 μmol/L, p=0.02). This early pregnancy preterm-term difference was unaffected when adjusted for BMI, race, and smoking (19.2 μmol/L, p=0.02), and again, was almost doubled when limited to women with triglycerides above the median (33.6 nmol/L, p=0.03).
Finally, we considered the potential effects of race on our results. Overall, compared with White women, Black women had lower levels of VLDL particles (total, large and small VLDL-P) and higher levels of HDL-C, but other lipid and lipoprotein levels were not different (Table 2). However, when evaluated in race-PTB groups, both Black and White women with PTB had lower levels of VLDL-P compared to their counterparts with term births, adjusted for BMI (Figure 2, panel A). Black women with PTB had higher concentrations of large HDL-P and GlycB compared to their counterparts with term births (Figure 2, panels B and C). In contrast, neither HDL-P nor GlycB was elevated in White women with preterm v. term births. There were no other race specific differences in lipids or lipoproteins.
Table 2.
Lipoprotein subclasses and size and traditional lipids in pregnant women, according to Race/Ethnicity
| African American (n=26) | White (n=23) | p | |
|---|---|---|---|
| Lipoproteins | |||
| VLDL particles (nmol/L) | |||
| Total VLDL and chylomicrons | 35.6 (29.6, 49.1) | 50.7 (45.3, 63.1) | 0.01 |
| Large LVDL and chylomicrons | 1.0 (0.6, 1.6) | 1.5 (1.0, 1.9) | 0.06 |
| Medium VLDL | 5.6 (3.1, 7.9) | 4.6 (2.3, 6.2) | 0.49 |
| Small VLDL | 29.3 (22.7, 42.0) | 44.9 (34.3, 56.4) | <0.01 |
| IDL particles (nmol/L) | 126 (84.7) | 137.7 (87.8) | 0.64 |
| LDL particles (nmol/L) | |||
| Total | 797 (302.1) | 890 (335.5) | 0.32 |
| Large | 336 (197) | 363 (269) | 0.69 |
| Small | 384 (220) | 428 (196) | 0.48 |
| HDL particles (μmol/L) | |||
| Total | 28.3 (7.0) | 28.8 (4.8) | 0.79 |
| Large | 7.5 (2.9) | 6.4 (3.0) | 0.19 |
| Medium | 7.0 (3.7) | 8.4 (3.0) | 0.14 |
| Small | 13.8 (4.1) | 13.9 (13.9) | 0.90 |
| VLDL size (nm) | 42.8 (4.9) | 43.5 (4.9) | 0.65 |
| LDL size (nm) | 20.8 (0.4) | 20.8 (0.4) | 0.89 |
| HDL size (nm) | 9.8 (0.4) | 9.6 (0.5) | 0.66 |
| Lipoprotein IR index | 25.5 (12.0, 36.0) | 33.0 (27.0, 39.0) | 0.36 |
| Glycoprotein A (μmol/L) | 383 (61.7) | 369 (50.5) | 0.40 |
| Glycoprotein B (μmol/L) | 143.8 (24.9) | 144.3 (23.9) | 0.94 |
| Cholesterol content | |||
| Total cholesterol (mg/dl) | 162.5 (28.7) | 159.2 (142.7) | 0.75 |
| HDL-cholesterol (mg/dl) | 53.3 (13.0) | 42.7 (12.2) | <.01 |
| LDL-cholesterol (mg/dl) | 79.1 (17.8) | 85.5 (24.0) | 0.31 |
| Triglycerides (mg/dl) | 74.0 (62.3, 100.0) | 86.6 (66.3, 137.0) | 0.10 |
Values are mean (SD) unless noted with * as median (IQR)
Abbreviations: VLDL, Very Low-density Lipoprotein; IDL, Intermediate-density Lipoprotein; LDL, Low-density Lipoprotein; HDL, High-density Lipoprotein; IR, Insulin Resistance.
Discussion
In our exploratory nested case control study, evaluation of lipoproteins and inflammation early in pregnancy revealed what may be important differences related to subsequent PTB. Overall, Black women had lower levels of total and small VLDL-P compared to White women, yet both Black and White women with PTBs had lower total and small VLDL-P at <18 weeks gestation than those without PTB. Furthermore, for both Black and White women, concentrations of LDL particles (LDL-P), but not LDL cholesterol (LDL-C), were positively associated with gestational age. In addition, among Black women, those with PTB had higher levels of the inflammatory marker GlycB and also higher levels of large HDL-P. These differences not detected in White women.
We can only speculate about the mechanisms that may explain our results. VLDL particles are precursors to LDL particles in the circulation, so lower concentrations may signal an impaired lipid metabolism required for placentation and fetal growth. This is consistent with a previous report of lower concentrations of small, dense VLDL particles in the third trimester among women who delivered growth restricted infants. 20 We and others have reported that triglycerides may be elevated in women with PTB. 7–9,11 Our results here suggest that the lipoprotein and inflammatory differences in women with PTB were particularly robust when evaluated in women with triglycerides above the median, providing some additional insight into the lipid-PTB association.
One study reported excess risk of recurrent PTB associated with larger VLDL-P size assessed at 16–21 weeks’ gestation. 21 While we detected no difference in VLDL-P size between women with and without PTB, the lipoprotein characteristics assessed in our study were evaluated in samples collected much earlier in gestation (mean gestational age 9.1 weeks’), likely before the most profound VLDL-P changes occur. There is evidence of a blunted triglyceride response in the first half of pregnancy in women with subsequent PTB22,23 and thus lipoprotein changes across gestation may also be informative.
Our cross-sectional data suggest that LDL-P but not LDL-C were positively associated with gestational week in the first half of pregnancy (range 5–18 weeks). Our results raise the possibility that evaluating lipoprotein particle concentration and size may reveal significant changes that may be masked when only measuring cholesterol content. How these changes, along with low VLDL-P, may be related to PTB are also important, unanswered questions.
Our finding of elevated GlycB (which assesses sialic acid) in the 1st trimester among women who deliver preterm is consistent with one recent report of elevated sialic acid in maternal plasma collected at delivery among women who delivered preterm vs. term, 24 and another that reported excess NMR-measured N-acetyl glycoproteins in maternal plasma collected after delivery of very low birth weight preterm infants. 25 In our exploratory analysis, we found that the association of GlycB with preterm birth was stronger in women with triglycerides above vs. below the median, providing support for the notion that high triglycerides reflect a pro-inflammatory milieu.
Exploratory race-specific analyses showed that the association of preterm birth with lower VLDL-P was not confounded by race as associations were similar among black and white women. In contrast, the association of higher GlycB and higher large HDL-P with preterm vs. term birth was strong among black women, but appeared absent among white women. Potential mechanisms for this racial difference in the association of these markers with preterm birth are unclear. However, the Dallas Heart Study has reported a racial difference in the relation of HDL indices to atherosclerosis and incident cardiovascular events.26 Furthermore, an emerging body of evidence has suggested that high levels of inflammation are associated with “dysfunctional” HDL that may be related to atherosclerosis and cardiovascular disease. 27,28,29 Whether or how HDL function may be altered during pregnancy is unknown, but the association of high inflammation (GlycB) and high large HDL-P with PTB in our study mirrors a previous report that the combination of high C-reactive protein or high IL-6 with high HDL-C identified individuals at high risk for cardiovascular events.29,30
Our findings must be considered in light of limitations. The study population was small, and results must be replicated in larger populations. We evaluated many markers, and due to small sample size were not able to adjust for multiple comparisons. In addition, although our pregnancy samples were non-fasting, adults in modern society spend much of their waking hours in the non-fasting state, and non-fasting levels may better or similarly predict adverse non-pregnancy outcomes such as cardiovascular events than fasting levels. 29 We analyzed lipoproteins at one time point during the first half of pregnancy. The trajectory of change across gestation may also be informative. Larger studies are needed to examine if lipoprotein differences are detectable in cases of spontaneous and indicated preterm births, a distinction that our small study could not examine.
Our results suggest that early in pregnancy, women with subsequent PTB have lower levels of VLDL- particles than women without PTB. This finding was particularly apparent among women with high triglycerides. LDL-P, but not LDL-C, was positively associated with gestational age. These results raise the possibility that an impaired lipid response required for placentation and fetal growth is involved in the pathogenesis of PTB. Furthermore, Black-White differences in the lipoprotein profile in the 1st trimester (lower VLDL-P and higher HDL-C) were similar to findings in non-pregnant women. However, the race-specific associations of higher large HDL-P (but not HDL-C) and GlycB with PTB suggest a possible role for dysfunctional HDL that warrants further study.
Acknowledgments
The research was supported by a grant (5K12HD43441-09) from the National Institutes of Health’s National Institute of Child Health and Human Development; Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) Award.
Footnotes
The authors report no conflict of interest.
References
- 1.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 2.Hamilton B, Martin J, Ventura S. National Vital Statistics Report. 2009. Births: Preliminary data for 2007; p. 57. [PubMed] [Google Scholar]
- 3.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in Pre-pregnancy Obesity in Nine States, 1993–2003. Obesity. 2007;15:986–93. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 4.Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–9. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- 5.Culhane JF, Goldenberg RL. Racial Disparities in Preterm Birth. Seminars in Perinatology. 2011;35:234–9. doi: 10.1053/j.semperi.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Kant AK, Graubard BI. Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003–2006. The American Journal of Clinical Nutrition. 2012;96:601–12. doi: 10.3945/ajcn.112.035535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edison RJ, Berg K, Remaley A, et al. Adverse Birth Outcome Among Mothers With Low Serum Cholesterol. Pediatrics. 2007;120:723–33. doi: 10.1542/peds.2006-1939. [DOI] [PubMed] [Google Scholar]
- 8.Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy Lipids Related to Preterm Birth Risk: the Coronary Artery Risk Development in Young Adults Study. Journal of Clinical Endocrinology and Metabolism. 2010:2009–28. doi: 10.1210/jc.2009-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnussen EB, Vatten LJ, Myklestad K, Salvesen KÅ, Romundstad PR. Cardiovascular risk factors prior to conception and the length of pregnancy: population-based cohort study. American Journal of Obstetrics and Gynecology. 2011;204:526e1–8. doi: 10.1016/j.ajog.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Alleman BW, Smith AR, Byers HM, et al. A proposed method to predict preterm birth using clinical data, standard maternal serum screening, and cholesterol. American Journal of Obstetrics and Gynecology. 2013;208:472e1–e11. doi: 10.1016/j.ajog.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudd LM, Holzman CB, Catov JM, Senagore PK, Evans RW. Maternal lipids at mid-pregnancy and the risk of preterm delivery. Acta Obstet Gynecol Scand. 2012;91:726–35. doi: 10.1111/j.1600-0412.2012.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubel C, Shakir Y, Gallaher M, McLaughlin M, Roberts J. Low-density lipoprotein particle size decreases during normal pregnancy in association with triglyceride increases. Journal of the Society for Gynecologic Investigation. 1998;5:244–50. doi: 10.1016/s1071-5576(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 13.Hubel CA, Lyall F, Weissfeld L, Gandley RE, Roberts JM. Small low-density lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metabolism. 1998;47:1281–8. doi: 10.1016/s0026-0495(98)90337-7. [DOI] [PubMed] [Google Scholar]
- 14.Meyer BJ, Stewart FM, Brown EA, et al. Maternal Obesity Is Associated With the Formation of Small Dense LDL and Hypoadiponectinemia in the Third Trimester. Journal of Clinical Endocrinology & Metabolism. 2013;98:643–52. doi: 10.1210/jc.2012-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackey R, Kuller L, Sutton-Tyrrell K, Evans R, Holubkov R, Matthews K. Hormone therapy, lipoprotein subclasses, and coronary calcification: the Healthy Women Study. Arch Intern Med. 2005;165:510–5. doi: 10.1001/archinte.165.5.510. [DOI] [PubMed] [Google Scholar]
- 16.Otvos J, Mora S, Shalaurova I, Greenland P, Mackey R, Goff DJ. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–13. doi: 10.1016/j.jacl.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyarajah E, Cromwell W, Otvos J. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clinics in laboratory medicine. 2006;26:847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Festa A, Williams K, Hanley AJG, et al. Nuclear Magnetic Resonance Lipoprotein Abnormalities in Prediabetic Subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–72. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 19.Garvey WT, Kwon S, Zheng D, et al. Effects of Insulin Resistance and Type 2 Diabetes on Lipoprotein Subclass Particle Size and Concentration Determined by Nuclear Magnetic Resonance. Diabetes. 2003;52:453–62. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 20.Sattar N, Greer I, Galloway P, et al. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. Journal of Clinical Endocrinology and Metabolism. 1999;84:128–30. doi: 10.1210/jcem.84.1.5419. [DOI] [PubMed] [Google Scholar]
- 21.Thorp JM, Jr, Rice MM, Harper M, et al. Advanced lipoprotein measures and recurrent preterm birth. Am J Obstet Gynecol. 2013 doi: 10.1016/j.ajog.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scifres C, Catov J, Simhan H. Maternal race and gestational age dependent changes in maternal serum lipids. Am J Obstet Gynecol. 2013;208:S93–S4. [Google Scholar]
- 23.Tyroler H, Glueck C, Christensen B, Kwiterovich P. Plasma high-density lipoprotein cholesterol comparisons in black and white populations. The Lipid Research Clinics Program Prevalence Study. Circulation. 1980;62:99–107. [PubMed] [Google Scholar]
- 24.Kwiterovich P, Virgil D, Garrett E, et al. Lipoprotein heterogeneity at birth: influence of gestational age and race on lipoprotein subclasses and Lp (a) lipoprotein. Ethn Dis. 2004;14:351–9. [PubMed] [Google Scholar]
- 25.Sala F, Catapano A, Norata G. High density lipoproteins and atherosclerosis: emerging aspects. Journal of Geriatric Cardiology. 2012;9:401–7. doi: 10.3724/SP.J.1263.2011.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas M, Mooradian A. Inflammation, high-density lipoprotein and cardiovascular dysfunction. Current Opinion in Infectious Diseases. 2011;24:265–72. doi: 10.1097/QCO.0b013e328344b724. [DOI] [PubMed] [Google Scholar]
- 27.Ugur M, Kurtul N, Balat O, Ekici M, Kul S. Assessment of maternal serum sialic acid levels in preterm versus term labor: a prospective-controlled clinical study. Archives of Gynecology and Obstetrics. 2012;286:1097–102. doi: 10.1007/s00404-012-2423-2. [DOI] [PubMed] [Google Scholar]
- 28.Tea I, Le Gall G, Küster A, et al. 1H-NMR-Based Metabolic Profiling of Maternal and Umbilical Cord Blood Indicates Altered Materno-Foetal Nutrient Exchange in Preterm Infants. PLoS ONE. 2012;7:e29947. doi: 10.1371/journal.pone.0029947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM. Fasting versus Nonfasting Triglycerides and the Prediction of Cardiovascular Risk: Do We Need to Revisit the Oral Triglyceride Tolerance Test? Clin Chem. 2008;54:11–3. doi: 10.1373/clinchem.2007.097907. [DOI] [PubMed] [Google Scholar]