SummaryAs compared to uninfected controls with heart failure (HF) and sleep apnea (SA), those with human immunodeficiency virus (HIV) are more likely to have obstructive rather than central SA. CPAP use among persons with HIV and HF was associated with lower 30-day HF readmission.
Keywords: heart failure, sleep apnea, apnea–hypopnea index, human immunodeficiency virus, heart failure readmission
Abstract
Background
Sleep apnea (SA) is common and has prognostic significance among broad groups of patients with heart failure (HF). There are no data characterizing the presence, associations, and prognostic significance of SA among persons living with human immunodeficiency virus (PLHIV) with HF.
Methods
We conducted a single-center study of PLHIV with HF with reduced ejection fraction (HFrEF; left ventricular ejection fraction [LVEF] <50%) and analyzed the relationship of SA with 30-day HF hospital readmission rate.
Results
Our cohort included 1124 individuals admitted with HFrEF; 15% were PLHIV, and 92% were on antiretroviral therapy. SA was noted in 28% of PLHIV and 26% of uninfected controls. Compared to uninfected controls with HFrEF and SA, PLHIV with HFrEF and SA had a lower body mass index, lower LVEF, a higher pulmonary artery systolic pressure (PASP), were more likely to have obstructive rather than central SA (P < .05 for all). In a multivariable model, PASP, low CD4 count, high viral load (VL), and SA parameters (apnea–hypopnea index, CPAP use and duration) were predictors of 30-day HF readmission rate. Each 1-hour increase in CPAP use was associated with a 14% decreased risk of 30-day HF readmission among PLHIV.
Conclusions
Compared to uninfected controls, PLHIV were more likely to have obstructive SA than central SA. Apnea severity, low CD4 count, high VL, and cocaine use were positively associated with 30-day HF hospital readmission rate, whereas CPAP use and increased duration of CPAP use conferred protection.
(See the Viewpoints by Owens and Hicks on pages 472–7.)
The life expectancy of persons living with HIV (PLHIV) on antiretroviral therapy (ART) has increased and has resulted in HIV being considered a chronic disease [1–6]. In this context, PLHIV are facing a heightened risk of age-related comorbidities including heart failure (HF). Indeed, the risk of incident HF is increased more than 2-fold among PLHIV on ART [1, 3, 6]. There are 2 types of HF: HF with a preserved ejection fraction and HF with a reduced EF (HFrEF). Compared to uninfected controls with HF, PLHIV with both types of HF have worse cardiovascular outcomes [3, 7]. The mechanisms underlying the increased occurrence of adverse outcomes among PLHIV with HF remain unclear. Sleep apnea (SA) is particularly common among broad groups of patients with HFrEF and is associated with increased morbidity and mortality in that population [8–10]. Among PLHIV without known HF, sleep disturbances are reported in up to 70% [11, 12]. Therefore, as SA is a cause of increased morbidity and mortality among uninfected patients with HFrEF, outcomes among PLHIV with HF are poor, and SA may be common among PLHIV, we aimed to test the presence, characteristics, associations, and prognostic significance of SA among PLHIV with HFrEF.
Our aim in this study was to add to the limited knowledge about SA in PLHIV with HF. We hypothesized that SA was highly prevalent among PLHIV with HFrEF and that the presence of SA among PLHIV with HFrEF was associated with worse outcomes.
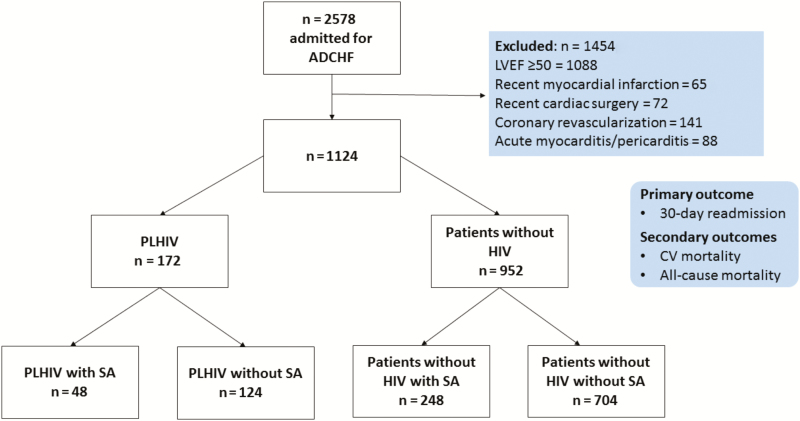
METHODS
Study Design and Patient Population
After obtaining institutional board review approval, we retrospectively analyzed data on the 2578 patients admitted to a US tertiary care hospital (Bronx-Lebanon Hospital Center of Mount Sinai School of Medicine, Bronx, New York) in 2011 with a primary diagnosis of acute decompensated HF. Individuals who had left ventricular ejection fraction (LVEF) ≥50% (n = 1088), recent (≤ 3 months) history of myocardial infarction (n = 65), cardiac surgery (n = 72), coronary revascularization (n = 141), acute myocarditis, or pericarditis (n = 88) were excluded from the study. The final study group of 1124 individuals hospitalized with HFrEF consisted of 172 PLHIV and 952 uninfected controls. The diagnoses of HIV, HF, and SA were ascertained through manual review of each individual’s electronic health record (EHR). An HF admission was defined per the American College of Cardiology and American Heart Association key data elements and definitions for cardiovascular endpoint events. An HF admission was defined as a hospital admission with a primary diagnosis of HF and length of stay of at least 24 hours, with new or worsening symptoms of HF on presentation, objective evidence of new or worsening HF, and initiation or intensification of treatment specifically for HF [13]. SA was diagnosed with polysomnography with an apnea–hypopnea index of ≥5 along with oxygen desaturation during sleep.
Outcomes
Our primary outcome was 30-day HF hospital readmission rate, defined according to European Society of Cardiology consensus criteria [14] and ascertained through physician-adjudicated individual EHR review. The follow-up period began on the date of discharge from the first HF hospitalization in 2011. Our secondary outcomes included rates of cardiovascular (CV) mortality and all-cause mortality. CV death was defined as death due to HF, sudden cardiac death, arrhythmias, or acute ischemic events [15]. Death was determined through the Social Security death index and confirmed by physician-adjudicated individual EHR review.
Covariates
Through EHR review, data were collected on traditional HF risk factors (including hypertension, dyslipidemia, diabetes mellitus, coronary artery disease [CAD], family history of CAD, body mass index [BMI], prior or active cigarette smoking, and prior or active cocaine use) as well as on presence, characteristics, and severity of SA; type of SA (central vs obstructive); apnea–hypopnea index (AHI); continuous positive airway pressure (CPAP) use; and daily duration of CPAP use [7, 16, 17]. Also through EHR review, data were collected on medication use at the time of discharge from the index HF hospitalization. Details on HIV-specific parameters (current ART, duration of ART, CD4 count, viral load [VL]) were recorded from those available closest to the time of discharge from the index HF hospitalization.
Statistical Analyses
Continuous variables are presented as mean and standard deviation or median (interquartile range [IQR]), as appropriate, based on normality, and categorical variables are presented as percentages. Continuous data were compared using unpaired Student t tests or Wilcoxon rank-sum tests, as appropriate. Categorical data were compared using the χ2 or Fisher exact test. Comparisons were made between the following groups: PLHIV with and without SA, HIV-uninfected controls with and without SA, PLHIV with SA and HIV-uninfected controls without SA, and PLHIV with SA with central sleep apnea (CSA) vs PLHIV with SA with obstructive sleep apnea (OSA). Survival curves were plotted using Kaplan-Meier curves. Univariate and multivariate regression analyses were performed to determine the association between baseline covariates and 30-day HF hospital readmission rate. Multivariate Cox proportional hazard regression analyses for the 30-day HF hospital readmission rate were constructed using a P value < .01 on the univariate analysis for entry. Otherwise, statistical significance was defined using a 2-tailed P value ≤ .05. Both VL and CD4 count were not included in the multivariate model together due to the overlap between those individuals with a low CD4 count and a high VL. Similarly, both CPAP use and CPAP duration were not included in the multivariate model together. PLHIV admitted with HFrEF who also had SA were further divided based on median AHI, the use of CPAP, and median nightly duration of CPAP. Statistical analyses were performed using SPSS software version 24.
RESULTS
Demographics and Baseline Characteristics
Among PLHIV with HFrEF, 92% (159/172) were on ART (median duration, 8.5 years; IQR, 4–16 years). In this group, the mean CD4 count was 293 cells/mm3 and the median VL was 279 copies/mL (range, <50 to 3000457). Fifty-three percent (91/172) had a CD4 count ≥200 cell/mm3, 37% (64/172) had a VL <50 copies/mL, and 60% (102/172) had VL <200 copies/mL. SA was noted in 28% of PLHIV (48/172) with HFrEF and 26% (248/952) of uninfected controls with HFrEF (Figure 1). The prevalence of SA was similar between PLHIV with HFrEF and uninfected controls with HFrEF (Table 1). Comparison of PLHIV with HFrEF with and without SA revealed that PLHIV with HFrEF and SA had a higher pulmonary artery systolic pressure (PASP) and BMI. However, HIV-specific parameters (CD4 count, VL, and ART use) were similar between these 2 groups. Comparison of HIV-uninfected controls with HFrEF with and without SA revealed that HIV-uninfected controls with HFrEF and SA had a higher LVEF, higher BMI, and lower percentage of spironolactone use (Table 1). When patients with HFrEF and SA with and without HIV were compared, those individuals with HIV had a lower BMI and LVEF, a higher PASP, were more likely to have OSA rather than CSA, and were more likely to use CPAP for a longer duration (Table 1). Finally, within the group of patients with HIV, HFrEF, and SA, those with CSA were compared to those with OSA: PLHIV with CSA had higher rates of hypertension (80% vs 60%), a lower BMI (29 ± 5.0 vs 34 ± 4.5 kg/m2), a lower LVEF (27 ± 6.8 vs 39 ± 4.5%), a higher PASP (57 ± 7.3 vs 46 ± 8 .3 mm Hg), and more severe SA (AHI, 36 vs 15/hour) compared to those with OSA (Table 2). Additionally, PLHIV with HFrEF and CSA had a lower CD4 count (117 vs 389 cells/mm3) and lower rates of viral suppression (13% vs 51%,) compared to PLHIV with HFrEF and OSA (Table 2).
Figure 1.
Consort diagram. Abbreviations: ADCHF, acute decompensated congestive heart failure; CV, cardiovascular; LVEF, left ventricular ejection fraction; PLHIV, persons living with human immunodeficiency virus; SA, sleep apnea.
Table 1.
Baseline Characteristics of People Living With Human Immunodeficiency Virus (HIV) and Non-HIV Individuals
| Characteristic | Group Aa (n = 48) | Group Ba n = 124 | Group Ca (n = 248) | Group Da (n = 704) | P Value A vs B | P Value C vs D | P Value A vs C |
|---|---|---|---|---|---|---|---|
| Females | 27 (56%) | 55 (44%) | 118 (49%) | 554 (58%) | .161 | .180 | .271 |
| Age (years)b | 61 ± 9.1 | 59 ± 9.6 | 60 ± 9.8 | 60 ± 9.5 | .117 | .822 | .353 |
| Race | |||||||
| Hispanic | 17 (35%) | 46 (37%) | 92 (37%) | 278 (40%) | .890 | .798 | .942 |
| African American | 22 (46%) | 52 (42%) | 107 (43%) | 294 (41%) | |||
| Others | 9 (19%) | 26 (21%) | 49 (19%) | 132 (19%) | |||
| Cardiovascular risk factors | |||||||
| Diabetes | 12 (25%) | 46 (37%) | 99 (39%) | 241 (34%) | .132 | .108 | .060 |
| Hypertension | 32 (67%) | 78 (63%) | 171 (68%) | 431 (61%) | .654 | .030 | .754 |
| Smoking | 28 (58%) | 65 (52%) | 122 (49%) | 354 (50%) | .485 | .768 | .246 |
| Ischemic heart disease | 21 (44%) | 50 (40%) | 91 (36%) | 261 (37%) | .682 | .915 | .356 |
| Implantable cardioverter defibrillator | 13 (27%) | 30 (24%) | 54 (24%) | 156 (16%) | .694 | .899 | .421 |
| Left ventricular ejection fraction (%)b | 36.6 ± 8.0 | 37.2 ± 8.9 | 41.0 ± 6.0 | 35.7 ± 8.9 | .690 | <.001 | <.001 |
| Pulmonary artery systolic pressure (mm Hg)b | 50.0 ± 9.5 | 45.5 ± 9.0 | 40.0 ± 9.0 | 38.9 ± 8.3 | .006 | .098 | .091 |
| Cocaine use | 17 (35%) | 45 (36%) | 59 (23%) | 133 (19%) | .914 | .098 | .091 |
| Heart failure medications | |||||||
| Beta blocker | 43 (90%) | 110 (89%) | 221(89%) | 631 (90%) | .869 | .819 | .923 |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 42 (87%) | 110 (89%) | 220 (89%) | 624 (88%) | .824 | .975 | .809 |
| Spironolactone | 8 (17%) | 30 (24%) | 44 (18%) | 178 (25%) | .286 | .020 | .857 |
| Furosemide | 42 (87%) | 101 (81%) | 211 (85%) | 565 (80%) | .342 | .092 | .663 |
| Human immunodeficiency virus parameters | |||||||
| CD4 count cells/mm3 b | 304 ± 246 | 289 ± 237 | … | … | .717 | … | … |
| VL c | 178 (<50-2000564) | 345 (<50-3000457) | … | … | .001 | … | … |
| VL | |||||||
| <50 copies/mL | 19 (39%) | 45 (36%) | … | … | .688 | … | … |
| <200 copies/mL | 29 (62%) | 73(59%) | … | … | .621 | … | … |
| ART | 42 (87%) | 117 (94%) | … | … | .194 | … | … |
| Duration of ART (years)c | 8 (4–16) | 9 (4–16) | … | … | .793 | … | … |
| SA parameters | |||||||
| Body mass index (kg/m2)b | 32 ± 5.4 | 25 ± 5.4 | 39 ± 4.6 | 33 ± 5.4 | <.001 | <.001 | <.001 |
| SA | 48 (28%) | … | 248 (26%) | … | … | … | .610 |
| Central sleep apnea | 14 (29%) | … | 112 (45%) | … | … | … | .033 |
| Obstructive sleep apnea | 32 (66%) | … | 111 (45%) | ||||
| Mixed SA | 2 (4%) | … | 25 (10%) | ||||
| Apnea–hypopnea indexc | 24 (10–40) | … | 22 (8–40) | … | … | … | .191 |
| CPAP treatment | 38 (79%) | … | 157 (64%) | … | … | … | .031 |
| CPAP duration (hours/day)c | 6 (0–8) | … | 4 (0–6) | … | … | … | .001 |
The bold values mean statistically significant difference.
Abbreviations: ART, antiretroviral therapy; CPAP, continuous positive airway pressure; SA, sleep apnea; VL, viral load.
aGroup A, people living with human immunodeficiency virus (PLHIV), SA+; group B, PLHIV, SA–; group C, non-HIV, SA+; and group D, non-HIV, SA–.
bMean ± standard deviation.
cMedian (interquartile range).
Table 2.
Baseline Characteristics of People Living With Human Immunodeficiency Virus (PLHIV) With Central Sleep Apnea vs PLHIV With Obstructive Sleep Apnea
| Characteristic | PLHIV and CSA (n = 15) | PLHIV and OSA (n = 33) | P Value |
|---|---|---|---|
| Female | 8 (53%) | 18 (55%) | .937 |
| Age (years)a | 60 ± 9.0 | 62 ± 7.6 | .537 |
| Race | |||
| Hispanic | 5 (33%) | 12 (36%) | … |
| African American | 7 (47%) | 15 (46%) | .956 |
| Others | 3 (20%) | 6 (18%) | … |
| Cardiovascular risk factors | |||
| Diabetes | 4 (27%) | 9 (27%) | .759 |
| Hypertension | 12 (80%) | 20 (60%) | .322 |
| Smoking | 9 (60%) | 19 (58%) | .874 |
| Ischemic heart disease | 7 (47%) | 14 (43%) | 0.783 |
| Implantable cardioverter defibrillator | 4 (26%) | 9 (27%) | .759 |
| Left ventricular ejection fraction (%)a | 27 ± 6.8 | 39 ± 4.5 | <.001 |
| Pulmonary artery systolic pressure (mm Hg)a | 57 ± 7.3 | 46 ± 8.3 | <.001 |
| Cocaine use | 5 (33%) | 12 (37%) | .838 |
| Heart failure medications | |||
| Beta blocker | 13 (86%) | 29 (88%) | .994 |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 14 (93%) | 28 (85%) | .649 |
| Spironolactone | 3 (20%) | 5 (15%) | .692 |
| Furosemide | 13 (86%) | 29 (88%) | 0.944 |
| Human immunodeficiency virus parameters | |||
| CD4 count cells/mm3 a | 117 ± 80 | 389 ± 250 | <.001 |
| Undetectable viral load (<50 copies/mL) | 2 (13%) | 17 (51%) | .023 |
| ART | 13 (87%) | 29 (88%) | .994 |
| Duration of ART (years)b | 8 (5–14) | 9 (4–16) | .601 |
| Sleep apnea parameters | |||
| Body mass index (kg/m2)a | 29 ± 5.0 | 34 ± 4.5 | .001 |
| Apnea–hypopnea indexb | 36 (31–40) | 15 (10–37) | <.001 |
| CPAP treatment | 11 (74%) | 27 (81%) | .703 |
| CPAP duration (hours/day)b | 5 (0–8) | 6 (0–7) | .695 |
The bold values mean statistically significant difference.
Abbreviations: ART, antiretroviral therapy; CPAP, continuous positive airway pressure; CSA, central sleep apnea; OSA, obstructive sleep apnea; PLHIV, people living with human immunodeficiency virus.
aMean ± standard deviation.
bMedian (interquartile range).
Outcomes
Among the entire cohort, 55% were re-admitted with decompensated HF within 30 days of discharge from the incident HF hospitalization. Among PLHIV with HFrEF, factors associated with an increased 30-day HF hospital readmission rate on univariate analysis included traditional HF risk factors (diabetes mellitus, a history of CAD, increased PASP), the use of traditional medications for HFrEF (beta blocker, angiotensin converting enzyme inhibitor/angiotensin receptor blocker), nontraditional HF risk factors (cocaine use), HIV-specific parameters (low CD4 count and high VL), and parameters relating to SA (AHI, CPAP use duration) (Supplementary Table 1). In multivariable modeling conducted among PLHIV with HFrEF, the following parameters remained independently associated with increased 30-day HF readmission rate among PLHIV with HFrEF: history of CAD, increased PASP, cocaine use, low CD4 count (or high VL), and high AHI. CPAP use and duration of CPAP use were associated with a lower 30-day HF hospital readmission rate in this group (Table 3 and Supplementary Tables 2 and 3).
Table 3.
Multivariate Predictors of 30-Day Heart Failure Rehospitalization Among People Living With Human Immunodeficiency Virus
| 30-Day Rehospitalization Outcome | Hazard Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Diabetes | 1.132 | 0.686 | 1.868 | .433 |
| Ischemic heart disease | 2.114 | 1.286 | 3.431 | <.001 |
| Cocaine use | 1.536 | 1.225 | 1.925 | <.001 |
| Pulmonary artery systolic pressure | 1.155 | 1.100 | 1.213 | <.001 |
| Beta blocker | 0.337 | 0.066 | 1.727 | .192 |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker | 0.390 | 0.041 | 3.737 | .414 |
| CD4 count | 0.995 | 0.991 | 0.999 | .021 |
| Apnea–hypopnea index | 1.147 | 1.075 | 1.223 | <.001 |
| Continuous positive airway pressure treatment | 0.317 | 0.139 | 0.720 | <.006 |
The bold values mean statistically significant difference.
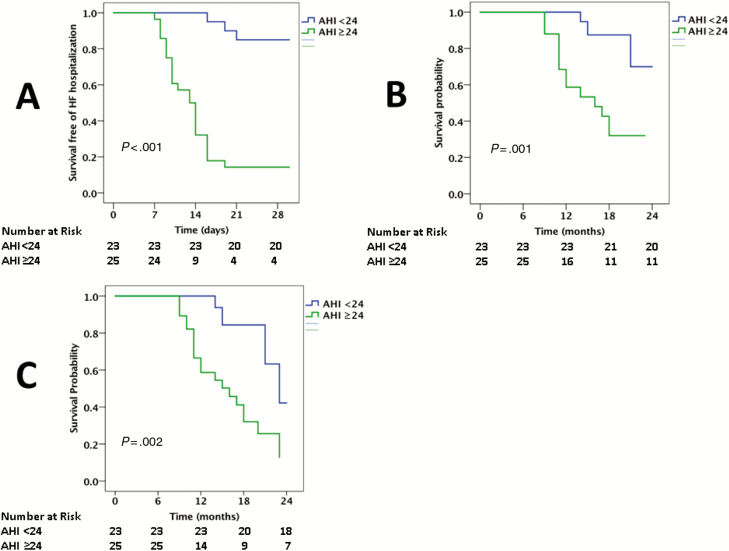
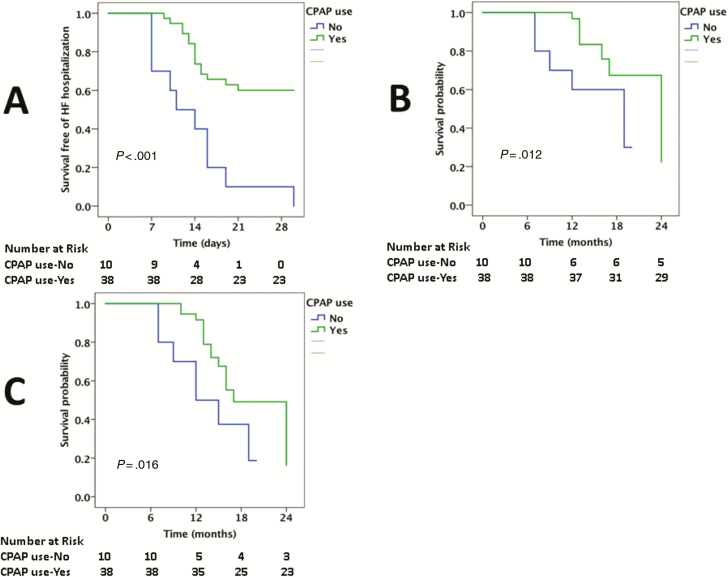
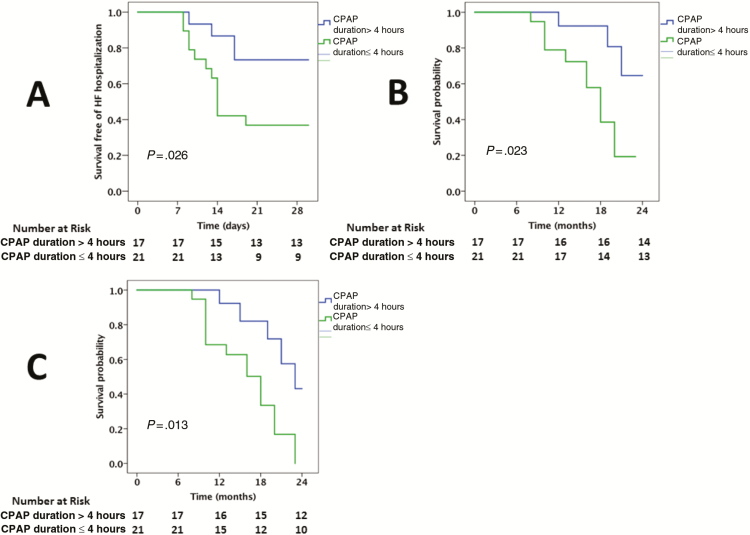
The median AHI was 24/hour among the PLHIV who were diagnosed with SA. The 30-day HF hospital readmission rate was compared between those above and below the median AHI. The 30-day hospital readmission rate was higher in PLHIV with HFrEF with an AHI ≥24/hour vs AHI <24/hour (84% vs 13%, P < .001; Figure 2). In a univariable model, the following factors were associated with an increased AHI among PLHIV with SA: a history of CAD, smoking, higher VL, lower CD4 count, lower LVEF, and higher PASP (Supplementary Table 4). In a multivariable model, smoking, CD4 count (or VL), and PASP were independently associated with an increased AHI (Supplementary Table 5). The 30-day HF hospital readmission rate was also compared between those who were and were not using CPAP. The 30-day HF hospital readmission rate was markedly increased among PLHIV with SA who were not using CPAP (100% vs 40%, P < .001; Figure 3). Finally, the 30-day HF hospital readmission rate was compared between those using CPAP with a duration of use of ≤4 hours per day compared to >4 hours per day. The longer duration of CPAP use among PLHIV with HFrEF was associated with a lower 30-day HF hospital readmission rate (27% vs 63%, P = .03; Figure 4).
Figure 2.
Kaplan-Meier survival curves comparing the 30-day readmission (A), cardiovascular mortality (B), and all-cause mortality (C) among people living with human immunodeficiency virus admitted with heart failure with reduced ejection fraction who have sleep apnea and an apnea-hypopnea index <24 vs ≥24. Abbreviations: AHI, apnea–hypopnea index; HF, heart failure.
Figure 3.
Kaplan-Meier survival curves comparing 30-day readmission (A), cardiovascular mortality (B), and all-cause mortality (C) among people living with human immunodeficiency virus admitted with heart failure with reduced ejection fraction who have sleep apnea and use continuous positive airway pressure (CPAP) vs do not use CPAP. Abbreviations: CPAP, continuous positive airway pressure; HF, heart failure.
Figure 4.
Kaplan-Meier survival curves comparing 30-day readmission (A), cardiovascular mortality (B), and all-cause mortality (C) among people living with human immunodeficiency virus admitted with heart failure with reduced ejection fraction who have sleep apnea on continuous positive airway pressure with daily duration of use >4 hours vs ≤4hours. Abbreviations: CPAP, continuous positive airway pressure; HF, heart failure.
Among PLHIV, CV and all-cause mortality rates were increased among PLHIV with HFrEF with an elevated AHI (CV mortality, 56% vs 13%, P = .002; all-cause mortality, 72% vs 22%, P < .001; Figure 2), among those who were not using CPAP (CV mortality, 50 vs 24%; all-cause mortality, 70% vs 39%; Figure 3), and among those with a shorter median duration of CPAP use (CV mortality, 42% vs 20%; all-cause mortality, 58% vs 33%; Figure 4).
DISCUSSION
Here, we aimed to assess how SA influences outcomes among PLHIV with HFrEF. While the presence of SA among PLHIV with HFrEF was similar to that in uninfected controls, there were notable differences.
When patients with HFrEF and SA with and without HIV were compared, those individuals with HIV were more likely to have OSA rather than CSA and were more likely to use CPAP for a longer duration. Among patients with HFrEF and SA, those individuals with HIV also had a lower BMI as well as a lower LVEF and a higher PASP. When PLHIV with SA were divided according to the type of SA, central vs obstructive, those with CSA had a lower BMI, a lower LVEF, a higher PASP, and an increased AHI compared to those with OSA. Additionally, PLHIV with HFrEF with CSA had a lower mean CD4 count and lower rates of viral suppression. Significant predictors of 30-day HF hospital readmission among PLHIV with HFrEF included not only traditional HF risk factors (CAD, PASP), nontraditional HF risk factors (cocaine use), and HIV-specific parameters (low CD4 count or high VL) but also factors related to SA. Indeed, SA severity was positively associated with 30-day HF hospital readmission rate. Each 1-hour increase in CPAP use was associated with a 14% decreased risk of 30-day HF hospital readmission. Similar findings of an effect of SA severity (AHI) and CPAP use and duration of CPAP were noted for CV and all-cause mortality.
The prevalence of SA was similar between HFrEF patients with SA noted in 28% of PLHIV with HFrEF compared to 26% of uninfected controls. There are no prior data detailing the prevalence of SA among PLHIV with HFrEF. However, there are data on the presence of SA among uninfected controls with HFrEF. These data show a range in prevalence of SA among patients with HF from 4% to 70% depending on whether routine sleep testing was performed [18–22]. However, the type of SA differed, and studies note a difference in the type of SA according to the presence or absence of HF [23]. Specifically, there are 3 types of SA: OSA, CSA, and mixed [23, 24]. Among patients without HF, OSA is the most common, and, among patients with HF, CSA is rare [8, 25]. However, studies among broad groups of individuals with HF show markedly increased rates of CSA [26]. For example, in a prospective study of 700 patients with HFrEF, Oldenburg et al noted CSA in 53% of HFrEF with SA [18]. We had similar findings among our HIV-uninfected control group with SA, with CSA noted in 45%. However, among PLHIV with HFrEF and SA, rates of CSA were lower and rates of OSA were higher in comparison to uninfected controls. CSA usually occurs because of transient reduction by the pontomedullary pacemaker while the breathing rhythm is being generated I response to the changes in the partial pressures of carbon dioxide that can fall below the apnea threshold. In contrast, OSA is defined by upper airway occlusion yielding reduced airflow as a result of upper airway edema, tongue falling posteriorly, and, most importantly, obesity [8]. In PLHIV, rates of OSA compared to CSA may be increased due to several factors. Primarily, ART may lead to lipodystrophy and fat deposition in areas including the neck, causing obstruction, as well as in the thorax and abdomen, decreasing end-expiratory lung volume [27–29].
This finding has prognostic importance. Among patients with HF without known HIV, CSA has been shown to be an independent risk marker for poor prognosis and death [30–32], and CSA in HF is associated with a worse outcome than OSA. The finding of a difference in the type of SA may also have therapeutic relevance. Specifically, despite concerns about compliance, we found that almost 80% of our HIV population with HF were using CPAP and that the median duration of nightly CPAP use was greater among PLHIV. In a multivariable model, both CPAP use and duration of CPAP use were independent predictors of HF rehospitalization. Findings of a beneficial effect of CPAP uses on broad cardiovascular outcomes have previously been demonstrated. For example, in a study involving 720 patients [33], the use of CPAP was associated with a lower risk of atrial fibrillation, with a greater benefit seen in those with longer duration of CPAP use. Similarly, in a study of 725 patients with SA, the use of CPAP for less than 4 hours per night was associated with an increase in CV events [34]. The effect of CPAP on outcomes among individuals with HF appears less consistent, but the variability is likely related to the type of HF. For example, in a prospective study involving 60 patients with HFrEF, Blanco et al showed that CPAP treatment improved the LVEF in patients with OSA by 9%; however, no significant improvement was noted in individuals with CSA [24]. In comparison, in adaptive servo-ventilation therapy to treat central sleep apnea in systolic HF patients, adaptive servo ventilation therapy among a HFrEF cohort with CSA was associated with an increase in overall mortality [35, 36]. When data from PLHIV with HFrEF with CSA and OSA were compared, PLHIV with HFrEF and SA with CSA had a higher AHI and prevalence of hypertension, lower BMI, lower LVEF, higher PASP, lower CD4 count, and higher VL.
The limitations to our study include its retrospective nature based on a single US urban tertiary care center. Therefore, these data may not be generalizable, and prospective studies of outcomes associated with HFrEF hospitalizations among large, diverse populations of individuals with HIV with SA are needed.
In conclusion, we are the first to report on the presence, associations, and prognostic implications of SA among PLHIV with HFrEF. Compared to uninfected controls with HFrEF and SA, PLHIV are more likely to have OSA rather than CSA. Apnea severity is associated with adverse HF outcomes, whereas CPAP use and increased duration of CPAP use conferred protection. Moreover, cocaine use, low CD4 count, and high VL were associated with the increased rate of 30-day HF readmission among PLHIV. Additional research on strategies that may improve outcomes among PLHIV hospitalized with HFrEF along with other outcomes that influence comorbidities is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. R. M. A. was supported by the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI; 5T32HL076136). M. V. Z. was supported in part by the NIH/NHLBI (1R01HL137562 - 01A1). V. T. was supported in part by the NIH/NHLBI (1R01HL132786 - 01A1). T. G. N. was supported in part by the Kohlberg Foundation, an American Heart Association Fellow to Faculty award (12FTF12060588), the NIH/NHLBI (1R01HL130539-01A1; 1RO1HL137562-01A1; K24HL113128-06), and the NIH/Harvard Center for AIDS Research (P30 AI060354).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. WHO data and Statistics. Available at: http://www.who.int/hiv/data/en/. Accessed 24 September 2017
- 2. Butt AA, Chang CC, Kuller L, et al. . Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011; 171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CH, Skanderson M, et al. . Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging cohort study. JAMA Cardiol 2017; 2:536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lohse N, Obel N. Update of survival for persons with HIV infection in Denmark. Ann Intern Med 2016; 165:749–50. [DOI] [PubMed] [Google Scholar]
- 5. Paisible AL, Chang CC, So-Armah KA, et al. . HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr 2015; 68:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janjua SA, Triant VA, Addison D, et al. . HIV infection and heart failure outcomes in women. J Am Coll Cardiol 2017; 69:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javaheri S, Barbe F, Campos-Rodriguez F, et al. . Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malhotra A, Patil S, Sands S, Ayas N. Central sleep apnoea in congestive heart failure. Lancet Respir Med 2015; 3:507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014; 383:736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goswami U, Baker JV, Wang Q, Khalil W, Kunisaki KM. Sleep apnea symptoms as a predictor of fatigue in an urban HIV clinic. AIDS Patient Care STDS 2015; 29:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patil SP, Brown TT, Jacobson LP, et al. . Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and -uninfected men. PLoS One 2014; 9:e99258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicks KA, Tcheng JE, Bozkurt B, et al. . 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015; 66:403–69. [DOI] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of European Society of Cardiology (ESC). Developed with the special contribution of Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18:891–975. [DOI] [PubMed] [Google Scholar]
- 15. Mehran R, Rao SV, Bhatt DL, et al. . Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–47. [DOI] [PubMed] [Google Scholar]
- 16. Addison D, Farhad H, Shah RV, et al. . Effect of late gadolinium enhancement on the recovery of left ventricular systolic function after pulmonary vein isolation. J Am Heart Assoc 2016; 5:e003570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neilan TG, Farhad H, Mayrhofer T, et al. . Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015; 8:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007; 9:251–7. [DOI] [PubMed] [Google Scholar]
- 19. Schulz R, Blau A, Börgel J, et al. ; Working Group Kreislauf und Schlaf of the German Sleep Society Sleep apnoea in heart failure. Eur Respir J 2007; 29:1201–5. [DOI] [PubMed] [Google Scholar]
- 20. Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009; 11:602–8. [DOI] [PubMed] [Google Scholar]
- 21. Sekizuka H, Osada N, Miyake F. Sleep disordered breathing in heart failure patients with reduced versus preserved ejection fraction. Heart Lung Circ 2013; 22:104–9. [DOI] [PubMed] [Google Scholar]
- 22. Padeletti M, Green P, Mooney AM, Basner RC, Mancini DM. Sleep disordered breathing in patients with acutely decompensated heart failure. Sleep Med 2009; 10:353–60. [DOI] [PubMed] [Google Scholar]
- 23. Dharia SM, Brown LK. Epidemiology of sleep-disordered breathing and heart failure: what drives what. Curr Heart Fail Rep 2017; 14:351–64. [DOI] [PubMed] [Google Scholar]
- 24. Blanco Pérez JJ, Zamarrón Sanz C, Almazán Ortega R, García García M, López Castro J, Tumbeiro Novoa M. Sleep apnea syndrome in heart failure. Effect of continuous positive airway pressure. An Med Interna 2008; 25:15–9. [DOI] [PubMed] [Google Scholar]
- 25. Cowie MR, Gallagher AM. Sleep disordered breathing and heart failure: what does the future hold?JACC Heart Fail 2017; 5:715–23. [DOI] [PubMed] [Google Scholar]
- 26. Javaheri S, Parker TJ, Liming JD, et al. . Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998; 97:2154–9. [DOI] [PubMed] [Google Scholar]
- 27. Taibi DM. Sleep disturbances in persons living with HIV. J Assoc Nurses AIDS Care 2013; 24:S72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dorey-Stein Z, Amorosa VK, Kostman JR, Lo Re V 3rd, Shannon RP. Severe weight gain, lipodystrophy, dyslipidemia, and obstructive sleep apnea in a human immunodeficiency virus-infected patient following highly active antiretroviral therapy. J Cardiometab Syndr 2008; 3:111–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Epstein LJ, Strollo PJ Jr, Donegan RB, Delmar J, Hendrix C, Westbrook PR. Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep 1995; 18:368–76. [DOI] [PubMed] [Google Scholar]
- 30. Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol 2007; 49:2028–34. [DOI] [PubMed] [Google Scholar]
- 31. Yumino D, Wang H, Floras JS, et al. . Relationship between sleep apnoea and mortality in patients with ischaemic heart failure. Heart 2009; 95:819–24. [DOI] [PubMed] [Google Scholar]
- 32. Bitter T, Westerheide N, Prinz C, et al. . Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011; 32:61–74. [DOI] [PubMed] [Google Scholar]
- 33. Neilan TG, Farhad H, Dodson JA, et al. . Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013; 2:e000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. ; Spanish Sleep and Breathing Network Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 307:2161–8. [DOI] [PubMed] [Google Scholar]
- 35. Woehrle H, Cowie MR, Eulenburg C, et al. . Adaptive servo ventilation for central sleep apnoea in heart failure: SERVE-HF on-treatment analysis. Eur Respir J 2017; 50:1601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magalang UJ, Pack AI. Heart failure and sleep-disordered breathing–the plot thickens. N Engl J Med 2015; 373:1166–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.