SUMMARY
G-protein-coupled receptors (GPCRs) constitute the largest superfamily of cell-surface signaling proteins. However, mechanisms underlying their surface targeting and sorting are poorly understood. Here, we screen the Rab family of small GTPases in the surface transport of multiple GPCRs. We find that manipulation of Rab43 function significantly alters the surface presentation and signaling of all GPCRs studied without affecting non-GPCR membrane proteins. Rab43 specifically regulates the transport of nascent GPCRs from the endoplasmic reticulum (ER) to the Golgi. More interestingly, Rab43 directly interacts with GPCRs in an activation-dependent fashion. The Rab43-binding domain identified in the receptors effectively converts non-GPCR membrane protein transport into a Rab43-dependent pathway. These data reveal a crucial role for Rab43 in anterograde ER-Golgi transport of nascent GPCRs, as well as the ER sorting of GPCR members by virtue of its ability to interact directly.
In Brief
Li et al. report that Rab43 GTPase controls anterograde ER-Golgi transport of nascent GPCRs, as well as their sorting from other membrane proteins, which is mediated via direct interaction with the receptors. Their results suggest a mechanism of targeting and sorting of the members of the GPCR superfamily.
INTRODUCTION
G-protein-coupled receptors (GPCRs) constitute the largest and the most structurally diverse superfamily of membrane receptors and modulate a wide variety of physiological and pathological functions; they represent therapeutic targets of approximately one-third of the drugs on the market (Bradley and Tobin, 2016; Kobilka, 2011; Pierce et al., 2002; Venkatakrishnan et al., 2013). The function of GPCRs can be mediated through coupling to heterotrimeric G proteins, arrestins, and other signaling proteins that in turn activate downstream effectors, such as protein kinases, adenylyl cyclases, phospholipases, and ion channels. One important factor that regulates the precise function of the receptors is their intracellular trafficking processes, which determine the amount of the receptors at the cell surface, the functional destination for most GPCRs.
Intracellular trafficking of GPCRs begins at the endoplasmic reticulum (ER), where they are synthesized. Correctly folded and properly assembled receptors are able to pass the ER quality-control system and move forward from the ER to the Golgi, where the receptors may undergo post-translational modifications, such as glycosylation, to attain mature status and then reach the cell surface, where they are available for binding to their cognate ligands. Upon agonist stimulation, the receptors at the cell surface may become internalized into the endosomal compartment. The internalized receptors in endosomes can be sorted to a recycling pathway for return to the plasma membrane, to a lysosome pathway for degradation, or to a retrograde pathway for transport to the Golgi. Over the past few decades, most studies of GPCR trafficking have focused on the events involved in internalization, recycling, and degradation (Hanyaloglu and von Zastrow, 2008; Kang et al., 2014; Marchese et al., 2008; Tan et al., 2004). However, the molecular mechanisms that govern the anterograde cell-surface export of GPCRs en route from the ER through the Golgi, as well as their sorting from other plasma membrane proteins during biosynthesis and maturation, remain poorly understood.
Rab GTPases form the largest branch of the Ras-related small GTPase superfamily and are the master regulators of vesicle-mediated membrane traffic in exocytic and endocytic pathways (Hutagalung and Novick, 2011; Pfeffer and Aivazian, 2004). Although there are many unanswered questions regarding how these Rab GTPases are orchestrated to ensure the transport of distinct cargoes to their final destinations, it is well known that each Rab has a distinct subcellular localization pattern that correlates with its function in directing cargo transport between specific subcellular compartments. Compared with many other secretory Rab GTPases, the function of Rab43 is poorly characterized. Rab43 localizes at the Golgi (Cox et al., 2016; Haas et al., 2005, 2007) and is important for the maintenance of Golgi structure and function (Haas et al., 2007), retrograde transport of Shiga toxin from the cell surface to the trans-Golgi (Haas et al., 2007), phagosome maturation (Seto et al., 2011), assembly of herpes simplex virus 1 (Zenner et al., 2011), and antigen cross-presentation by dendritic cells (Kretzer et al., 2016). As expression of its dominant-negative mutant induced the redistribution of GM130 to punctate structures adjacent to ER exit sites, Rab43 was suggested to regulate the early ER-Golgi secretory pathway (Dejgaard et al., 2008). However, the actual cargoes that use the Rab43-mediated pathway to traffic from the ER to the Golgi have not been identified. Here, we show that Rab43 specifically modulates the ER-to-Golgi transport of newly synthesized GPCRs and that this function of Rab43 is mediated via direct and activation-dependent interaction with the receptors. These data identify an important role for Rab43 in the sorting and biosynthesis of GPCRs and suggest a specific pathway that requires Rab43 and mediates the forward trafficking of nascent GPCRs.
RESULTS
Rab43 Regulates the Cell-Surface Transport, Subcellular Localization, and Function of α2B-AR
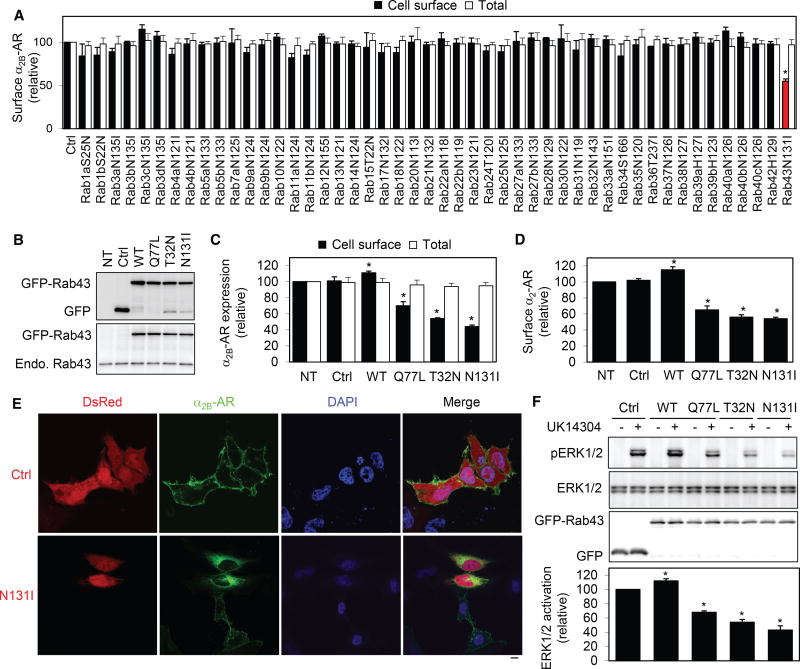
To systemically investigate the function of the Rab GTPase family in the anterograde transport of GPCRs, we first determined the effect of transient expression of 48 dominant-negative Rab mutants on the cell-surface expression of α2B-adrenergic receptor (AR), a prototypic GPCR, using stable HEK293 cells expressing N-terminal hemagglutinin (HA)-tagged α2B-AR. Rab mutants were tagged with GFP, and their expression was detected by fluorescent microscopy (Figure S1). Among these Rab GTPases examined, only Rab43 mutant markedly attenuated the cell-surface number of α2B-AR by ~45% compared with cells transfected with control vectors as measured by intact live-cell ligand binding using the cell-nonpermeable radioligand [3H]-RX821002 (Figure 1A). In contrast, expression of other 47 Rab mutants did not significantly influence the cell-surface expression of α2B-AR. Furthermore, none of these Rab mutants altered the overall synthesis of α2B-AR as measured by flow cytometry following staining with HA antibodies in permeabilized cells (Figure 1A).
Figure 1. Effect of Rab43 and Its Mutants on the Cell-Surface Expression and Signaling of α2B-AR.
(A) Screening for Rab GTPases involved in the surface transport of α2B-AR. HEK293 cells stably expressing HA-α2B-AR were transfected with individual GFP-Rab mutants. The surface expression of α2B-AR was determined by intact cell ligand binding using [3H]-RX821002. The mean value of specific radioligand binding was 25,652 ± 1,745 cpm from cells transfected with the control vector.
(B) Western blot analysis of expression of Rab43 and its mutants by using GFP (top) and Rab43 antibodies (bottom). NT, non-transfection.
(C) Effect of Rab43 and its mutants on the surface expression of stably expressed HA-α2B-AR in HEK293 cells.
(D) Effect of Rab43 on the surface expression of endogenous α2-AR in MCF-7 cells. The mean value of specific ligand binding was 563 ± 67 cpm in MCF-7 cells without transfection.
(E) Effect of Rab43N131I on the subcellular distribution of α2B-AR. HEK293 cells were transfected with α2B-AR-GFP together with the vector dsRed-C1 (Ctrl) or dsRed-Rab43N131I. The subcellular distribution of α2B-AR was revealed by confocal microscopy.
(F) Effect of Rab43 on α2B-AR-mediated ERK1/2 activation. HEK293 cells stably expressing α2B-AR were transfected with the pEGFP-C1 vector (Ctrl) or GFPRab43. The cells were stimulated with UK14304 at 1 µM for 5 min, and ERK1/2 activation was determined by immunoblotting.
Data represent mean ± SE (n = 3–4) (A, C, D, and F). The images shown in (E) are representatives of at least 5 experiments. Scale bar, 10 µm. *p < 0.05 versus Ctrl.
See also Figures S1 and S2.
To further characterize the function of Rab43 in the cell-surface transport of α2B-AR, we generated two additional Rab43 mutants: a constitutively active Q71L mutant and a dominant-negative T32N mutant. GFP-Rab43 and GFP-Rab43Q71L were partially colocalized with the ER exit site markers Sec23 and Sec24, the ER-Golgi intermediate marker ERGIC53, and the cis-Golgi marker GM130 (Figures S2A and S2B). Consistent with previous studies (Cox et al., 2016; Haas et al., 2005, 2007), the dominant-negative mutants Rab43T32N and Rab43N133I were expressed in the cytoplasm and caused a dispersal of the Golgi structure as indicated by staining of GM130 antibodies (Figures S2C and S2D). The expression levels of Rab43 and its mutants were very similar and were ~1.5 fold higher than endogenous Rab43 expression (Figure 1B). Transient expression of the negative mutants Rab43T32N and Rab43N133I was strongly inhibited, whereas Rab43 overexpression moderately but significantly enhanced the surface expression of stably transfected α2B-AR in HEK293 cells (Figure 1C). Furthermore, expression of the active mutant Rab43Q71L also inhibited the transport of α2B-AR (Figure 1C), suggesting that GTP hydrolysis and recycling of Rab43 are important events for Rab43 to perform its function in α2B-AR transport. Similar effects were observed on endogenous α2-AR in MCF-7 cells (Figure 1D).
To study the effect of Rab43 on the subcellular distribution of α2B-AR, α2B-AR-GFP and dsRed-Rab43N131I were coexpressed in HEK293 cells. As expected, confocal microscopy revealed that α2B-AR-GFP was robustly expressed at the cell surface in cells transfected with control vectors, whereas its surface expression was remarkably inhibited with an extensive accumulation in the perinuclear region in cells expressing Rab43N131I (Figure 1E). Altogether, these data strongly suggest that Rab43 plays an important role in the cell-surface transport of α2B-AR.
α2B-AR couples to the Gi/Go family of G proteins, and its activation inhibits adenylyl cyclases, stimulates ERK1/2, and suppresses voltage-gated calcium channels (Dong et al., 2011; Li et al., 1998, 2012; Li and Horn, 2008; Timmons et al., 2004). To determine whether regulation of α2B-AR transport by Rab43 influences the function of the receptor in cells, we measured the effect of manipulating Rab43 on ERK12/ activation. In parallel with their effects on the surface expression of α2B-AR, expression of individual Rab43 mutants significantly compromised ERK1/2 activation in response to stimulation with the α2-AR agonist UK14304, whereas expression of wild-type Rab43 slightly enhanced ERK1/2 activation as compared with cells expressing α2B-AR alone (Figure 1F). These data are consistent with the effects of Rab43 on the surface expression of α2B-AR.
Rab43 Controls α2B-AR Transport from the ER to the Golgi
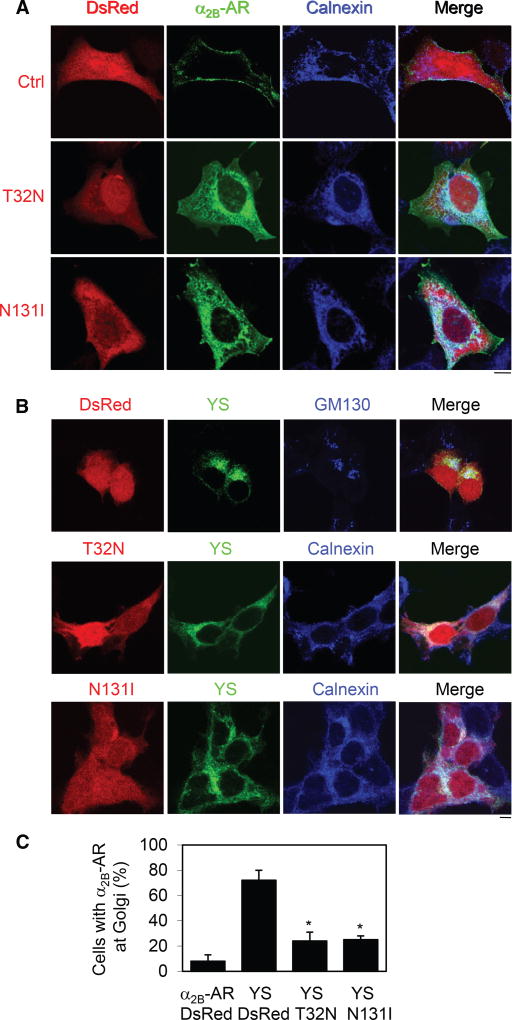
To define the intracellular compartments in which Rab43 regulates receptor transport, GFP-tagged α2B-AR was expressed together with dsRed-tagged Rab43 mutants, and colocalization of α2B-AR with different intracellular organelle marker proteins was revealed by confocal microscopy. The cell-surface expression of α2B-AR was remarkably attenuated and the receptors were strongly colocalized with the ER marker calnexin (Figure 2A), but not the cis-Golgi marker GM130 (Figure S3A), in cells expressing Rab43 mutants.
Figure 2. Rab43 Regulates α2B-AR Transport from the ER to the Golgi.
(A) Colocalization of α2B-AR with the ER marker calnexin. HEK293 cells were transfected with α2B-AR-GFP together with dsRed-C1 or dsRed-Rab43 mutants and then stained with calnexin antibodies.
(B) Effect of Rab43 on the ER-to-Golgi transport of the YS mutant. HEK293 cells were transfected with GFP-tagged YS mutant together with dsRed-C1 or dsRed-Rab43 mutants. The cells were then stained with GM130 or calnexin antibodies.
(C) Quantitation of the data shown in (B). Approximately 100 cells were counted in each experiment. The data are the mean ± SE (n = 3). *p < 0.05 versus YS plus dsRed.
The images are representative of 3 experiments. Scale bars, 10 µm. See also Figures S3 and S4.
We next took advantage of our previously characterized Golgilocalized YS mutant of α2B-AR (Dong and Wu, 2006) to determine whether Rab43 mutants block its transport from the ER to the Golgi. In contrast to the cell-surface expression of α2B-AR, the mutant YS-AA was retained in the Golgi (Figure S3B) and colocalized with GM130 (Figure 2B). Transient expression of Rab43 mutants strongly arrested the YS-AA mutant in the perinuclear region (Figure S3B), and the receptors were colocalized with calnexin (Figure 2B). Quantitative data showed that more than 70% of control cells had YS-AA at the Golgi, whereas less than 20% of cells expressing Rab43 mutants contained Golgi-localized YS-AA mutant (Figure 2C). These data indicate that expression of Rab43 mutants induces defective ER-to-Golgi transport of α2B-AR.
We then determined the effect of Rab43 on the ER export kinetics of α2B-AR and vesicular stomatitis virus glycoprotein (VSVG) in brefeldin A (BFA) treatment and washout experiments. BFA is a fungal metabolite that is well known to inhibit ER-Golgi cargo transport. BFA treatment markedly blocked α2B-AR and VSVG export from the ER (Figures S4A and S4C). At 1 hr washout, α2B-AR and VSVG exported from the ER in ~70% and 97% cells, respectively (Figures S4B and S4D), and they were clearly detected at the cell surface (Figures S4A and S4C). At 4 hr washout, both α2B-AR and VSVG had been fully exported from the ER. In contrast, α2B-AR was almost completely unable to move out of the ER and transport to the Golgi and cell surface even at 24 hr washout in cells expressing Rab43N131I (Figures S4A and S4B). Consistent with previous reports showing Rab43-independent transport of VSVG (Cox et al., 2016; Dejgaard et al., 2008), Rab43N131I did not affect the ER or cell-surface transport of VSVG (Figures S4C and S4D).
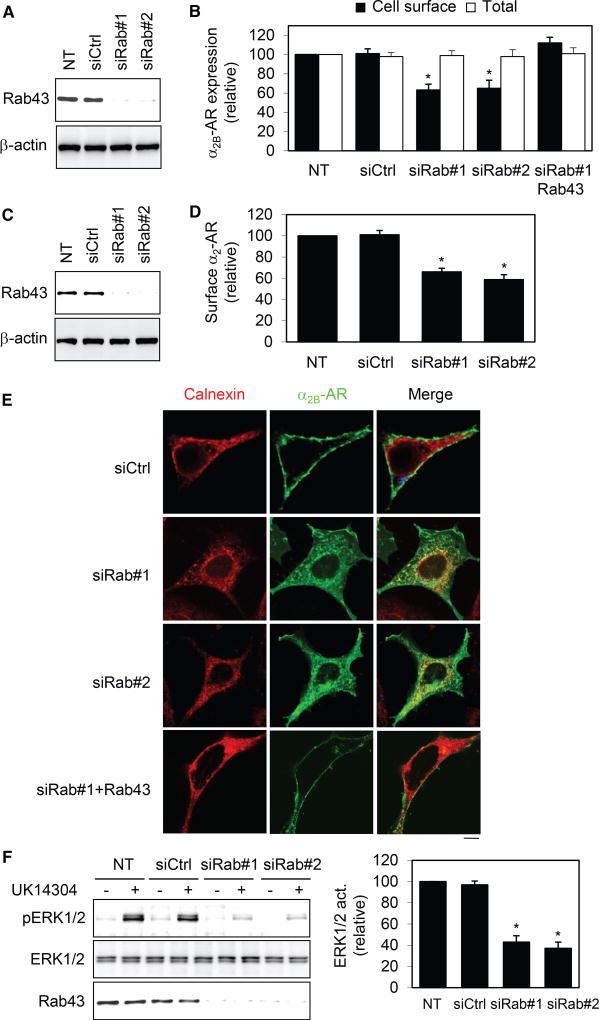
siRNA-Mediated Depletion of Rab43 Inhibits ER-to-Cell-Surface Transport of α2B-AR
A small interfering RNA (siRNA)-mediated depletion approach was then used to determine the role of Rab43 in the cell-surface transport of α2B-AR. Similar to Rab43 mutants, siRNA targeting Rab43 reduced the cell-surface expression of stably expressed α2B-AR in HEK293 cells (Figures 3A and 3B). Overexpression of Rab43 completely reversed the inhibitory effect of Rab43 siRNA on cell-surface transport of α2B-AR (Figure 3B). Expression of Rab43 siRNA also inhibited the cell-surface expression of endogenous α2-AR in MCF-7 cells (Figures 3C and 3D) by ~40%. siRNA-mediated depletion of Rab43 caused an extensive colocalization of α2B-AR with calnexin, but not GM130 (data not shown), whereas α2B-AR was strongly expressed at the cell surface in cells expressing control siRNA or Rab43 siRNA plus Rab43 (Figure 3E). Furthermore, Rab43 siRNA significantly inhibited α2B-AR-mediated ERK1/2 activation compared with cells expressing control siRNA (Figure 3F).
Figure 3. Effect of siRNA-Mediated Depletion of Rab43 on the Surface Expression and Signaling of α2B-AR.
(A) Western blot analysis of Rab43 expression in HEK293 cells.
(B) Effect of Rab43 siRNA on the surface expression of stably expressed HA-α2B-AR, and the rescue of their effect by Rab43.
(C) siRNA-mediated knockdown of Rab43 in MCF-7 cells.
(D) Effect of siRNA-mediated knockdown of Rab43 on the surface expression of endogenous α2-AR in MCF-7 cells.
(E) Colocalization of α2B-AR with calnexin in cells transfected with siRNA targeting Rab43.
(F) Effect of Rab43 siRNA on α2B-AR-mediated ERK1/2 activation.
Data represent mean ± SE (n = 3–4) (B, D, and F). *p < 0.05 versus NT. The images are representative of 3 experiments. Scale bar, 10 µm.
Rab43 Is Likely a Specific Regulator for the Transport of GPCRs
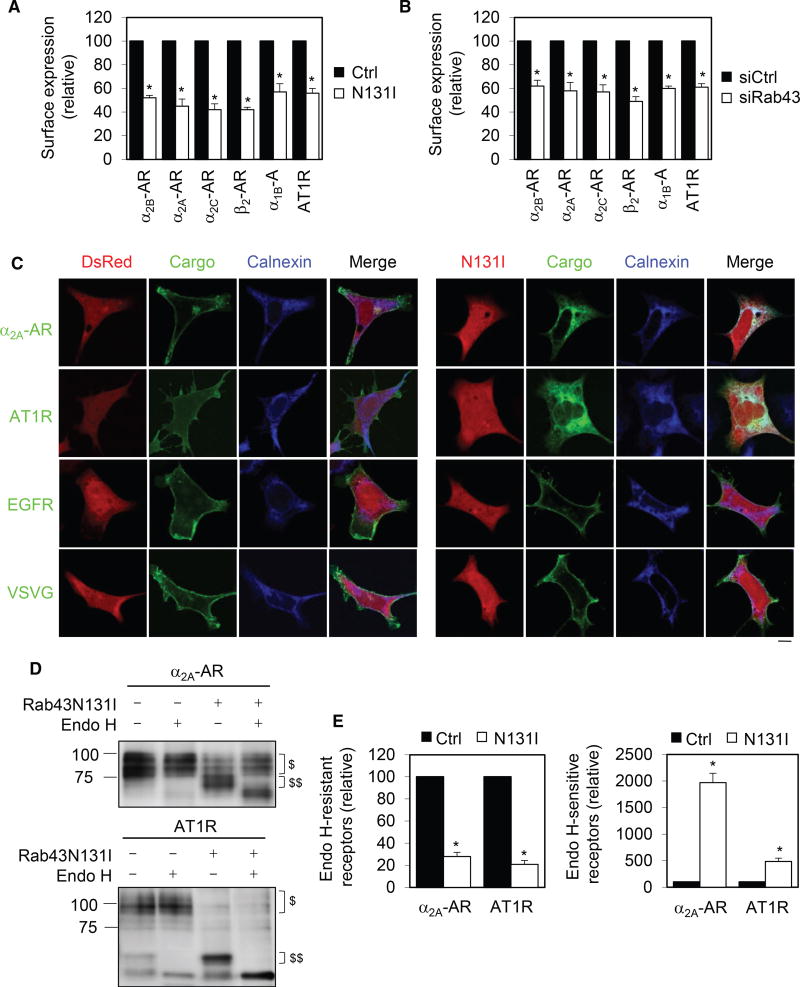
We next determined the effects of Rab43N131I on the cell-surface expression of six GPCRs (including α2A-AR, α2B-AR, α2C-AR, β2-AR, α1B-AR, and angiotensin II type 1 receptor [AT1R]), and two non-GPCR membrane proteins (epidermal growth factor receptor [EGFR], and VSVG). All of these six GPCRs belong to the family A, and they are prototypic GPCRs. Expression of Rab43N133I and Rab43 siRNA significantly attenuated the cell-surface number of all six GPCRs examined (Figures 4A and 4B). Further studies showed that overexpression of Rab43 slightly enhanced, whereas all three Rab43 mutants (Figure S5A) and two Rab43 siRNAs (Figure S5B) markedly reduced, the cell-surface expression of α2A-AR as measured by intact cell ligand binding.
Figure 4. Rab43 Modulates the ER-Surface Transport of Multiple GPCRs.
(A) Effect of Rab43N131I on the surface transport of GPCRs. HEK293 cells were transfected with individual GPCRs together with dsRed-C1 (Ctrl) or dsRed-Rab43N131I, and the cell-surface expression of each receptor was measured by radioligand binding or flow cytometry.
(B) Effect of siRNA-mediated depletion of Rab43 on the surface transport of GPCRs.
(C) Effect of Rab43 on the subcellular localization of different membrane proteins. Scale bar, 10 µm.
(D) Deglycosylation of α2A-AR (top) and AT1R (bottom) by Endo H treatment. $ indicates Endo-H-resistant bands and $$ Endo-H-sensitive bands.
(E) Quantitative data showing the effect of Rab43 on the formation of Endo-H-resistant (left) and Endo-H-sensitive glycosylation (right).
Data represent mean ± SE (n = 3–5) (A, B, and E). *p < 0.05 versus Ctrl. See also Figure S5.
Confocal microscopy revealed that Rab43N131I induced an extensive ER accumulation of α2A-AR and AT1R as indicated by colocalization with calnexin (Figure 4C). In contrast, Rab43N131 did not affect the surface expression of EGFR (Figure 4C) and the activation of ERK1/2 after stimulation with EGF (Figure S5C and S5D). Rab43N133I also did not influence the surface transport of VSVG (Figure 4C) These data suggest that Rab43 may specifically regulate the surface transport of GPCRs.
To further study the effect of Rab43 on the ER-Golgi transport of GPCRs, we measured the effect of Rab43 on the conversion from simple to complex N-linked glycosylation of α2A-AR and AT1R. α2B-AR does not have any N-linked glycosylation sites, whereas α2A-AR and AT1R have 2 and 3 N-linked glycosylation sites at their N termini, respectively. Expression of Rab43N131I dramatically reduced the acquisition of complex N-linked sugars (endoglycosidase H [Endo H] resistant) of both receptors (Figures 4D and 4E), which was further confirmed by PNGase F treatment to remove both simple and complex glycosylation (Figures S5E and S5F). In parallel, the formation of simple or Endo-H-sensitive glycosylation of both receptors was markedly enhanced by Rab4N131I (Figures 4D and 4E). These data further demonstrate that Rab43 regulates GPCR transport from the ER to the Golgi.
The Function of Rab43 in the Anterograde Transport of Newly Synthesized GPCRs, but Not Internalization
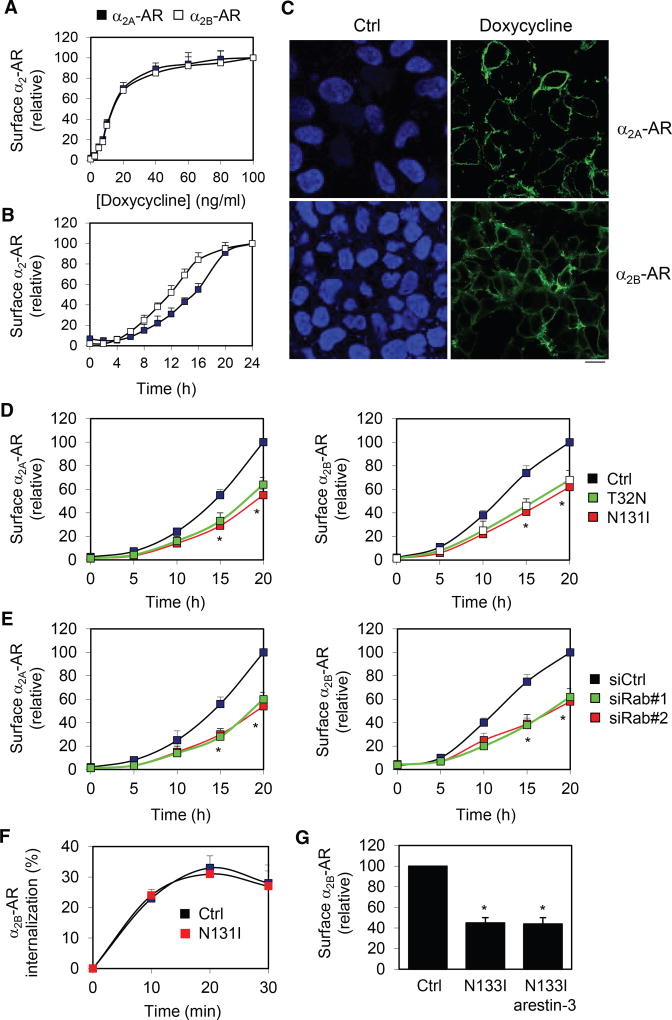
We next used the Tet-On 3G tetracycline-inducible gene expression system (Clontech Laboratories) to generate stable HEK293 cells inducibly expressing HA-α2A-AR and HA-α2B-AR and characterize the role of Rab43 in the surface transport of newly synthesized GPCRs. Although α2A-AR and α2B-AR have almost identical responses to different concentrations of doxycycline (Figure 5A), α2B-AR requires less time to reach the cell surface than α2A-AR (Figure 5B). Robust surface expression of both receptors was detected by confocal microscopy following staining with anti-HA antibodies in nonpermeabilized cells after treatment with doxycycline (Figure 5C).
Figure 5. Effect of Rab43 on the Surface Transport of Newly Synthesized GPCRs.
(A and B) Doxycycline dose- (A) and time-dependent (B) induction of the surface expression of α2A-AR and α2B-AR. In a typical experiment, the maximal specific binding of 3H-RX821002 is 25,673 cpm/well (24-well dish) in cells expressing α2A-AR (1.52 × 106 receptors/cell) and 22,459 cpm/well in cells expressing α2B-AR (1.46 × 106 receptors/cell).
(C) Representative images showing the expression of α2A-AR and α2B-AR after induction of doxycycline (40 ng/mL) for 24 hr followed by HA antibody staining in nonpermeabilized cells.
(D) Effect of Rab43 mutants on the surface expression of inducibly expressed α2A-AR and α2B-AR after incubation with doxycycline (40 ng/mL) for up to 20 hr.
(E) Effect of siRNA-mediated knockdown of Rab43 on the surface expression of inducibly expressed α2A-AR and α2B-AR.
(F) Effect of Rab43N133I on the internalization of α2B-AR. HEK293 cells stably expressing α2B-AR were transfected with Rab43N133I and arrestin-3 and then stimulated with epinephrine (100 µM) for 10–30 min.
(G) Effect of dominant-negative mutant arrestin-3(201–409) on the surface expression of α2B-AR. In each panel, the cell surface expression of α2-AR was determined by intact cell ligand binding, and the data are presented as the mean ± SE (n = 3–5). *p < 0.05 versus Ctrl.
Rab43 mutants and siRNA targeting Rab43 significantly inhibited the cell-surface expression of both α2A-AR and α2B-AR after doxycycline induction for 15 and 20 hr, and the inhibitory effects between Rab43 mutants and siRNA and between α2A-AR and α2B-AR were very similar (Figures 5D and 5E). These data clearly demonstrate that the normal function of Rab43 is required for cell-surface transport of nascent α2A-AR and α2B-AR.
Because previous studies suggested a role for Rab43 in retrograde transport from the cell surface to the Golgi (Haas et al., 2007), we studied whether Rab43 regulates the internalization of GPCRs in response to agonist stimulation. Rab43N131I did not affect the internalization of α2B-AR in response to epinephrine stimulation in HEK293 cells expressing arrestin-3, which was shown to enhance the internalization process of α2B-AR (DeGraff et al., 1999) (Figure 5F). Furthermore, dominant-negative mutant arrestin-3(201–409) could not reverse the inhibitory effect of Rab43N131I on the surface expression of α2B-AR (Figure 5G), suggesting that the effect of Rab43 on the surface expression of GPCRs was unlikely induced by constitutive internalization of the receptors.
Direct and Activation-Dependent Interaction of Rab43 with GPCRs
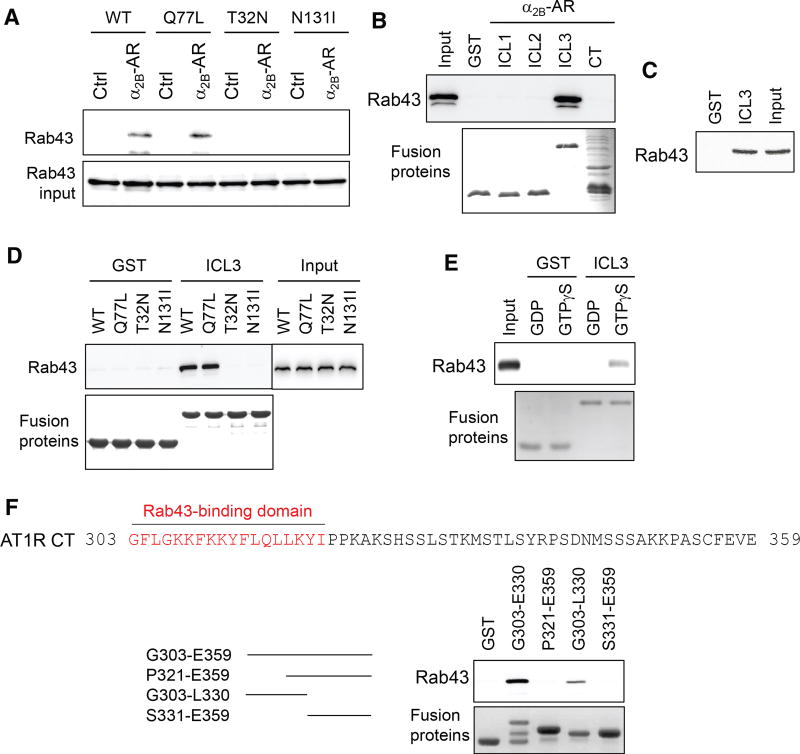
To elucidate the molecular mechanism underlying the function of Rab43 in GPCR transport, we determined whether Rab43 was able to associate with the receptors. In co-immunoprecipitation (co-IP) assays, HEK293 cells stably expressing HA-α2B-AR were transiently transfected with GFP-Rab43 or its mutants, and the receptors were pulled down by α2B-AR antibodies. The amount of Rab43 in the IP was similar in cells expressing Rab43 and its active mutant, Q77L, whereas it was undetectable in cells expressing the dominant-negative mutants T32N and N131I (Figure 6A).
Figure 6. Interaction of Rab43 with α2B-AR and AT1R.
(A) Interaction of α2B-AR with Rab43 as measured by co-IP. HEK293 cells without (Ctrl) or with stably expressed HA-α2B-AR were transfected with GFP-Rab43, and the receptors were immunoprecipitated with α2B-AR antibodies. The level of Rab43 was determined by immunoblotting.
(B) Interaction of the intracellular domains of α2B-AR with Rab43. The ICL1, ICL2, ICL3, and CT of α2B-AR were generated as GST fusion proteins and incubated with total cell homogenates expressing GFP-Rab43. Bound Rab43 was revealed by immunoblotting.
(C) Interaction of the α2B-AR ICL3 with endogenous Rab43 using total cytosolic proteins prepared from HEK293 cells.
(D) Interaction of the α2B-AR ICL3 with Rab43 and its mutants.
(E) Direct and activation-dependent interaction of Rab43 with the α2B-AR ICL3. Rab43 prepared by the in vitro translation system was incubated with 100 µM GTPγS or GDP for 1 hr and then incubated with GST or GST-ICL3.
(F) Rab43’s interaction with the AT1R CT and its fragments generated as GST fusion proteins. In each panel, similar results were obtained in at least 3 experiments. See also Figure S6.
We next sought to identify specific domains that mediated α2B-AR interaction with Rab43. For this purpose, the first intracellular loop (ICL1), ICL2, ICL3, and C terminus (CT) of α2B-AR (Figure S6A) were generated as glutathione S-transferase (GST) fusion protein and incubated with total cell lysates expressing GFP-Rab43. ICL3, but not ICL1, ICL2, or the CT, bound strongly to Rab43 (Figure 6B). However, the ICL3 fragments containing the N-terminal half, C-terminal half, and middle portion did not bind to Rab43 (Figures S6B and S6C), suggesting that the overall structure of the loop may be important for Rab43 interaction. ICL3 also bound to endogenous Rab43 (Figure 6C). The interaction of Rab43 and its active mutant Q77L with the ICL3 was comparable, whereas the negative mutants T32N and N131I had no detectable binding to ICL3 (Figure 6D).
To determine whether the interaction between ICL3 and Rab43 is direct or indirect, Rab43 was generated in an in vitro translation system and then incubated with GST-ICL3 in the presence of GTPγS or guanosine diphosphate (GDP). The interaction between Rab43 and GSTICL3 was more potent in the present of GTPγS than in the presence of GDP (Figure 6E). These data demonstrate that Rab43 is able interact with α2B-AR (specifically its ICL3) in a direct and activation-dependent manner.
We next studied whether Rab43 physically associated with other GPCRs. Similar to the α2B-AR ICL3, the AT1R CT generated as GST fusion proteins bound strongly to Rab43 (Figure 6F). Furthermore, the N-terminal half retained the ability to bind to Rab43, whereas the C-terminal fragments did not, suggesting that the Rab43-binding domain is likely localized within the N-terminal region, which contains helix VIII (Zhang et al., 2015) (Figure 6F).
The Sorting Function of Rab43 in GPCR Transport
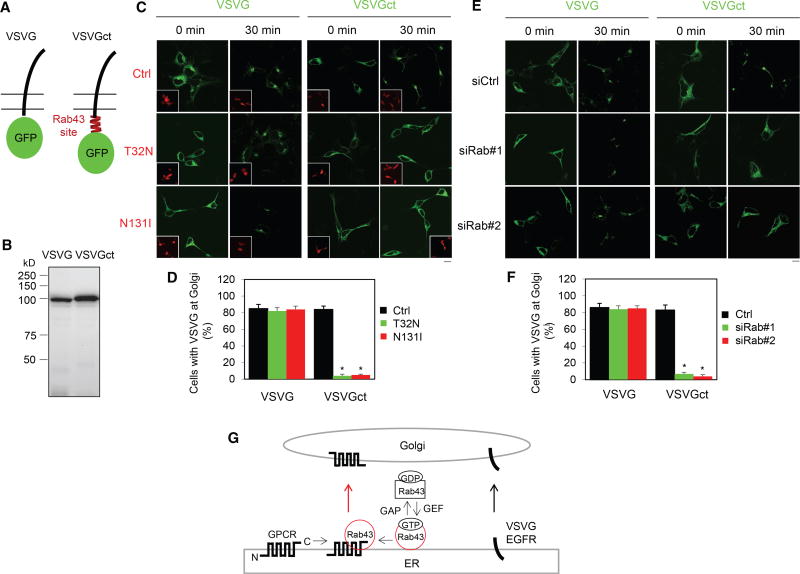
We next investigated wither the interaction between Rab43 and GPCRs is important for GPCR sorting from other plasma membrane proteins. For this purpose, we used the temperature-sensitive mutant VSVGtsO45, which was misfolded and retained within the ER at restrictive temperature and correctly delivered to the Golgi and plasma membrane at a permissive temperature (Presley et al., 1997). VSVG was fused at its CT with the AT1R C-terminal Rab43-binding domain G303-L330 (VSVGct) (Figure 7A). As expected, immunoblotting revealed that the apparent molecular weight of the chimera VSVGct was slightly larger than that of VSVG alone (Figure 7B). VSVG and VSVGct were similarly expressed and retained in the ER in cells cultured at 40°C and transported to the Golgi after a shift to 32°C for 30 min (Figure 7C). Although the expression of Rab43 mutants had no effect on the transport of VSVG from the ER to the Golgi, both mutants remarkably arrested VSVGct in the ER and almost abolished its transport to the Golgi (Figures 7C and 7D). VSVGct expression in the ER was observed in ~85% cells expressing Rab43 mutants after incubation at 32°C for 30 min (Figure 7D), and prolonged incubation up to 180 min did not enhance the export of VSVGct from the ER (data not shown). Similarly, the expression of siRNA targeting Rab43 markedly inhibited the transport from the ER to the Golgi of VSVGct but had no effect on VSVG (Figures 7E and 7F). These data indicate that the Rab43-binding domain identified in the AT1R CT is sufficient to dictate VSVG transport in a Rab43-dependent fashion.
Figure 7. Effect of the Rab43-Binding Domain Identified in the AT1R CT on the ER-Golgi Transport of VSVG.
(A) A diagram showing the generation of the VSVG chimera containing the Rab43-binding domain identified in the AT1R CT (VSVGct).
(B) Expression of VSVG and VSVGct in HEK293 cells by immunoblotting using GFP antibodies.
(C) Effect of Rab43 mutants on the ER-to-Golgi transport of VSVG and VSVGct. HEK293 cells were transfected with VSVG-GFP or VSVGct-GFP together with dsRed-C1 or dsRed-Rab43 mutants. The cells were cultured at 40°C for 24 hr (0 min) and then shifted to 32°C for 30 min.
(D) Quantitation of the data shown in (C).
(E) The effect of Rab43 siRNA on the ER-to-Golgi transport of VSVG and VSVGct.
(F) Quantitation of the data shown in (E).
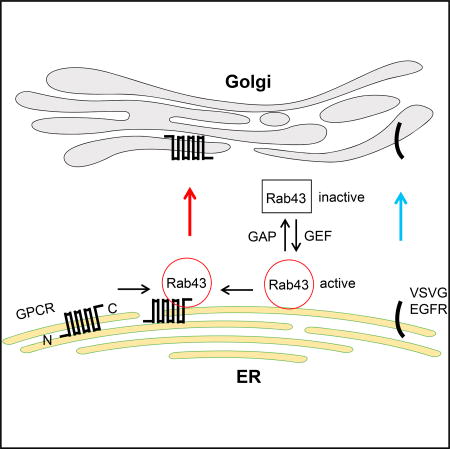
(G) A model depicting the roles of Rab43 in the ER-to-Golgi transport of GPCRs as well as their sorting at the ER after synthesis by virtue of its ability to interact with the receptors in an activation-dependent manner (see text for details).
The data shown in (D) and (F) are percentage of cells with VSVG expression at the Golgi, with a total of 100 cells counted in each experiment, and are presented as the mean ± SE (n = 3–5). Scale bars, 10 µm. *p < 0.05 versus Ctrl.
DISCUSSION
In this study, we have identified Rab43 as an important and likely specific regulator of anterograde ER-to-Golgi transport of nascent GPCRs (Figure 7G). Although a previous report suggested a possible role for Rab43 in ER-to-Golgi trafficking (Dejgaard et al., 2008), the molecules that utilize a Rab43-mediated route of transport from the ER to the Golgi remain unknown. The function of Rab43 in GPCR trafficking was first identified in a screening assay measuring the effect of 48 dominant-negative Rab mutants on the cell-surface expression of stably expressed α2B-AR at steady state, which was further supported by different strategies used to manipulate Rab43 function. Consistently, expression of Rab43 mutants and siRNA targeting Rab43 markedly inhibited the surface presentation of α2B-AR in different cell systems in which the receptor was endogenously, transiently, stably, or inducibly expressed, whereas Rab43 overexpression produced the opposite effect. In addition to α2B-AR, the cell-surface expression of several other GPCRs was also sensitive to the manipulation of Rab43 function. However, the transport of two non-GPCR cargoes, EGFR and VSVG, was not affected by Rab43, which is consistent with other reports showing that VSVG transport is independent of Rab43 (Cox et al., 2016; Dejgaard et al., 2008). These data suggest that Rab43 is a common mediator for the transport of multiple GPCRs. These studies have also identified GPCRs as the first cargoes that use the Rab43-mediated pathway to move forward.
Our studies have also provided direct evidence indicating that Rab43 modulates GPCR transport from the ER to the Golgi (Figure 7G). This became evident as Rab43 mutants and siRNA targeting Rab43 arrested all GPCRs studied, as well as the Golgi-localized α2B-AR YS mutant, in the ER. Another important line of evidence suggesting that Rab43 works at the level of the ER came from the analysis of the glycosylation status of GPCRs, which showed that Rab43N131I markedly reduced the acquisition of complex N-linked sugars of α2A-AR and AT1R and enhanced the formation of simple glycosylation. In addition, in BFA washout experiments, α2B-AR remained in the ER, unable to recover its export ability to move to the Golgi and the cell surface in cells expressing Rab43 mutants. These data demonstrate that reducing the function of Rab43 induces an accumulation of GPCRs in the ER and indicate an important function of Rab43 in regulating ER-Golgi transport. Rab43 was demonstrated to modulate retrograde transport of Shiga toxin from the cell surface to the trans-Golgi (Haas et al., 2007) but had no effect on the internalization of α2B-AR, suggesting that Rab43 can coordinate the transport of different cargo proteins along different transport routes.
We have demonstrated direct and activation-dependent interactions between Rab43 and GPCRs, providing a possible molecular mechanism responsible for the function of Rab43 in regulating GPCR trafficking (Figure 7G). The physical association of Rab43 with α2B-AR and AT1R, together with the opposing effects of gain and loss of Rab43 function on the cell-surface export of receptors and the selectivity of Rab43-mediated GPCR transport discussed above, strongly suggests that Rab43’s regulation of GPCR trafficking is likely specific. Our recent studies have demonstrated that Rab26 regulates the post-Golgi transport of α2B-AR and interacts with the ICL3 of α2B-AR in an activation-dependent fashion (Li et al., 2012). These studies suggest that α2B-AR may utilize the same intracellular domain, ICL3, to sequentially interact with distinct Rab GTPases to modulate its transport at distinct steps (i.e., Rab43 from the ER to the Golgi, and Rab26 from the Golgi to the plasma membrane). It is also interesting to note that the ICL3 of α2B-AR, via a positively charged triple-Arg motif, binds to Sec24C/D (Dong et al., 2012) and GGA3 (Zhang et al., 2016a), which are important components of ER-derived COPII- and Golgi-derived clathrin-coated transport vesicles, respectively. Whereas the interaction with Sec24 is important for α2B-AR transport from the ER to the Golgi (Dong et al., 2012), the interaction with GGA regulates receptor transport from the Golgi to the plasma membrane (Zhang et al., 2016a, 2016b). These data support a notion that cargo GPCRs may physically bind to components of the transport machinery, including Rab GTPases, to control their own anterograde transport to the cell surface.
Another important finding presented here is the sorting function of Rab43, which is likely responsible for selecting GPCRs to be transported in a Rab43-dependent manner. Although GPCRs share a common structural topology, how they are separated from other plasma membrane proteins during maturation processing is poorly understood. We have demonstrated that Rab43 specifically regulates the cell-surface transport of several GPCRs, but not the non-GPCR cargoes VSVG and EGFR. Direct evidence indicating the sorting function of Rab43 in GPCR traffic is the finding that VSVGct, a VSVG chimera containing the Rab43-binding domain identified in AT1R, was highly responsive to the functional inhibition of Rab43. VSVGct was almost completely unable to transport to the Golgi in cells with functional inhibition of Rab43. These data indicate that the Rab43-binding domain is sufficient to sort VSVG to be transported in the Rab43-dependent pathway. A previous study suggests that Rab34 may be able to sort membrane proteins at the medial Golgi (Cox et al., 2016). As attenuation of Rab43 function via the expression of its mutants and siRNA induces an extensive accumulation of GPCRs in the ER, the sorting of GPCRs likely occurs as early as in the ER, where these receptors are synthesized and assembled. Nevertheless, our studies suggest that direct interaction with Rab43 is a prerequisite for the selection of GPCRs to the Rab43-mediated pathway (Figure 7G). These data provide important evidence suggesting that Rab GTPases may directly participate in cargo selection via physical association with the cargo (Carroll et al., 2001).
Our previous and current studies have demonstrated that both Rab43 and Sec24 are involved in regulation of the sorting of GPCRs at the ER. However, their relationship needs further investigation. The fact that mutation of the Sec24-binding motif RRR does not disrupt Rab43’s interaction with α2B-AR implies that Rab43 and Sec24 have distinct requirements for interacting with the receptor and that they may interact with the receptor sequentially and/or cooperatively. It is also possible that Rab43, Sec24, and α2B-AR may form a ternary complex that significantly contributes to the recruitment of α2B-AR onto the COPII vesicles and subsequent forward transport of the vesicles. These data also suggest that the ICL3 of α2B-AR is a scaffolding domain that mediates the receptor’s interaction with multiple transport machinery proteins and controls export trafficking of the receptor along the biosynthetic pathway.
GPCRs regulate a variety of cell functions under physiological and pathological conditions and are the most heavily investigated drug targets (Allen and Roth, 2011; Cooke et al., 2015; Sato et al., 2015). Enhanced or reduced cell-surface expression of GPCRs has been reported in a number of human diseases without a description of the exact mechanisms involved (Meana et al., 1992). Emerging evidence from the past decade suggests that the cell-surface transport of GPCRs is regulatable and mediated through multiple pathways in a cell-type- and receptor-specific manner (Bie et al., 2010; Filipeanu et al., 2006a; Wu et al., 2003). A number of studies have identified several highly conserved export motifs embedded within the receptors that direct receptor export from the ER and the Golgi (Dong et al., 2012; Dong and Wu, 2006; Duvernay et al., 2009; Zhang et al., 2011). Many regulatory proteins, such as receptor activity modifying proteins (RAMPs), chaperones, escort proteins, and gatekeepers, have also been demonstrated to stabilize receptor conformation, facilitate receptor maturation, and promote receptor delivery to the plasma membrane (Björk et al., 2013; Chen et al., 2009; Colley et al., 1991; Doly et al., 2015; Dwyer et al., 1998; Ferreira et al., 1996; Hay and Pioszak, 2016; McLatchie et al., 1998; Rodrigues et al., 2017; Sauvageau et al., 2014; Tai et al., 1999). As the master regulators of vesicle-mediated membrane traffic, Rab GTPases play an important role in the biosynthetic processing of GPCRs. Therefore, a thorough elucidation of the regulatory mechanisms controlling the export of GPCRs to their functional destinations and their sorting from other membrane proteins will not only enhance our understanding of fundamental biology of GPCRs but also provide an important foundation for the development of new therapeutic strategies by targeting GPCR export trafficking.
In summary, our present study has identified Rab43 as a crucial regulator of the anterograde transport and sorting of nascent GPCRs and suggests a novel Rab43-coordinated ER-Golgi transport route specific for the forward movement of GPCRs. These functions of Rab43 are likely mediated through its direct and activation-dependent interaction with the receptors. Overall, this study provides important insights into the regulation of GPCR targeting to the functional destination and their sorting from other membrane proteins, which are poorly explored areas in the study of the GPCR superfamily as well as general membrane trafficking.
EXPERIMENTAL PROCEDURES
Materials
Antibodies against GFP, calnexin, β-actin, and α2B-AR were purchased from Santa Cruz Biotechnology, Rab43 from Abcam, GM130 from BD Transduction, ERK1/2 and phospho-ERK1/2 from Cell Signaling Technology, and HA from Roche Applied Science. Alexa-Fluor-488-, Alexa-Fluor-594-, and Alexa-Fluor-647-labeled secondary antibodies were from Life Technologies. UK14304 and BFA were obtained from Sigma-Aldrich. Endo H and PNGase F were from BioLabs. The radioligands [3H]-RX821002 (50 Ci/mmol), [3H]-CGP12177 (51 Ci/mmol), and [7-methoxy-3H]-prazosin (70 Ci/mmol) were from PerkinElmer Life Sciences. All other materials were obtained as described elsewhere (Dong et al., 2012; Wu et al., 2003)
Plasmids and Constructions
GFP- and HA-tagged GPCRs were generated as described previously (Dong et al., 2010, 2012; Li et al., 2012). Rab GTPases were cloned by RT-PCR using total RNA extracted from HeLa or HEK293 cells or generated as described previously (Filipeanu et al., 2006b; Matsui et al., 2011; Tsuboi and Fukuda, 2006; Wu et al., 2003). Rab43 and its mutants were also cloned into the pdsRedmonomer-C1 vector at the EcoRI and BamHI restriction sites. Rab mutants were generated using QuikChange site-directed mutagenesis using the primers listed in Table S1. For generation of VSVGct, in which the AT1R C-terminal fragment G303-L320 was fused to the VSVG CT, the fragment G303-L320 carrying the sticky ends of EcoRI and BamHI was generated by PCR and ligated into VSVGtsO45, which was cloned at the BglII and EcoRI restriction sites in the pEGFP-N1 vector.
Cell Culture and Transient Transfection
HEK293 cells and MCF-7 cells that express endogenously α2B-AR were cultured and transient transfection was carried out using Lipofectamine 2000 as described previously (Wu et al., 2003). Transfection efficiency was estimated to be greater than 75% based on microscopy detecting the fluorescence of tagged proteins.
Generation of Stable and Inducible Cell Lines Expressing α2-AR
HEK293 cell lines stably expressing HA-α2B-AR were generated as described previously (Li et al., 2012). The cells stably expressing HA-α2B-AR were confirmed by immunoblotting and intact cell ligand binding. The cell line expressing 6.9 × 105 α2B-AR per cell was used in the current study.
The Tet-On 3G tetracycline inducible gene expression system (Clontech Laboratories) was utilized to generate stable cell lines inducibly expressing HA-α2A-AR and HA-α2B-AR in HEK293 cells as described previously (Zhang et al., 2016a). The cell lines expressing 8.3 × 105 α2A-AR/cell and 8.5 × 105 α2B-AR/cell were utilized in the current study.
siRNA-Mediated Depletion of Rab43
Two Stealth RNAi duplexes (siRNA) targeting to human Rab43 (NCBI: NM_198490; #1, 5′-CAGGGCUGCGUCGGUUUGUGGUCUA-3′ [non-coding sequence]; #2, 5′-GGGACCCGGACGAGCAGUACGAUUU-3′) and a negative control med GC duplex were purchased from Invitrogen. siRNA-mediated knockdown of Rab43 was carried out as described previously (Wu et al., 2003). MCF-7 cells were transfected with siRNA twice, and all experiments were performed at 72 hr after the first siRNA transfection.
Radioligand Binding of Intact Live Cells
The cell-surface expression of α2-, β- and α1-AR was measured by ligand binding of intact live cells as described previously (Dong et al., 2012; Li et al., 2012) using [3H]-RX821002, [3H]-CGP12177, and [7-methoxy-3H]-prazosin, respectively. For measurement of endogenous α2-AR, MCF-7 cells were cultured on 6-well dishes and transfected with Rab43 constructs and siRNA targeting Rab43. All radioligand-binding assays were performed in triplicate.
Flow Cytometry
For measurement of receptor expression at the cell surface, HEK293 cells transfected with HA-tagged receptors were suspended in PBS containing 1% fetal bovine serum (FBS) and incubated with high-affinity anti-HA-fluorescein (3F10) at 2 µg/mL for 30 min at 4°C. For measurement of total receptor expression, HEK293 cells were permeabilized with 0.2% Triton X-100 in PBS for 5 min before incubation with anti-HA antibodies. The fluorescence was analyzed on a flow cytometer (Dickinson FACSCalibur) as described previously (Wu et al., 2003).
Fluorescence Microscopy
HEK293 cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% saponin for 20 min, and blocked with PBS containing 5% BSA, 0.1% gelatin, and 0.1% saponin for 30 min. The cells were sequentially stained with primary antibodies (1:200 dilution for GM130 and 1:500 dilution for calnexin) and Alexa-Fluor-conjugated secondary antibodies (1:500 dilution). In BFA treatment and washout assays, HEK293 cells were transfected with α2B-ARGFP and then incubated with BFA (6 µg/mL) at 37°C for 2.5 hr. BFA washout was carried out by removing, rinsing, and incubating cells with fresh medium containing cycloheximide (100 µg/mL) for 1, 4, or 24 hr at 37°C.
Images were captured using a Zeiss LSM780 confocal microscope.
Deglycosylation
Deglycosylation of GPCRs by treatments with Endo H and PNGase F enzymes was performed following the manufacturer’s instructions with modifications. Briefly, cell lysates prepared from HEK293 cells transfected with individual receptors with or without Rab43N131I were incubated in denaturing buffer containing 0.5% SDS and 40 mM DTT for 30 min at 37°C and the denatured proteins were digested with 1–2 µL enzymes in GlycoBuffer for 1 hr at 37°C.
Co-IP
HEK293 cells stably expressing HA-α2B-AR were cultured on 100-mm dishes and transfected with 8 µg of the pEGFP-C1 vector or Rab43 for 48 hr. The cells were washed twice with PBS, harvested, and lysed with 500 µL of buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 1% Nonidet P-40. After centrifugation, the supernatants were incubated with 1 µg HA antibody overnight at 4°C followed by incubation with 50 µL protein G agarose beads for 5 hr. Resin was collected by centrifugation and washed 4 times. Immunoprecipitated receptors were eluted with 30 µL of 1× SDS gel loading buffer and GFP-Rab43 detected.
GST Fusion Protein Pull-Down Assays
GST fusion protein pull-down assays were carried out using the MagneGST pull-down system as described previously (Li et al., 2012; Zhang et al., 2016a). Briefly, GST fusion proteins were incubated with total HEK293 cell homogenates in 500 µL binding buffer containing 20mMTris-HCl (pH 7.5), 140m NaCl, 1% Nonidet P-40, 0.5% BSA, and 10% glycerol for 4 hr at 4°C. The bound proteins were solubilized and detected by immunoblotting.
Translation of Rab43 In Vitro
Rab43 in the pcDNA3.1 vector was translated using the TnT T7 quick in vitro translation system (Promega). Briefly, the reaction was carried out in a total volume of 50 µL containing 40 µL TnT T7 Quick Master Mix, 1 µL of 1 mM methionine, and 1 µg of the Rab43 plasmid at 30°C for 90 min. After translation, 20 µL of the reaction mixtures was diluted into 200 µL binding buffer containing 20mMTris-HCl (pH 7.5), 140mNaCl,1% Nonidet P-40, and 0.5% BSA. Rab43 was then incubated in binding buffer containing 100 µMGTPγS or GDP for 1 hr followed by incubation with GST fusion proteins as described above.
Measurement of VSVG Transport from the ER to the Golgi
HEK293 cells grown on coverslips in 6-well dishes were transfected with 0.25 µg VSVG-GFP or VSVGct-GFP constructs together with 1.25 µg Rab43 mutants or siRNA targeting Rab43 as described above. The cells were cultured for 24 hr at 40°C to induce the accumulation of VSVG in the ER and then transferred to 32°C for 30 min to allow VSVG to transport to the Golgi. The cells were fixed, and the subcellular localization of VSVG was visualized by confocal microscopy as described above. The cells with VSVG expression at the Golgi were counted, and ~100 cells were counted in each experiment.
Measurement of ERK1/2 Activation
HEK293 cells stably expressing α2B-AR were cultured on 6-well dishes and transfected with Rab43 mutants or siRNA. The cells were starved for 3 hr before stimulation with UK14304 at 1 µM for 5 min or EGF at 10 ng/mL. Stimulation was terminated by the addition of 200 µL ice-cold cell lysis buffer. ERK1/2 activation was determined by measuring ERK1/2 phosphorylation by immunoblotting.
Statistical Analysis
Differences were evaluated using Student’s t test, and p < 0.05 was considered as statistically significant. The data are expressed as the means ± SE.
Supplementary Material
Highlights.
Rab43 specifically regulates the surface transport and signaling of GPCR family members
Rab43 controls the forward ER-to-Golgi traffic of nascent GPCRs
Rab43 directly interacts with GPCRs in an activation-dependent fashion
Rab43-binding domain of GPCRs sufficiently changes the non-GPCR protein transport pathway
Acknowledgments
This work was supported by the NIH (grants GM118915 and CA204315 to G.W.).
Footnotes
Supplemental Information includes six figures and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2017.10.011.
AUTHOR CONTRIBUTIONS
C.L. and G.W. conceived the study and designed the experiments. C.L., Z.W., Y.F., W.H., M.K., and G.W. performed the experiment. C.L., Z.W., Y.F., W.H., M.K., Y.S., H.L., Z.D., M.F., M.K., and G.W. analyzed the data. M.F. provided reagents and materials. C.L. and G.W. wrote the manuscript with input from all authors.
References
- Allen JA, Roth BL. Strategies to discover unexpected targets for drugs active at G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2011;51:117–144. doi: 10.1146/annurev-pharmtox-010510-100553. [DOI] [PubMed] [Google Scholar]
- Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, Lowenstein CJ, Weinman EJ, Pan ZZ. Nerve growth factor-regulated emergence of functional delta-opioid receptors. J. Neurosci. 2010;30:5617–5628. doi: 10.1523/JNEUROSCI.5296-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk S, Hurt CM, Ho VK, Angelotti T. REEPs are membrane shaping adapter proteins that modulate specific g protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS ONE. 2013;8:e76366. doi: 10.1371/journal.pone.0076366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Tobin AB. Design of next-generation G protein-coupled receptor drugs: Linking novel pharmacology and in vivo animal models. Annu. Rev. Pharmacol. Toxicol. 2016;56:535–559. doi: 10.1146/annurev-pharmtox-011613-140012. [DOI] [PubMed] [Google Scholar]
- Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 2001;292:1373–1376. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen C, Kotsikorou E, Lynch DL, Reggio PH, Liu-Chen LY. GEC1-kappa opioid receptor binding involves hydrophobic interactions: GEC1 has chaperone-like effect. J. Biol. Chem. 2009;284:1673–1685. doi: 10.1074/jbc.M808303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Cooke RM, Brown AJ, Marshall FH, Mason JS. Structures of G protein-coupled receptors reveal new opportunities for drug discovery. Drug Discov. Today. 2015;20:1355–1364. doi: 10.1016/j.drudis.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Cox JV, Kansal R, Whitt MA. Rab43 regulates the sorting of a subset of membrane protein cargo through the medial Golgi. Mol. Biol. Cell. 2016;27:1834–1844. doi: 10.1091/mbc.E15-03-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraff JL, Gagnon AW, Benovic JL, Orsini MJ. Role of arrestins in endocytosis and signaling of alpha2-adrenergic receptor subtypes. J. Biol. Chem. 1999;274:11253–11259. doi: 10.1074/jbc.274.16.11253. [DOI] [PubMed] [Google Scholar]
- Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R, Simpson JC, Presley JF. Rab18 and Rab43 have key roles in ER-Golgi trafficking. J. Cell Sci. 2008;121:2768–2781. doi: 10.1242/jcs.021808. [DOI] [PubMed] [Google Scholar]
- Doly S, Shirvani H, Gata G, Meye FJ, Emerit MB, Enslen H, Achour L, Pardo-Lopez L, Yang SK, Armand V, et al. GABA receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol. Psychiatry. 2015;21:480–490. doi: 10.1038/mp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Wu G. Regulation of anterograde transport of alpha2-adrenergic receptors by the N termini at multiple intracellular compartments. J. Biol. Chem. 2006;281:38543–38554. doi: 10.1074/jbc.M605734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H, Wu G. Rab8 interacts with distinct motifs in alpha2B- and beta2-adrenergic receptors and differentially modulates their transport. J. Biol. Chem. 2010;285:20369–20380. doi: 10.1074/jbc.M109.081521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Li C, Wu G. Regulation of α(2B)-adrenergic receptor-mediated extracellular signal-regulated kinase 1/2 (ERK1/2) activation by ADP-ribosylation factor 1. J. Biol. Chem. 2011;286:43361–43369. doi: 10.1074/jbc.M111.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple arg motif mediates α(2B)-adrenergic receptor interaction with Sec24C/D and export. Traffic. 2012;13:857–868. doi: 10.1111/j.1600-0854.2012.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Dong C, Zhang X, Robitaille M, Hébert TE, Wu G. A single conserved leucine residue on the first intracellular loop regulates ER export of G protein-coupled receptors. Traffic. 2009;10:552–566. doi: 10.1111/j.1600-0854.2009.00890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Troemel ER, Sengupta P, Bargmann CI. Odorant receptor localization to olfactory cilia is mediated by ODR-4, a novel membrane-associated protein. Cell. 1998;93:455–466. doi: 10.1016/s0092-8674(00)81173-3. [DOI] [PubMed] [Google Scholar]
- Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–640. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol. Pharmacol. 2006a;69:1571–1578. doi: 10.1124/mol.105.019984. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Zhou F, Lam ML, Kerut KE, Claycomb WC, Wu G. Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J. Biol. Chem. 2006b;281:11097–11103. doi: 10.1074/jbc.M511460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas AK, Fuchs E, Kopajtich R, Barr FA. A GTPase-activating protein controls Rab5 function in endocytic trafficking. Nat. Cell Biol. 2005;7:887–893. doi: 10.1038/ncb1290. [DOI] [PubMed] [Google Scholar]
- Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J. Cell Sci. 2007;120:2997–3010. doi: 10.1242/jcs.014225. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hay DL, Pioszak AA. Receptor activity-modifying proteins (RAMPs): new insights and roles. Annu. Rev. Pharmacol. Toxicol. 2016;56:469–487. doi: 10.1146/annurev-pharmtox-010715-103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Tian X, Benovic JL. Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr. Opin. Cell Biol. 2014;27:63–71. doi: 10.1016/j.ceb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol. Sci. 2011;32:213–218. doi: 10.1016/j.tips.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzer NM, Theisen DJ, Tussiwand R, Briseño CG, Grajales-Reyes GE, Wu X, Durai V, Albring J, Bagadia P, Murphy TL, Murphy KM. RAB43 facilitates cross-presentation of cell-associated antigens by CD8α+ dendritic cells. J. Exp. Med. 2016;213:2871–2883. doi: 10.1084/jem.20160597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Horn JP. Differential inhibition of Ca2+ channels by alpha2-adrenoceptors in three functional subclasses of rat sympathetic neurons. J. Neurophysiol. 2008;100:3055–3063. doi: 10.1152/jn.90590.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YW, Guyenet PG, Bayliss DA. Voltage-dependent calcium currents in bulbospinal neurons of neonatal rat rostral ventrolateral medulla: modulation by alpha2-adrenergic receptors. J. Neurophysiol. 1998;79:583–594. doi: 10.1152/jn.1998.79.2.583. [DOI] [PubMed] [Google Scholar]
- Li C, Fan Y, Lan TH, Lambert NA, Wu G. Rab26 modulates the cell surface transport of α2-adrenergic receptors from the Golgi. J. Biol. Chem. 2012;287:42784–42794. doi: 10.1074/jbc.M112.410936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Itoh T, Fukuda M. Small GTPase Rab12 regulates constitutive degradation of transferrin receptor. Traffic. 2011;12:1432–1443. doi: 10.1111/j.1600-0854.2011.01240.x. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Meana JJ, Barturen F, García-Sevilla JA. Alpha 2-adrenoceptors in the brain of suicide victims: increased receptor density associated with major depression. Biol. Psychiatry. 1992;31:471–490. doi: 10.1016/0006-3223(92)90259-3. [DOI] [PubMed] [Google Scholar]
- Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat. Rev. Mol. Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott- Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rodrigues AR, Sousa D, Almeida H, Gouveia AM. Cell surface targeting of the Melanocortin 5 Receptor (MC5R) requires serine-rich terminal motifs. Biochim. Biophys. Acta. 2017;1864:1217–1226. doi: 10.1016/j.bbamcr.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 2015;95:377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau E, Rochdi MD, Oueslati M, Hamdan FF, Percherancier Y, Simpson JC, Pepperkok R, Bouvier M. CNIH4 interacts with newly synthesized GPCR and controls their export from the endoplasmic reticulum. Traffic. 2014;15:383–400. doi: 10.1111/tra.12148. [DOI] [PubMed] [Google Scholar]
- Seto S, Tsujimura K, Koide Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic. 2011;12:407–420. doi: 10.1111/j.1600-0854.2011.01165.x. [DOI] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Bode C, Wolfrum U, Sung CH. Rhodopsin’s carboxy-terminal cytoplasmic tail acts as a membrane receptor for cytoplasmic dynein by binding to the dynein light chain Tctex-1. Cell. 1999;97:877–887. doi: 10.1016/s0092-8674(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE. Membrane trafficking of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004;44:559–609. doi: 10.1146/annurev.pharmtox.44.101802.121558. [DOI] [PubMed] [Google Scholar]
- Timmons SD, Geisert E, Stewart AE, Lorenzon NM, Foehring RC. alpha2-Adrenergic receptor-mediated modulation of calcium current in neocortical pyramidal neurons. Brain Res. 2004;1014:184–196. doi: 10.1016/j.brainres.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J. Cell Sci. 2006;119:2196–2203. doi: 10.1242/jcs.02962. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J. Biol. Chem. 2003;278:47062–47069. doi: 10.1074/jbc.M305707200. [DOI] [PubMed] [Google Scholar]
- Zenner HL, Yoshimura S, Barr FA, Crump CM. Analysis of Rab GTPase-activating proteins indicates that Rab1a/b and Rab43 are important for herpes simplex virus 1 secondary envelopment. J. Virol. 2011;85:8012–8021. doi: 10.1128/JVI.00500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Dong C, Wu QJ, Balch WE, Wu G. Di-acidic motifs in the membrane-distal C termini modulate the transport of angiotensin II receptors from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2011;286:20525–20535. doi: 10.1074/jbc.M111.222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Unal H, Gati C, Han GW, Liu W, Zatsepin NA, James D, Wang D, Nelson G, Weierstall U, et al. Structure of the angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015;161:833–844. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Davis JE, Li C, Gao J, Huang W, Lambert NA, Terry AV, Jr, Wu G. GGA3 interacts with a G protein-coupled receptor and modulates its cell surface eExport. Mol. Cell. Biol. 2016a;36:1152–1163. doi: 10.1128/MCB.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Huang W, Gao J, Terry AV, Wu G. Regulation of alpha2B-adrenergic receptor cell surface transport by GGA1 and GGA2. Sci. Rep. 2016b;6:37921. doi: 10.1038/srep37921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.