We found no difference in occurrence of chronic Q-fever between patients with or without a newly detected valvulopathy at time of acute Q-fever diagnosis. Thus, universal screening is not justified and would lead to unnecessary and undesirable long-term antibiotic use.
Keywords: Q-fever, Coxiella burnetii, echocardiography, heart valve disease, endocarditis
Abstract
Background
Echocardiographic screening of acute Q-fever patients and antibiotic prophylaxis for patients with cardiac valvulopathy is considered an important approach to prevent chronic Q-fever-related endocarditis. During a large Q-fever epidemic in the Netherlands, routine screening echocardiography was discontinued, raising controversy in the international literature. We followed a cohort of acute Q-fever patients to estimate the risk for developing chronic Q-fever, and we evaluated the impact of screening in patients who were not yet known to have a valvulopathy.
Methods
The study population consisted of patients diagnosed with acute Q-fever in 2007 and 2008. We retrospectively reviewed all screening echocardiographs and checked for development of chronic Q-fever 8 years after the acute episode. Risks of developing chronic Q-fever in relation to the presence or absence of valvulopathy were analyzed with logistic regression.
Results
The cohort included 509 patients, of whom 306 received echocardiographic screening. There was no significant difference (P-value = .22) in occurrence of chronic Q-fever between patients with a newly detected valvulopathy (2/84, 2.4%) and those with no valvulopathy (12/202, 5.9%). Two patients with a newly detected valvulopathy, who did not receive antibiotic prophylaxis, developed chronic Q-fever at a later stage.
Conclusions
We found no difference in outcome between patients with and without a valvulopathy newly detected by echocardiographic screening. In retrospect, the 2 above-mentioned patients could have benefitted from antibiotic prophylaxis, but its omission must be weighed against the unnecessary large-scale and long-term use of antibiotics that would have resulted from universal echocardiographic screening.
Q-fever is a zoonosis caused by the bacterium Coxiella burnetii [1]. Acute Q-fever mainly presents as febrile illness, atypical pneumonia, or hepatitis. However, almost 60% of acute C. burnetii infections remain asymptomatic, and 1–5% of acute Q-fever infections will develop into chronic Q-fever [1, 2].
In the Netherlands, vascular infection is the most common clinical presentation of chronic Q-fever, in contrast to other countries, such as France, where endocarditis predominates [3, 4]. Clinical manifestations leading to the diagnosis of endocarditis are not specific, posing a challenge to the clinician [1]. Early detection and treatment of endocarditis and other presentations of chronic infection may prevent prolonged morbidity, complications, and fatal outcomes [2, 5, 6]. Main risk factors for developing endocarditis are older age and underlying cardiac valvulopathy [7, 8]. In symptomatic acute Q-fever patients with valvulopathy, the estimated risk of developing endocarditis is 39% [8]. Progression to endocarditis has been reported in patients with undiagnosed and clinically silent valvulopathies [9]. Physicians are therefore encouraged to detect at-risk patients by echocardiographic screening of all acute Q-fever patients for presence of valvulopathy [8, 9]. In France, indeed, echocardiography is part of the standard work-up for all acute Q-fever patients.
Initially, when the Netherlands faced its first outbreak of Q-fever in 2007, referral of all acute Q-fever patients for echocardiography was recommended to all treating physicians. However, when the number of acute Q-fever patients sharply increased in 2008, cardiologists became increasingly reluctant to continue routine screening, as it drained a lot of resources and yielded many valvulopathies classified as “minor,” with no clinical significance. Additionally, none of the patients with mostly minor valvulopathies were diagnosed with chronic Q-fever [10, 11]. Thus, screening was discontinued, and French studies estimate that 100 Dutch cases of endocarditis may have been missed and that half of them could have been prevented with antibiotic prophylaxis [12].
In the present study, we followed a large cohort of acute Q-fever patients over 8 years to estimate the risk for developing chronic Q-fever [13]. Our aim was to evaluate the impact of echocardiographic screening in patients who were not yet known to have a valvulopathy. Our results may help to improve screening policies in future outbreaks.
METHODS
Patient Enrollment
We invited patients who were diagnosed with Q-fever in 2007 and 2008 and who had participated in the Q-HORT study. This was a 4-year follow-up study of patients diagnosed with acute Q-fever in 2007–2009 that aimed to detect chronic Q-fever cases [13]. All patients enrolled in the Q-HORT study were diagnosed with Q-fever at the Laboratory of Medical Microbiology of the Jeroen Bosch Hospital, the regional diagnostic facility serving Bernhoven Hospital in Uden, Jeroen Bosch Hospital in ‘s-Hertogenbosch, and most general practitioners in the catchment areas of these 2 hospitals. The laboratory is located in the epicenter of the 2007–2010 outbreak, where approximately 80% of all reported Q-fever cases in 2007 and 2008 were diagnosed [14]. The present study included Q-HORT patients diagnosed in 2007 and 2008, because only during this period were clinicians instructed to refer acute Q-fever patients to a cardiologist for a single screening by echocardiography.
During the outbreak, the standard work-up after diagnosis of acute Q-fever consisted of serological testing at 3, 6, and 12 months. After 4 years, an additional test was performed in the context of Q-HORT. Before Q-HORT enrolment, 4 years after their acute Q-fever diagnosis, potential participants received an informed consent form. Next, the persons who participated in the Q-HORT study filled in a questionnaire. The questionnaire gathered data on general demographics and risk factors for chronic Q-fever. Patients who were diagnosed with chronic Q-fever based on the first blood sample submitted to the laboratory were excluded, as it was too late to follow their course of Q-fever development. Patients with an immunoglobulin G (IgG) phase II titer ≤1:32 in all follow-up samples were likewise excluded, as they did not meet the case definition of a laboratory-confirmed acute Q-fever case.
Acute Q-Fever Diagnosis
One of 3 laboratory criteria had to be met for the diagnosis of acute Q-fever [13]:
(1) Both immunoglobulin M (IgM) and IgG phase II antibody titers ≥1:32 at diagnosis as determined by immunofluorescence assay (IFA; Focus Diagnostics, Inc., Cypress, CA, USA), with IgG phase II ≥1:64 during follow-up;
(2) IgM phase II positive and IFA IgG phase II ≥1:32 at diagnosis by enzyme-linked immunosorbent assay (Virion\Serion, Würzburg, Germany), with IgG phase II ≥1:64 during follow-up;
(3) a positive polymerase chain reaction (PCR; in-house assay [15]) result preceding seroconversion in IFA, with IgG phase II ≥1:64 during follow-up.
Echocardiography
During the Q-fever outbreak in 2007, public health authorities informed treating physicians as to the need for echocardiographic screening after every notification of acute Q-fever [8, 9].
Patients were screened with transthoracic echocardiography (TTE), and transoesophageal echocardiography was performed when the TTE results were inconclusive. The cardiologists of both involved hospitals interpreted all echocardiographs according to the European Society of Cardiology guidelines. The aortic, mitral, pulmonic, and tricuspid valves were examined for regurgitation and stenosis. Per diagnosis (e.g. aortic regurgitation, aortic stenosis, mitral regurgitation), patients were subdivided into groups with no, mild, moderate, or severe valvulopathy [16]. For the present study, we collected echocardiographic results by reviewing medical records, excluding patients with an echocardiograph taken more than 1 year after acute Q-fever diagnosis.
Definitions of Baseline Characteristics and Chronic Q-Fever
For baseline characteristics, we used the Q-HORT questionnaire data, which were collected 4 years after the acute Q-fever diagnosis. For the patients who died between this diagnosis and the Q-HORT invitation, and for whom therefore no questionnaire was available, we obtained baseline characteristics from medical files at both hospitals. Additionally, serological results at time of diagnosis, at 3-, 6-, and 12-month follow-up, and at 4 years after diagnosis were obtained from the Q-HORT database. In the Netherlands, chronic Q-fever is not a notifiable disease. However, since 2007, all chronic Q-fever cases are included in a national chronic Q-fever database, which is maintained by the University Medical Centre in Utrecht. In 2016, approximately 8 years after the diagnosis, we checked the national chronic Q-fever database to see whether more patients had been diagnosed with chronic Q-fever. For the patients who underwent echocardiography at time of diagnosis, we checked whether they were known to have valvulopathy before the Q-fever diagnosis. Next, we searched the medical records for information about the chronic Q-fever cases, such as additional risk factors and antibiotic treatment. Based on all available information, cases were subdivided into no, possible, probable, or proven chronic Q-fever, as suggested by the Dutch Q-fever Consensus Group. See supplementary Table 1 [17]. We categorized patients into the highest classification they received during follow-up.
Statistical Analysis
In order to prevent survivor bias, we included patients who died between the diagnosis of acute Q-fever and the Q-HORT invitation 4 years later. We performed the χ2 test, Fisher exact test, and the unpaired T-test to compare the characteristics of patients with and without echocardiographic screening. We performed univariable and multivariable logistic regression analysis, corrected for sex and age at time of diagnosis, to investigate whether the development of chronic Q-fever differed significantly between patients with and without valvulopathy. In this analysis, we excluded patients with known valvulopathy before Q-fever diagnosis, as echocardiographic screening was not intended for that patient group. Lastly, we performed univariable and multivariable analyses restricted to probable and proven chronic Q-fever cases. A P-value of <.05 was considered statistically significant. SAS version 9.4 was used for the analyses (SAS Institute Inc., USA).
Ethical Permission
The Medical Ethical Committee Brabant (METC Brabant) approved the Q-HORT study. The Internal Review Board of Jeroen Bosch Hospital approved the present study. The Q-HORT informed consent form included permission to access medical data. We enrolled only patients who gave permission to review all available echocardiographic, clinical, and laboratory data. We obtained information about deceased patients in accordance with the Medical Treatment Contracts Act (article 458).
RESULTS
In 2011 and 2012, 519 acute Q-fever patients, who were diagnosed in 2007 or 2008, participated in the Q-HORT study. We excluded 10 persons from analyses, as 1 already had chronic Q-fever at time of the diagnosis; 3 had an IgG phase I and/or IgG phase II titer ≤1:32 in all follow-up samples; 3 underwent echocardiography >1 year after diagnosis, and for 3 the echocardiography report could not be traced.
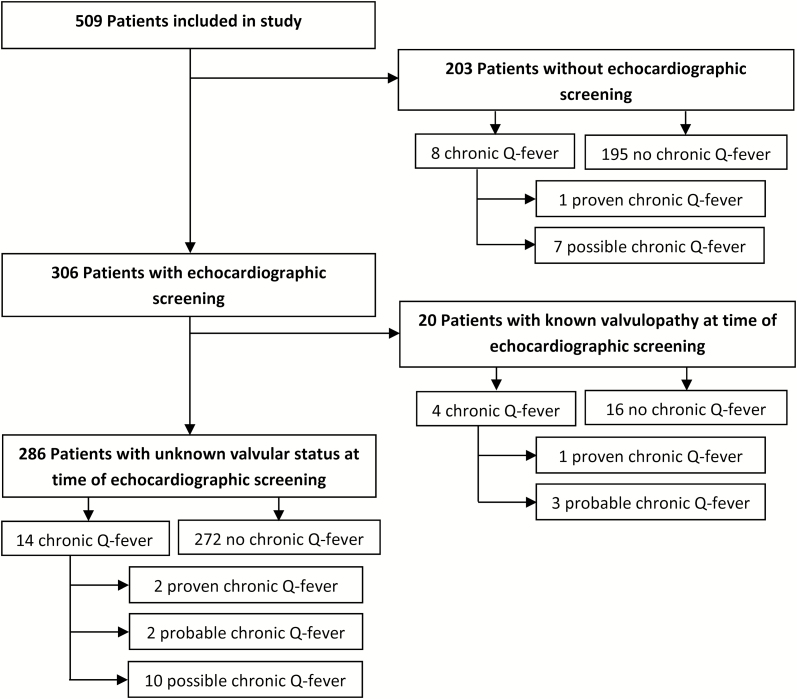
Table 1 shows baseline characteristics for the remaining 509 patients, 4 years after their acute Q-fever diagnosis. Of the total, 306 (60.1%) patients underwent echocardiographic screening at time of diagnosis. Of these 306, 20 were already diagnosed with 1 or more valvulopathies before the acute Q-fever episode (Figure 1). In those not screened, 8 of the 203 (3.9%) were diagnosed with chronic Q-fever, 1 of whom had proven chronic Q-fever and 7 had possible chronic Q-fever. In screened patients with unknown valvulopathy status at time of acute Q-fever diagnosis, 14 of 286 (4.9%) patients were diagnosed with chronic Q-fever during 4-year follow-up. Of these, 10 had no valvulopathy or other risk factor at time of screening and were classified as possible chronic Q-fever based on serological findings. Of the other 4 patients, 2 were classified as probable chronic Q-fever, and 2 as proven chronic Q-fever. Additionally, among 20 screened patients with known valvulopathy at time of acute Q-fever diagnoses, 4 (20.0%) were diagnosed with chronic Q-fever, of whom 1 had proven and 3 had probable chronic Q-fever. Of the 5 probable chronic Q-fever cases in this cohort of 509 acute Q-fever patients, 1 was immunocompromised and the other 4 had a valvulopathy. Approximately 8 years after the diagnosis, or 4 years after Q-HORT participation, no additional patients were diagnosed with chronic Q-fever. One person in the entire cohort received antibiotic prophylaxis. This person was known to have a valvulopathy (mild stenosis of aortic valve after valve replacement with a mechanical prosthesis) at the time of acute Q-fever diagnosis and did not develop chronic Q-fever. None of the patients with a newly detected valvulopathy was treated prophylactically with antibiotics.
Table 1.
Baseline Characteristics of Acute Q-Fever Patients, 4 Years After Their Diagnosis in 2007 or 2008
| Characteristic | Patients Without Echocardiography (N = 203) Number/N (%) | Patients With Echocardiography (N = 306)a Number/N (%) | P-Valueb |
|---|---|---|---|
| Sex, male | 117/203 (57.6) | 178/306 (58.2) | .905 |
| Age at time acute Q-fever diagnosis, years (± SD) | 49.9 ± 13.1c | 53.0 ± 12.3c | .008d |
| Aortic aneurysm | 3/203 (1.5) | 2/305 (0.7) | .393e |
| Vascular prosthesis | 4/203 (2.0) | 5/305 (1.6) | 1.000e |
| Heart valve prosthesis | 1/203 (0.5) | 5/305 (1.6) | .410e |
| Myocardial infarction | 13/203 (6.4) | 14/305 (4.6) | .372 |
| Coronary artery proceduref | 15/203 (7.4) | 22/305 (7.2) | .940 |
| Peripheral arterial procedureg | 8/203 (3.9) | 1/305 (0.3) | .004e |
| Pacemaker | 1/203 (0.5) | 9/305 (3.0) | .057e |
| Rheumatoid arthritis | 15/203 (7.4) | 16/305 (5.3) | .323 |
| Crohn’s disease/ulcerative colitis | 1/203 (0.5) | 3/305 (1.0) | 1.000e |
| Malignancy in last 5 years | 19/203 (9.4) | 23/305 (7.5) | .466 |
| Chronic renal disease | 1/203 (0.5) | 4/305 (1.3) | .653e |
| Asthma | 7/203 (3.5) | 11/305 (3.6) | .925 |
| COPD | 9/203 (4.4) | 12/305 (3.9) | .782 |
| Diabetes | 18/203 (8.9) | 24/305 (7.9) | .689 |
| Organ transplantation | 0/203 (0.0) | 1/304 (0.3) | 1.000e |
| Pregnancy in last 5 yearsh | 4/82 (4.9) | 1/124 (0.8) | .083e |
Abbreviations: COPD, chronic obstructive pulmonary disease; N, total number of that group; SD, standard deviation.
a Data are missing for 1 patient, as no questionnaire was completed.
b P-values are calculated by χ2 test unless otherwise indicated.
c Age is shown as mean ± SD, as age was normally distributed.
d P-values are calculated by unpaired T-test.
e P-values are calculated by Fisher exact test.
f Coronary artery procedure includes bypass surgery, percutaneous coronary intervention, or a stent.
g Peripheral arterial procedure includes bypass surgery, angioplasty, or a stent.
h Calculated for women only.
Figure 1.
Flowchart of included patients.
Of the 4 proven chronic Q-fever cases in the cohort of 509 acute Q-fever patients, 1 had an endocarditis, and 3 had an infected aneurysm (Table 2).
Table 2.
Details of Proven Chronic Q-Fever Patients
| Sex | Age | Known Valvulopathya | Valvulopathyb | Diagnosis Chronic Q-Fever (Time After Acute Q-Fever)c | IgG Phase Id | Clinical Presentation Chronic Q-Fever | PCR Result | Antibiotic Treatmente | Antibiotic Prophylaxis |
|---|---|---|---|---|---|---|---|---|---|
| Mh | 70–79 | No | No | 19 months | 1:2048 | Infected aneurysm | + | Yes | No |
| F | 70–79 | No | Yes; mild AoS | 2 months | 1:2048 | Endocarditis | - | Yesf | No |
| M | 60–69 | Yes | Yes; moderate MR, mild TR | 3 months | 1:4096 | Infected aneurysm | + | Yes | No |
| Mg,h | 50–59 | Unknown (no screening echo made) | Unknown (no screening echo made) | 3 months | 1:4096 | Infected aneurysm | + | Yes | No |
Abbreviations: AoS, aortic stenosis; echo, echocardiography; IgG, immunoglobulin G; MR, mitral regurgitation; PCR, polymerase chain reaction; TR, tricuspid regurgitation.
a Already diagnosed with valvulopathy before diagnosis of acute Q-fever.
b Valvulopathy detected at time of screening.
c Time between acute Q-fever diagnosis and when IgG phase I titer was for the first time ≥ 1:1024, or other clinical sign of chronic infection.
d Highest IgG phase I during follow-up after diagnosis of acute Q-fever.
e Consisted of doxycyclin and hydroxychloroquine.
f Treated with claritromycin because of an intolerance to doxycyclin, hydroxychloroquine, moxifloxacin, and ciproxin.
g Patient also known to have Klinefelter syndrome.
h Died within four years after acute Q-fever diagnosis.
In Table 3, we present the results of echocardiographic screening after acute Q-fever diagnosis, combined for patients with known and unknown valvulopathy at the time of the diagnosis. The prevalence of valvulopathy in this group was 33.7% (103/306). Of the 103 patients with valvulopathy, 85 (82.5%) were classified with 1 or more mild valvulopathies, 16 (15.5%) with 1 or more moderate valvulopathies, and 2 (2.0%) with a severe valvulopathy. Chronic Q-fever developed at a later stage in 3 of the 85 patients (3.5%) with mild valvulopathy, 2 of the 16 (12.5%) with moderate valvulopathy, and 1 of the 2 patients (50%) with severe valvulopathy.
Table 3.
Valvulopathy per Category of Chronic Q-Fever Infection, Including Patients With Known Valvulopathy at Time of Echocardiographic Screening, in Acute Q-Fever Patients Diagnosed in 2007 or 2008a
| Chronic Q-Fever, Number | Total Number (%) | ||||
|---|---|---|---|---|---|
| No | Possible | Probable | Proven | ||
| Aortic regurgitation | |||||
| No | 272 | 10 | 5 | 3 | 290 (94.8) |
| Mild | 15 | 0 | 0 | 0 | 15 (4.9) |
| Moderate | 1 | 0 | 0 | 0 | 1 (0.3) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Aortic stenosis | |||||
| No | 281 | 10 | 4 | 2 | 297 (97.1) |
| Mild | 6 | 0 | 0 | 1 | 7 (2.3) |
| Moderate | 1 | 0 | 0 | 0 | 1 (0.3) |
| Severe | 0 | 0 | 1 | 0 | 1 (0.3) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Mitral regurgitation | |||||
| No | 232 | 10 | 1 | 2 | 245 (80.1) |
| Mild | 46 | 0 | 3 | 0 | 49 (16.0) |
| Moderate | 9 | 0 | 1 | 1 | 11 (3.6) |
| Severe | 1 | 0 | 0 | 0 | 1 (0.3) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Mitral stenosis | |||||
| No | 286 | 10 | 5 | 3 | 304 (99.4) |
| Mild | 1 | 0 | 0 | 0 | 1 (0.3) |
| Moderate | 1 | 0 | 0 | 0 | 1 (0.3) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Tricuspid regurgitation | |||||
| No | 234 | 10 | 4 | 2 | 250 (81.7) |
| Mild | 50 | 0 | 1 | 1 | 52 (17.0) |
| Moderate | 4 | 0 | 0 | 0 | 4 (1.3) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Tricuspid stenosis | |||||
| No | 287 | 10 | 5 | 3 | 305 (99.7) |
| Mild | 1 | 0 | 0 | 0 | 1 (0.3) |
| Moderate | 0 | 0 | 0 | 0 | 0 (0.0) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Pulmonic regurgitationb | |||||
| No | 286 | 10 | 5 | 3 | 304 (99.4) |
| Mild | 2 | 0 | 0 | 0 | 2 (0.6) |
| Moderate | 0 | 0 | 0 | 0 | 0 (0.0) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
| Pulmonic stenosisb | |||||
| No | 288 | 10 | 5 | 3 | 306 (100.0) |
| Mild | 0 | 0 | 0 | 0 | 0 (0.0) |
| Moderate | 0 | 0 | 0 | 0 | 0 (0.0) |
| Severe | 0 | 0 | 0 | 0 | 0 (0.0) |
| Total | 288 | 10 | 5 | 3 | 306 |
a Multiple valvulopathies are possible in 1 patient.
b The pulmonic valve is difficult to visualize and not described in every report.
Of the 84 screened patients with newly detected valvulopathy at time of acute Q-fever diagnosis, two patients developed chronic Q-fever (2.4%) during follow-up (Table 4). In the group of 202 patients who had no newly detected valvulopathy at time of screening, 12 were diagnosed with chronic Q-fever (5.9%). In univariable analysis, valvulopathy was not significantly associated with chronic Q-fever (taking all levels together) (odds ratio [OR] = 0.39, 95% confidence interval [CI]: 0.09–1.76, P-value = .22). In multivariable logistic regression analysis corrected for differences in age and sex, valvulopathy was again no risk factor for developing chronic Q-fever (OR = 0.26, 95% CI: 0.06–1.24, P-value = .09). Additionally, we performed univariable analysis for only the probable and the proven cases, as the possible cases are less likely to be true chronic infections. Again, no statistically significant difference was found between the patients with and without newly detected valvulopathy (OR = 2.32, 95% CI: 0.32–16.73, P-value = .40). We were not able to perform multivariable analysis for this subgroup, due to the low number of chronic cases.
Table 4.
Number of Chronic Q-Fever Cases, by Presence of Valvulopathy During Echocardiographic Screening at the Time of Acute Q-Fever Diagnosis in 2007 or 2008
| Number Chronic Q-Fever | Total | ||
|---|---|---|---|
| No | Yes | ||
| Valvulopathy | |||
| No | 190 | 12 | 202 |
| Yes | 82 | 2 | 84 |
| Total | 272 | 14 | 286 |
DISCUSSION
We found no statistically significant difference in development of chronic Q-fever between acute Q-fever patients with and without valvulopathy detected with screening echocardiography. However, 2 patients with a newly detected valvulopathy did not receive antibiotic prophylaxis and were diagnosed with chronic Q-fever later on.
Early in the large Q-fever epidemic in the Netherlands, public health authorities instructed clinicians to perform echocardiographic screening in all reported acute Q-fever patients. However, the responsibility of referring patients for echocardiography lay with the treating physician, and cardiologists were sometimes reluctant to perform echocardiography without clear clinical indication. Therefore, even at that stage, only slightly more than half of all patients received echocardiographic screening. Furthermore, as no professional guideline existed on how to prevent chronic infection in patients with newly detected valvulopathy, no such patients were prophylactically treated with antibiotics. Later in the epidemic, a small cohort study found no chronic Q-fever in patients with newly detected valvulopathy, and echocardiographic screening was not further promoted [10, 11].
The present study seems to confirm that vascular infection is more common than endocarditis as clinical manifestation of chronic infection [3]. Therefore, screening for aortic aneurysms in patients who present with a primary infection, could be considered as Eldin et al. has suggested [18]. However, in France, where many chronic Q-fever research is performed, endocarditis seems to predominate [4], due possibly to differences in the virulence of circulating C. burnetii strains [1, 19] and different case definitions for chronic Q-fever [20]. Additionally, reports from a national reference centre are prone to selection bias, whereas we studied a non-selected group of acute Q-fever patients, eliminating sampling and selection bias. Moreover, in our study the time of acute illness was established, and how the patients were diagnosed was well described.
The difference in the incidence of endocarditis may further be explained by a lack of specificity in describing valvular defect severity in retrospective reports [2, 8, 21]. In one study, the risk for endocarditis in patients with valvulopathy was estimated to be 39%, but many of these patients had a prosthetic valve [8]. In our study, a much lower percentage had prosthetic valves, and mainly minor valvulopathies were diagnosed. Minor, clinically insignificant valvulopathy has a high prevalence in any unselected population [22]. Finally, the vascular infections diagnosed in the Netherlands may be an embolic consequence of clinically silent endocarditis, as Million et al. suggested [4].
Our results have reduced the likelihood of 2 other possible explanations for the difference between the Dutch and French literature. First, given the 8-year follow-up of this study, a possibly prolonged incubation period for endocarditis to become manifest is unlikely to explain the difference. Second, we performed a systematic screening and still found more vascular infections than endocarditis. It seems therefore unlikely that a lack of systematic screening led to underdiagnoses of endocarditis in the Netherlands. However, the numbers are low because the number of chronic Q-fever cases in this study was smaller than anticipated.
The primary strength of this study is the large sample size. The Q-fever epidemic in the Netherlands provides the largest group of acute Q-fever patients ever reported, and the largest one with echocardiographic results. Second, we included a prolonged follow-up with serological results at 3, 6, 12 months, and 4 years. After approximately 8 years, we checked to see if additional acute Q-fever patients had been diagnosed with chronic Q-fever. Finally, we included all deceased patients to avoid survival bias.
Among study limitations are the potential that the risk of proven chronic Q-fever was underestimated, as long-term antibiotic treatment was initiated in 4 patients (1 possible and 3 probable cases). If left untreated, proven chronic Q-fever might have developed. However, the risk of chronic Q-fever may have been overestimated, as our patients were categorized into the highest classification they received during the 4-year follow-up. For example, 12 patients were classified as possible chronic Q-fever based on an IgG phase I titer ≥1:1024, without a risk factor for chronic Q-fever. During the follow-up, the titers showed a spontaneous decline in phase I IgG titers (data not shown). Arguably, these patients should not be classified as having a chronic infection. Another limitation is that although the screened and not-screened acute Q-fever patients were largely comparable with respect to baseline characteristics, we had no information on the decision process of doctors and patients with respect to screening. Therefore, we cannot rule out some degree of selection bias. Finally, almost 60% of acute Q-fever patients are known to remain asymptomatic. These as-yet-unidentified acute Q-fever patients could of course not be included in our study despite being at risk for progression to chronic infection [23].
To decide whether a new screening approach should be implemented, often the Wilson and Jungner criteria are used [24]. Some criteria support screening. First, chronic Q-fever is a serious health problem that may lead to death. Next, Dutch hospitals have the health infrastructure to implement this possible screening. Last, an echocardiography is not invasive and is therefore acceptable as screening test. However, some criteria are not supportive. First, if screening is implemented, many acute Q-fever patients will receive antibiotic prophylaxis even if chronic Q-fever is unlikely to develop without this prophylaxis. Second, as in an earlier study performed in the Netherlands, mostly minor valvulopathies were diagnosed in our study [10]. In Dutch guidelines drawn after the large Q-fever outbreaks, definitions are unclear as to which valvular defect and which grade of defect are important risk factors for developing chronic Q-fever [17]. Because of our low number of diagnosed chronic Q-fever patients, this study can provide no new insight on this issue. Third, the treatment (antibiotic prophylaxis) is not generally accepted, having been investigated only in France in a selected group of patients [12]. Next, long-term treatment with doxycycline and hydroxychloroquine is not without potential complications. Drug-induced photosensitivity is a notorious adverse effect of doxycycline, and long-term use of hydroxychloroquine can lead to retinopathy [25, 26]. Last, the cost-effectiveness of the screening has not been investigated.
In conclusion, we found no difference in Q-fever outcome between patients with or without a newly detected valvulopathy at the time of their acute Q-fever episode. Additionally, echocardiographic screening would lead to an unnecessary long-term antibiotic use, which is not desirable and which must be included in cost-benefit analysis of screening in future outbreaks. We recommend that Dutch guidelines regarding chronic Q-fever should further specify the types and grades of valvulopathy that are most important to prognosis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank cardiologist Monique Jacobs of the Jeroen Bosch Hospital for providing insight into cardiologic management of acute Q-fever patients of that hospital, Prof. Roel Coutinho of the Julius Center UMC Utrecht for critically reviewing the manuscript, and Maren Ouborg for her help with the data collection. English language editing was provided by Lucy Phillips.
Financial support. This study was financed from the regular budget of the Centre for Infectious Disease Control Netherlands of the National Institute for Public Health and the Environment made available by the Ministry of Health, Welfare, and Sport, project number V/150207/17/RI.
Potential conflicts of interest. All authors: No reported conflicts of Interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999; 12:518–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Million M, Thuny F, Richet H, Raoult D. Long-term outcome of Q fever endocarditis: a 26-year personal survey. Lancet Infect Dis 2010; 10:527–35. [DOI] [PubMed] [Google Scholar]
- 3. Kampschreur LM, Delsing CE, Groenwold RH, et al. Chronic Q fever in the Netherlands 5 years after the start of the Q fever epidemic: results from the Dutch chronic Q fever database. J Clin Microbiol 2014; 52:1637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Million M, Raoult D. No such thing as chronic Q fever. Emerg Infect Dis 2017; 23:856–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Botelho-Nevers E, Fournier PE, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis 2007; 26:635–40. [DOI] [PubMed] [Google Scholar]
- 6. Wegdam-Blans MC, Vainas T, van Sambeek MR, et al. Vascular complications of Q-fever infections. Eur J Vasc Endovasc Surg 2011; 42:384–92. [DOI] [PubMed] [Google Scholar]
- 7. Million M, Walter G, Thuny F, Habib G, Raoult D. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin Infect Dis 2013; 57:836–44. [DOI] [PubMed] [Google Scholar]
- 8. Fenollar F, Fournier PE, Carrieri MP, Habib G, Messana T, Raoult D. Risks factors and prevention of Q fever endocarditis. Clin Infect Dis 2001; 33:312–6. [DOI] [PubMed] [Google Scholar]
- 9. Fenollar F, Thuny F, Xeridat B, Lepidi H, Raoult D. Endocarditis after acute Q fever in patients with previously undiagnosed valvulopathies. Clin Infect Dis 2006; 42:818–21. [DOI] [PubMed] [Google Scholar]
- 10. Limonard GJ, Nabuurs-Franssen MH, Dekhuijzen PN, Groot CA. Prevention of Q fever endocarditis. Lancet Infect Dis 2011; 11:82–3. [DOI] [PubMed] [Google Scholar]
- 11. Limonard GJ, Nabuurs-Franssen MH, Weers-Pothoff G, et al. One-year follow-up of patients of the ongoing Dutch Q fever outbreak: clinical, serological and echocardiographic findings. Infection 2010; 38:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edouard S, Million M, Royer G, Giorgi R, Grisoli D, Raoult D. Reduction in incidence of Q fever endocarditis: 27 years of experience of a national reference center. J Infect 2014; 68:141–8. [DOI] [PubMed] [Google Scholar]
- 13. Wielders CC, van Loenhout JA, Morroy G, et al. Long-term serological follow-up of acute Q-fever patients after a large epidemic. PLoS One 2015; 10:e0131848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van der Hoek W, Morroy G, Renders NH, et al. Epidemic Q fever in humans in the Netherlands. Adv Exp Med Biol 2012; 984:329–64. [DOI] [PubMed] [Google Scholar]
- 15. Schneeberger PM, Hermans MH, van Hannen EJ, Schellekens JJ, Leenders AC, Wever PC. Real-time PCR with serum samples is indispensable for early diagnosis of acute Q fever. Clin Vaccine Immunol 2010; 17:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European Society of Cardiology. Eur Heart J 2007; 28:230–68. [DOI] [PubMed] [Google Scholar]
- 17. Wegdam-Blans MC, Kampschreur LM, Delsing CE, et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect 2012; 64:247–59. [DOI] [PubMed] [Google Scholar]
- 18. Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 2017; 30:115–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samuel JE, Frazier ME, Mallavia LP. Correlation of plasmid type and disease caused by Coxiella burnetii. Infect Immun 1985; 49:775–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wielders CC, Morroy G, Wever PC, Coutinho RA, Schneeberger PM, van der Hoek W. Strategies for early detection of chronic Q-fever: a systematic review. Eur J Clin Invest 2013; 43:616–39. [DOI] [PubMed] [Google Scholar]
- 21. Tissot-Dupont H, Vaillant V, Rey S, Raoult D. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 2007; 44:232–7. [DOI] [PubMed] [Google Scholar]
- 22. Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999; 83:897–902. [DOI] [PubMed] [Google Scholar]
- 23. Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis 2005; 5:219–26. [DOI] [PubMed] [Google Scholar]
- 24. Wilson JM, Jungner G.. Principles and practice of screening for disease. Geneva: World Health Organization Public Health Papers; 1968:34. [Google Scholar]
- 25. Drucker AM, Rosen CF. Drug-induced photosensitivity: culprit drugs, management and prevention. Drug Saf 2011; 34:821–37. [DOI] [PubMed] [Google Scholar]
- 26. Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol 2008; 23:201–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.