Abstract
Death of adult cardiac myocytes and supportive tissues resulting from cardiovascular diseases such as myocardial infarction (MI) is the proximal driver of pathological ventricular remodeling that often culminates in heart failure. After MI the heart may lose up to a billion myocytes1, resulting in impaired pump function, persistent sympathetic neurohormonal activation, pathological hypertrophy of surviving myocytes, and replacement fibrosis2. Despite advances in the diagnosis and early treatment of acute MI, the lifetime risk and overall health burden from heart failure remain profoundly high3. This is partially because no currently available therapeutic, barring heart transplantation, can directly replenish myocytes lost from the injured heart. For decades, the field has struggled to define the intrinsic capacity and cellular sources for endogenous myocyte turnover in pursuing more innovative therapeutic strategies aimed at regenerating the injured heart. While controversy persists to this day as to the best therapeutic regenerative strategy to employ, a growing consensus has been reached that the very limited capacity for new myocyte formation in the adult mammalian heart is due to proliferation of existing cardiac myocytes, but not due to the activity of an endogenous progenitor cell source of some sort. Hence, future therapeutic approaches should take into consideration the fundamental biology of myocyte renewal in designing strategies to potentially replenish these cells from the injured heart.
Keywords: cardiac regeneration, myocardial infarction, stem cell, proliferation
Subject Terms: Myocardial Biology, Myocardial Regeneration, Stem Cells
New Myocyte Formation Across Development, Homeostasis, and Injury
Myocyte formation in the adult non-mammalian heart
Studies in amphibians and teleost fish such as the zebrafish were among the first to demonstrate experimentally that the postnatal heart could generate new myocytes4, 5. In adult zebrafish, surgical resection of up to 20% of the ventricle resulted in the formation of a transient fibrin-rich clot, followed by a robust increase in cycling myocytes as indicated by BrdU incorporation6. A similar response has been documented in amphibians such as the newt7 and the axolotl8. The expansion of new myocytes following partial resection of adult zebrafish or amphibian hearts can result in virtually complete structural and functional recovery of the ventricle, although some studies have demonstrated that this regenerative response is inadequate in the face of a more severe injury9. Genetic fate mapping studies in the zebrafish have concluded that pre-existing myocytes are the predominant cellular source for new myocyte formation in non-mammalian hearts10, 11. However, the precise mechanisms governing the generation of new myocytes in these systems have not been fully delineated and may involve direct cell cycle re-entry of differentiated myocytes10, dedifferentiation to a more plastic state which precedes proliferation12, 13, or even cellular fusion14. Determining the biological distinctions underlying the profound cardiac regenerative capacity in select non-mammalian species, as opposed to the limited capacity observed in mammalian hearts, is an area of active investigation15, 16. Perhaps most striking, fundamental differences in cardiovascular physiology, tissue oxygen content, or growth kinetics, which are considerable across amphibians, fish, and mammals, may not fully explain disparities in regeneration. For example, recent studies comparing zebrafish to a related freshwater teleost fish, the medaka, have revealed a near-complete loss of cardiac regenerative capacity in the latter17, 18, suggesting that an even more subtle mechanism may be involved.
Myocyte formation in the fetal mammalian heart
Cardiomyogenesis during mammalian heart development is initiated with the commitment of cardiogenic precursor cells derived from the embryonic mesoderm19, 20, which themselves are specified through a series of paracrine and autocrine gene regulatory signals culminating in activation of the transcription factor Mesp121. During the early stages of heart development, Mesp1+ mesodermal progenitors are further specified into cardiac progenitor cells of the first and second heart fields19, 22–24. These committed cardiac progenitors undergo rapid proliferation and differentiation to generate and expand the early heart tube25, 26. Coordination of this process is tightly regulated by several key cardiogenic transcription factors including Nkx2.5, Tbx5, and Gata420, 27. In terms of proliferative capacity, fate mapping and BrdU pulse-label studies suggest that cardiac progenitors are more proliferative than the early cardiac myocytes they give rise to28, 29; however, development of the early linear heart tube into the four-chambered heart is driven by substantial proliferation of differentiated myocytes30, 31. This second wave of proliferation from differentiated myocytes is essential for expansion of the ventricular chambers and establishment of the cardiac trabeculae32, consistent with the increase in myocyte proliferation that drives chamber formation during development of the zebrafish heart15.
Genetic manipulations in mice have revealed the extensive capacity of the fetal mammalian heart for new myocyte generation. In one study, mice with partial myocyte loss during development were generated via Nkx2.5-Cre-driven excision of holocytochrome c synthase (Hccs)33. Hcss is an X-linked gene required for mitochondrial function and cell viability. By taking advantage of random X chromosome inactivation, this model resulted in a roughly 50% loss of cardiac myocytes in a cell-autonomous fashion in females carrying Nkx2.5-Cre and one loxP targeted Hccs allele. Male mice of this same genotype were not viable beyond embryonic day 14.5. By coupling this genetic system with a dual reporter strategy that allowed for differential fate mapping of X-inactivated cells with either LacZ or GFP, this study demonstrated a compensatory 27% increase in proliferation in the remaining myocytes that were spared via X-inactivation of the Hccs floxed allele, which allowed the majority of these animals to develop normally into adulthood33. A more recent approach used Nkx2.5-Cre-driven diphtheria toxin coupled with blastocyst complementation to generate a series of mouse lines with quantifiable embryonic myocyte ablation ranging from 10% to roughly 95%34. This study demonstrated that mice depleted for up to 60% of myocytes during embryogenesis could survive into adulthood with no overt cardiac phenotype, by rapidly upregulating proliferation of the remaining myocytes. Thus, the embryonic mammalian heart retains a remarkable degree of plasticity, driven by myocyte cell cycle activity35.
Myocyte formation in the neonatal mammalian heart
During the first days of postnatal life, the mammalian heart undergoes substantial growth and physiological remodeling to accommodate an increased demand for cardiac output. Hypertrophic growth of cardiac myocytes is the predominant effector in this process36 but additional, temporally restricted myocyte hyperplasia also plays a role. In rodents, myocyte cell volume increases more than two-fold within the first week of postnatal life37 with concomitant increases in myocyte DNA synthesis rates and myocyte binucleation that peak by postnatal day 730. Estimates of the amount of myocyte proliferation and the absolute increase in myocyte number that occurs during this period can vary, largely due to technical issues related to identifying proliferation events in vivo from histological sections. Using thymidine analog (BrdU or EdU)-based detection methods, several investigators reported rates of 5–10% of myocytes undergoing active DNA synthesis within postnatal days 3–4 in rodent hearts, which rapidly declined to ~1% after this point38. More recent studies have examined isolated myocyte nuclei by flow cytometry39, 40 and have documented largely comparable rates. These rates are also in agreement with data obtained using quantitative non-radioactive multi-isotope mass spectrometry to track cellular turnover of myocytes using 15N-thymidine41 which demonstrated a 1% myocyte turnover rate in the postnatal day 4 mouse heart. Collectively these results suggest that postnatally, new myocyte formation is highest within the first four days after birth but then rapidly declines as cells enter mitotic arrest and terminal differentiation around postnatal day 742. Early postnatal cardiomyogenesis also differs between the left and right ventricle, as chamber-specific rapid proliferation is responsible for the marked expansion of left ventricular myocardium in mammals soon after birth43–45. Differences in regional signaling cues likely underlie the preferential induction of proliferation in the left versus right ventricles; for example, loss of Fgf10 results in myocyte hypoplasia only in the right ventricle46. This chamber-specific distinction in myocyte proliferative capacity or responsiveness to proliferative signals may also play a role in the ability of the left ventricle to regenerate following injury in neonatal mammals, which we discuss next.
Some of the strongest evidence for myocyte proliferation in the postnatal heart comes from models of cardiac injury in mice within the first week after birth. Surgical resection of the left ventricular apex in 1 day-old neonates resulted in a robust upregulation of myocyte proliferation and near complete regeneration of the injury site by day 21 47. This enhanced regenerative capacity of p1 neonatal mouse hearts was also observed by multiple different investigators using coronary artery ligation-induced MI48, 49 or cryoinjury50, 51. However, other groups have reported a limited or completely absent capacity for neonatal heart regeneration in mice52, 53. Technical variations in surgical technique or mode of injury have been suggested as reasons for the negative results54–56, thus ongoing efforts to standardize experimental models of neonatal heart injury will be needed, although the positive results reported would appear to be undeniable and indicate that regeneration is indeed observed in the postnatal mouse heart with the greatest capacity immediately after birth 57–59.
A second round of increased myocyte cell cycle activity and proliferation has been described in the postnatal mouse heart, occurring between postnatal days 10–14 through a burst of thyroid hormone signaling, and accounting for almost a doubling in myocyte number60, 61. However, attempts to replicate this finding using either nuclear LacZ-targeted transgenic mice, or radiolabeled thymidine uptake failed62, 63. In contrast, a marked increase in myocyte DNA content was observed during this time62, but this was instead associated with an increase in ploidy. A recently developed lineage tracing system to fate map cycling myocytes in the mouse also did not demonstrate an increase in proliferative cells at postnatal day 15 64. Thus, reactivation of myocyte DNA synthesis pathways may be occurring during the second week of postnatal growth in the mouse, but this appears to be unlikely to reflect myocyte cell division leading to new myocyte formation.
Myocyte formation in the adult mammalian heart
As previously discussed, significant myocyte cell cycle withdrawal occurs by the first week of postnatal life in the mammalian heart, concurrent with an increase in the number of binucleated myocytes due to a final round of karyokinesis without cytokinesis, at least in rodent models42. As a result, new myocyte formation during adulthood is highly limited. Multi-isotope mass spectrometry showed a frequency of 0.015% 15N-thymidine labeled cardiac myocyte nuclei in young adult mice over an eight-week period41, consistent with previous reports that demonstrated a sharp decline in myocyte DNA synthesis during postnatal life30, 38. Myocardial infarction (MI) injury resulted in a significant increase in labeled myocytes. However, only a rare fraction of these showed evidence for completion of cytokinesis, demonstrating that cardiac myocyte DNA synthesis and true cellular division are not directly correlative, and that endogenous cardiomyogenesis in the adult heart post-MI remains extremely modest. Advances in genetic lineage tracing systems have also allowed for more precise quantitation of myocyte turnover and dynamics in the mouse heart under homeostatic conditions and with aging. A 2014 report used a Cre-dependent dual GFP or RFP labeling system to distinguish myocytes resulting from complete cytokinesis versus binucleation65. Combining this approach with an inducible Myh6-driven Cre recombinase demonstrated that approximately 0.01%–0.02% adult myocyte renewal could be observed in adult mice. Of note, this study did not identify an appreciable increase in new myocytes following MI, in contrast to other reports with alternative fate mapping approaches that have observed an increase in new myocytes derived from pre-existing myocytes in the peri-infarct zone after MI41. Collectively, these studies demonstrate a detectable but considerably reduced capacity for new myocyte formation in adult mammalian hearts soon after birth. Strategies to enhance the limited capacity of myocyte derived new myocyte formation should be pursued as a therapeutic strategy aimed at improving the function of the injured heart.
Myocyte formation in the healthy and diseased human heart
Evidence for myocyte turnover in human hearts during aging or after disease stimuli comes primarily from retrospective analyses. However recent novel approaches have provided considerable insight. Specifically, a landmark series of studies took advantage of pre-existing 14C incorporation found in postmortem heart samples from subjects living during the sharp rise in atmospheric 14C associated with nuclear bomb testing from the early 1950s until 196366, 67. Mathematical modeling using levels of 14C incorporation in isolated human myocyte nuclei demonstrated a maximal turnover rate of about 1% myocyte DNA per year in non-diseased adult human hearts, which declines by more than half with advancing age66. Moreover, juvenile and early adolescent hearts showed the largest annual rates of new myocyte DNA labeling, reaching a peak of 5% per year, which sharply declined after 10 years of age67. However, this approach only indirectly measures cellular turnover and does not detect or report actual cytokinesis, so it remains likely that the true rates of new myocyte formation in the human heart are lower. Moreover, these rates of cardiac myocyte formation via 14C incorporation are highly dependent on assumptions and parameters used in the mathematical models. Independent analysis of these data has suggested that variations in parameters such as atmospheric 14C levels or myocyte attrition rates can confound the interpretation of human cardiomyogenesis via 14C incorporation68. The modeling approaches using 14C incorporation also concluded that new myocyte formation ceases in the early postnatal human heart, whereas other studies have documented indices of myocyte proliferation in young human heart tissues69, albeit only at the post-mortem histological level. Despite these caveats, Cold War-era 14C incorporation does provide compelling evidence that the adult human heart retains a finite but detectable degree of cardiac myocyte DNA turnover throughout postnatal life, and that this rate is highest early after birth and during early adolescence, with a sharp decline thereafter and a continual decrease with advanced age. These findings are in accordance with experimental data from mammalian model systems in which actual cytokinesis can also be evaluated.
Evidence also supports the potential for an increase in endogenous cardiac myocyte turnover in certain human disease contexts. A small cohort of patients with advanced heart failure who received left ventricular assist devices showed significant increases in myocytes positive for histological markers of cell cycle activation and cytokinesis70. Clinical presentations in newborns with severe cardiac damage, perinatal MI, or genetic cardiomyopathies have also demonstrated evidence for myocyte cell cycle re-entry71 and in some cases complete structural and functional recovery72, 73, suggesting that the neonatal human heart may retain some inherent cardiac plasticity, as observed in small animal models.
Endogenous Sources of New Myocytes in the Adult Mammalian Heart
The longstanding historical view of the adult heart as a terminally differentiated organ lacking considerable myocyte turnover74 was challenged over the last 15 years with reports of profound intrinsic regenerative capacity via resident cardiac stem cells. However, based on the sum of available data as of 2018 the original historical model appears to have been correct all along; that new myocyte formation in the adult heart is very limited. After many studies in animal models and more recent analyses of human tissue samples, the consensus view in the field is that new myocyte formation in healthy adult hearts occurs at a rate around 0.5%–1% per year75. Here we review the available evidence that addresses the potential cell sources for cardiac myocyte turnover; either proliferation of pre-existing myocytes, or differentiation from a non-myocyte cell type.
In vivo evidence for endogenous myocyte proliferation in mammalian hearts
Much of the foundational work for assessing cardiac myocyte proliferation was based on measures of mitotic figures and DNA synthesis in rodent hearts30, 38, 39, 76, 77. These studies suggested that myocytes were themselves the source of identified cycling cells, but only the adoption of genetic lineage tracing models allowed the field to definitively address this question. Pulse-labeling of pre-existing myocytes with a GFP reporter using the αMHC-driven MerCreMer demonstrated that the proportion of GFP+ labeled myocytes did not change over physiological aging out to one year78, suggesting that a non-myocyte (GFP−) cell source did not replenish myocytes. An extensive follow-up study from the same group combined this αMHC-driven lineage tracing model with mass cytometry of labeled thymidine to track cycling cells in a highly sensitive way, which showed an annual myocyte turnover rate in the mouse of 0.76% under physiological conditions41, a rate consistent with total myocyte DNA turnover in humans66, 67. Of note, these thymidine-labeled cycling myocytes were GFP+, having been previously labeled by αMHC-MerCreMer lineage tracing, indicating they were pre-existing myocytes. A more recent report has also used this thymidine incorporation and mass cytometry protocol to track new myocyte formation after prolonged voluntary exercise in mice79. Voluntary exercise was associated with a striking 4-fold increase in thymidine incorporation and an increase in mononuclear diploid myocytes. Of note, the determination of myocyte ploidy in this study was limited to in situ hybridization labeling of serial tissue sections, so future confirmatory experiments will be needed to directly assess the extent that increased DNA synthesis after exercise reflects new myocyte formation. Additionally, a lineage tracing study that coupled inducible Cre recombinase to a hypoxia-regulated element of Hif-1α found that myocytes under local tissue hypoxia preferentially re-enter the cell cycle and undergo proliferation80, while a newly developed cell-cycle-specific reporter system in the mouse heart also demonstrated early postnatal myocyte proliferative capacity in vivo64. Taken together these findings are consistent with numerous studies demonstrating that the hearts of zebrafish6, 10 and other lower vertebrates8 as well as neonatal rodent hearts34, 47 all are repopulated with new myocytes via proliferation of pre-existing myocytes after various injuries.
Isolation of human myocytes from transplant or cadaveric hearts demonstrated the presence of mitotic markers, as well as markers of cytokinesis, which correlated with patient age69. Specifically, myocytes showing phosphorylation of histone H3 and assembly of the contractile ring required for cytokinesis were most frequently present in patients less than one year of age (roughly 0.01–0.02%), while myocytes from patients after age 20 showed rates roughly half that (0.005%), which was estimated in this same study to reflect an approximate yearly myocyte turnover of 1.9% in human hearts ranging from ages 19 to 2369. An age-dependent decrease in myocyte proliferation is also in agreement with retrospective carbon dating studies, which have demonstrated that postnatal myocyte turnover in humans is highest soon after birth and precipitously diminishes with age66, 67. In line with this, individual case studies in human neonates have also documented histological indices suggestive of myocytes going through the cell cycle71, 73.
In vivo evidence for myocyte differentiation from an endogenous non-myocyte cell type in mammalian hearts
In the early 2000s, studies reporting the presence of endogenous cardiac stem cells in adult hearts that could generate de novo myocytes81–83 transformed the field and quickly led to clinical application. Follow-up studies would report that cardiac stem cells, identified predominantly by surface marker expression of the receptor tyrosine kinase c-Kit84 or stem cell antigen-1 (Sca-185), or side population (SP) phenotype cells85, 86, could robustly generate new myocardium when isolated, expanded in culture, and delivered into infarcted hearts in rodents. However, if the adult heart contained biologically active endogenous stem cells with cardiomyogenic capacity, it stands to reason that these cells could act in vivo without culture manipulation to replenish the cardiac myocyte pool after injury, as is the case for other known adult stem cells and their respective tissues of residence87. We have previously discussed at length the limitations of using cells in culture as a surrogate for the in vivo biological activity of those cells in their native environment88, 89. Thus, here we focus on the in vivo evidence that the adult mammalian heart does (or does not) contain an endogenous stem cell that contributes to new myocyte formation.
One of the initial observations that led to the hypothesis of myocyte formation from non-myocytes was the presence of sex-mismatched myocyte nuclei in human hearts following heart transplant. A study published in 2002 analyzed post-mortem heart tissue from male patients who had received a female donor heart and found that up to 10% of the cardiac myocytes and vascular cells in these patients carried the Y chromosome, suggesting that they were newly formed cells derived from the patient after transplant90. Similar analyses were carried out by other investigators, but these studies showed rates of myocyte chimerism of less than 0.05%91, 92 or none at all93. Nonetheless, the conclusion made from these studies was that non-myocyte cell types derived from outside the heart had migrated into the tissue and differentiated into new myocytes. The presumption that these were stem cells was based largely on the immunohistochemical detection of surface markers such as c-Kit and Sca-1, also present on hematopoietic stem cells90, although Sca-1 is a murine gene with no known human homologue94, making it uncertain which cell types in the human heart were detected using a Sca-1 antibody in that previous study. However, a more fundamental limitation of these human studies was the inability to rule out heterotypic cell fusion as the source of these chimeric myocytes. Studies in mice that received myocardial infarction demonstrated that circulating immune cells as well as endothelial cells or skeletal muscle myoblasts could fuse with pre-existing cardiac myocytes in vivo95, 96. A genetic approach was also used to label mouse or hamster donor hearts with nuclear LacZ in a xenograft model of heterotopic heart transplant into GFP+ transgenic rats97. demonstrating that donor cell myocyte nuclei were due to fusion of circulating cells with a pre-existing recipient myocyte. Thus, the presence of sex-mismatched myocyte nuclei following heart transplantation, which is exceptionally rare, is not an indicator of new myocyte formation from a non-myocyte stem cell.
Following these initial descriptions, several studies performed in the setting of myocardial injury documented myocyte proliferation events that may have arisen from a non-myocyte source78, 84, 98. Based on this and a series of papers demonstrating cardiomyogenic potential of cultured c-Kit cells using cellular transplantation, a conclusion was drawn that endogenous c-Kit cells underlie the turnover of myocytes observed under physiological conditions99. However, there was no direct evidence documenting the origin of these new myocytes as derived from c-Kit stem cells until the development of genetic fate mapping tools to track the contribution of endogenous c-Kit cells in the heart. One of the first approaches, using transgenic mice containing a bacterial artificial chromosome (BAC) system to drive eGFP in Kit expressing cells, demonstrated a limited degree of cardiomyogenesis from c-Kit cells only in the neonatal heart50, 100, but this system was limited by the inability to permanently mark Kit expressing cells and the artificial nature of the transgenic approach in faithfully recapitulating endogenous Kit allele gene expression. Thus, several groups independently generated knock-in mice with Cre recombinase under the control of the endogenous Kit promoter, which were used with Cre-dependent reporter alleles to irreversibly mark c-Kit expressing cells and their progeny with genetically encoded fluorescent indicator proteins101–103. In agreement with prior reports using the BAC approach, these studies demonstrated a modest but detectable contribution of c-Kit cells to cardiomyogenesis during heart development and in the neonatal heart. However, in the adult heart these studies all demonstrated that the cardiac myocyte contribution from endogenous c-Kit cells remained less than 0.03% during physiological growth, aging, or after injury by myocardial infarction or isoproterenol infusion101–104. Moreover, up to 80% of this already low myocyte contribution was not from c-Kit cell differentiation but the result of cell fusion between existing myocytes and circulating hematopoietic cells from bone marrow-derived c-Kit cells105, 106. As a result, the field at large has concluded that c-Kit cells are not a physiological source of new myocyte formation in the adult heart75.
Although cells marked by c-Kit are not cardiac myocyte forming stem cells107, other purported stem cell classes remain less defined at the present day. One recent study performed lineage tracing of Sca-1 cells in mice using a partial Sca-1 (gene name Ly6a) promoter fragment108. This study observed that roughly 2–4% of all Sca-1 lineage-traced cells in the adult heart were myocytes. The conclusion drawn was that at least a few percent of myocytes were replenished from a Sca-1-derived source throughout aging, although this would be above the consensus myocyte turnover rate of maximally 1% per year documented by numerous approaches as discussed above. An alternate explanation of these findings is that the Ly6a promoter fragment that was employed does not accurately recapitulate endogenous Ly6a gene expression. Indeed, the use of a transgenic line that employed a partial promoter construct to track c-Kit cells also precluded definitive analysis of endogenous c-Kit cell fates because such constructs do not contain the full sequence of gene regulatory elements and are often ectopically expressed in other cell types109 including differentiated myocytes110. Even the more rigorous “knock-in” lineage tracing strategy introduces heterozygosity of the targeted allele, which might produce confounding effects110. For example, a recent report argued that Kit allele heterozygosity due to introduction of Cre impairs ex vivo functionality and/or in vivo labeling efficiency of c-Kit resident cardiac stem cells111. Genetic lineage tracing also depends on the chosen marker gene not being expressed in other differentiated cell types, which is not always the case. For example, ABCG2, the ATP-binding cassette transporter that confers the side population (SP) phenotype to another population of cells with purported cardiomyogenic capacity83, 85, 86, is also expressed in fully differentiated adult cardiac myocytes112.
In light of these concerns, a new series of genetic tools was developed to allow for fate mapping and quantitation of cardiomyogenesis from any non-myocyte cell sources, without the need for marker-specific lineage tracing113, 114. This model used four distinct mouse models and combined Cre-lox and Dre-rox dual recombinases with interleaved cell fate reporters to irreversibly and differentially label all pre-existing cardiac myocytes, as well as all cardiac non-myocytes with a different fluorescent protein. This approach demonstrated that non-myocytes of any source do not contribute to the new myocyte formation in the adult heart at baseline or after infarction injury114. Importantly, none of the four models used in this paper relied on targeted “knock-ins” for any of the stem cell marker genes, eliminating concerns of Kit allele heterozygosity raised previously. This study also demonstrated that differentiated non-myocyte cell types, such as epicardial cells, fibroblasts or endothelial cells, do not transdifferentiate into cardiac myocytes in vivo, which had been previously proposed115, 116. The results of Zhou and colleagues 113, 114 are in support of previous lineage tracing studies that did not observe cardiomyogenesis or transdifferentiation from differentiated non-myocyte cell sources in vivo117–119.
Thus, more than 15 years after the initial reports of endogenous cardiac stem cells, rigorous genetic lineage tracing efforts from numerous investigators have demonstrated that non-myocyte cell types have essentially no contribution to endogenous myocyte turnover in the adult mammalian heart107. Proliferation from pre-existing myocytes is the mechanism by which the adult heart appears to replace new myocytes under homeostatic conditions and diseased conditions, albeit at very low levels75. While we are certain that the controversy will continue for years to come and more papers will be published suggesting the existence of some “flavor” of adult stem cell that can repair the heart, we believe that the rigorous lineage tracing results discussed above, especially the definitive approach of Zhou and colleagues,113, 114, are collectively indisputable and definitely show that the heart is not repaired by any sort of progenitor cell, and that the adult mammalian heart simply lacks a myocyte producing stem cell.
Therapeutically Augmenting New Myocyte Formation in Adult Mammalian Hearts
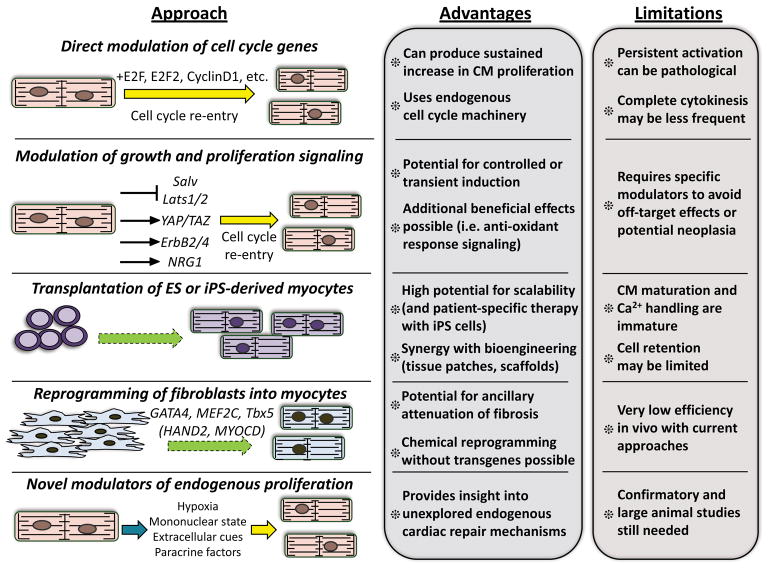
The consensus rate of 1% myocyte DNA synthesis in the adult mammalian heart each year is several orders of magnitude too small to produce sufficient numbers of new myocytes and replace those lost by injury and thereby impart functional benefit 2, 120. Cell therapy was originally proposed as a solution to this limitation, as transplanted adult stem cells were at first proposed to engraft, differentiate into myocytes and thus repopulate the infarcted heart with new myocardium99. However, the functional benefits observed following transplant of most adult cell types has now been attributed to indirect mechanisms and not de novo cardiomyogenesis121. Several of these mechanisms, such as revascularization, amelioration of fibrosis, or release of cardioprotective paracrine factors have been extensively reviewed previously121–123. Thus, here we will discuss strategies that have been proposed specifically with the goal of increasing total myocyte numbers in the injured heart through one or more cellular mechanisms. We also assess the potential advantages for each strategy in terms of therapeutic potential, as well as the inherent limitations and risks of each (Figure).
Figure. Therapeutic sources for new myocyte generation in the adult heart.
Summary of current and proposed strategies discussed throughout the review that aim to directly replenish cardiac myocytes lost following ischemic injury or in the setting of progressive heart failure. Potential strategies include direct reactivation of the myocyte cell cycle program through genetic or pharmacological manipulations (yellow arrows), reprogramming of non-myocytes by manipulation of cellular states (green dashed arrows), or indirect cell cycle reactivation through myocyte-autonomous or extracellular mechanisms (blue arrow). Therapeutic and mechanistic advantages to each strategy are highlighted, as well as crucial limitations, risks, or other considerations.
Reactivation or enhancement of endogenous myocyte cell cycle activity
The sharp decline in proliferative capacity in the postnatal heart has been directly correlated to downregulation and repression of cell cycle regulators in myocytes42, 124. However, adult cardiac myocytes do retain some capacity for DNA synthesis and to a lesser extent, cytokinesis. Therefore, one major avenue that has been pursued for potential therapeutic application is the reactivation of myocyte cell cycle activity in the injured adult heart (Figure). In terms of in vivo feasibility, mouse models that employed over-expression of known proliferative genes, or direct cell cycle regulators, laid much of the groundwork. An early study using transgenic over-expression of the oncogenic transcription factor c-myc in the heart demonstrated approximately a 40% increase in myocardial mass and a doubling of both myocyte DNA content and absolute myocyte numbers125. Likewise, transgenic expression of the SV40 large T-antigen in mice also produced a profound hyperplasia of atrial126 or ventricular127 myocytes in vivo. While such results established that adult myocytes could be coaxed back into cell cycle, use of cell cycle genes to mediate such events also produces the potential for tumorigeicity128. Moreover, induction of cell cycle activity in this way was not purely a proliferative signal, as additional studies of c-myc over-expression in adult hearts demonstrated myocyte hypertrophy and multinucleation instead of overt proliferation129, suggesting that the hyperplasia seen in earlier models was predominantly via enhanced cell cycle activity during development or early postnatal growth. As a result, efforts shifted towards a more systematic induction of cell cycle checkpoint pathways to achieve adult myocyte proliferation. Using viral-mediated gene delivery, over-expression of the transcription factor E2F1, which governs the G1/S cell cycle transition, was shown to drive adult myocytes into S-phase and promote myocyte DNA synthesis in vivo130. However, E2F1 induction also caused rapid myocyte apoptosis and early-onset lethality. In contrast, this was not observed with overexpression of E2F2 by viral-mediated gene delivery131, suggesting that the cell cycle regulator chosen, and the mode of overexpression, are important points of control. In line with this, another study using transgenic over-expression of cyclin D1 demonstrated a 41% increase in cardiac mass and a sustained increase in DNA synthesis as measured by thymidine incorporation assays, without lethality132. Similarly, over-expression of CDK2 enhanced total cardiac mass in neonatal mouse hearts but resulted in a greater percentage of small, mononucleated myocytes133. Numerous additional studies over the last decade have provided confirmatory evidence that adult myocytes can re-enter cell cycle upon introduction of cell cycle activators using a transgenic approach in the mouse134, 135 (also summarized in 42, 136). More recently, combinatorial approaches by introducing multiple regulators simultaneously have demonstrated that synergistic increases in myocyte cell cycle activity are achievable, which may provide further benefit137. However, a key observation drawn from these studies is that enhancing cell cycle activity in adult myocytes is not a direct surrogate for new myocyte formation. In many cases, enhanced DNA synthesis results in myocyte hypertrophy or multinucleation without complete cell division. An ongoing challenge in the field is to decipher the molecular roadblocks that prevent cellular division even in the context of enhanced cell cycle activity. For this, multi-step manipulation of several distinct but convergent pathways may be required, to both enhance myocyte cycling and facilitate cytokinesis.
Modulation of endogenous signaling pathways governing myocyte growth and division
During embryonic development and postnatal growth, cardiac myocyte proliferation, differentiation, and cell cycle exit are all controlled by coordinated signal transduction, the details of which have been thoroughly dissected both in vitro and in vivo20. In the context of new myocyte formation in adult hearts, many of these pathways have been implicated as a means to promote endogenous proliferation, thereby possibly recapitulating the signals present during development and early heart growth (Figure). Here we will highlight some of the recent developments and experimental findings that suggest reactivation of endogenous signaling cascades in the adult heart as a means to promote therapeutic new myocyte formation. We would also direct the reader to comprehensive past reviews on some of these key pathways138–141.
The Hippo-YAP/TAZ kinase cascade
The Hippo-YAP signaling pathway is an evolutionarily conserved central regulator of organ size and organ growth142. In mammals, the core Hippo signaling pathway acts via the kinases Mst1 and Mst2, homologues of Drosophila Hippo, which phosphorylate Lats1/2 in order to induce Lats1/2-dependent phosphorylation of the transcriptional co-activators yes-associated protein (YAP) and TAZ. Phosphorylation of YAP or TAZ triggers export of these proteins from the nucleus and therefore loss of YAP/TAZ-mediated TEAD transcription factor binding143. TEAD transcription factors govern expression of core proliferation gene programs, so the overall result of active Hippo signaling is repression of proliferation.
Coordinated Hippo-YAP signaling is required for proper fetal heart growth, as conditional deletion of YAP1 during early heart development resulted in significantly impaired myocyte proliferation and lethal hypoplasia144. Moreover, cardiac-specific deletion of salvador (Salv), a scaffold protein that facilitates Lats1/2 phosphorylation by Mst1 and Mst2, resulted in severe cardiomegaly and hyperproliferation of myocytes, due to loss of YAP/TAZ repression145. Because active Hippo-YAP/TAZ signaling suppresses proliferation and is regulated at several nodal points, the notion that removal of key components of the pathway could diminish Hippo signaling and thus reactivate proliferation in the adult heart has received considerable attention. Myocyte deletion of either Salv or Lats1/2 specifically in adult mice promoted cell cycle re-entry and increased indices of proliferation in vivo146, and this was associated with enhanced reparative ability of hearts after MI, as well as after apical resection at postnatal day 8, a time-point at which the mouse heart normally is unable to mount a regenerative response. Induction of YAP-mediated proliferation via over-expression of a constitutively active mutant YAP was also sufficient to enhance myocyte proliferation and improve heart function after MI in adult mice147. Similar benefits were observed with adeno- associated virus (AAV)-mediated delivery of human YAP in mice post-MI148.
These studies discussed above have encouraged the use of therapeutics that modulate YAP/TAZ signaling. However, there are still key steps before this approach can be realized. Like all strategies that attempt to enhance cell cycle activity in the heart, the potential for tumorigenesis or other off-target effects will necessitate an approach that can be rigorously controlled temporally and spatially, ideally enhancing YAP/TAZ-mediated proliferation in areas of injury and for a limited period of time. However, a more fundamental question relates to the endogenous signals that serve to repress myocyte proliferation via YAP/TAZ, either by retaining Hippo pathway activity, or directly regulating YAP/TAZ. Several recent studies have implicated a structural segregation of YAP in the myocyte as an additional layer of control. Combined deletion of the myocyte intercalated disk components αE and αT catenin resulted in enhanced myocyte proliferation, and this was shown to be driven by release of sequestered YAP from the intercalated disk149, suggesting that cytoskeletal remodeling might be an important downstream step toward myocyte proliferative potential. Indeed, YAP-meditated target gene profiling in the heart is enriched in regulators of the actin cytoskeleton150, and inhibition of RhoA-mediated cytoskeletal remodeling prevented the nuclear accumulation of YAP in isolated myocytes151. As a final point of future consideration, beneficial effects of YAP activity might not be limited to proliferation, as YAP also has been implicated as an anti-apoptotic factor in the heart152, in part via modulation of the oxidative stress response153, 154. Thus Hippo-YAP/TAZ signaling remains an intriguing candidate pathway for enhancing myocyte proliferation in the adult heart.
The neuregulin-ErbB signaling pathway
The ErbB family of receptor tyrosine kinases encompasses four family members, the prototypical member being the epidermal growth factor receptor (EGFR), or ErbB1. Activating ligands for ErbB family members include EGF itself, as well as a subfamily of four ligands called neuregulins (NRGs), which predominantly activate ErbB3 and ErbB4155, 156. NRG-ErbB signaling has diverse biological functions but is intricately linked to enhanced cell proliferation, perhaps best highlighted by the discovery of activating mutations in ErbB2, also called HER2/neu in a large subset of breast cancers156. During cardiac development NRG-ErbB signaling acts primarily in the formation of the valves, as well as the ventricular trabeculae157. However, during heart development NRG-ErbB signaling also acts to promote differentiation158, 159 as opposed to proliferation.
Much of the focus on NRG-ErbB signaling in the adult heart stemmed from observations that breast cancer patients receiving the HER2-targeted therapeutic trastuzumab showed elevated incidence of cardiotoxicity, which was exacerbated in patients also receiving anthracyclines160. Studies in gene-targeted mice lacking myocyte ErbB2 or ErbB4, which are the highest-expressed family members161 also revealed developmental phenotypes consistent with cardiomyopathy162, 163, collectively signifying that NRG-ErbB signaling might also be involved in postnatal cardiac homeostasis. Subsequently, a study that employed cardiac myocyte-specific over-expression of ErbB4, or administration of recombinant NRG1, suggested that activation of NRG-ErbB signaling could improve cardiac function and lessen injury post-MI164. In line with this finding, increasing NRG1 levels also promoted myocyte proliferation and tissue repair in the injured zebrafish heart165. Enhanced mammalian myocyte proliferation and subsequent reparative effects were also observed with over-expression of a constitutively active form of ErbB2166 or delivery of recombinant NRG1167. However, a second study failed to demonstrate enhancement of myocyte proliferation in mice treated with recombinant NRG1168. NRG-ErbB in the heart is remarkably pleiotropic, having effects on vascular tone, cellular metabolism, and signaling at neuromuscular junctions157. Thus, the benefits reported in injured hearts treated with NRG may in fact be due to multiple mechanisms of action in addition to myocyte proliferation. Currently, recombinant NRG peptides are in clinical trial for chronic heart failure patients169 with some initial encouraging results. Future studies will need to address the proper duration and magnitude at which elevated NRG-ErbB signaling might benefit the injured heart. However, persistent/constitutive NRG activation can ultimately lead to cardiac hyperplasia and heart failure in animal models170, suggesting that targeting the NRG-ErbB pathway will not be straightforward171.
Exogenous Cellular Therapeutics for New Myocyte Formation
As discussed earlier in this review, a number of cell types initially proposed for direct myocyte formation and regeneration in the adult heart, such as freshly isolated bone marrow mononuclear cells172, 173 or cardiac c-Kit cells174, are now known to lack the ability to generate myocytes. Other classes of adult-derived cells, such as bone marrow mesenchymal stem cells, were reported to possess a modest degree of cardiomyogenic potential in animal models175, 176, albeit at levels insufficient to directly repopulate an infarcted heart. Hence, there are currently no reproducible data between laboratories to demonstrate the existence of any sort of adult progenitor cell that can be isolated or grown in culture and then injected into the adult heart to create physiologically meaningful de novo myocytes. Thus, to generate therapeutically relevant numbers of new myocytes directly, the field has largely turned to embryonic stem (ES) cells or more recently, to a variety of strategies intended to coax adult cell types back to a pluripotent-like state first (Figure), then differentiated into myocytes in culture before injecting into the heart.
Differentiation of embryonic stem cells into myocytes
Embryonic stem (ES) cells have been an attractive cell source for studying cardiac myocyte differentiation and potentially generating large numbers of myocytes for therapeutic application, as ES cell technology is relatively mature and well-established177 compared to more recent work with reprogrammed cell types. Human ES cells will form embryoid bodies that contain beating myocytes when cultured in suspension178 or when co-cultured with endodermal cells179. ES cell-derived myocytes express characteristic transcription factors such as Nkx2.5, GATA4, and MEF2, as well as sarcomeric genes, and can be propagated in extended culture180. Moreover, ES cell-derived myocytes are heterogeneous, consisting of cells with atrial, ventricular, and nodal-like properties181. However, this plasticity is a double-edged sword, as one of the most pressing concerns with use of ES cell-derived myocytes has been the potential for arrhythmias due to immature or dysregulated electrical coupling182. As a result, studies have explored the use of modified protocols to preferentially generate more highly differentiated atrial or ventricular ES cell-derived myocytes by modulation of defined signaling pathways or with modified culture conditions183, 184.
Delivery of embryoid body-derived ES cells selected for cardiomyogenic potential into infarcted rodent hearts was shown to produce sustained improvements in cardiac function with evidence for cell engraftment and differentiation in vivo185. Human ES cells were also able to engraft into the hearts of pigs with ablation-induced complete heart block and restore normal cardiac rhythm186, suggesting that ES-derived myocytes have the potential to electrically couple to host myocardium. Currently, studies in non-human primates have provided the most relevant evidence to-date that generation of new myocardium from transplanted ES cells may be clinically feasible187–189. For example, it has been shown that human ES cell-derived myocytes could be delivered into the infarcted macaque heart, and that these cells formed cardiac muscle grafts with mature characteristics in vivo189. These data provide evidence that cardiac remuscularization from human ES cells may be possible. However, much more work remains to optimize the therapeutic potential of ES cell-derived myocytes, particularly in the areas of graft survival and immunogenicity, electrical coupling, and maturation of the ES cell derived myocytes that remain poorly differentiated190. Indeed, transplant of ES cell-derived myocytes is particularly challenging in chronically infarcted hearts, as the benefits can be transient and limited to acute functional improvement191, 192. Arrhythmogenesis from poorly or incompletely coupled ES cell-derived myocardium to host muscle is also a major concern, and this was particularly evident in the macaque model with human ES cell transplant, as all transplanted animals showed arrhythmia189. Another known long-term concern with an ES cell-derived myocyte approach is the potential that some cells escape through the rigorous myocyte differentiation procedure and retain pluripotency, potentially resulting in teratoma formation. As a result, better-defined and more progressively differentiated ES cell-derived myocytes will be needed before this approach could be attempted in humans193, 194.
Reprogramming of adult cells into myocytes
Technical and ethical hurdles associated with ES cells have driven an exhaustive search for alternate sources of newly generated myocytes. To this end, the development of induced pluripotent stem cell (iPS) technology, allowing for the directed conversion of adult somatic cells into an embryonic-like pluripotent state, transformed cardiac regenerative medicine approaches and opened up a wealth of diagnostic and potential therapeutic options195, 196. Currently, strategies to generate new myocytes via cellular reprogramming are being explored through multiple approaches – including the differentiation of converted iPS cells directly into myocytes or the direct conversion of adult fibroblasts into myocytes, bypassing an iPS or progenitor-like intermediate (Figure). These strategies all possess both advantages and limitations and at present there is no clear frontrunner, although we will attempt to highlight the progress and future questions that must be addressed in each instance.
Differentiation of iPS cells into myocytes
Introduction of the transcription factors c-Myc, Oct3/4, Sox2, and Klf4 into mouse or human fibroblasts converts them into a pluripotent cell type (iPS cell) with the potential to generate all three germ layers in vitro, teratogenicity in vivo, and the ability to generate fully penetrant mouse chimeras when injected into blastocysts197, 198. iPS cells can be differentiated into beating myocytes in culture via addition of BMP4 and activin A, mirroring observations in ES cells198. Similar results could be obtained when culturing iPS cells as embryoid bodies199, 200 or on a collagen IV matrix, although myocyte differentiation in this latter case was suggested to occur through an Flk-1+ mesodermal precursor intermediate cell stage201. As is the case for all proposed therapies that begin with undifferentiated pluripotent cell types, the potential for tumorigenicity remains a major concern. Determining approaches to mitigate this risk without removing the differentiation potential of iPS cells is ongoing but still requires extensive development. Also, like ES cells, myocytes from iPS cells are structurally and functionally immature compared to adult myocytes, particularly in terms of sarcomeric protein organization and calcium handling properties202. Hence, novel strategies that enhance iPS-derived myocyte maturation203, bioengineering modifications204 and/or addition of defined chemical factors205, 206 have shown promise and will likely be required to achieve fully functional myocytes that integrate more effectively into a host myocardium.
Comprehensive characterization and reliable approaches for differentiation of myocytes from ES cells or iPS cells207 should be part of future experimentation. When these issues are resolved the field can turn to development of approaches that include vascularizing areas seeded with new myocytes and improving electromechanical synchrony of transplanted and host myocytes. It is important to keep in mind that even if a robust and uniform ability to generate new cardiac muscle from previously undifferentiated pluripotent cells in vivo was developed, the ability to provide functional vascularization to this graft would still be required to allow for full tissue restoration. In addition, the proper alignment of myocytes within the graft would be beneficial in better coordinating contractility activity of bands of cells within the ventricle.
Another key possibility to consider is that ES or iPS cells may impart functional benefits to the injured heart that are not due to direct remuscularization. Indeed, delivery of human ES or iPS-derived myocytes into MI-injured immunodeficient mice has been shown to significantly improve contractile performance and lessen remodeling even with negligible retention of the transplanted cells208, 209. Cell delivery in these studies was associated with an upregulation of circulating angiogenic and cardioprotective cytokines, suggesting that paracrine-mediated repair pathways may have been activated with ES or iPS cell therapy. A recent large study of non-human primates treated with human ES cells that had been differentiated into cardiovascular progenitors also showed improvement in cardiac function, despite very limited cell retention and no evidence for remuscularization210. Thus, the mechanisms of action for ES- and iPS-derived myocyte cell therapy may be multifaceted and not purely due to contractile activity of the injected nascent myocytes.
Direct reprogramming of fibroblasts into myocytes
The discovery that fully differentiated adult fibroblasts could be forced into a pluripotent cell state with defined transcription factors was an exciting recent development in the field. Efforts in the area began with the observation that transfection of cultured embryonic mouse mesoderm with the cardiomyogenic transcription factors Gata4 and Tbx5 along with the chromatic remodeling factor Baf60c was able to redirect non-myogenic mesodermal tissue towards a cardiac fate211. This demonstrated that cardiomyogenic capacity could be conferred by defined factors, although whether this was possible in fully differentiated cells or tissues was not known. However, in 2010 a systematic reductionist approach using a collection of candidate transcription factors revealed that combinatorial delivery of Gata4, Mef2c, and Tbx5 (GMT) could reprogram adult fibroblasts isolated from the heart or the tail tip of mice into cardiac myocytes212. Moreover, fibroblasts carrying the GMT cocktail expressed cardiac myocyte genes and activated an αMHC-driven GFP reporter when injected into the mouse heart. Myocyte conversion from fibroblasts by GMT was limited to 20% of cells in vitro, suggesting that additional improvements could be made. Addition of the transcription factor Hand2 was shown to synergistically improve fibroblast to myocyte conversion with GMT transduction in vitro213. Importantly, in vivo viral-mediated delivery of either GMT214 or GHMT (GMT+Hand2)213 ameliorated cardiac dysfunction in a rodent infarct model. However, these data were surprisingly not accompanied by a rigorous assessment of true myocyte conversion rates within the adult heart, making it impossible to conclude that the improvement in cardiac function was due to new myocyte formation in vivo.
While technical challenges remain for adopting this approach as a strategy for new myocyte generation in vivo, direct fibroblast reprogramming has several theoretical advantages (Figure). It avoids the use of an undifferentiated or pluripotent cell state, which should curtail tumorigenicity. The conversion of fibroblasts to myocytes may also serve a dual role of reducing pathological fibrosis while generating new contractile tissue. However, several key questions require further investigation before this approach can be considered for therapeutic myocyte generation. The major limitation presently is that the conversion rate in vivo appears too low to provide sufficient new myocytes to achieve an improvement in cardiac pump function215. Moreover, as fibroblast to myocyte conversion appears to occur without inducing additional cellular proliferation, the absolute number of new myocytes generated may be less than what could be achieved with other strategies, particularly in situations such as chronic ischemia where fibroblast activation is still ongoing216. Human fibroblasts are also more refractory to reprogramming versus those from mouse, requiring the two additional transcription factors, Mesp1 and Myocardin217. Therefore, it is not clear that this approach will work in the adult human heart. Another concern relates to the use of viral gene delivery to achieve reprogramming in vivo. Recent studies have suggested alternative reprogramming approaches by using microRNAs or chemical mediators to indirectly induce the expression of reprogramming factors218–220 while the use of non-integrating viral constructs could also mitigate some safety concerns221. However, any of these potential approaches would most likely need to be made specific to the fibroblasts in the heart, and no other cardiac cell types or fibroblasts of other tissues. Overall, the ability to reprogram adult fibroblasts into myocytes in vitro or in vivo provides another potential avenue towards achieving meaningful therapeutic myocyte replacement once the molecular pathways have been carefully defined and optimal methods for efficient reprogramming have been determined.
Tissue engineering approaches to new myocyte formation
The collective experience of many labs over the past decades has revealed that injecting isolated cells into an infarcted heart in the hopes they will restore tissue architecture and generate new beating heart muscle is a tall order. To improve the overall situation, the field has increasingly adopted an interdisciplinary approach by integrating cell biology with tissue engineering222. Many of the cell types discussed above are now being deployed not in an isolated suspension, but in pre-fabricated biomaterial or chemical scaffolds or as engineered heart tissue patches. Seeding of human ES cell-derived smooth muscle and endothelial cells in a fibrin patch resulted in functional improvement in a swine model of myocardial infarction223. This patch lacked ES cell-derived myocytes, suggesting that the beneficial effects were achieved via revascularization or paracrine-mediated effects on the host myocardium. Addition of myocytes, in this case derived from iPS cells, as well as a slow-release delivery of IGF-1, provided a synergistic benefit224, highlighting the ability to deliver growth factors or protective cytokines via biological scaffolds as an additional advantage.
Delivery of pre-fabricated cardiac tissue patches using hydrogel as the scaffolding material is also under active investigation. Initial proof-of-concept studies using neonatal rat myocytes demonstrated that engineered heart tissues could be constructed in vitro that displayed many of the hallmark features of mature myocardium, including structural maturity of sarcomeres and T-tubules and uniform inotropic responsiveness225. Implantation of these tissues onto infarcted rat hearts demonstrated a capacity for electrical coupling, improved heart function, and ameliorated adverse remodeling226. Newer iterations of this technology have used human ES cell-derived myocytes227 or iPS-derived myocytes228. These studies have also demonstrated feasibility and functional benefits when grafted onto infarcted hearts. However, the precise mechanisms of functional improvement from engineered heart tissues remain undefined and are likely multifaceted. Remuscularization from patch-derived cells has been observed in some studies228, suggesting that direct cardiomyogenesis may be occurring to provide functional benefit, but this has not been the case uniformly. Moreover, it remains uncertain how a large cell-derived graft could be completely revascularized when incorporated into the host environment, which would be required for sustained viability of the graft. Of note, a recent analysis of ES cell-derived engineered heart tissues revealed that tissues lethally irradiated prior to transplant (to ablate functional myocytes) were equally protective in a rodent infarction model as their living counterparts227. This important control experiment suggests that engineered heart tissues or biomaterial patches may be providing benefits irrespective of myocyte recellularization, such as structural stability or modulation of inflammatory cell activation. Despite this, preliminary clinical assessment in a very small number of patients has begun with several of these technologies, including fibrin patches seeded with ES cells229. Still in its infancy, the merger of cell therapeutics with bioengineering has invariably benefited the field and fueled basic discovery in cardiac repair230. The challenge now will be to develop scalable and therapeutically amenable approaches that will reduce or even overcome the inherent limitations of unmodified cell delivery.
Emerging Paradigms and Future Directions for Therapeutic Myocyte Generation
The fundamental questions regarding cardiac myocyte renewal and the search for cardiac regenerative therapies are deeply rooted in the field and have been intensely pursued for over a century120, 231. But in the last two decades, new genetic tools, experimental techniques, and a continuing effort to refine measures of myocyte proliferation and cell division232, 233, combined with an aging heart failure patient population, have fueled an upsurge in attempts to increase myocyte content in the heart. As a result, the field continues to delineate the fundamental molecular pathways that govern or suppress myocyte turnover in various contexts. Here we will briefly touch on emerging and newly uncovered pathways that are likely to provide additional mechanistic insights and may lead to novel therapeutic strategies (Figure).
Role of oxidative and metabolic states in postnatal myocyte generation
Following a newborns first breath, the oxygen content of the arterial blood increases to adult levels. Soon after this event, the mammalian heart undergoes a rapid switch in energy utilization and metabolism, from a largely glycolytic, carbohydrate-driven program to one that primarily utilizes fatty acids234. This process is tightly coordinated with myocardial mitochondrial expansion, myocyte terminal differentiation, and cell cycle exit, required for the enhanced pumping requirements of the mature heart235. In contrast, lower vertebrates, primarily those living in aqueous environments, retain a more immature, developmental metabolic phenotype16, raising the question of whether cardiac myocyte plasticity and proliferative capacity are tied to metabolic state and requirements of contractile rigor. To this end, a recent study compared mitochondrial content and ultrastructure across the hearts of zebrafish and neonatal mice from p1 to p7 and found that in mice mitochondrial expansion and cristae formation occurred coincident with the window of time at which myocyte proliferative capacity is lost236. In a comprehensive series of experiments, local myocyte hypoxia was shown to promote cell cycle re-entry, and that the expansion of mitochondrial oxidative metabolism and the subsequent reactive oxygen species elevation that occurs postnatally is a driver of reduced myocyte proliferation in vivo236, 237. Extending this paradigm, the same group recently reported that systemic gradual hypoxia in adult mice could produce a proliferative response in the heart at baseline or after injury, suggesting that modulating metabolism or tissue oxygen levels could be a novel approach to new myocyte formation. In potentially translating these findings, conformational studies are needed and the potential risks associated with a hypoxic state will need to be considered238. Translating these basic findings into a large animal model (Porcine) context would also seem critical.
Role of myocyte nuclear content and ploidy
The majority of terminally differentiated adult cardiac myocytes are binucleated and/or polyploid in rodents, due to karyokinesis without cytokinesis during cell cycle withdrawal30, 42. In many regenerative species such as zebrafish, myocytes remain mononucleated and diploid throughout life10. Further, mononucleated myocytes in mammals are smaller than binucleated cells, and have been proposed to retain more proliferative potential239. To address this possibility, a recent study performed an exhaustive in vivo screen of 120 inbred mouse lines and measured the frequency of mononucleated diploid myocytes in hearts from these lines240. Mouse strains selected for analysis that had a greater proportion of mononucleated diploid myocytes showed improved recovery after MI and had increased indices of myocyte cell cycle activity such as EdU uptake. Availability of high-density SNP maps for these lines allowed the group to perform forward genetic screening, from which they identified TNNI3K, a cardiac-specific protein kinase that adversely regulates ischemic injury and pathological hypertrophy in the adult heart241, 242, as a candidate effector of mononucleated versus binucleated myocytes levels. Regulation of myocyte proliferation by ploidy and nucleation status was also demonstrated in a recent zebrafish study using transgenic mosaicism243, revealing that diploid myocytes proliferated more readily than those with polyploid nuclei. Moreover, transgenic fish lacking the proper complement of diploid myocytes were unable to mount a regenerative response following heart injury. Taken together these studies suggest that proliferation may proceed more readily in mononucleated diploid myocytes versus those with polyploidy.
Structural cues in provoking or suppressing myocyte proliferation
Perhaps the most thought-provoking and unresolved issue regarding endogenous proliferation of adult cardiac myocytes is that it appears to happen in the setting of an exceptionally rigid and complex cellular architecture; namely, the sarcomere. In order to divide, mammalian cells must rapidly and accurately disassemble and reassemble cytoskeletal and nuclear structural protein complexes. In addition to this intracellular restraint, cell-cell junction and cell-matrix interactions must be disassembled and then properly restored. Cardiac myocyte proliferation during embryogenesis or early postnatal life occurs in an immature and more plastic tissue environment with lower pressures and functional requirements, which is also the case for lower vertebrates such as the zebrafish12. To address the question of how adult hearts might achieve a similar feat, or if structural disassembly is required, a recent study took advantage of time-lapse video microscopy to document myocyte proliferation in adult mouse myocytes, cocultured with neonatal rat ventricular myocytes (NRVMs)244. Proliferative adult myocytes showed a sequential progression of sarcomere disassembly, induction of cell cycle markers, and then re-organization of sarcomeric structure and restoration of contractility. Mechanistically, physical connection between adult myocytes and NRVMs via connexin 43 was required for this restoration of differentiated myocyte structure244. These data suggest that physical interactions are a determinant for coordinated proliferation-differentiation in adult myocytes that undergo cell cycle re-entry. This emerging paradigm is made more evident from a recent series of studies demonstrating key roles for extracellular matrix proteins in promoting endogenous myocyte proliferation. In a pair of reports, coupling of myocytes to the extracellular matrix via agrin, as well as to the dystrophin-glycoprotein (DGC) complex via dystroglycan 1, were shown to be responsible for regulating the intracellular distribution of YAP and coordinating proliferative vs anti-proliferative signals in the cell245, 246. Agrin, via binding to dystroglycan 1, triggers DGC disassembly and release of YAP, promoting myocyte proliferation245, while the assembled DGC can sequester YAP and confines proliferation, even in the setting of Hippo pathway modulation246. Thus, the molecular events underlying complete myocyte proliferation have emerged as even more multifaceted, requiring coordinated steps to both disconnect the dividing cell from its extracellular anchors, and remodel intracellular components to produce functional myocytes as a result. However, the need for dedifferentiation of the adult myocytes in the heart before they are permissive to proliferation poses a known problem related to the loss of functional rigor, as if a significant number of these cells de-differentiated at once, it would surely lead to reduced cardiac functional performance and likely heart failure. Thus, long-term it will be necessary to selectively and temporally control myocyte cell cycle re-entry in coordinating therapeutic regenerative approaches.
Conclusions
Conclusive evidence collected over the last several years across diverse animal models and in humans has demonstrated a clear capacity for new myocyte generation in the adult mammalian heart. Maximal cardiac myocyte turnover during homeostasis in the adult is limited to roughly 1% per year, while in the face of cardiac injury or disease, this turnover slightly increases but cannot compensate for extensive myocyte loss. In both cases, proliferation of pre-existing myocytes is the overwhelming cellular contributor to new myocyte formation.
Proliferation of adult cardiac myocytes involves the reactivation or modulation of numerous signaling pathways central to embryonic and early postnatal heart growth. In contrast, the extent of the intrinsic barriers limiting endogenous proliferation in adult hearts remains unclear, as is the extent to which easily measured DNA proliferative indexes reflect true cellular proliferation with bonafide new myocyte formation. Therapeutic manipulations aimed at enhancing the limited regenerative capacity of the adult heart by directly targeting new myocyte formation can include direct or indirect stimulation of endogenous myocyte cell cycle activity, or the use of exogenous ES cells or pluripotent cells or tissues first directed towards a cardiomyogenic fate. The strategies we have highlighted here are by no means exhaustive, and the broader field of cardiac regeneration is exploring numerous avenues to achieve meaningful heart repair, including direct modulation of tissue fibrosis, stimulating revascularization, and inflammation-based tissue healing and resolution247–251. All of these approaches must be thoroughly vetted in small and large animal model systems, and in particular strategies for therapeutic new myocyte generation must be designed with the overarching goal of restoring myocardial tissue that is structurally and functionally equivalent to that which was lost. Achieving this will require uncovering additional cellular and molecular mechanisms that control physiological and pathological cardiac myocyte turnover at the cell, tissue, and whole-organ level.
Acknowledgments
Sources of Funding
S.R.H., J.D.M. and R.J.V. were supported by grants from the National Institutes of Health (F32 HL128083 to R.J.V). J.D.M. was also supported by the Howard Hughes Medical Institute.
Footnotes
Disclosures
None.
Nonstandard Abbreviations and Acronyms: None.
References
- 1.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 4.Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249–53. doi: 10.1002/jez.1401870208. [DOI] [PubMed] [Google Scholar]
- 5.Bader D, Oberpriller JO. Repair and reorganization of minced cardiac muscle in the adult newt (Notophthalmus viridescens) J Morphol. 1978;155:349–57. doi: 10.1002/jmor.1051550307. [DOI] [PubMed] [Google Scholar]
- 6.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–90. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 7.Laube F, Heister M, Scholz C, Borchardt T, Braun T. Re-programming of newt cardiomyocytes is induced by tissue regeneration. J Cell Sci. 2006;119:4719–29. doi: 10.1242/jcs.03252. [DOI] [PubMed] [Google Scholar]
- 8.Flink IL. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat Embryol (Berl) 2002;205:235–44. doi: 10.1007/s00429-002-0249-6. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Rosa JM, Burns CE, Burns CG. Zebrafish heart regeneration: 15 years of discoveries. Regeneration (Oxf) 2017;4:105–123. doi: 10.1002/reg2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–5. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development. 2011;138:2895–902. doi: 10.1242/dev.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jopling C, Sleep E, Raya M, Marti M, Raya A, Izpisua Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–9. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Han P, Yang H, Ouyang K, Lee D, Lin YF, Ocorr K, Kang G, Chen J, Stainier DY, Yelon D, Chi NC. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawamiphak S, Kontarakis Z, Filosa A, Reischauer S, Stainier DYR. Transient cardiomyocyte fusion regulates cardiac development in zebrafish. Nat Commun. 2017;8:1525. doi: 10.1038/s41467-017-01555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development. 2016;143:729–40. doi: 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vivien CJ, Hudson JE, Porrello ER. Evolution, comparative biology and ontogeny of vertebrate heart regeneration. NPJ Regen Med. 2016;1:16012. doi: 10.1038/npjregenmed.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, Kudo A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev Dyn. 2014;243:1106–15. doi: 10.1002/dvdy.24154. [DOI] [PubMed] [Google Scholar]
- 18.Lai SL, Marin-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DY. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife. 2017:6. doi: 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 20.Galdos FX, Guo Y, Paige SL, VanDusen NJ, Wu SM, Pu WT. Cardiac Regeneration: Lessons From Development. Circ Res. 2017;120:941–959. doi: 10.1161/CIRCRESAHA.116.309040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell. 2008;3:69–84. doi: 10.1016/j.stem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 23.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garry DJ, Olson EN. A common progenitor at the heart of development. Cell. 2006;127:1101–4. doi: 10.1016/j.cell.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res. 2009;104:179–88. doi: 10.1161/CIRCRESAHA.108.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly RG, Buckingham ME, Moorman AF. Heart fields and cardiac morphogenesis. Cold Spring Harb Perspect Med. 2014:4. doi: 10.1101/cshperspect.a015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development. 2003;130:3877–89. doi: 10.1242/dev.00580. [DOI] [PubMed] [Google Scholar]
- 29.de Boer BA, van den Berg G, de Boer PA, Moorman AF, Ruijter JM. Growth of the developing mouse heart: an interactive qualitative and quantitative 3D atlas. Dev Biol. 2012;368:203–13. doi: 10.1016/j.ydbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. Am J Physiol. 1996;271:H2183–9. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- 31.Soufan AT, van den Berg G, Ruijter JM, de Boer PA, van den Hoff MJ, Moorman AF. Regionalized sequence of myocardial cell growth and proliferation characterizes early chamber formation. Circ Res. 2006;99:545–52. doi: 10.1161/01.RES.0000239407.45137.97. [DOI] [PubMed] [Google Scholar]
- 32.Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. Circ Res. 2010;107:1428–44. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drenckhahn JD, Schwarz QP, Gray S, Laskowski A, Kiriazis H, Ming Z, Harvey RP, Du XJ, Thorburn DR, Cox TC. Compensatory growth of healthy cardiac cells in the presence of diseased cells restores tissue homeostasis during heart development. Dev Cell. 2008;15:521–33. doi: 10.1016/j.devcel.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Sturzu AC, Rajarajan K, Passer D, Plonowska K, Riley A, Tan TC, Sharma A, Xu AF, Engels MC, Feistritzer R, Li G, Selig MK, Geissler R, Robertson KD, Scherrer-Crosbie M, Domian IJ, Wu SM. Fetal Mammalian Heart Generates a Robust Compensatory Response to Cell Loss. Circulation. 2015;132:109–21. doi: 10.1161/CIRCULATIONAHA.114.011490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakada Y, Kimura W, Sadek HA. Defining the Limit of Embryonic Heart Regeneration. Circulation. 2015;132:77–8. doi: 10.1161/CIRCULATIONAHA.115.017070. [DOI] [PubMed] [Google Scholar]
- 36.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–46. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 38.Soonpaa MH, Field LJ. Survey of studies examining mammalian cardiomyocyte DNA synthesis. Circ Res. 1998;83:15–26. doi: 10.1161/01.res.83.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Walsh S, Ponten A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010;86:365–73. doi: 10.1093/cvr/cvq005. [DOI] [PubMed] [Google Scholar]
- 40.Bergmann O, Zdunek S, Alkass K, Druid H, Bernard S, Frisen J. Identification of cardiomyocyte nuclei and assessment of ploidy for the analysis of cell turnover. Exp Cell Res. 2011;317:188–94. doi: 10.1016/j.yexcr.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–44. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hislop A, Reid L. Weight of the left and right ventricle of the heart during fetal life. J Clin Pathol. 1972;25:534–6. doi: 10.1136/jcp.25.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guerreiro D, Lennox SC, Anderson RH. Postnatal development of the pig heart. Cardiovasc Res. 1980;14:675–9. doi: 10.1093/cvr/14.11.675. [DOI] [PubMed] [Google Scholar]
- 45.Beinlich CJ, Rissinger CJ, Morgan HE. Mechanisms of rapid growth in the neonatal pig heart. J Mol Cell Cardiol. 1995;27:273–81. doi: 10.1016/s0022-2828(08)80026-0. [DOI] [PubMed] [Google Scholar]
- 46.Rochais F, Sturny R, Chao CM, Mesbah K, Bennett M, Mohun TJ, Bellusci S, Kelly RG. FGF10 promotes regional foetal cardiomyocyte proliferation and adult cardiomyocyte cell-cycle re-entry. Cardiovasc Res. 2014;104:432–42. doi: 10.1093/cvr/cvu232. [DOI] [PubMed] [Google Scholar]
- 47.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4:966–77. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bryant DM, O’Meara CC, Ho NN, Gannon J, Cai L, Lee RT. A systematic analysis of neonatal mouse heart regeneration after apical resection. J Mol Cell Cardiol. 2015;79:315–8. doi: 10.1016/j.yjmcc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK, Kotlikoff MI. c-kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A. 2012;109:13380–5. doi: 10.1073/pnas.1208114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol Biol. 2013;1037:343–53. doi: 10.1007/978-1-62703-505-7_20. [DOI] [PubMed] [Google Scholar]
- 52.Andersen DC, Ganesalingam S, Jensen CH, Sheikh SP. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Reports. 2014;2:406–13. doi: 10.1016/j.stemcr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darehzereshki A, Rubin N, Gamba L, Kim J, Fraser J, Huang Y, Billings J, Mohammadzadeh R, Wood J, Warburton D, Kaartinen V, Lien CL. Differential regenerative capacity of neonatal mouse hearts after cryoinjury. Dev Biol. 2015;399:91–9. doi: 10.1016/j.ydbio.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konfino T, Landa N, Ben-Mordechai T, Leor J. The type of injury dictates the mode of repair in neonatal and adult heart. J Am Heart Assoc. 2015;4:e001320. doi: 10.1161/JAHA.114.001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadek HA, Martin JF, Takeuchi JK, Leor J, Nie Y, Giacca M, Lee RT. Multi-investigator letter on reproducibility of neonatal heart regeneration following apical resection. Stem Cell Reports. 2014;3:1. doi: 10.1016/j.stemcr.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sen S, Sadek HA. Neonatal heart regeneration: mounting support and need for technical standards. J Am Heart Assoc. 2015;4:e001727. doi: 10.1161/JAHA.114.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoud AI, Porrello ER, Kimura W, Olson EN, Sadek HA. Surgical models for cardiac regeneration in neonatal mice. Nat Protoc. 2014;9:305–11. doi: 10.1038/nprot.2014.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polizzotti BD, Ganapathy B, Haubner BJ, Penninger JM, Kuhn B. A cryoinjury model in neonatal mice for cardiac translational and regeneration research. Nat Protoc. 2016;11:542–52. doi: 10.1038/nprot.2016.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haubner BJ, Schuetz T, Penninger JM. A reproducible protocol for neonatal ischemic injury and cardiac regeneration in neonatal mice. Basic Res Cardiol. 2016;111:64. doi: 10.1007/s00395-016-0580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naqvi N, Singh R, Iismaa SE, Li M, Calvert JW, Martin DI, Harvey RP, Graham RM, Husain A. Cardiomyocytes Replicate and their Numbers Increase in Young Hearts. Cell. 2015;163:783–4. doi: 10.1016/j.cell.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell. 2015;163:1026–36. doi: 10.1016/j.cell.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 63.Soonpaa MH, Zebrowski DC, Platt C, Rosenzweig A, Engel FB, Field LJ. Cardiomyocyte Cell-Cycle Activity during Preadolescence. Cell. 2015;163:781–2. doi: 10.1016/j.cell.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 64.Hirai M, Chen J, Evans SM. Tissue-Specific Cell Cycle Indicator Reveals Unexpected Findings for Cardiac Myocyte Proliferation. Circ Res. 2016;118:20–8. doi: 10.1161/CIRCRESAHA.115.307697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111:8850–5. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–75. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 68.Elser JA, Margulies KB. Hybrid mathematical model of cardiomyocyte turnover in the adult human heart. PLoS One. 2012;7:e51683. doi: 10.1371/journal.pone.0051683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A. 2013;110:1446–51. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP, Sadek HA. Human ventricular unloading induces cardiomyocyte proliferation. J Am Coll Cardiol. 2015;65:892–900. doi: 10.1016/j.jacc.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakagama Y, Inuzuka R, Ichimura K, Hinata M, Takehara H, Takeda N, Kakiuchi S, Shiraga K, Asakai H, Shindo T, Hirata Y, Saitoh M, Oka A. Accelerated Cardiomyocyte Proliferation in the Heart of a Neonate With LEOPARD Syndrome-Associated Fatal Cardiomyopathy. Circ Heart Fail. 2018;11:e004660. doi: 10.1161/CIRCHEARTFAILURE.117.004660. [DOI] [PubMed] [Google Scholar]
- 72.Farooqi KM, Sutton N, Weinstein S, Menegus M, Spindola-Franco H, Pass RH. Neonatal myocardial infarction: case report and review of the literature. Congenit Heart Dis. 2012;7:E97–102. doi: 10.1111/j.1747-0803.2012.00660.x. [DOI] [PubMed] [Google Scholar]
- 73.Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C, Stein JI, Penninger JM. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ Res. 2016;118:216–21. doi: 10.1161/CIRCRESAHA.115.307017. [DOI] [PubMed] [Google Scholar]
- 74.Zak R. Cell proliferation during cardiac growth. Am J Cardiol. 1973;31:211–9. doi: 10.1016/0002-9149(73)91034-5. [DOI] [PubMed] [Google Scholar]
- 75.Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisen J, Giacca M, Hare JM, Houser S, Lee RT, Marban E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–5. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, Anversa P. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 78.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–4. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vujic A, Lerchenmuller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML, Lee RT, Rosenzweig A. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. doi: 10.1038/s41467-018-04083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature. 2015;523:226–30. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 81.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 82.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh H, Chi X, Bradfute SB, Mishina Y, Pocius J, Michael LH, Behringer RR, Schwartz RJ, Entman ML, Schneider MD. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci. 2004;1015:182–9. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- 84.Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M, Agosti V, Viglietto G, Condorelli G, Indolfi C, Ottolenghi S, Torella D, Nadal-Ginard B. Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827–42. doi: 10.1016/j.cell.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 85.Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2010;138:40–9. doi: 10.1016/j.ijcard.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 86.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 87.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molkentin JD, Houser SR. Are resident c-Kit+ cardiac stem cells really all that are needed to mend a broken heart? Circ Res. 2013;113:1037–9. doi: 10.1161/CIRCRESAHA.113.302564. [DOI] [PubMed] [Google Scholar]
- 89.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SJ, Middleton RC, Marban E, Molkentin JD. van Berlo et al. reply. Nature. 2018;555:E18. doi: 10.1038/nature25772. [DOI] [PubMed] [Google Scholar]
- 90.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 91.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–40. doi: 10.1161/01.res.0000014822.62629.eb. [DOI] [PubMed] [Google Scholar]
- 92.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–8. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 93.Glaser R, Lu MM, Narula N, Epstein JA. Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation. 2002;106:17–9. doi: 10.1161/01.cir.0000021923.58307.8f. [DOI] [PubMed] [Google Scholar]
- 94.Loughner CL, Bruford EA, McAndrews MS, Delp EE, Swamynathan S, Swamynathan SK. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics. 2016;10:10. doi: 10.1186/s40246-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 96.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, Akazawa H, Molkentin JD, Kasanuki H, Komuro I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–63. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dedja A, Zaglia T, Dall’Olmo L, Chioato T, Thiene G, Fabris L, Ancona E, Schiaffino S, Ausoni S, Cozzi E. Hybrid cardiomyocytes derived by cell fusion in heterotopic cardiac xenografts. FASEB J. 2006;20:2534–6. doi: 10.1096/fj.06-6586fje. [DOI] [PubMed] [Google Scholar]
- 98.Angert D, Berretta RM, Kubo H, Zhang H, Chen X, Wang W, Ogorek B, Barbe M, Houser SR. Repair of the injured adult heart involves new myocytes potentially derived from resident cardiac stem cells. Circ Res. 2011;108:1226–37. doi: 10.1161/CIRCRESAHA.110.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–13. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Zhang L, Qiao Z, Wang QD, Lui KO, Zhou B. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell Res. 2016;26:119–30. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wallner M, Duran JM, Mohsin S, Troupes CD, Vanhoutte D, Borghetti G, Vagnozzi RJ, Gross P, Yu D, Trappanese DM, Kubo H, Toib A, Sharp TE, 3rd, Harper SC, Volkert MA, Starosta T, Feldsott EA, Berretta RM, Wang T, Barbe MF, Molkentin JD, Houser SR. Acute Catecholamine Exposure Causes Reversible Myocyte Injury Without Cardiac Regeneration. Circ Res. 2016;119:865–79. doi: 10.1161/CIRCRESAHA.116.308687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nat Med. 2014;20:1386–93. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maliken BD, Kanisicak O, Karch J, Khalil H, Fu X, Boyer JG, Prasad V, Zheng Y, Molkentin JD. Gata4-Dependent Differentiation of c-Kit(+) Derived Endothelial Cells Underlies Artefactual Cardiomyocyte Regeneration in the Heart. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.033703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai CL, Molkentin JD. The Elusive Progenitor Cell in Cardiac Regeneration: Slip Slidin’ Away. Circ Res. 2017;120:400–406. doi: 10.1161/CIRCRESAHA.116.309710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, Braun T. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cairns LA, Moroni E, Levantini E, Giorgetti A, Klinger FG, Ronzoni S, Tatangelo L, Tiveron C, De Felici M, Dolci S, Magli MC, Giglioni B, Ottolenghi S. Kit regulatory elements required for expression in developing hematopoietic and germ cell lineages. Blood. 2003;102:3954–62. doi: 10.1182/blood-2003-04-1296. [DOI] [PubMed] [Google Scholar]
- 110.Gude NA, Firouzi F, Broughton KM, Ilves K, Nguyen KP, Payne CR, Sacchi V, Monsanto MM, Casillas AR, Khalafalla FG, Wang BJ, Ebeid D, Alvarez R, Jr, Dembitsky WP, Bailey BA, van Berlo JH, Sussman MA. Cardiac c-Kit Biology Revealed by Inducible Transgenesis. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.117.311828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vicinanza C, Aquila I, Cianflone E, Scalise M, Marino F, Mancuso T, Fumagalli F, Giovannone ED, Cristiano F, Iaccino E, Marotta P, Torella A, Latini R, Agosti V, Veltri P, Urbanek K, Isidori AM, Saur D, Indolfi C, Nadal-Ginard B, Torella D. Kit(cre) knock-in mice fail to fate-map cardiac stem cells. Nature. 2018;555:E1–E5. doi: 10.1038/nature25771. [DOI] [PubMed] [Google Scholar]
- 112.Solbach TF, Paulus B, Weyand M, Eschenhagen T, Zolk O, Fromm MF. ATP-binding cassette transporters in human heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:231–43. doi: 10.1007/s00210-008-0279-6. [DOI] [PubMed] [Google Scholar]
- 113.He L, Li Y, Li Y, Pu W, Huang X, Tian X, Wang Y, Zhang H, Liu Q, Zhang L, Zhao H, Tang J, Ji H, Cai D, Han Z, Han Z, Nie Y, Hu S, Wang QD, Sun R, Fei J, Wang F, Chen T, Yan Y, Huang H, Pu WT, Zhou B. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat Med. 2017;23:1488–1498. doi: 10.1038/nm.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y, He L, Huang X, Issa Bhaloo S, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, Yu W, Zhang L, Liu X, Liu K, Tang J, Zhang H, Cai D, Adams RH, Xu Q, Lui KO, Zhou B. Genetic Lineage Tracing of Non-Myocyte Population by Dual Recombinases. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.034250. [DOI] [PubMed] [Google Scholar]
- 115.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–4. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fioret BA, Heimfeld JD, Paik DT, Hatzopoulos AK. Endothelial cells contribute to generation of adult ventricular myocytes during cardiac homeostasis. Cell Rep. 2014;8:229–41. doi: 10.1016/j.celrep.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou B, Honor LB, Ma Q, Oh JH, Lin RZ, Melero-Martin JM, von Gise A, Zhou P, Hu T, He L, Wu KH, Zhang H, Zhang Y, Pu WT. Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J Mol Cell Cardiol. 2012;52:43–7. doi: 10.1016/j.yjmcc.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Banerjee I, Zhang J, Moore-Morris T, Lange S, Shen T, Dalton ND, Gu Y, Peterson KL, Evans SM, Chen J. Thymosin beta 4 is dispensable for murine cardiac development and function. Circ Res. 2012;110:456–64. doi: 10.1161/CIRCRESAHA.111.258616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.He L, Huang X, Kanisicak O, Li Y, Wang Y, Li Y, Pu W, Liu Q, Zhang H, Tian X, Zhao H, Liu X, Zhang S, Nie Y, Hu S, Miao X, Wang QD, Wang F, Chen T, Xu Q, Lui KO, Molkentin JD, Zhou B. Preexisting endothelial cells mediate cardiac neovascularization after injury. J Clin Invest. 2017;127:2968–2981. doi: 10.1172/JCI93868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kishore R, Khan M. More Than Tiny Sacks: Stem Cell Exosomes as Cell-Free Modality for Cardiac Repair. Circ Res. 2016;118:330–43. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lafontant PJ, Field LJ. The cardiomyocyte cell cycle. Novartis Found Symp. 2006;274:196–207. doi: 10.1002/0470029331.ch12. discussion 208–13, 272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jackson T, Allard MF, Sreenan CM, Doss LK, Bishop SP, Swain JL. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol. 1990;10:3709–16. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Field LJ. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988;239:1029–33. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- 127.Katz EB, Steinhelper ME, Delcarpio JB, Daud AI, Claycomb WC, Field LJ. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol. 1992;262:H1867–76. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- 128.Simpson PC. Proto-oncogenes and cardiac hypertrophy. Annu Rev Physiol. 1989;51:189–202. doi: 10.1146/annurev.ph.51.030189.001201. [DOI] [PubMed] [Google Scholar]
- 129.Xiao G, Mao S, Baumgarten G, Serrano J, Jordan MC, Roos KP, Fishbein MC, MacLellan WR. Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ Res. 2001;89:1122–9. doi: 10.1161/hh2401.100742. [DOI] [PubMed] [Google Scholar]
- 130.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, Schneider MD. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100:2722–8. doi: 10.1172/JCI119817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ebelt H, Zhang Y, Kampke A, Xu J, Schlitt A, Buerke M, Muller-Werdan U, Werdan K, Braun T. E2F2 expression induces proliferation of terminally differentiated cardiomyocytes in vivo. Cardiovasc Res. 2008;80:219–26. doi: 10.1093/cvr/cvn194. [DOI] [PubMed] [Google Scholar]
- 132.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, Kim KK, Field LJ. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–54. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, Lorell BH, Izumo S. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88:443–50. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- 134.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiang FL, Guo M, Yutzey KE. Overexpression of Tbx20 in Adult Cardiomyocytes Promotes Proliferation and Improves Cardiac Function After Myocardial Infarction. Circulation. 2016;133:1081–92. doi: 10.1161/CIRCULATIONAHA.115.019357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 137.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018;173:104–116 e12. doi: 10.1016/j.cell.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ikeda S, Sadoshima J. Regulation of Myocardial Cell Growth and Death by the Hippo Pathway. Circ J. 2016;80:1511–9. doi: 10.1253/circj.CJ-16-0476. [DOI] [PubMed] [Google Scholar]
- 139.Lin Z, Pu WT. Harnessing Hippo in the heart: Hippo/Yap signaling and applications to heart regeneration and rejuvenation. Stem Cell Res. 2014;13:571–81. doi: 10.1016/j.scr.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wadugu B, Kuhn B. The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol. 2012;302:H2139–47. doi: 10.1152/ajpheart.00063.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pentassuglia L, Sawyer DB. The role of Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res. 2009;315:627–37. doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–46. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 144.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–9. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–90. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–44. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–63. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, van Roy F, Radice GL. Alpha-catenins control cardiomyocyte proliferation by regulating Yap activity. Circ Res. 2015;116:70–9. doi: 10.1161/CIRCRESAHA.116.304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morikawa Y, Zhang M, Heallen T, Leach J, Tao G, Xiao Y, Bai Y, Li W, Willerson JT, Martin JF. Actin cytoskeletal remodeling with protrusion formation is essential for heart regeneration in Hippo-deficient mice. Sci Signal. 2015;8:ra41. doi: 10.1126/scisignal.2005781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vite A, Zhang C, Yi R, Emms S, Radice GL. alpha-Catenin-dependent cytoskeletal tension controls Yap activity in the heart. Development. 2018:145. doi: 10.1242/dev.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–88. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. A functional interaction between Hippo-YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun. 2014;5:3315. doi: 10.1038/ncomms4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matsuda T, Zhai P, Sciarretta S, Zhang Y, Jeong JI, Ikeda S, Park J, Hsu CP, Tian B, Pan D, Sadoshima J, Del Re DP. NF2 Activates Hippo Signaling and Promotes Ischemia/Reperfusion Injury in the Heart. Circ Res. 2016;119:596–606. doi: 10.1161/CIRCRESAHA.116.308586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–16. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 156.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 157.Odiete O, Hill MF, Sawyer DB. Neuregulin in cardiovascular development and disease. Circ Res. 2012;111:1376–85. doi: 10.1161/CIRCRESAHA.112.267286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lai D, Liu X, Forrai A, Wolstein O, Michalicek J, Ahmed I, Garratt AN, Birchmeier C, Zhou M, Hartley L, Robb L, Feneley MP, Fatkin D, Harvey RP. Neuregulin 1 sustains the gene regulatory network in both trabecular and nontrabecular myocardium. Circ Res. 2010;107:715–27. doi: 10.1161/CIRCRESAHA.110.218693. [DOI] [PubMed] [Google Scholar]
- 159.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A. 2002;99:10464–9. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Groarke J, Tong D, Khambhati J, Cheng S, Moslehi J. Breast cancer therapies and cardiomyopathy. Med Clin North Am. 2012;96:1001–19. doi: 10.1016/j.mcna.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–9. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 162.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8:459–65. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–60. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 164.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 165.Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife. 2015:4. doi: 10.7554/eLife.05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–38. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- 167.Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennett DG, dos Remedios CG, Haubner BJ, Penninger JM, Kuhn B. Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci Transl Med. 2015;7:281ra45. doi: 10.1126/scitranslmed.aaa5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reuter S, Soonpaa MH, Firulli AB, Chang AN, Field LJ. Recombinant neuregulin 1 does not activate cardiomyocyte DNA synthesis in normal or infarcted adult mice. PLoS One. 2014;9:e115871. doi: 10.1371/journal.pone.0115871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–14. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 170.Sysa-Shah P, Xu Y, Guo X, Belmonte F, Kang B, Bedja D, Pin S, Tsuchiya N, Gabrielson K. Cardiac-specific over-expression of epidermal growth factor receptor 2 (ErbB2) induces pro-survival pathways and hypertrophic cardiomyopathy in mice. PLoS One. 2012;7:e42805. doi: 10.1371/journal.pone.0042805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yutzey KE. Regenerative biology: Neuregulin 1 makes heart muscle. Nature. 2015;520:445–6. doi: 10.1038/520445a. [DOI] [PubMed] [Google Scholar]
- 172.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 173.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 174.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-Term Outcome of Administration of c-kit(POS) Cardiac Progenitor Cells After Acute Myocardial Infarction: Transplanted Cells Do not Become Cardiomyocytes, but Structural and Functional Improvement and Proliferation of Endogenous Cells Persist for at Least One Year. Circ Res. 2016;118:1091–105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Weil BR, Suzuki G, Leiker MM, Fallavollita JA, Canty JM., Jr Comparative Efficacy of Intracoronary Allogeneic Mesenchymal Stem Cells and Cardiosphere-Derived Cells in Swine with Hibernating Myocardium. Circ Res. 2015;117:634–44. doi: 10.1161/CIRCRESAHA.115.306850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 178.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 180.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 181.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 182.Anderson ME, Goldhaber J, Houser SR, Puceat M, Sussman MA. Embryonic stem cell-derived cardiac myocytes are not ready for human trials. Circ Res. 2014;115:335–8. doi: 10.1161/CIRCRESAHA.114.304616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang Q, Jiang J, Han P, Yuan Q, Zhang J, Zhang X, Xu Y, Cao H, Meng Q, Chen L, Tian T, Wang X, Li P, Hescheler J, Ji G, Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–87. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez-Terzic C, Alekseev AE, Puceat M, Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–9. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 186.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 187.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, Bellamy V, Rucker-Martin C, Barbry P, Bel A, Bruneval P, Cowan C, Pouly J, Mitalipov S, Gouadon E, Binder P, Hagege A, Desnos M, Renaud JF, Menasche P, Puceat M. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–39. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, Bellabas L, Brinon B, Vanneaux V, Pradeau P, Peyrard S, Larghero J, Pouly J, Binder P, Garcia S, Shimizu T, Sawa Y, Okano T, Bruneval P, Desnos M, Hagege AA, Casteilla L, Puceat M, Menasche P. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122:S118–23. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 189.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Don CW, Murry CE. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med. 2013;17:1355–62. doi: 10.1111/jcmm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 192.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49:941–9. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dubois NC, Craft AM, Sharma P, Elliott DA, Stanley EG, Elefanty AG, Gramolini A, Keller G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat Biotechnol. 2011;29:1011–8. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J, Kamp TJ. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–36. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 196.Yoshida Y, Yamanaka S. Induced Pluripotent Stem Cells 10 Years Later: For Cardiac Applications. Circ Res. 2017;120:1958–1968. doi: 10.1161/CIRCRESAHA.117.311080. [DOI] [PubMed] [Google Scholar]
- 197.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 198.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 199.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507–17. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 200.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Schenke-Layland K, Rhodes KE, Angelis E, Butylkova Y, Heydarkhan-Hagvall S, Gekas C, Zhang R, Goldhaber JI, Mikkola HK, Plath K, MacLellan WR. Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells. 2008;26:1537–46. doi: 10.1634/stemcells.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hwang HS, Kryshtal DO, Feaster TK, Sanchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bedada FB, Chan SS, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3:594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2015;117:995–1000. doi: 10.1161/CIRCRESAHA.115.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–10. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tonnessen T, Kryshtal DO, Louch WE, Knollmann BC. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res. 2017;121:1323–1330. doi: 10.1161/CIRCRESAHA.117.311920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–58. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ong SG, Huber BC, Lee WH, Kodo K, Ebert AD, Ma Y, Nguyen PK, Diecke S, Chen WY, Wu JC. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation. 2015;132:762–771. doi: 10.1161/CIRCULATIONAHA.114.015231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, Goldstone AB, Wang M, Fukushi M, Ebert AD, Woo YJ, Rulifson E, Yang PC. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ Res. 2017;121:e22–e36. doi: 10.1161/CIRCRESAHA.117.310803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhu K, Wu Q, Ni C, Zhang P, Zhong Z, Wu Y, Wang Y, Xu Y, Kong M, Cheng H, Tao Z, Yang Q, Liang H, Jiang Y, Li Q, Zhao J, Huang J, Zhang F, Chen Q, Li Y, Chen J, Zhu W, Yu H, Zhang J, Yang HT, Hu X, Wang J. Lack of Remuscularization Following Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitor Cells in Infarcted Nonhuman Primates. Circ Res. 2018;122:958–969. doi: 10.1161/CIRCRESAHA.117.311578. [DOI] [PubMed] [Google Scholar]
- 211.Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–11. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–86. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res. 2012;111:50–5. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’Rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, McKinsey TA, Song K. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat Commun. 2015;6:8243. doi: 10.1038/ncomms9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, Tohyama S, Yuasa S, Kokaji K, Aeba R, Yozu R, Yamagishi H, Kitamura T, Fukuda K, Ieda M. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci U S A. 2013;110:12667–72. doi: 10.1073/pnas.1304053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, Nakashima H, Akiyama M, Wada R, Inagawa K, Nishiyama T, Kaneda R, Fukuda T, Takeda S, Tohyama S, Hashimoto H, Kawamura Y, Goshima N, Aeba R, Yamagishi H, Fukuda K, Ieda M. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. EMBO J. 2014;33:1565–81. doi: 10.15252/embj.201387605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Singh VP, Mathison M, Patel V, Sanagasetti D, Gibson BW, Yang J, Rosengart TK. MiR-590 Promotes Transdifferentiation of Porcine and Human Fibroblasts Toward a Cardiomyocyte-Like Fate by Directly Repressing Specificity Protein 1. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mohamed TM, Stone NR, Berry EC, Radzinsky E, Huang Y, Pratt K, Ang YS, Yu P, Wang H, Tang S, Magnitsky S, Ding S, Ivey KN, Srivastava D. Chemical Enhancement of In Vitro and In Vivo Direct Cardiac Reprogramming. Circulation. 2017;135:978–995. doi: 10.1161/CIRCULATIONAHA.116.024692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miyamoto K, Akiyama M, Tamura F, Isomi M, Yamakawa H, Sadahiro T, Muraoka N, Kojima H, Haginiwa S, Kurotsu S, Tani H, Wang L, Qian L, Inoue M, Ide Y, Kurokawa J, Yamamoto T, Seki T, Aeba R, Yamagishi H, Fukuda K, Ieda M. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell. 2018;22:91–103 e5. doi: 10.1016/j.stem.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 222.Yanamandala M, Zhu W, Garry DJ, Kamp TJ, Hare JM, Jun HW, Yoon YS, Bursac N, Prabhu SD, Dorn GW, 2nd, Bolli R, Kitsis RN, Zhang J. Overcoming the Roadblocks to Cardiac Cell Therapy Using Tissue Engineering. J Am Coll Cardiol. 2017;70:766–775. doi: 10.1016/j.jacc.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xiong Q, Hill KL, Li Q, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS, Zhang J. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells. 2011;29:367–75. doi: 10.1002/stem.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–61. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 226.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 227.Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ Res. 2015;117:720–30. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weinberger F, Breckwoldt K, Pecha S, Kelly A, Geertz B, Starbatty J, Yorgan T, Cheng KH, Lessmann K, Stolen T, Scherrer-Crosbie M, Smith G, Reichenspurner H, Hansen A, Eschenhagen T. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med. 2016;8:363ra148. doi: 10.1126/scitranslmed.aaf8781. [DOI] [PubMed] [Google Scholar]
- 229.Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 230.Weinberger F, Mannhardt I, Eschenhagen T. Engineering Cardiac Muscle Tissue: A Maturating Field of Research. Circ Res. 2017;120:1487–1500. doi: 10.1161/CIRCRESAHA.117.310738. [DOI] [PubMed] [Google Scholar]
- 231.Carvalho AB, de Carvalho AC. Heart regeneration: Past, present and future. World J Cardiol. 2010;2:107–11. doi: 10.4330/wjc.v2.i5.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Soonpaa MH, Rubart M, Field LJ. Challenges measuring cardiomyocyte renewal. Biochim Biophys Acta. 2013;1833:799–803. doi: 10.1016/j.bbamcr.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Leone M, Magadum A, Engel FB. Cardiomyocyte proliferation in cardiac development and regeneration: a guide to methodologies and interpretations. Am J Physiol Heart Circ Physiol. 2015;309:H1237–50. doi: 10.1152/ajpheart.00559.2015. [DOI] [PubMed] [Google Scholar]
- 234.Lopaschuk GD, Collins-Nakai RL, Itoi T. Developmental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26:1172–80. doi: 10.1093/cvr/26.12.1172. [DOI] [PubMed] [Google Scholar]
- 235.Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114:717–29. doi: 10.1161/CIRCRESAHA.114.301863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–79. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sharma A, Wu SM. Members Only: Hypoxia-Induced Cell-Cycle Activation in Cardiomyocytes. Cell Metab. 2015;22:365–6. doi: 10.1016/j.cmet.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 238.Guimaraes-Camboa N, Evans SM. Redox Paradox: Can Hypoxia Heal Ischemic Hearts? Dev Cell. 2016;39:392–394. doi: 10.1016/j.devcel.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen X, Wilson RM, Kubo H, Berretta RM, Harris DM, Zhang X, Jaleel N, MacDonnell SM, Bearzi C, Tillmanns J, Trofimova I, Hosoda T, Mosna F, Cribbs L, Leri A, Kajstura J, Anversa P, Houser SR. Adolescent feline heart contains a population of small, proliferative ventricular myocytes with immature physiological properties. Circ Res. 2007;100:536–44. doi: 10.1161/01.RES.0000259560.39234.99. [DOI] [PubMed] [Google Scholar]
- 240.Patterson M, Barske L, Van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JG, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR, Sucov HM. Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet. 2017;49:1346–1353. doi: 10.1038/ng.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tang H, Xiao K, Mao L, Rockman HA, Marchuk DA. Overexpression of TNNI3K, a cardiac-specific MAPKKK, promotes cardiac dysfunction. J Mol Cell Cardiol. 2013;54:101–11. doi: 10.1016/j.yjmcc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Vagnozzi RJ, Gatto GJ, Jr, Kallander LS, Hoffman NE, Mallilankaraman K, Ballard VL, Lawhorn BG, Stoy P, Philp J, Graves AP, Naito Y, Lepore JJ, Gao E, Madesh M, Force T. Inhibition of the cardiomyocyte-specific kinase TNNI3K limits oxidative stress, injury, and adverse remodeling in the ischemic heart. Sci Transl Med. 2013;5:207ra141. doi: 10.1126/scitranslmed.3006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gonzalez-Rosa JM, Sharpe M, Field D, Soonpaa MH, Field LJ, Burns CE, Burns CG. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell. 2018;44:433–446 e7. doi: 10.1016/j.devcel.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, Zhou L, Zhou B, Duan DD, Chen X, Houser SR, Zeng C. Dedifferentiation, Proliferation, and Redifferentiation of Adult Mammalian Cardiomyocytes After Ischemic Injury. Circulation. 2017;136:834–848. doi: 10.1161/CIRCULATIONAHA.116.024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, Udi Y, Sarig R, Sagi I, Martin JF, Bursac N, Cohen S, Tzahor E. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. 2017;547:227–231. doi: 10.1038/nature22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016;119:91–112. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017:24. doi: 10.1111/micc.12305. [DOI] [PubMed] [Google Scholar]
- 249.Sattler S, Rosenthal N. The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochim Biophys Acta. 2016;1863:1813–21. doi: 10.1016/j.bbamcr.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 250.Broughton KM, Wang BJ, Firouzi F, Khalafalla F, Dimmeler S, Fernandez-Aviles F, Sussman MA. Mechanisms of Cardiac Repair and Regeneration. Circ Res. 2018;122:1151–1163. doi: 10.1161/CIRCRESAHA.117.312586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Gourdie RG, Dimmeler S, Kohl P. Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat Rev Drug Discov. 2016;15:620–38. doi: 10.1038/nrd.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]