Summary
Background
Hepatitis-related liver diseases are a leading cause of mortality and morbidity among people with HIV/AIDS taking combination antiretroviral therapy. We assessed the effect of hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infection on HIV outcomes in patients in China.
Methods
We did a nationwide retrospective observational cohort study with data from the China National Free Antiretroviral Treatment Program from 2010–11. Patients older than 18 years starting standard antiretroviral therapy for HIV who had tested positive for HBV and HCV were followed up to Dec 31, 2012. We used Kaplan-Meier analysis and Cox proportional hazard models to evaluate survival, and logistic regression models to estimate virological failure, immunological response, and retention in care.
Findings
33 861 patients with HIV met eligibility criteria. 2958 (8·7%) participants had HBV co-infection, 6149 (18·2%) had HCV co-infection, and 1114 (3·3%) had triple infection. All-cause mortality was higher in participants with triple infection (adjusted hazard ratio 1·90, 95% CI 1·53–2·37) and HCV co-infection (1·46, 1·25–1·70) than in those with HIV only, but not in those with HBV co-infection (1·06, 0·89–1·26). People with triple infection were also more likely to have virological failure (adjusted odds ratio [OR] 1·26, 95% CI 1·02–1·56) than were those with HIV only, whereas the difference was not significant for those with HBV co-infection (0·93, 0·80–1·10) or HCV co-infection (1·10, 0·97–1·26). No co-infection was significantly associated with a difference in CD4 cell count after 1 year of treatment. Loss to follow-up was more common among participants with triple infection (OR 1·37, 95% CI 1·16–1·62) and HCV co-infection (1·30, 1·17–1·45), but not HBV co-infection (0·93, 0·82–1·05), than among those with HIV only.
Interpretation
Screening for viral hepatitis is important in individuals diagnosed as HIV positive. Effective management for viral hepatitis should be integrated into HIV treatment programmes. Long-term data are needed about the effect of hepatitis co-infection on HIV disease progression.
Funding
The National Center for AIDS/STD Control and Prevention, China Center for Disease Control and Prevention.
Introduction
HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) contribute substantially to the global burden of disease.1,2 Worldwide, an estimated 31·4–35·9 million people have HIV/AIDS, 350–400 million people have HBV, and 150–170 million have HCV.3–5 These viruses share routes of transmission and many people with HIV are co-infected with HBV and HCV, especially in some populations such as injection drug users and men who have sex with men.6–8 The prevalence of HBV co-infection ranges from 5% to 20% and HCV co-infection ranges from 5% to 15% in resource-limited regions.3 In New York City, the prevalence of HIV and HBV co-infection is 4·5%, that of HIV and HCV co-infection is 25·0%, and that of triple infection (HIV, HBV, and HCV) is 1·6%.9 The transmission routes and epidemiology of HBV and HCV in people with HIV differ between low-income and high-income countries.10,11 In low-income countries, most HBV infections occur in infancy or childhood through perinatal transmission or close household contact, and the main route of transmission for HCV is unsafe injection.1,12 In high-income countries, HBV is commonly transmitted through sexual contact and HCV through injection drug use and sex between men who have sex with men.10,12
The introduction of combination antiretroviral therapy has greatly increased survival and decreased morbidity of people with HIV.13,14 As the life expectancy of people with HIV increases, chronic hepatitis-related liver diseases have become a growing concern.15,16 Additionally, hepatitis co-infection can complicate clinical management of HIV and increase hepatotoxicity.17,18
HIV can accelerate hepatitis-related liver diseases.15,19 By contrast, the effect of HBV or HCV on HIV clinical progression, immunological response, and virological response has not been well established.20–22 Some researchers report that HIV and HBV co-infection increases the risk of virological failure and death23 and that HIV and HCV co-infection decreases survival.24 However, others found no effect of HBV or HCV on virological or immunological responses to combination antiretroviral therapy or mortality.25 Moreover, very few data exist for the effect of triple viral co-infection on outcomes.
We assessed the effect of HBV and HCV co-infection on HIV clinical progression, virological and immunological responses, and retention in care in people with HIV receiving combination antiretroviral therapy in China.
Methods
Study design and participants
We did a nationwide clinical observational cohort study with data from the National Free Antiretroviral Treatment Program database, established and managed by the National Center for AIDS/STD Control and Prevention, China Center for Disease Control and Prevention (China CDC). The organisation, management, and workings of the database have been previously described.26,27 In brief, local health workers complete standardised reporting forms at baseline (initiation of antiretroviral therapy) and follow-up (0·5, 1, 2, and 3 months after initiation antiretroviral therapy, and every 3 months thereafter). Forms are entered into a web-based electronic data collection system and sent to the national office for data-cleaning and analysis.28
We included participants if they were older than 18 years, treatment naive, started a standard treatment regimen between Jan 1, 2010, and Dec 31, 2011, and had HBV surface antigen and HCV antibody results recorded in the database. We included follow-up data up to Dec 31, 2012.
Since 2002, people with HIV in China with a CD4 count of 200 cells per µL or less, total lymphocyte count of less than 1200 cells per µL, or WHO disease stage 3 or 4 have been eligible for free combination antiretroviral therapy. In 2008, the CD4 count threshold for treatment was increased to 350 cells per µL.29 Between 2003 and 2005, in agreement with WHO recommendations, the first-line HIV treatment regimen recommended in China was zidovudine or stavudine plus didanosine and nevirapine or efavirenz.29 After 2005, these regimens were gradually replaced with zidovudine or stavudine plus lamivudine and nevirapine. Since 2010, stavudine has been phased out and tenofovir has been introduced in the first-line regimen, as recommended by WHO.30
This study was approved by the Institutional Review Boards of the National Center for AIDS/STD Control and Prevention, China CDC in Beijing, and the Center for Global Health at the US CDC in Atlanta, GA, USA.
Procedures
We assessed four outcomes: survival, HIV virological response, HIV immunological response, and retention in treatment. We calculated survival as time from treatment initiation to death (all-cause and non-injury) or censoring. Patients were censored at dropout, loss to follow-up, or on Dec 31, 2012, whichever came first. We defined HIV virological suppression as plasma HIV RNA viral load less than 400 copies per mL after 6–18 months of combination antiretroviral therapy. We considered viral load of 400 copies per mL or more after 6–18 months of combination antiretroviral therapy as virological failure.31 We also assessed virological failure in three subgroups (400–1000 copies per mL, 1001–9999 copies per mL, and ≥10 000 copies per mL). We defined HIV immunological response as a CD4 count increase of more than 30% compared with baseline after 9–15 months of combination antiretroviral therapy. We categorised retention status as retention, death, treatment withdrawal, or loss to follow-up (>90 days since a missed schedule visit).
We compared these four outcomes in four co-infection status groups: HIV monoinfection (HBV negative, HCV negative), HIV and HBV co-infection (HBV positive, HCV negative), HIV and HCV co-infection (HBV negative, HCV positive), and triple infection (HBV positive, HCV positive). We analysed covariates including sex, age (18–24, 25–29, 30–34, 35–39, 40–44, 45–54, and ≥55 years), self-reported mode of HIV transmission (blood transfusion or former plasma donor, injection drug use, sex with men who have sex with men, heterosexual sex), baseline CD4 count (<50, 50–199, 200–349, and ≥350 cells per µL), baseline alanine aminotransferase concentration (<40, 40–79, and ≥80 U/L), and baseline total bilirubin concentration (<17·1, ≥17·1 µmol/L).
HIV infection status was based on positive test results from two peripheral blood samples by HIV ELISA and confirmed by western blot. All HIV testing sites were certified by the National AIDS Reference Laboratory at China CDC. HBV chronic infection was defined by the presence in serum samples of HBsAg according to ELISA. We assessed serum HCV antibody positivity by second-generation or third-generation ELISA techniques; no information for HCV RNA testing was collected in the database.
Statistical analysis
We assessed characteristics by co-infection status with χ2 tests (for categorical data) and non-parametric Wilcoxon tests (for continuous data). We assessed all-cause mortality and non-injury-related mortality by co-infection status with Kaplan-Meier analysis. We used log-rank tests to assess significance. We used Cox proportional hazard models to calculate unadjusted and adjusted hazard ratios (HRs) and 95% CIs. We tested deviations from the proportional hazard assumption by log-log survival plots. Because of the relation between HCV co-infection and injection drug use, for each multivariable analysis, we adjusted or stratified by transmission route (injection drug use or sexual contact).
We used logistic regression to estimate unadjusted and adjusted odds ratios (ORs) and 95% CIs for virological failure, immunological response, and treatment retention. As a sensitivity analysis for virological failure, we used two viral load cutoffs (>1000 and ≥10 000 copies per mL). We selected covariates a priori for inclusion in multivariable models on the basis of biological importance.27,31 We deemed a p value less than 0·05 as statistically significant for all analyses. We did the analyses with SAS (version 9.2).
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
70 694 people with HIV started combination antiretroviral therapy between 2010 and 2011, 36 833 of whom we excluded either because they lacked HBV or HCV testing results (n=35 855), or because they had not started a standard initial treatment regimen (n=978). 33 861 patients met eligibility criteria. 2958 (8·7%) participants had HBV co-infection, 6149 (18·2%) had HCV co-infection, and 1114 (3·3%) had triple infection. Two-thirds of participants were men with a median age of 37 years (table 1). About two-thirds of participants were infected through heterosexual transmission and almost half received antiretroviral treatment at a county or lower level hospital. Median CD4 count at treatment initiation was 195 cells per µL and most patients received zidovudine, lamivudine, and nevirapine or efavirenz as the initial regimen (table 1). Patients with HCV or triple infection, compared with those with HBV or HIV monoinfection, were more likely to be male (p<0·001), to have been infected by injection drug use (p<0·001), and to have initiated antiretroviral treatment at a county or lower level hospital (p<0·001). Median CD4 cell counts at baseline were higher in patients with triple infection and HCV infection than in those with HBV or HIV monoinfection (table 1).
Table 1.
Baseline characteristics
| Total (n=33 861) | Triple infection (n=1114) |
HCV–HIV co- infection(n=6149) |
HBV–HIV co- infection (n=2958) |
HIV only (n=23 640) |
|
|---|---|---|---|---|---|
| Age at start of antiretroviral therapy (years) | 37 (31–46) | 35 (31–40) | 36 (32–41) | 37 (31–46) | 38 (30–48) |
| 18–24 | 2135 (6·3%) | 33 (3·0%) | 188 (3·1%) | 180 (6·1%) | 1734 (7·4%) |
| 25–29 | 4854 (14·3%) | 146 (13·1%) | 699 (11·4%) | 439 (14·8%) | 3570 (15·1%) |
| 30–34 | 6354 (18·8%) | 324 (29·1%) | 1524 (24·8%) | 579 (19·6%) | 3927 (16·6%) |
| 35–39 | 6537 (19·3%) | 329 (29·5%) | 1841 (29·9%) | 506 (17·1%) | 3861 (16·3%) |
| 40–44 | 4669 (13·8%) | 170 (15·3%) | 1188 (19·3%) | 402 (13·6%) | 2909 (12·3%) |
| 45–54 | 4887 (14·4%) | 90 (8·0%) | 574 (9·3%) | 461 (15·6%) | 3762 (15·9%) |
| ≥55 | 4425 (13·1%) | 22 (2·0%) | 135 (2·2%) | 391 (13·2%) | 3877 (16·4%) |
|
| |||||
| Sex | |||||
| Male | 22 534 (66·6%) | 942 (84·6%) | 4951 (80·5%) | 2064 (69·8%) | 14 577 (61·7%) |
| Female | 11 327 (33·4%) | 172 (15·4%) | 1198 (19·5%) | 894 (30·2%) | 9063 (38·3%) |
|
| |||||
| Transmission mode | |||||
| Blood transfusion or previous plasma donation | 418 (1·3%) | 14 (1·3%) | 168 (2·9%) | 43 (1·5%) | 193 (0·9%) |
| Injection drug use | 6959 (22·0%) | 803 (74·8%) | 4457 (75·9%) | 316 (11·3%) | 1383 (6·3%) |
| MSM | 3159 (10·0%) | 8 (0·8%) | 61 (1·0%) | 317 (11·4%) | 2773 (12·7%) |
| Heterosexual | 21 128 (66·7%) | 248 (23·1%) | 1187 (20·2%) | 2113 (75·8%) | 17 580 (80·1%) |
|
| |||||
| Type of treatment centre | |||||
| Infectious disease hospital | 2356 (7·0%) | 53 (4·8%) | 370 (6·0%) | 199 (6·7%) | 1734 (7·4%) |
| Provincial or prefectural hospital | 11 407 (33·8%) | 313 (28·3%) | 2017 (33·0%) | 1126 (38·3%) | 7951 (33·8%) |
| Center for Disease Control and Prevention | 5145 (15·3%) | 89 (8·0%) | 474 (7·8%) | 475 (16·2%) | 4107 (17·4%) |
| County or lower level hospital | 14 809 (43·9%) | 652 (58·9%) | 3253 (53·2%) | 1142 (38·8%) | 9762 (41·4%) |
|
| |||||
| Baseline CD4 count (cells per µL) | 195 (82–283) | 223 (110–306) | 216 (118–301) | 178 (58–271) | 190 (74–278) |
| ≥350 | 2610 (7·9%) | 135 (12·5%) | 773 (12·9%) | 180 (6·2%) | 1522 (6·6%) |
| 200–349 | 13 547 (40·9%) | 459 (42·5%) | 2494 (41·8%) | 1100 (38·0%) | 9494 (40·9%) |
| 50–199 | 11 054 (33·4%) | 341 (31·6%) | 2083 (34·9%) | 967 (33·4%) | 7663 (33·0%) |
| 0–49 | 5927 (17·8%) | 144 (13·4%) | 619 (10·4%) | 647 (22·4%) | 4517 (19·5%) |
|
| |||||
| Alanine aminotransferase concentration (U/L) | |||||
| <40 | 24 358 (72·6%) | 581 (52·7%) | 3338 (54·6%) | 1975 (67·4%) | 18 464 (78·9%) |
| 40–79 | 6768 (20·2%) | 344 (31·2%) | 1800 (29·5%) | 711 (24·3%) | 3913 (16·7%) |
| ≥80 | 2417 (7·2%) | 177 (16·1%) | 970 (15·9%) | 245 (8·4%) | 1025 (4·4%) |
|
| |||||
| Total bilirubin concentration (µmol/L) | |||||
| <17·1 | 28 271 (87·0%) | 827 (79·4%) | 4836 (82·9%) | 2410 (83·9%) | 20 198 (88·8%) |
| ≥17·1 | 4233 (13·0%) | 214 (20·6%) | 999 (17·1%) | 462 (16·1%) | 2558 (11·2%) |
|
| |||||
| Initial antiretroviral regimen | |||||
| AZT+3TC+NVP/EFV | 22 340 (66·0%) | 652 (58·5%) | 3876 (63·0%) | 1646 (55·7%) | 16 166 (68·4%) |
| D4T+3TC+NVP/EFV | 8811 (26·0%) | 286 (25·7%) | 1480 (24·1%) | 731 (24·7%) | 6314 (26·7%) |
| TDF+3TC+NVP/EFV | 2710 (8·0%) | 176 (15·8%) | 793 (12·9%) | 581 (19·6%) | 1160 (4·9%) |
Data are median (IQR) or n (%). All variables were significantly associated with hepatitis co-infection groups (p<0·05). HBV=hepatitis B virus. HCV=hepatitis C virus. MSM=men who have sex with men. AZT=zidovudine. 3TC=lamivudine. NVP=nevirapine. EFV=efavirenz. D4T=stavudine. TDF=tenofovir.
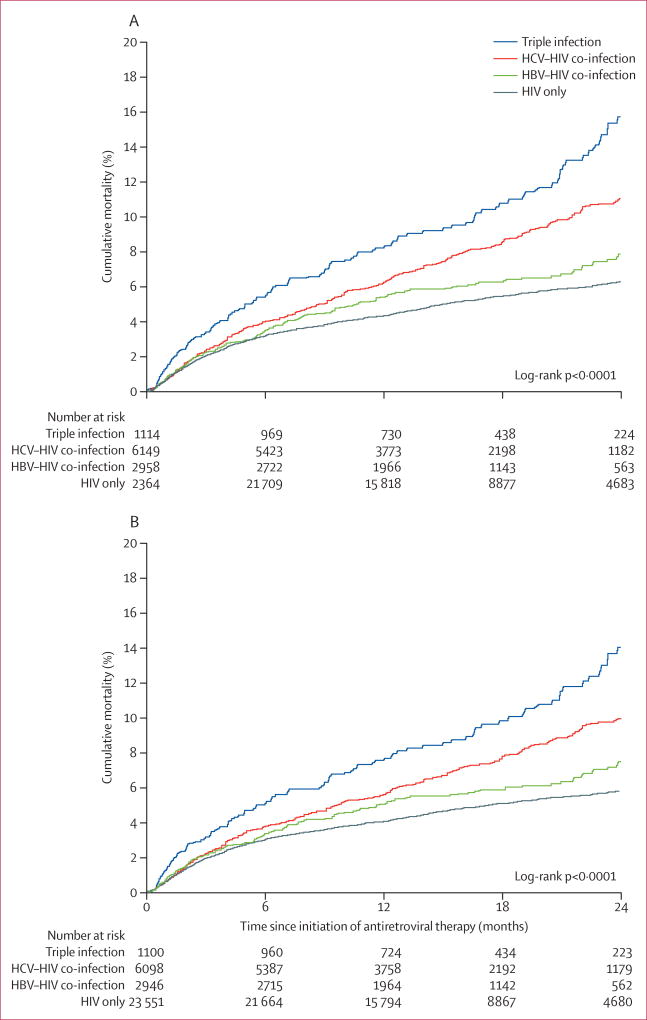
2054 participants died from any cause during 556 400 person-months of follow-up. Median follow-up was 15 months (IQR 12–23). Median follow-up for participants with HCV (14 months) was slightly lower than that in the other three co-infection groups (15 months). The cumulative probability of all-cause death was 3·4% at 6 months, 5·0% at 12 months, and 7·6% at 24 months. In univariable analyses, compared with monoinfection, people with triple infection had the highest risk of death, followed by those with HCV co-infection, and HBV co-infection (table 2, figure 1A). A multivariable analysis adjusting for all covariates (table 2, appendix) showed that patients with HIV, HBV, and HCV were more likely to die than were those with HIV only (p<0·0001), as were those with HIV and HCV (p<0·0001). Participants with HIV and HBV did not have a significantly higher risk of death than those with HIV only (p=0·51; table 2). When stratified by transmission route, we found much the same associations, although the association of HCV co-infection with all-cause death was not significant for patients who had contracted HIV through injection drug use (table 2).
Table 2.
Associations between hepatitis co-infection and time to death
| Triple infection | HCV–HIV co-infection | HBV–HIV co-infection | HIV only | |
|---|---|---|---|---|
| All-cause mortality (n=33 861) | ||||
|
| ||||
| Number of events | 131 | 501 | 186 | 1236 |
| Time on antiretroviral treatment (months) | 17 892 | 96 680 | 49 007 | 392 821 |
| Unadjusted HR (95% CI), p value | 2·32 (1·94–2·77), <0·0001 | 1·62 (1·46–1·80), <0·0001 | 1·21 (1·03–1·41), 0·02 | ·· |
| HR adjusted by age and sex (95% CI), p value | 2·49 (2·07–2·99), <0·0001 | 1·72 (1·54–1·92), <0·0001 | 1·20 (1·03–1·40), 0·02 | ·· |
| HR adjusted by all factors but not transmission mode (95% CI), p value | 2·63 (2·16–3·20), <0·0001 | 2·03 (1·80–2·28), <0·0001 | 1·09 (0·92–1·29), 0·31 | ·· |
| HR adjusted by all factors and transmission mode (95% CI), p value | 1·90 (1·53–2·37), <0·0001 | 1·46 (1·25–1·70), <0·0001 | 1·06 (0·89–1·26), 0·51 | ·· |
| HR adjusted by all factors, stratified by transmission mode (95% CI), p value Injection drug use | 1·63 (1·23–2·17), 0·0008 | 1·21 (0·97–1·51), 0·09 | 0·97 (0·61–1·54), 0·91 | ·· |
| Sexual transmission | 1·99 (1·31–3·02), 0·001 | 1·84 (1·47–2·29), <0·0001 | 1·04 (0·86–1·26), 0·71 | ·· |
|
| ||||
| Non-injury mortality (n=33 695) | ||||
|
| ||||
| Number of events | 117 | 450 | 174 | 1147 |
| Time on antiretroviral treatment (months) | 17 736 | 96 170 | 48 906 | 392 107 |
| Unadjusted HR (95% CI), p value | 2·25 (1·86–2·72), <0·0001 | 1·58 (1·41–1·76), <0·0001 | 1·22 (1·04–1·43), 0·02 | ·· |
| HR adjusted by age and sex (95% CI), p value | 2·43 (2·00–2·95), <0·0001 | 1·68 (1·50–1·89), <0·0001 | 1·21 (1·03–1·42), 0·02 | ·· |
| HR adjusted by all factors but not transmission mode (95% CI), p value | 2·57 (2·09–3·15), <0·0001 | 2·00 (1·77–2·26), <0·0001 | 1·09 (0·92–1·29), 0·34 | ·· |
| HR adjusted by all factors and transmission mode (95% CI), p value | 1·94 (1·54–2·44), <0·0001 | 1·47 (1·25–1·73), <0·0001 | 1·06 (0·89–1·27), 0·51 | ·· |
| HR adjusted by all factors, stratified by transmission mode (95% CI), p value Injection drug use | 1·68 (1·24–2·27), 0·001 | 1·25 (0·99–1·59), 0·06 | 0·96 (0·59–1·58), 0·88 | ·· |
| Sexual transmission | 2·04 (1·33–3·13), 0·001 | 1·79 (1·42–2·26), <0·0001 | 1·06 (0·87–1·29), 0·57 | ·· |
HBV=hepatitis B virus. HCV=hepatitis C virus. HR=hazard ratio.
Figure 1. Cumulative probability of death, by hepatitis virus infection status.
(A) All-cause mortality and (B) non-injury mortality. HBV=hepatitis B virus. HCV=hepatitis C virus.
After excluding 166 participants who died of injuries, 33 695 remained in the analysis of non-injury death. The cumulative probability of non-injury death at each point was similar but lower than all-cause death (figure 1B). The associations between co-infection and non-injury death in univariable and multivariable analyses were consistent with the analyses of all-cause death (table 2).
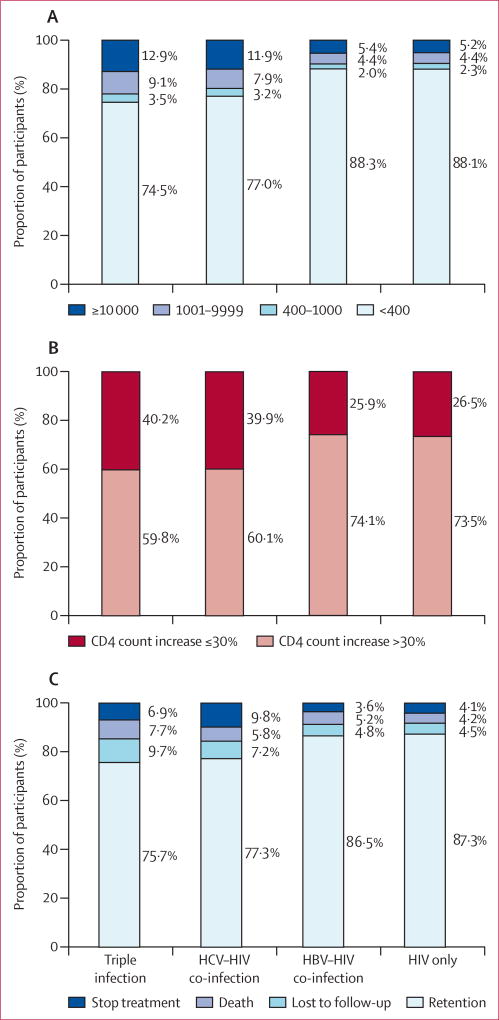
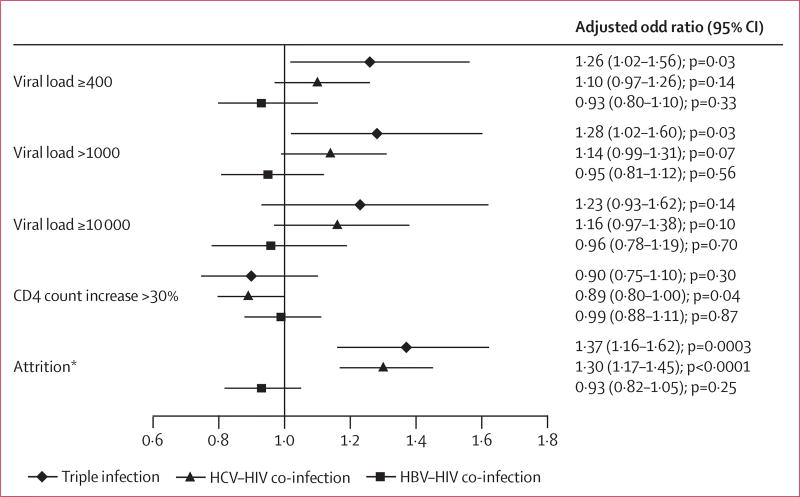
23 991 participants had viral load test results. Viral suppression was rarest among people with triple infection (492 of 660; 74·5%), and more common among people with HCV and HIV (3023 of 3926; 77·0%), HIV and HBV (1873 of 2122; 88·3%), and HIV only (15 223 of 17 283; 88·1%; figure 2A). 12·9% of people with triple infection had a viral load of 10 000 copies per mL or more, compared with 11·9% of those with HCV–HIV co-infection, and 5·4% with HBV–HIV co-infection. In multivariable analysis, people with triple infection were more likely to have virological failure than were those with monoinfection (figure 3). Patients co-infected with HCV were not significantly more likely to have virological failure. Virological failure occurred at much the same rate in participants co-infected with HIV and HBV, and those with HIV only (figure 3). Sensitivity analyses with different cutoffs for virological failure showed much the same results (figure 3).
Figure 2. Outcomes after 12 months of combination antiretroviral therapy.
(A) Viral load response, (B) change of CD4 cell count, and (C) retention status. HBV=hepatitis B virus. HCV=hepatitis C virus.
Figure 3. Multivariable analyses of association between hepatitis co-infection and outcomes.
Controlled for age, sex, treatment centre, CD4 cell count, HIV transmission mode, and initial antiretroviral treatment regimens. *Includes death and stopping.
25 303 patients had CD4 cell count test results. An immunological response occurred in 427 (59·8%) of 714 patients with triple infection, 2478 (60·1%) of 4125 with HIV and HCV, 1652 (74·1%) of 2230 with HIV and HBV, and 13 400 (73·5%) of 18 234 with HIV only (figure 2B). Change in CD4 cell count did not differ significantly between hepatitis co-infection groups and patients with HIV only (figure 3).
People with triple infection were most likely to be lost to follow-up, followed by those with HCV co-infection, those with HBV co-infection, and those with HIV only (figure 2C). In adjusted logistic regression analysis, non-retention in treatment was more likely in people with triple infection and HCV co-infection than in those with HIV infection only (figure 3). No significant difference occurred between those with HBV and HIV, and those with HIV only (figure 3).
Discussion
In this cohort of people being treated for HIV in China, 8·7% had HBV co-infection, 18·2% had HCV co-infection, and 3·3% had triple infection. Those with triple infection and HCV co-infection had a higher risk of death and not being retained in treatment. Triple infection also increased the risk of virological failure. Co-infection with HCV did not affect virological or immunological responses. Co-infection with HBV did not significantly affect any outcomes.
The prevalence of HBV co-infection in this study is lower than previously reported in Asia (14·6–21·7%),23,32 but higher than reported in the USA (4·5%).9 This difference is partly because of a greater proportion of men who have sex with men in previous studies. By contrast, the proportion of HCV co-infection in our study is higher than that previously reported in the Asia–Pacific region (7·2–10·4%).25,33 The predominate mode of HIV transmission in participants with HBV was heterosexual sex, which accords with other studies in Asia,32,33 but it was less common than reported in the USA and Europe.9,34 The primary mode of transmission of HIV among patients with HCV co-infection and triple infection was injection drug use, which occurred in much the same proportions as reported in the USA, Canada, and Europe.35,36 Patients infected from blood donation or transfusion were the largest known HIV-infected population in the early to mid-1990s in China.37 However, few patients in our analysis were infected through this route, showing the benefits of blood donation screening policies. The prevalence of hepatitis co-infection varied by population characteristics in our study, and previous studies have reported variation by geographic location within China.25
Our findings support results from most previous studies, showing that people with HIV and HCV have shorter survival even after starting antiretroviral treatment.22,38,39 By contrast, one report showed no significant effect of HCV, which might be because of a smaller number of deaths than in our study.25 Our findings also accord with previous studies showing no difference in survival between people with HIV and HBV, and those with HIV only,25,33 which could be related to the inclusion of lamivudine in the initial HIV treatment regimen.40
People with triple infection had significantly worse virological responses than those with HIV only, a finding which has not previously been well documented. We recorded no other differences in virological or immunological responses, which is consistent with most previous studies.19,20,24,25,32,38 One study suggested that HBV infection could increase HIV virological failure,23 but it was done in a single hospital with a high proportion of men who have sex with men so might not be generalisable.
Participants with triple infection or with HIV–HCV co-infection were less likely to be retained in treatment than were other participants. This finding is a concern, as maintenance of treatment and care is crucial to improve individual and public health outcomes.41 Low retention in these groups might, in part, be the result of them having a high proportion of injection drug users, who have a higher risk of leaving care and of death than other patients in China.41,42 However, other factors might also be involved. Additional research is needed to develop interventions aimed at increasing retention.
Our results should be interpreted within the study’s limitations. First, HBV infection was diagnosed by HBsAg testing, which only measures presence or absence of antigen. We did not measure biomarkers that represent HBV activity status, and thus we were unable to identify acute HBV infection or HBV stage. This shortcoming might mean some individuals with acute infection were classified as having chronic disease, leading to overestimation of chronic HBV infection. However, this bias is somewhat balanced by missing the diagnosis of occult HBV infection (undetectable HBsAg in serum with the presence of HBV DNA), with proportions of occult HBV infection ranging from 3% to 11% in people with HIV.43,44 Second, HCV diagnosis was based on antibodies, not RNA. With the high prevalence of interleukin 28B genotype CC in Asia,45 patients who might have cleared the infections were included in the HCV group, overestimating the prevalence of HCV infection.20 Third, because of the little information for cause of death, we could not differentiate between non-AIDS-related and AIDS-related deaths. Hepatitis-related liver-disease deaths are common in people with HIV, therefore we might have overestimated the effect of hepatitis on HIV progression. However, we excluded patients with injury-related deaths, partly removing the effect of non-AIDS deaths. Fourth, we could not control for other factors, such as socioeconomic status, tobacco and alcohol use, and time since diagnosis, because these data were not collected in the database. Fifth, injection drug use might be under-reported, leading to confounding related to misclassification of transmission mode. Finally, ascertainment of death was not linked with a centralised death registry, thus mortality might be underestimated.46
To the best of our knowledge, this is the first large, national cohort study to comprehensively assess survival, virological and immunological responses, and retention in care of people with HIV co-infected with HBV or HCV, or both (panel). Our findings emphasise the importance of screening for viral hepatitis when individuals are identified as HIV positive. Moreover, effective management for viral hepatitis should be integrated into HIV treatment programmes, including effective treatment for triple infection and co-infection with HCV or HBV, and HBV vaccination for HBV-negative people with HIV to reduce HIV-related morbidity and mortality in regions in China where hepatitis is endemic. Long-term data are needed about the effect of hepatitis co-infection on HIV disease progression.
Panel: Research in context.
Systematic review
We searched Medline with the terms “HIV”, “HBV”, “HCV”, and “co-infection”. We found several studies that show that HIV can accelerate hepatitis-related liver diseases.15,19 By contrast, the effect of infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) on HIV clinical progression, immunological response, and viral response has not been well established.20–22 Some studies have found no effect of hepatitis virus infection on viral and immunological response to combination antiretroviral therapy or mortality.25 However, others have shown that HIV-HBV co-infection increases the risk of virological failure and death,23 although our findings in the present study do not support this. Our study results support previous findings that HIV and hepatitis C virus co-infection decreases survival.24 Very few data exist for the effect of triple viral infection. Our study suggests worse outcomes for those with triple infection compared with those with HIV alone.
Interpretation
These viruses share routes of transmission and many people with HIV are co-infected with HBV and HCV in China, particularly in injection drug users, sex workers, and men who have sex with men. Of an estimated 700 000 people with HIV in China about 10% also have HBV.47 A systematic review of methadone maintenance treatment clinics in China showed 4·6% of attendees had HCV–HIV co-infection.48 Our findings emphasise the importance of screening for viral hepatitis when individuals are diagnosed with HIV. Moreover, effective management of viral hepatitis should be integrated into HIV treatment programmes.
Acknowledgments
We thank the staff of the local counties’ Centers for Disease Control, who spent numerous hours and great effort working with us in obtaining, verifying, and cleaning the data used in this study.
Footnotes
See Online for appendix
Contributors
HS, FZ, HZ, and MB designed the study. DZ and HF collected the data. ZD and HZ analysed the data. YW, YZ, YM, XL, and JL interpreted the data. MB, W-PC, and ZW suggested additional analyses. ZD and HZ designed the figures. HZ and NK drafted the report. All authors reviewed, revised, and approved the final report.
Declaration of interests
We declare no competing interests.
References
- 1.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection—a global challenge. N Engl J Med. 2012;366:1749–52. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Easterbrook P, Sands A, Harmanci H. Challenges and priorities in the management of HIV/HBV and HIV/HCV coinfection in resource-limited settings. Semin Liver Dis. 2012;32:147–57. doi: 10.1055/s-0032-1316476. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. [accessed Aug 26, 2014];Global report: UNAIDS report on the global AIDS epidemic. 2012 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf.
- 5.Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010;14:e1024–31. doi: 10.1016/j.ijid.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Rockstroh JK, Spengler U. HIV and hepatitis C virus co-infection. Lancet Infect Dis. 2004;4:437–44. doi: 10.1016/S1473-3099(04)01059-X. [DOI] [PubMed] [Google Scholar]
- 7.Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin Infect Dis. 2012;55(suppl 1):S33–42. doi: 10.1093/cid/cis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS. 2005;19(suppl 3):S41–46. doi: 10.1097/01.aids.0000192069.95819.aa. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol. 2008;14:6689–93. doi: 10.3748/wjg.14.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–09. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 11.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May M, Boulle A, Phiri S, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–57. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 15.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–26. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 16.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 17.Nunez M. Clinical syndromes and consequences of antiretroviral-related hepatotoxicity. Hepatology. 2010;52:1143–55. doi: 10.1002/hep.23716. [DOI] [PubMed] [Google Scholar]
- 18.Labarga P, Soriano V, Vispo ME, et al. Hepatotoxicity of antiretroviral drugs is reduced after successful treatment of chronic hepatitis C in HIV-infected patients. J Infect Dis. 2007;196:670–76. doi: 10.1086/520092. [DOI] [PubMed] [Google Scholar]
- 19.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 20.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 21.Stebbing J, Waters L, Mandalia S, Bower M, Nelson M, Gazzard B. Hepatitis C virus infection in HIV type 1-infected individuals does not accelerate a decrease in the CD4+ cell count but does increase the likelihood of AIDS-defining events. Clin Infect Dis. 2005;41:906–11. doi: 10.1086/432885. [DOI] [PubMed] [Google Scholar]
- 22.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–05. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 23.Sheng WH, Chen MY, Hsieh SM, et al. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clin Infect Dis. 2004;38:1471–77. doi: 10.1086/420744. [DOI] [PubMed] [Google Scholar]
- 24.Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39:1507–13. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM. Hepatitis B and C virus coinfection in The TREAT Asia HIV Observational Database. J Gastroenterol Hepatol. 2007;22:1510–18. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang F, Dou Z, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11:516–24. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 28.Mao Y, Wu Z, Poundstone K, et al. Development of a unified web-based national HIV/AIDS information system in China. Int J Epidemiol. 2010;39(suppl 2):79–89. doi: 10.1093/ije/dyq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F. China Free Antiretroviral Therapy Manual, 2012. Beijing: People’s Medical Publishing House; 2012. [Google Scholar]
- 30.WHO. [accessed Aug 26, 2014];Antiretroviral therapy for HIV infection in adults and adolescents. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf?ua=1.
- 31.Ma Y, Zhao D, Yu L, et al. Predictors of virologic failure in HIV-1-infected adults receiving first-line antiretroviral therapy in 8 provinces in China. Clin Infect Dis. 2010;50:264–71. doi: 10.1086/649215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Li Y, Zhang C, et al. Immunological and virological responses to cART in HIV/HBV co-infected patients from a multicenter cohort. AIDS. 2012;26:1755–63. doi: 10.1097/QAD.0b013e328355ced2. [DOI] [PubMed] [Google Scholar]
- 33.Law WP, Duncombe CJ, Mahanontharit A, et al. Impact of viral hepatitis Co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169–77. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- 34.Miailhes P, Trabaud MA, Pradat P, et al. Impact of highly active antiretroviral therapy (HAART) on the natural history of hepatitis B virus (HBV) and HIV coinfection: relationship between prolonged efficacy of HAART and HBV surface and early antigen seroconversion. Clin Infect Dis. 2007;45:624–32. doi: 10.1086/520752. [DOI] [PubMed] [Google Scholar]
- 35.Spradling PR, Richardson JT, Buchacz K, et al. Trends in hepatitis C virus infection among patients in the HIV Outpatient Study, 1996–2007. J Acquir Immune Defic Syndr. 2010;53:388–96. doi: 10.1097/QAI.0b013e3181b67527. [DOI] [PubMed] [Google Scholar]
- 36.Landes M, Newell ML, Barlow P, et al. Hepatitis B or hepatitis C coinfection in HIV-infected pregnant women in Europe. HIV Med. 2008;9:526–34. doi: 10.1111/j.1468-1293.2008.00599.x. [DOI] [PubMed] [Google Scholar]
- 37.Dou Z, Chen RY, Wang Z, et al. HIV-infected former plasma donors in rural Central China: from infection to survival outcomes, 1985–2008. PLoS One. 2010;5:e13737. doi: 10.1371/journal.pone.0013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weis N, Lindhardt BO, Kronborg G, et al. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42:1481–87. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- 39.Lumbreras B, Jarrin I, del Amo J, et al. Impact of hepatitis C infection on long-term mortality of injecting drug users from 1990 to 2002: differences before and after HAART. AIDS. 2006;20:111–16. doi: 10.1097/01.aids.0000196164.71388.3b. [DOI] [PubMed] [Google Scholar]
- 40.Puoti M, Cozzi-Lepri A, Paraninfo G, et al. Impact of lamivudine on the risk of liver-related death in 2,041 HBsAg- and HIV-positive individuals: results from an inter-cohort analysis. Antivir Ther. 2006;11:567–74. doi: 10.1177/135965350601100509. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Napravnik S, Eron J, et al. Attrition among human immunodeficiency virus (HIV)-infected patients initiating antiretroviral therapy in China, 2003–2010. PLoS One. 2012;7:e39414. doi: 10.1371/journal.pone.0039414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu E, Rou K, McGoogan JM, et al. Factors associated with mortality of HIV-positive clients receiving methadone maintenance treatment in China. J Infect Dis. 2013;208:442–53. doi: 10.1093/infdis/jit163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opaleye OO, Oluremi AS, Atiba AB, et al. Occult hepatitis B virus infection among HIV positive patients in Nigeria. J Trop Med. 2014;2014:796121. doi: 10.1155/2014/796121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khamduang W, Ngo-Giang-Huong N, Gaudy-Graffin C, et al. Prevalence, risk factors, and impact of isolated antibody to hepatitis B core antigen and occult hepatitis B virus infection in HIV-1-infected pregnant women. Clin Infect Dis. 2013;56:1704–12. doi: 10.1093/cid/cit166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao XW, Ling Y, Li XH, et al. Association of genetic variation in IL28B with hepatitis C treatment-induced viral clearance in the Chinese Han population. Antivir Ther. 2011;16:141–47. doi: 10.3851/IMP1703. [DOI] [PubMed] [Google Scholar]
- 46.Zhu H, Napravnik S, Eron JJ, et al. Decreasing excess mortality of HIV-infected patients initiating antiretroviral therapy: comparison with mortality in general population in China, 2003–2009. J Acquir Immune Defic Syndr. 2013;63:e150–57. doi: 10.1097/QAI.0b013e3182948d82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rong-Rong Y, Xi-En G, Shi-Cheng G, Yong-Xi Z. Interaction of hepatitis B and C viruses in patients infected with HIV. J Acquir Immune Defic Syndr. 1999;48:505–06. doi: 10.1097/QAI.0b013e31816de23c. [DOI] [PubMed] [Google Scholar]
- 48.Zhuang X, Liang Y, Chow EP, Wang Y, Wilson DP, Zhang L. HIV and HCV prevalence among entrants to methadone maintenance treatment clinics in China: a systematic review and meta-analysis. BMC Infect Dis. 2012;12:130. doi: 10.1186/1471-2334-12-130. [DOI] [PMC free article] [PubMed] [Google Scholar]