Abstract
A series of postpartum Streptococcus pyogenes infections prompted an investigation to rule out potential transmission by a health care worker. None of the hospital staff screened were colonized. All isolates were determined to be unrelated by molecular methods, including whole-genome sequencing. Thus, nosocomial transmission was considered unlikely.
Keywords: hospital epidemiology, postpartum infection, streptococcus pyogenes
Invasive infections caused by Streptococcus pyogenes (group A streptococcus [GAS]) cause significant global morbidity and mortality. For postpartum women, the risk of acquiring invasive GAS is 20-fold higher than in nonpregnant women, resulting in 220 cases per year in the United States as of 2002 [1, 2]. Although the infection occurs in otherwise healthy women, it carries significant risk of mortality, with a case fatality rate of 3.5% and a 6- to 20-fold increased incidence of neonatal death [1, 2].
GAS infections can occur in clusters and may be transmitted by an asymptomatic health care worker (HCW), potentially causing infections up to even more than a year apart [1, 3, 4]. Thus, any case of postpartum GAS warrants investigation to rule out possible transmission by an HCW to prevent potential additional cases. As part of routine surveillance for Caesarean section surgical site infections (SSIs), 5 cases of postpartum GAS were identified at a large academic medical center over 14 months, from February 2015 to March 2016. With the identification of the first case, an investigation transpired to ensure that cases were not connected through carriage by an HCW.
METHODS
Case Definition
The 2002 Centers for Disease Control and Prevention (CDC) guideline identifies the following as a case of an invasive health care–associated postpartum GAS infection: isolation, during the hospital stay or within the first 7 days after discharge, of GAS from a sterile site or a surgical wound [5].
Investigation Methodology
For each case, the electronic medical record was reviewed using a standardized data collection form to identify demographics, potential risk factors for infection, possible source, clinical course, and treatment. HCWs who had cared for the patients were also identified. Potential patient colonization was assessed by an Ob-Gyn physician who interviewed each patient to identify sick contacts and history of reported skin/soft tissue infections. Patients were screened at nonsterile sites (ie, oropharynx, vagina, and perirectal area) for GAS colonization. The HCWs associated with the first case were also screened for GAS at the same nonsterile sites, and wounds if present.
GAS strains from each patient were retained by the hospital microbiology lab and sent to the Ohio Department of Health for comparison by pulsed field gel electrophoresis (PFGE). Two isolates that were indistinguishable by PFGE were sent to the Department of Pathology and Genomic Medicine, Houston Methodist Hospital, for whole-genome sequencing (WGS), performed as previously described [6, 7].
RESULTS
Case 1
In February 2015, a 37-year-old female (F) was identified 10 days after a Caesarian delivery with an infected incision that grew GAS. Screening cultures from the oropharynx, vagina, and rectum were negative (Table 1), suggesting that she was not colonized with GAS. Blood and urine cultures were not done. An investigation was begun to ensure that no health care–associated transmission had occurred. Seventeen HCWs who cared for her were identified; none screened positive for GAS carriage at any site.
Table 1.
Demographics, Risk Factors, Cultures From 5 Postpartum Females
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| Age, y | 37 | 26 | 39 | 31 | 34 |
| Ethnicity | White | Black | White | Black | White |
| Weight, kg | 65.5 | 60.9 | 98.2 | 88.6 | 81.8 |
| Smoking status | Former | Current | Former | Current | Former |
| GBS status | Not performed | Negative | Negative | Positive | Negative |
| Gestational age at delivery | 28w 1d | 36w 1d | 39w 3d | 38w 4d | 40w 4d |
| Delivery method | C-section | Vaginal | C-section | C-section | Vaginal |
| Days from delivery to GAS culture | 10 | 1 | 20 | 7 | 2 |
| Days from discharge to GAS culture | 6 | 0 | 17 | 4 | 1 |
| Comorbid illnesses and possible risk factor for GAS | Chronic kidney disease | Sick contact with fever, sore throat | Congenital coagulopathy | Clitoral piercing removed 1 day before delivery | None |
| Endometritis | No | Yes | No | No | Yes |
| Primary site of infection | C-section incisional | Endometritis | No clinical infection; vaginal colonization only | Bacteremia | Endometritis |
| Blood culture | Not performed | Positive | Not performed | Positive | Positive |
| Urine culture | Not performed | Positive | Negative | Positive | Negative |
| Wound culture | Positive | No wound | No wound | No wound | No wound |
| Oropharyngeal culture | Negative | Positive | Not performed | Negative | Negative |
| Vaginal culture | Negative | Positive | Positive | Positive | Positive |
| Perirectal culture | Negative | Negative | Not performed | Negative | Negative |
Abbreviations: C-section, Cesarean section; GAS, group A streptococcus; GBS, group B streptococcus.
Case 2
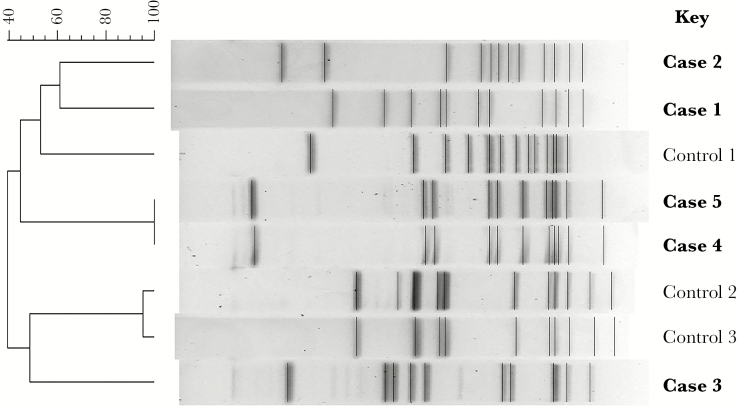
A 26-year-old F was identified 3 months after case 1, when she presented with endometritis and GAS bacteremia 1 day after an uncomplicated vaginal delivery. A urine culture and GAS screening cultures from the oropharynx and vagina were positive, indicating likely colonization before delivery. PFGE results (Figure 1) showed that case 1 and 2 were different, supporting the hypothesis that HCW transmission had not occurred. No additional staff were screened then.
Figure 1.
Pulsed field gel electrophoresis.
Case 3
Two months after case 2, a 39-year-old F presented with vaginal bleeding on postoperative day 20 after a Caesarian section delivery. A vaginal culture was positive for GAS, and all other screening sites were culture-negative. She was judged to be colonized, rather than having a true infection.
Case 4
A 31-year-old F re-presented 1 day after case 3 with septic shock due to GAS bacteremia. She was on postoperative day 7 from a Caesarian section delivery. Cultures were positive for GAS from blood, urine, and vagina. Her condition quickly improved with antibiotics and supportive care. Further questioning revealed that she had a clitoral ring removed 1 day before delivery. Although she did not have overt signs of clitoral infection, because she was positive for vaginal colonization, this was considered the most likely source. Notably, her prenatal group B streptococcus (GBS) screen was positive, and she had been treated with ampicillin 6 hours before delivery. She also received cefazolin perioperatively and for 24 hours after delivery. PFGE showed that cases 3 and 4 were distinct from each other and also from the prior 2 patients. There was no overlap in HCWs caring for these 4 patients, so health care–related transmission was thought unlikely.
Case 5
Eight months after case 4, in March 2016, a 34-year-old F presented with endometritis and GAS bacteremia 2 days after an uncomplicated vaginal delivery. The patient was culture-positive for vaginal carriage. The PFGE pattern of both her isolates was identical to the strain from case 4, prompting further investigation. A single HCW had cared for both patients, and this HCW screened negative for GAS carriage in the oropharynx, vagina, and rectum. Isolates from cases 4 and 5 were both type emm3. WGS showed that these 2 isolates differed by 16 single nucleotide polymorphisms. This level of genetic difference strongly argues against HCW-related transmission or a common source of transmission [8].
DISCUSSION
Cases 1, 2, 4, and 5 met the CDC case definition for invasive postpartum GAS infection. Case 3 presented beyond the 7-day postdischarge window required to be considered health care associated. In addition, this patient was judged to be colonized with GAS rather than truly infected. Index case 1 was not colonized with GAS, so her case was initially concerning for health care–associated transmission; however, an exhaustive review of HCWs who cared for her did not reveal any colonization. Cases 2, 4, and 5 also met CDC criteria for postpartum invasive GAS, but the isolates from these 3 patients were genetically distinct from each other, as assessed by a combination of PFGE and WGS analysis, and did not appear to have been transmitted by a shared HCW. Other than case 1, all had vaginal carriage of GAS, which likely predisposed them to an invasive GAS infection. No other shared risk factors were identified, and all 5 patients recovered quickly with appropriate treatment. Due to the potentially severe nature of invasive GAS infections, the identification of a single case, especially in an otherwise healthy postpartum patient, mandates a thorough epidemiologic investigation. In this case series, there was no evidence indicating that infections were cross-transmitted, hospital acquired, or associated with an HCW.
It is well established that prenatal screening for GBS has dramatically reduced the incidence of neonatal infections [9], but screening for GAS is not routinely performed. GAS vaginal colonization is a risk factor for developing an invasive infection, although compared with GBS, GAS colonization is far less frequent. A surveillance study performed in 2000 indicated that the rate of GAS colonization late in pregnancy was 0.03%, vs 20.1% for GBS [10]; however, in our small series, 4 of the 5 patients were colonized with GAS. There were 5661 deliveries at our institution during the study period, so based on only 4 positive patients, the rate was 0.071%. This is double the previously reported rate without screening any of the asymptomatic patients; thus we presume our local rate of GAS colonization is much higher. This is supported by surveillance data from the Ohio Department of Health indicating that the total rate of invasive GAS has increased 7-fold in the period from 1996 to 2008 [11], although unfortunately the percentage of postpartum infections was not specifically quantified. In the last 3 years in the county where our facility is located, invasive GAS case rates have steadily increased from 3.0 (2014) to 3.8 (2015) to 4.3 per 100 000 population in 2016 [12]. Furthermore, in 2015, there were 5 community outbreaks of GAS in the county, involving 133 total patients, compared with just 2 outbreaks involving 22 total patients during the preceding 3 years [11]. Thus, local rates are on the rise and coincided with our cases. With this increase, screening for GAS colonization may be useful, especially if peripartum antibiotics reduced colonization rates and risk for invasive infection. In our cases, only 1 patient (case 4) had GBS colonization before delivery. This patient was treated with peripartum ampicillin but remained colonized with GAS upon presentation, with an invasive infection 7 days later, suggesting that GAS colonization was not eradicated.
In conclusion, although the incidence of GAS vs GBS remains low, current data show that both community-acquired disease and invasive infections are increasing. Additional surveillance data are necessary to confirm rates of vaginal GAS carriage and to assess if targeted peripartum antibiotic treatment of carriers would alter the risk of postpartum invasive GAS. Given the potential for high morbidity and mortality with GAS infection, it is important for hospital epidemiology programs to remain vigilant. Importantly, no additional cases have been identified through calendar year 2017. Although no HCW transmission was identified in this investigation, Caesarian section SSI surveillance is ongoing.
Acknowledgments
A special thanks to Eric Brandt at the Ohio Department of Health for assistance with PFGE. We also thank Mathew Ojeda Saavedra at the Houston Methodist Research Institute for assistance with WGS.
Prior presentations. These data were presented in part at 2016 IDWeek in New Orleans, Louisiana.
Financial support. R.J.O. and J.M.M. were supported in part by funds from the Fondren Foundation.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chuang I, Van Beneden C, Beall B, Schuchat A. Population-based surveillance for postpartum invasive group a streptococcus infections, 1995-2000. Clin Infect Dis 2002; 35:665–70. [DOI] [PubMed] [Google Scholar]
- 2. Deutscher M, Lewis M, Zell ER, et al. ; Active Bacterial Core Surveillance Team Incidence and severity of invasive Streptococcus pneumoniae, group A streptococcus, and group B streptococcus infections among pregnant and postpartum women. Clin Infect Dis 2011; 53:114–23. [DOI] [PubMed] [Google Scholar]
- 3. Viglionese A, Nottebart VF, Bodman HA, Platt R. Recurrent group A streptococcal carriage in a health care worker associated with widely separated nosocomial outbreaks. Am J Med 1991; 91:329–33S. [DOI] [PubMed] [Google Scholar]
- 4. Mastro TD, Farley TA, Elliott JA, et al. . An outbreak of surgical-wound infections due to group A streptococcus carried on the scalp. N Engl J Med 1990; 323:968–72. [DOI] [PubMed] [Google Scholar]
- 5. Beall B, Besser J, Bisno A, et al. . Prevention of invasive group A streptococcal disease among household contacts of case patients and among postpartum and postsurgical patients: recommendations from the Centers for Disease Control and Prevention. Clin Infect Dis 2002; 35:950–9. [DOI] [PubMed] [Google Scholar]
- 6. Olsen RJ, Fittipaldi N, Kachroo P, et al. . Clinical laboratory response to a mock outbreak of invasive bacterial infections: a preparedness study. J Clin Microbiol 2014; 52:4210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long SW, Olsen RJ, Eagar TN, et al. . Population genomic analysis of 1,777 extended-spectrum beta-lactamaseproducing Klebsiella pneumoniae isolates, Houston, Texas: unexpected Abundance of Clonal Group 307. MBio 2017; 8:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beres SB, Carroll RK, Shea PR, et al. . Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci U S A 2010; 107:4371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 2010; 59:1–36. [PubMed] [Google Scholar]
- 10. Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol 2000; 8:217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohio Department of Health. Reported cases of selected notifiable diseases - Ohio, 2008–2016 2017. https://www.odh.ohio.gov/en/healthstats/disease/idann/idann. Accessed 6 April 2018.
- 12. Columbus Public Health. Annual summary of reportable diseases 2016 2017. https://www.columbus.gov/publichealth/programs/Office-of-Epidemiology/Infectious-Disease-Reports/. Accessed 6 April 2018.