Abstract
In this paper, we present a novel method for the correction of motion artifacts that are present in fetal magnetic resonance imaging (MRI) scans of the whole uterus. Contrary to current slice-to-volume registration (SVR) methods, requiring an inflexible anatomical enclosure of a single investigated organ, the proposed patch-to-volume reconstruction (PVR) approach is able to reconstruct a large field of view of non-rigidly deforming structures. It relaxes rigid motion assumptions by introducing a specific amount of redundant information that is exploited with parallelized patchwise optimization, super-resolution, and automatic outlier rejection. We further describe and provide an efficient parallel implementation of PVR allowing its execution within reasonable time on commercially available graphics processing units, enabling its use in the clinical practice. We evaluate PVR’s computational overhead compared with standard methods and observe improved reconstruction accuracy in the presence of affine motion artifacts compared with conventional SVR in synthetic experiments. Furthermore, we have evaluated our method qualitatively and quantitatively on real fetal MRI data subject to maternal breathing and sudden fetal movements. We evaluate peak-signal-to-noise ratio, structural similarity index, and cross correlation with respect to the originally acquired data and provide a method for visual inspection of reconstruction uncertainty. We further evaluate the distance error for selected anatomical landmarks in the fetal head, as well as calculating the mean and maximum displacements resulting from automatic non-rigid registration to a motion-free ground truth image. These experiments demonstrate a successful application of PVR motion compensation to the whole fetal body, uterus, and placenta.
Keywords: Motion correction, fetal magnetic resonance imaging, GPU acceleration, image reconstruction, super-resolution
I. Introduction
The advent of single shot fast spin echo (ssFSE) T2-weighted sequences has enabled spin echo image formation principles [1] in magnetic resonance imaging (MRI) to play an essential role in fetal diagnosis [2] and research [3]. In particular, cases for which ultrasound (US) fails to acquire conclusive image data benefit from fetal MRI [4], [5]. Recent advances in motion compensation for fetal MRI [6] facilitate advanced image-based diagnostics and lead to novel insights about the human development.
Fetal MRI enables an ability to distinguish between individual fetal structures such as brain, lung, kidney and liver, as well as pregnancy structures such as the placenta, umbilical cord and amniotic sac. It provides improved visualization and structural information of the fetal anatomy, which enables to study abnormalities during pregnancy such as neuro-developmental disorders [7], placental pathologies [8], fetuses with congenital lung masses [9], and conjoined twins [10]. MRI is considered to be safe after the first trimester [11] for 1.5T [12] and 3T [13] without the use of contrast agents, which may have teratogenic effects. Furthermore, this technology paves the way for researchers and clinicians to analyze correlations between childhood development and prenatal abnormalities.
During image acquisition the fetus is not sedated and moves freely as well as the mother breathes normally. As a result, movements are likely to corrupt the scans, hiding pathology and causing overlap between different anatomical regions. In order to limit these artifacts, fast scanning sequences such as ssFSE [14] allow for the rapid acquisition of single slices at high in-plane resolution in a large field of view and good tissue contrast of the uterus. However, when acquiring a 3D volume through a stack of slices, inter-slice artifacts in the out-of-plane views are highly likely. Consequently, this restricts reliable diagnostics to individual slices in the current clinical practice. Fig. 1 depicts a typical example of motion related artifacts in a fetal ssFSE scan. The observed motion (cf. Fig. 1 b & c) is of unpredictable nature and consists of a combination of maternal respiration, bowel and fetal movements.
Fig. 1.
Three view-planes for raw 3D data acquired through stacks of ssFSE images covering the whole uterus. The transverse (a) is the in-plane view, i.e., native 2D slice scan orientation. Motion causes streaky artifacts for multi-planar reconstructions (MPR) in orthogonal views (b) and (c) caused by both maternal and fetal movements between the acquisition of individual slices.
Slice-to-volume registration (SVR) combined with super-resolution image reconstruction techniques [15] can be applied to compensate motion between single slices by reconstructing a high-resolution (HR) image from multiple, overlapping low-resolution (LR) images, as shown in Fig. 2. To provide a sufficiently high number of samples for such an approach, multiple stacks of 2D-slices need to be acquired, ideally in orthogonal orientations. A simple LR 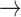 HR reconstruction model [15] can be formalized as:
HR reconstruction model [15] can be formalized as:
 |
where  denotes the
denotes the  -th LR image of total
-th LR image of total 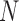 images, and
images, and  is the HR image. The matrix
is the HR image. The matrix 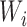 combines motion, sub-sampling and degradation effects:
combines motion, sub-sampling and degradation effects: 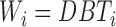 , where
, where  is the sub-sampling matrix,
is the sub-sampling matrix,  is the blurring matrix, and
is the blurring matrix, and  is the transformation matrix of observation
is the transformation matrix of observation 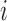 . The noise of observation
. The noise of observation  is represented by
is represented by 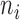 . LR images can be considered as a down-sampled, motion corrupted, blurred, and noisy version of the HR image. The resulting reconstruction can be divided into two main parts: motion correction (estimating
. LR images can be considered as a down-sampled, motion corrupted, blurred, and noisy version of the HR image. The resulting reconstruction can be divided into two main parts: motion correction (estimating 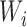 ) and super-resolution (estimating
) and super-resolution (estimating  ). Image registration can be used to estimate motion, interpolation to obtain a uniformly spaced HR image, and regularized super resolution with automatic outlier rejection to remove blur and noise. Volumetric fetal MR image reconstruction is more challenging than typical image reconstruction problems due to unconstrained random motion during slice acquisitions. Slice misalignments can lead to a loss of spatial coherence and typically introduce anisotropic voxel sizes and intensity inhomogeneities.
). Image registration can be used to estimate motion, interpolation to obtain a uniformly spaced HR image, and regularized super resolution with automatic outlier rejection to remove blur and noise. Volumetric fetal MR image reconstruction is more challenging than typical image reconstruction problems due to unconstrained random motion during slice acquisitions. Slice misalignments can lead to a loss of spatial coherence and typically introduce anisotropic voxel sizes and intensity inhomogeneities.
Fig. 2.
Illustration of the basic ideas behind reconstruction [15]: A simplified example of a 2D 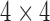 HR grid sampling from a 2D
HR grid sampling from a 2D 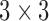 LR grids (left) and a practical example of 3D fetal MRI using multiple overlapping stacks of slices, by reconstructing a 3D HR image with an isotropic voxel size from LR images with anisotropic voxel size.
LR grids (left) and a practical example of 3D fetal MRI using multiple overlapping stacks of slices, by reconstructing a 3D HR image with an isotropic voxel size from LR images with anisotropic voxel size.
A. Practical Limitations
SVR methods have been successfully employed to address these problems in fetal MR and are typically applied to small regions and organs with rigid body characteristics that are identified by manual annotations [16]–[19] or less precise, automated segmentations [20]–[22]. Such approaches are prohibitive to whole body and uterus reconstruction because of the assumption of rigid motion in the 2D to 3D registration step of SVR. As a result, different areas in each slice that are likely to move in different directions will break this assumption, e.g., the head and thorax. Further, an extension of 2D-3D registration to include non-rigid deformations is only well defined with each slice and not well-constrained in 3D. Current SVR approaches will fail in the presence of non-rigid deformations and unpredictable organ shapes. This restricts the application of SVR to regions that are manually or automatically annotated. Thus, most of the previous SVR methods have been limited to the fetal brain as the main region of interest for fetal reconstruction due to the high incidence of neuro-developmental disability in premature infants. Only recently, [23], [24] proposed a motion corrected 3D reconstruction of fetal thoracic structures from prenatal MRI. Moreover, SVR is computationally expensive due to the exponential increase of computation with the size of the target area. This leads to prohibitive post-processing times in the clinical practice. Parallelized implementations [25] can address run-time problems, however, methodologically SVR is still restricted to small, rigid body areas.
B. Reconstruction of Large-Scale Anatomy
MRI has further been shown to be useful for the evaluation of the Whole uterus and structures like the placenta. During both normal and high-risk pregnancies, the whole uterine appearance and the condition of the placenta are considered to be an indicator for fetal health after birth [26]. Placental functions affect the birth weight as it controls the transmission of nutrients from the maternal to the fetal circulation [27]. However, the whole fetal body and secondary uterine parts can be inherently inconsistent. Different fetal body parts can move independently from the uterus. This makes the application of SVR and 2D-3D registration to the full uterus impossible in the presence of fetal motion and maternal respiration.
Besides, multiple births is a case where classical SVR pipelines, based on preprocessing steps to identify consistent rigid regions, will likely fail. The presence of multiple instances of the same fetal structure is usually not considered in previous methods. Therefore, a fully automatic motion correction method for the whole uterus, as it is presented in this paper, is very desirable and will enable the application of standard 3D image analysis techniques, e.g., [28], [29].
C. Related Work
Most motion compensation approaches for fetal MRI are based on SVR techniques that aim to obtain a motion-free and high resolution volume of a fetal target region. Registration of individual 2D slices with a higher resolution 3D volume [30] is the core approach of these algorithms. SVR methods assume that all acquired images are centered at a specific organ (e.g., brain, thorax) and cover three orthogonal image directions. Fig. 3 shows the core elements of SVR and the contribution of previous frameworks from the literature.
Fig. 3.
Overview of the required modules of state-of-the-art SVR methods and main components introduced by previous work.
The first SVR-based reconstruction framework for fetal MRI was introduced by Rousseau et al. [16]. It includes steps to correct 2D slice misalignments, intensity inhomogeneity distortions, and reconstructs an isotropic HR fetal brain from sets of LR images. Motion correction is done by applying a global 3D rigid alignment between the LR images using one image as a reference to define the global coordinate system. Then every slice is aligned to the initial reconstructed HR volume. Normalized mutual information is maximized using the gradient ascent method for both registration steps. A narrow Gaussian kernel is applied as a point spread function (PSF) for volume reconstruction and empty voxels are filled using the mean of the surrounding voxels. The image contrast is corrected using one LR image as a reference.
Jiang et al. [31] introduced the acquisition of many thin slices to provide sufficient sampling of the region of interest. Cross correlation is used as a cost function for the SVR steps assuming that the data have consistent contrast properties. After that, multilevel B-splines are applied to the volumetric reconstruction for data interpolation, which has the advantage of reducing blurring of the reconstructed image supported by including the thin slices.
Kim et al. [17] proposed a method for slice intersection motion correction (SIMC) of multi-slice MRI for 3D fetal brain image formation. The method is based on slice-to-slice registration using spatially weighted mean square intensity differences (MSD) of the signal between slices as an energy, assuming that the MRI contrasts are identical. Maternal tissues are excluded from the energy computations using a windowing function of a parametric ellipsoid model. Similar to [16], temporally adjacent slices are grouped together then divided into half iteratively. The splitting process is performed using discrete cosine basis functions.
Gholipour et al. [18] were the first to introduce a mathematical model for super-resolution (SR) volume reconstruction from slice acquisitions of fetal brains. The main difference to previous methods is that it includes knowledge of the slice acquisition model and the SR reconstruction is performed based on maximum likelihood and a robust M-estimation minimization for an error norm function. A Tikhonov regularization term is added to the cost function in order to enforce a solution when the number of acquired samples is not high enough for solving the reconstruction problem.
Rousseau et al. [32], [33] proposed to use a variational regularization including an approximation of Total Variation (TV) to better preserve edges. Later, Tourbier et al. [34] introduced an adaptive regularization by applying novel fast convex optimization techniques to design an efficient optimization algorithm for the super-resolution problem using edge-preserving TV regularization.
Murgasova et al. [19] were able to reconstruct the fetal brain using intensity matching and complete outlier removal. The main steps of their reconstruction method are: (i) 3D registration of the acquired stacks using a template stack; (ii) extracting region of interest (the fetal head) from all the stacks; (iii) intensity matching and bias correction between the slices based on an EM framework, where the differential bias fields and slice-dependent scaling factors are estimated during the reconstruction; (iv) motion correction using [16] based on the normalized cross correlation as a similarity measure and an approximated 3D Gaussian PSF similar to [31]. A posterior probability is used to define the inlier and outlier voxels within the EM framework in order to remove the motion-corrupted artifacts and misaligned data. Blurring in reconstructed images is reduced by integrating edge-preserving regularization based on anisotropic diffusion within the SR reconstruction framework.
Kainz et al. [25] developed a fast multi-GPU accelerated implementation for the method presented in [19], which is based on 2D-3D registration, SR with automatic outlier rejection and an optional intensity bias correction. They extended the reconstruction framework by automatically selecting the stack with least motion as the reference stack and using a fully flexible and accurate PSF instead of approximated functions. Using a multi-GPU framework enabled the SR reconstruction process to be approximately five to ten times faster than using a multi-CPU framework.
To our knowledge, modeling non-rigid transformations with multiple rigid transformations and without an initial registration target has only been preliminarily explored for fetal MRI in [35]. Other works regarding non-linear 3D-3D registration between an outlier-free, regularly sampled source and target volume, constrain parts of an image to move independently and rigidly (e.g. bones through manual segmentation and landmark annotation), while allowing other parts to deform [36]. In 3D-3D settings globally continuous 3D deformation fields, which should not be assumed for 2D-3D fetal motion compensation problems, can be approximated by fusing multiple local rigid transformations as shown in [37] and [38].
D. Contributions
In this paper we propose and evaluate a new paradigm for motion correction based on SVR and flexible subdivision of the input space into overlapping, highly redundant and partly rigid image patches [35], thus solving the motion compensation problem for large field of view reconstructions. We split the input into small overlapping areas and find these, which contain rigid components. This allows to iteratively learn their consistency compared to a global reconstruction optimization volume. Corrupted and inconsistent data is automatically identified and excluded using robust statistics. Our approach facilitates the automatic reconstruction of whole collections of motion corrupted stacks without the need of corresponding image segmentations. By treating rigid image patches as piecewise constant segments of organs further allows limited correction of non-rigid tissue motion. We also evaluate the non-rigid deformations that may result from PVR using anatomical landmarks annotated in the fetal head and non-rigid registration. The presented patch-to-volume reconstruction (PVR) finds automatically rigidly connected areas, which can be used as segmentation prior for further refinement using conventional SVR in small regions of interest. In contrast to [25], we further introduce a multi-scale patch approach and thoroughly evaluate the reconstruction quality of the whole uterus including the fetal brain and placenta. We test the breaking points of SVR and variations of PVR on synthetically motion corrupted brain phantom data. The presented approach is the only currently available method that is able to reconstruct fetal organs and detailed 3D volumes of secondary, non-rigidly moving structures such as the placenta.
II. Method
SVR-based motion compensation methods make use of the assumption that rigid regions, e.g., brain and thorax, of 2D input slices deforms rigidly, where a global 3D volume is reconstructed by iteratively registering these 2D input slices. We propose to increase the granularity of the input data by using 2D image patches of arbitrary shape instead of whole slices for SVR reconstruction. We explore square patches and dilated superpixels [39] for the definition of the patch shape. Superpixels provide a method to define semantically meaningful regions while reducing the required data redundancy and computational overhead.
PVR relies on the fact that certain regions of the scanned anatomy are rigid and can be reconstructed with SVR algorithms. However, unlike SVR, it is fully automatic and provides a full field of view reconstruction. Data consistency is obtained by oversampling a region of interest at different scan orientations. Robust statistics can be used to identify mis-registered or heavily corrupted data [18], [19]. Fig. 4 depicts a schematic overview of the proposed PVR framework.
Fig. 4.
A schematic and modular overview of the proposed patch-to-volume reconstruction (PVR) framework. The key parts are 3D-3D registration, patch extraction, 2D-3D registration, super-resolution, and EM-based outlier removal. Core contributions of PVR are written in red and marked with asterisk.
A. Input Data and Initialization
A template stack is either randomly or automatically chosen from available input stacks by detecting the stack with fewest motion artifacts [25]. Global intensity matching is applied to normalize intensity values of all input images followed by global 3D-3D alignments to spatially initialize the reconstruction target. Input data can be represented as stacks of 2D images (patches) consisting of
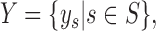 |
where 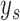 is a patch of arbitrary 2D-shape and indexed by the location
is a patch of arbitrary 2D-shape and indexed by the location 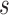 .
. 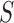 is the set of all locations in all
is the set of all locations in all 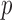 stacks,
stacks, 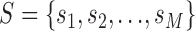 , and
, and 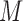 is total number of patches.
is total number of patches.
B. Patch Extraction
In the simplest, naïve case the shape of 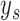 is square defined via its edge length
is square defined via its edge length  and stride
and stride  . This definition is generally applicable to any kind of oversampled motion corrupted data. If
. This definition is generally applicable to any kind of oversampled motion corrupted data. If 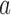 and
and 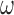 are fixed, no prior knowledge about the data is assumed. However, ideally each
are fixed, no prior knowledge about the data is assumed. However, ideally each 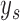 corresponds to a meaningful subregion of the volume in which motion can be characterized as rigid. Typically, square patches are overlapping to provide redundant representations of the same locations. The computational expense of such an approach increases with patch number and size
corresponds to a meaningful subregion of the volume in which motion can be characterized as rigid. Typically, square patches are overlapping to provide redundant representations of the same locations. The computational expense of such an approach increases with patch number and size 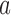 . Additional consideration must include the inherent trade-off between
. Additional consideration must include the inherent trade-off between 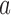 and the assumption of it containing rigid motion.
and the assumption of it containing rigid motion.
An alternative to naïve shape definitions of 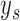 is to find correlation between voxel locations and their neighbors, which can be found by unsupervised image segmentation techniques such as superpixels (SP) [39]. These techniques allow similar-sized segments to be obtained from local intensity information (see Fig. 5) instead of employing dense sampling of overlapping patches, enabling the image reconstruction with fewer but more useful data blocks. Further, reducing the total amount of required data blocks for reconstruction lowers the computational overhead, positively impacting the overall run-time. Additionally, larger rigid areas require less computational effort for image registration and super-resolution, and more importantly less dependency on inherent image data parameters (e.g., voxel spacing, organ size, subject size).
is to find correlation between voxel locations and their neighbors, which can be found by unsupervised image segmentation techniques such as superpixels (SP) [39]. These techniques allow similar-sized segments to be obtained from local intensity information (see Fig. 5) instead of employing dense sampling of overlapping patches, enabling the image reconstruction with fewer but more useful data blocks. Further, reducing the total amount of required data blocks for reconstruction lowers the computational overhead, positively impacting the overall run-time. Additionally, larger rigid areas require less computational effort for image registration and super-resolution, and more importantly less dependency on inherent image data parameters (e.g., voxel spacing, organ size, subject size).
Fig. 5.
An illustrative figure showing both square patches and superpixels methods for the patch extraction step. A 2D superpixel shows more flexibility than a square patch in extracting rigid regions or similar voxels. In practice, superpixels are dilated with few pixels to include some contextual information in order to increase the accuracy of the patch to volume registration step.
While there are several techniques for generating SP in the literature [39]–[41], a fast and efficient SP approach is desirable for the clinical practice. Simple linear iterative clustering (SLIC) [39] obtains regular SP based on minimizing the distance  between the centroids of SP with an initial step size
between the centroids of SP with an initial step size  .
.  is defined as:
is defined as:
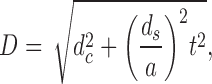 |
where  and
and 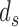 are the intensity and spatial Euclidean distances that are controlled by the adaptive compactness parameter
are the intensity and spatial Euclidean distances that are controlled by the adaptive compactness parameter 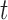 for each SP. In our previous work [35], we use a defined number of SP centers
for each SP. In our previous work [35], we use a defined number of SP centers 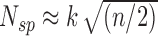 , where
, where 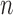 is the number of voxels in an image of size
is the number of voxels in an image of size 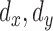 and
and 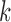 a constant factor. Here, we use a regular grid for initialization with a constant distance
a constant factor. Here, we use a regular grid for initialization with a constant distance 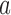 between SP centroids so that
between SP centroids so that  and dilate by
and dilate by 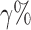 . Thus, instead of providing a target number of SP,
. Thus, instead of providing a target number of SP, 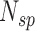 is defined by the image size and
is defined by the image size and 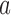 controls the relative size of the SP.
controls the relative size of the SP.
C. Multi-Scale Patches
Although larger patch regions are less likely to include rigidly connected regions, they may perform better during 2D-3D registration due to the additional contextual information of each patch. In contrast, smaller patch sizes are more likely to represent rigidly deformed regions, but provide less contextual information, potentially affecting the 2D-3D registration. A good trade-off between the size of the patch region and the likelihood of rigid motion needs to be found. Here, we propose the use of multi-scale patches for reconstruction to exploit the advantages of different patch sizes. We represent input data as stacks of 2D patches:
 |
where, instead of using the same 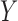 as a unique input, a different scale of
as a unique input, a different scale of 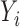 is used for each iteration
is used for each iteration 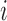 at
at 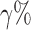 of its original size. Similar to Eq. 2,
of its original size. Similar to Eq. 2, 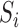 is the set of all locations in all
is the set of all locations in all 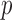 stacks but with different size for each iteration
stacks but with different size for each iteration 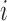 . This is done by re-calling the patch extraction module with a different patch size (see Fig. 4). Additionally, to increase contextual information for estimating the transformations, we compute overlapping
. This is done by re-calling the patch extraction module with a different patch size (see Fig. 4). Additionally, to increase contextual information for estimating the transformations, we compute overlapping 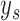 superpixel patches and dilate each by
superpixel patches and dilate each by 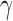 pixels using a flat structuring element
pixels using a flat structuring element  with a fixed neighborhood (26 pixels in our case), hence
with a fixed neighborhood (26 pixels in our case), hence 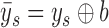 . Smaller
. Smaller 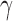 values result in a faster reconstruction. Ideally
values result in a faster reconstruction. Ideally 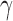 is > 50% of
is > 50% of 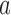 , ensuring that every pixel is covered by multiple samples.
, ensuring that every pixel is covered by multiple samples.
D. Patch to Volume Registration
An HR-image 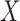 is reconstructed from a number of motion corrupted patches
is reconstructed from a number of motion corrupted patches 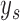 using 2D-3D registration-based super-resolution similar to [19] and [25], where an accurate PSF calculation is used to generate a gradually improving approximation of
using 2D-3D registration-based super-resolution similar to [19] and [25], where an accurate PSF calculation is used to generate a gradually improving approximation of 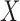 and further employed to initialize the 2D-3D registration and computation of robust statistics. In [19] and [25], the PSF is a sinc function for the in-plane and is the slice profile for the through-plane, measured for the employed MRI sequence (ssFSE), according to [31].
and further employed to initialize the 2D-3D registration and computation of robust statistics. In [19] and [25], the PSF is a sinc function for the in-plane and is the slice profile for the through-plane, measured for the employed MRI sequence (ssFSE), according to [31].
We employ an implementation of the PSF function by applying a Taylor series for a better approximation of small values close to 0. We truncate the series after several terms and bound the remainder based on relative error 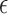 . The Taylor series approximation of the sinc function is defined as
. The Taylor series approximation of the sinc function is defined as 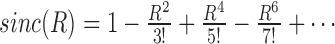 . The proposed approximate PSF achieves qualitative improvement of the reconstructed image compared to the sinc implementation. An example from the first iteration of a fetal brain reconstruction is shown in Fig. 6. This is expected since Taylor series approximation is common for computations requiring a high level of accuracy close to the machine precision limits of floating point computations [42].
. The proposed approximate PSF achieves qualitative improvement of the reconstructed image compared to the sinc implementation. An example from the first iteration of a fetal brain reconstruction is shown in Fig. 6. This is expected since Taylor series approximation is common for computations requiring a high level of accuracy close to the machine precision limits of floating point computations [42].
Fig. 6.
Example for the observed differences in the first iteration of a fetal brain MRI reconstruction (a). (b) shows a magnified region using a sinc function for the PSF similar to [35] and (c) shows the result from using a Taylor series approximation of the sinc function as used in this work. Taylor series approximation allows a better approximating of small values close to zero. (d) shows the difference between both images.
During the optimization process, individual 2D patches are continuously rigidly registered to the current 3D reconstruction of 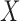 and reintegrated into
and reintegrated into 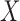 using iterative super-resolution with gradient descent optimization. Any similarity metric can be used as a cost function for the registration step such as mutual information [16], [19], cross correlation (CC) [25], [31], or mean square intensity differences [17], [18]. Choosing the best similarity metric for reconstruction depends on the input data. CC has been found to be effective for input data with similar intensity distribution [43]. In our experiments, we employ CC as the similarity metric for 2D-3D registration, after rescaling the intensities between the input stacks.
using iterative super-resolution with gradient descent optimization. Any similarity metric can be used as a cost function for the registration step such as mutual information [16], [19], cross correlation (CC) [25], [31], or mean square intensity differences [17], [18]. Choosing the best similarity metric for reconstruction depends on the input data. CC has been found to be effective for input data with similar intensity distribution [43]. In our experiments, we employ CC as the similarity metric for 2D-3D registration, after rescaling the intensities between the input stacks.
E. Super-Resolution
Given that the position and orientation of each patch 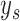 relative to
relative to 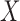 is known at any point, patches
is known at any point, patches 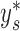 can be simulated from sampling the current approximation of
can be simulated from sampling the current approximation of 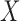 using the PSF.
using the PSF. 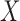 can subsequently be iteratively improved by minimizing the error between
can subsequently be iteratively improved by minimizing the error between 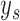 and
and 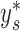 . Gradient decent is used for the optimization. The optimization has to be regularized to prevent amplification of registration error, noise and under-sampling artifacts. Adaptive regularization is applied to reduce smoothing effects. Therefore the objective function is
. Gradient decent is used for the optimization. The optimization has to be regularized to prevent amplification of registration error, noise and under-sampling artifacts. Adaptive regularization is applied to reduce smoothing effects. Therefore the objective function is
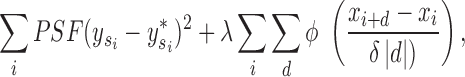 |
with 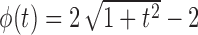 , voxel locations
, voxel locations  ,
, 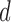 a vector between the index of a voxel and one of its 26 neighbors, and
a vector between the index of a voxel and one of its 26 neighbors, and  a parameter that controls when the gradient between neighboring voxels is considered to be an edge. For our data a gradient magnitude between 100 and 400 yields good results. The regularization term represents edge preserving filtering [44] and is used similarly as presented in [25].
a parameter that controls when the gradient between neighboring voxels is considered to be an edge. For our data a gradient magnitude between 100 and 400 yields good results. The regularization term represents edge preserving filtering [44] and is used similarly as presented in [25]. 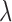 is decreased after each iteration. Following the experimental findings in [19] a suitable starting value for
is decreased after each iteration. Following the experimental findings in [19] a suitable starting value for 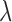 is
is 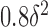 , halved after every iteration.
, halved after every iteration.
F. EM-Based Outlier Removal
Correctly registered patches  should provide a higher contribution to the final reconstruction, presenting a low error
should provide a higher contribution to the final reconstruction, presenting a low error 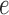 when compared to the original image data. [18] initially introduced an approach to account for outliers during super-resolution based on Huber function statistics. Similar to [19], we employ the expectation maximization algorithm for outlier removal by classifying
when compared to the original image data. [18] initially introduced an approach to account for outliers during super-resolution based on Huber function statistics. Similar to [19], we employ the expectation maximization algorithm for outlier removal by classifying 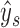 and the included pixels into an inlier and outlier class. A zero-mean Gaussian distribution
and the included pixels into an inlier and outlier class. A zero-mean Gaussian distribution 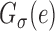 with variance
with variance  is used for the inliers and a uniform distribution with constant density
is used for the inliers and a uniform distribution with constant density
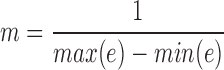 |
for the outliers. This makes use of available, highly redundant information (i.e., overlapping  ), to find partly matching patches and to depreciate or fully reject erroneous voxels. We aim to maximize the log-likelihood for each patch
), to find partly matching patches and to depreciate or fully reject erroneous voxels. We aim to maximize the log-likelihood for each patch
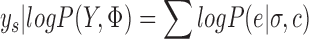 |
to be part of a region of rigid motion.  is the current estimate of the reconstructed volume
is the current estimate of the reconstructed volume 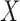 , the variance
, the variance  of the errors
of the errors 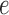 , and the proportion of correctly matched voxels
, and the proportion of correctly matched voxels 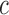 . The posterior probability for a pixel
. The posterior probability for a pixel 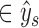 being identified as inlier is
being identified as inlier is
 |
We perform the updates of  and
and 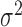 according to [19]:
according to [19]:
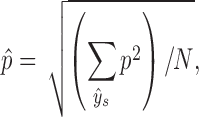 |
where 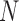 is the number of pixels in
is the number of pixels in 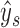 . We further define an inlier and outlier probability for each
. We further define an inlier and outlier probability for each 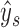 and exclude it from processing if classified as an outlier (e.g., if it contains structures moving in opposite directions during scanning, such as the fetal head and thorax). Only if information in
and exclude it from processing if classified as an outlier (e.g., if it contains structures moving in opposite directions during scanning, such as the fetal head and thorax). Only if information in 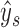 is consistent with the originally acquired data, will the registered patch contribute to the SR reconstruction of
is consistent with the originally acquired data, will the registered patch contribute to the SR reconstruction of 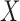 .
.
G. Identification of Rigid Regions and SVR Refinement
The rigidity of regions is measured by keeping track of the probability  of each pixel of every
of each pixel of every 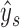 . This allows the identification of locations best fitting the rigid 2D-3D registration constraints. Candidate regions, that contain rigid motion components, can be identified by combining
. This allows the identification of locations best fitting the rigid 2D-3D registration constraints. Candidate regions, that contain rigid motion components, can be identified by combining 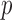 and
and 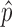 into a 3D volume utilizing the same PSF as used in the reconstruction. This can further initialize the rigid SVR reconstruction and visualize the data uncertainty during reconstruction.
into a 3D volume utilizing the same PSF as used in the reconstruction. This can further initialize the rigid SVR reconstruction and visualize the data uncertainty during reconstruction.
III. Implementation
A. Parallelization
The high data redundancy required for the proposed approach makes conventional single threaded implementation practically not feasible. Computational complexity of PVR is exponentially higher than SVR, depending on the employed patch overlap. For optimal performance we implemented our approach via General-Purpose Programming on Graphics Processing Units (GPGPU) using the Compute Unified Device Architecture (CUDA, NVIDIA, Santa Clara, CA) language [45], [46]. CUDA is a highly evolved single instruction multiple data (SIMD) programming language, which allows a large part of the proposed framework to be mapped onto GPU hardware. Currently, CUDA is the only high-level general purpose GPU language that provides bi-directional texture access via surfaces in a kernel, which is essential for the efficient implementation of certain parts of our framework. In this section we discuss the key implementation details.
We use a modular design to allow experimentation with the separate components of the algorithm. An overview of this design is shown in Fig. 7. The modules are encapsulated in a CUDA library, which can be used independently from the instantiating framework. We employ the successor of IRTK1 for interfacing with medical image data.
Fig. 7.
The software modules defined for the implementation of the proposed approach. For implementation details, please refer to the provided source code.
PVR is parallelized on three levels:
-
I.
Patch-level: Individual patches are mapped to blocks of a CUDA computing grid and the contained voxels are mapped to individual threads. Depending on the used GPU hardware, patch processing can also be mapped directly to the computing grid, such that each thread works on a complete patch (limited by the employed patch size). The resulting thread divergence provides opportunities for advanced GPU scheduling strategies [47] and for a direct translation of optimization strategies for image registration, for example patch-wise gradient descent.
-
II.
Voxel-level: For the parallelization of PSF-based super-resolution and robust statistics we follow a similar three-fold procedure definition as used in [25]. The voxels within each patch are processed using kernel level parallelization and parallel pixel-volume, volume-pixel, and volume-volume procedures are applied.
-
III.
Patch-batch: PVR scales to multiple GPUs through distributing independent subsets of patches over the desired number of devices. Synchronization is done through averaging of the resulting sub reconstruction volumes on the master GPU. Initial 3D-3D registration is performed on a single master GPU, which allows optimal coalesced memory access.
B. Availability of Source Code
We provide the source code of a C++/CUDA implementation of the proposed method, including parallelization strategies, in a publicly available software repository.2 The source code for the implementation of PVR is licensed under MIT license.
IV. Evaluation & Experiments
A. Evaluation of Global Image Quality
1). Evaluation of Adult Brain MRI Reconstruction:
We evaluate the performance and limitations of PVR in terms of accuracy and robustness with synthetic deformations of adult brain data. Similar to [18], an isotropic 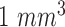 T2-weighted adult brain phantom with no noise obtained from the Brainweb database [48] is used for this experiment. Three experiments with different deformations are performed, namely:
T2-weighted adult brain phantom with no noise obtained from the Brainweb database [48] is used for this experiment. Three experiments with different deformations are performed, namely:
-
I.
Rigid Transformation: Synthetic rigid motion artifacts are generated by translating the axial, sagittal and coronal images with
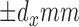 in the
in the 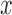 -direction.
-direction. -
II.
Bulk Transformation: Synthetic bulk motion artifacts are generated by rotating the upper half of the original image with
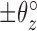 around the
around the 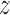 -axis.
-axis. -
III.Non-rigid deformation: Synthetic non-rigid motion artifacts are generated by skewing (shearing) the axial, sagittal, coronal images using:
where we use one combined skewing value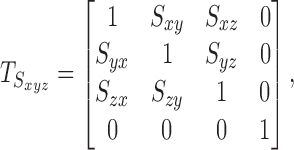
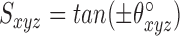 in the
in the 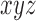 -direction.
-direction.
Following image deformation, a motion-corrupted 3D stack is constructed by sampling 2D images from both motion-free and motion-corrupted stacks in an interleaved manner similar to fetal MRI acquisition [7]. Where for the bulk motion experiment a 3D stack is constructed by only rotating the upper half of the brain. Three stacks are used for the reconstructions where each stack is sampled with a voxel size of 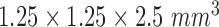 . We use standard axial, sagittal, and coronal orientations as shown in Fig. 8. An HR image with isotropic voxel size
. We use standard axial, sagittal, and coronal orientations as shown in Fig. 8. An HR image with isotropic voxel size 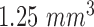 is reconstructed using SVR [25], square patch- and superpixel-based PVR.
is reconstructed using SVR [25], square patch- and superpixel-based PVR.
Fig. 8.
Strong synthetic non-rigid motion artifacts caused by skewing an adult brain phantom with an angle of ( ). Rows: MRI in standard orientations: coronal, axial, and sagittal. Columns: original scan (
). Rows: MRI in standard orientations: coronal, axial, and sagittal. Columns: original scan ( ), and sampled (
), and sampled (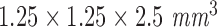 ) axially, coronally and sagittally, and SVR, PVR-square patches and PVR-superpixel reconstructed isotropic images (
) axially, coronally and sagittally, and SVR, PVR-square patches and PVR-superpixel reconstructed isotropic images (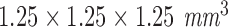 ).
).
2). Evaluation of Fetal Organ MRI Reconstructions:
Evaluating the quality of reconstructed fetal MRI is challenging due to the absence of motion-free ground truth data. For this purpose, we introduce a novel approach for the evaluation problem based on the originally acquired slice images. Assuming that 2D in-plane patches extracted from the original stacks contain no motion artifacts, we use them as gold standard and compare with corresponding simulated patches from the reconstructed volume. Evaluation metrics (see Sec. IV-A.3) are computed between the reconstructed input stacks and the final motion corrected image and averaged over the whole volume. The fetal brain is typically used to assess the quality of reconstruction as it moves rigidly, fulfilling the rigid motion assumption for SVR-based methods in the 2D-3D registration step. However, soft tissue organs such as the placenta deform non-rigidly. For this reason, we additionally chose to reconstruct the placenta and whole uterus as challenging test cases for PVR and SVR.
3). Evaluation Metrics:
We employ the following metrics for measuring the quality of the reconstructed image: Cross-correlation (CC) to measure the similarity between the intensities of input 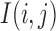 and reconstructed image
and reconstructed image 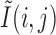 at the location
at the location 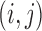 , which is defined as:
, which is defined as:
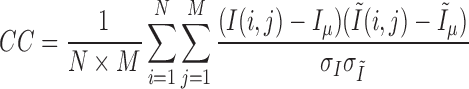 |
where N and M are the dimensions of a 2D slice.
The peak signal-to-noise ratio (PSNR) is used to measure the error introduced by motion and is based on the mean squared error (MSE) between the original 2D in-plane patch and the reconstructed image. PSNR is defined as:
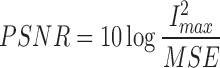 |
where  is the maximum intensity in the original image. An improved reconstruction quality usually results in higher PSNR. However, PSNR does not reflect well subjective human perception of image quality as it is mainly based on estimating absolute errors between individual pixels.
is the maximum intensity in the original image. An improved reconstruction quality usually results in higher PSNR. However, PSNR does not reflect well subjective human perception of image quality as it is mainly based on estimating absolute errors between individual pixels.
The structural similarity index (SSIM) accounts for image degradation as perceived changes in structural information [49]. It measures the structural similarity by comparing normalized local patterns of pixel intensities, which is similar to the human visual system’s abilities to extract information based on structure. The SSIM is defined as:
 |
where 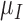 ,
, 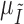 ,
, 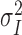 and
and  are the average and variance of the intensities of the original 2D in-plane slice and the reconstructed slice respectively.
are the average and variance of the intensities of the original 2D in-plane slice and the reconstructed slice respectively.  is the covariance of
is the covariance of 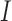 and
and 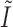 .
. 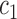 and
and  are defined as
are defined as  and
and 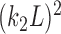 in order to balance the division with weak denominator, where
in order to balance the division with weak denominator, where 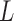 is the dynamic range of the intensities in image
is the dynamic range of the intensities in image 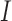 . Similar to [49],
. Similar to [49], 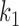 and
and 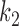 are equal to 0.01 and 0.03 respectively.
are equal to 0.01 and 0.03 respectively.
Structural dissimilarity (DSSIM) heat maps are calculated in order to visualize the dissimilarities between original and reconstructed images. DSSIM is calculated as a distance metric derived from SSIM:
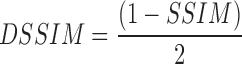 |
B. Evaluation of Local Deformations
Compared to SVR, PVR relaxes rigidity constraints and may therefore introduce deformation artifacts like shrinking of anatomical structures. Hence, in order to assess the geometric integrity of the 3D image reconstruction in terms of mm we conduct two experiments:
1). Evaluation With Anatomical Landmarks:
We use a set of 3D anatomical landmarks that can be clearly defined in 3D reconstructions of real fetal MRI head scans to assess the accuracy of nonrigid deformations resulting from PVR compared to SVR. In particular, we have selected 10 clinically relevant, anatomical landmarks in the eyes and sub-cortical region of the fetal brain, which define stable anatomical structures of the corpus callosum and the lateral ventricles. A clinical expert has chosen a set of 11 fetal MRI scans with the best reconstruction quality of the fetal head using SVR. We have repeated the reconstruction of this set from the raw scan data using PVR with patches of size 32 and stride 16 pixels. Two different observers have annotated the selected anatomical landmarks independently across the 11 subjects. The accuracy is assessed by calculating the average 3D Euclidean distance errors between PVR and SVR (mean annotation points of the two observers), measured for each landmark for all 11 subjects.
2). Evaluation of Displacement:
In this experiment we seek to evaluate the quality of PVR in cases of non-rigid motion between the scanned images using an approximated displacement error measured on the simulated skewing motion experiment of the brain phantom. The reconstructed images are first rigidly and then non-rigidly (b-spline [50]) registered to the motion-free ground truth image. The transformations resulting from non-rigid registration are used to transform a set of points sampled uniformly on a 3D grid (5% of the image size) inside the brain area. To ensure a fair comparison, we exclude reconstructed images from high motion skewing angles > 1.5°, which may affect registration to the reference image if the reconstructed image is blurred or distorted.
V. Results
A. Global Image Quality
1). Reconstruction of Adult Brain MRI:
Experiments on adult brain MR data using the Brainweb database [48] includes introducing synthetic non-rigid motion artifacts as described in Sec. IV-A.1. Example slices of standard planes of original and corrupted data are depicted in Fig. 8. Comparative experimental results of SVR and PVR reconstruction methods are shown in Fig. 9 for PSNR, SSIM and CC. For all metrics, PVR shows an improved performance over SVR, particularly in the presence of deformations with higher skewing angles. Further, we observe that superpixel-based PVR achieves performance similar to PVR using arbitrary square patches, while requiring a lower number of input patches.
Fig. 9.
Comparative reconstruction performance of SVR and PVR methods on synthetically corrupted Brainweb [48] data using rigid translational transformations (1st column), bulk transformations (2nd column) and non-rigid skewing deformations (3rd column). Left to right: PSNR, SSIM and CC over skew angle in degrees and translations in mm for SVR (blue), superpixel-based PVR ( , yellow) and PVR using square patches (
, yellow) and PVR using square patches (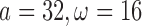 , red).
, red).
2). Reconstruction of Fetal Organs:
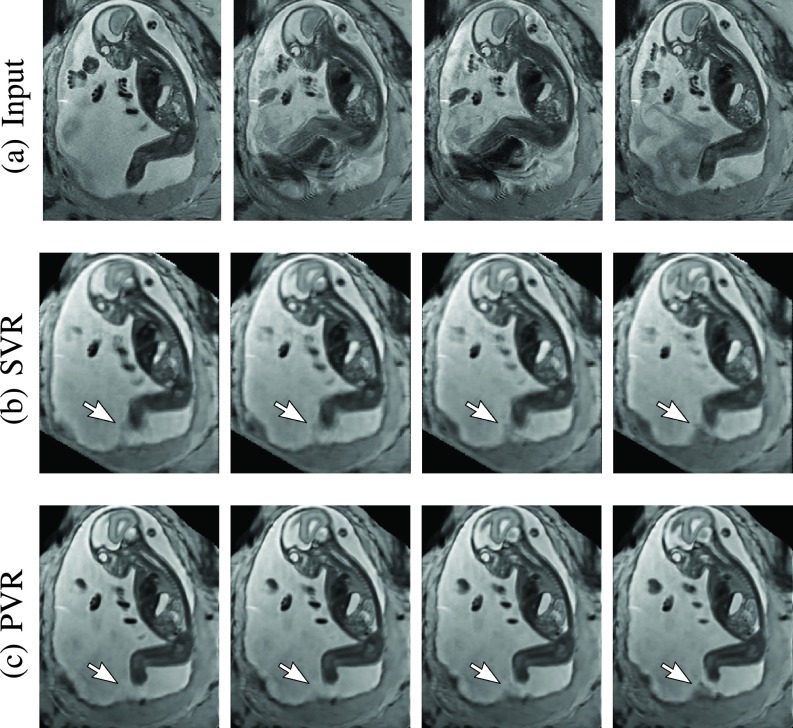
Exemplary PVR and SVR reconstructions under motion introduced by kicking of the fetus are shown in Fig. 10. PVR reconstruction results show an improved visual appearance and less blurring in the region with severe motion artifacts (arrow).
Fig. 10.
Example reconstructions of consecutive MR scans of a moving fetus (kicking): input data (a) and corresponding cutting planes through an SVR- (b) and PVR-reconstructed (c) volumes. SVR produces blurry but readable results because of high data redundancy and outlier rejection through robust statistics. PVR with square patches of 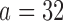 and
and 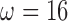 appears visually superior. The arrow points at an area of substantial quality differences caused by independent rapid movements of the leg.
appears visually superior. The arrow points at an area of substantial quality differences caused by independent rapid movements of the leg.
An example of a challenging clinical case with a kidney malformation in one of twin fetuses, is shown in Fig. 11. Our clinical partners confirmed that such complications are easier to examine and to quantify after PVR-based reconstruction.
Fig. 11.
Three viewing planes through the original motion corrupted scan of a moving twin with a gestational age of 28 weeks (a) and PVR reconstruction using multi-scale superpixels (b). For this dataset we masked the uterus to save unnecessary computation time in areas containing maternal tissue. The white arrow points at a unilateral multicystic kidney of one of the twins.
Comparative experiments of PVR variants were carried out on 32 fetal MR scans at gestational ages of approximately 20 weeks with voxel size around 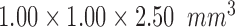 and
and  , constituting challenging image corruption samples. The ssFSE T2-weighted sequences data have been acquired on a Philips Achieva 1.5T, the mother lying 20° tilt on the left side to avoid pressure on the inferior vena cava and aligned to the main axes of the fetus. Table I (a) & (b) show numerical results of evaluating individual stacks before reconstruction (baseline), and the final reconstructed image from 3–6 stacks using square patches (
, constituting challenging image corruption samples. The ssFSE T2-weighted sequences data have been acquired on a Philips Achieva 1.5T, the mother lying 20° tilt on the left side to avoid pressure on the inferior vena cava and aligned to the main axes of the fetus. Table I (a) & (b) show numerical results of evaluating individual stacks before reconstruction (baseline), and the final reconstructed image from 3–6 stacks using square patches (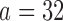 and
and 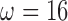 ), superpixels (
), superpixels (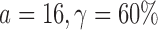 ) and multi-scale superpixels (with initial
) and multi-scale superpixels (with initial 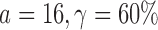 and
and  scaling factor for every new iteration). Statistical testing between baseline and PVR variants was carried out using paired T-Tests and differences between using fixed or multi-scale and between square patches or superpixels were assessed via Two-factor ANOVA with repeated measures. In Table I (a) & (b) the names of PVR variants are marked in bold if statistically significant differences have been found during analysis, i.e., FS and MS and/or Square Patches and Superpixel pairs are bold if the results between them differ significantly.
scaling factor for every new iteration). Statistical testing between baseline and PVR variants was carried out using paired T-Tests and differences between using fixed or multi-scale and between square patches or superpixels were assessed via Two-factor ANOVA with repeated measures. In Table I (a) & (b) the names of PVR variants are marked in bold if statistically significant differences have been found during analysis, i.e., FS and MS and/or Square Patches and Superpixel pairs are bold if the results between them differ significantly.
TABLE I. Average (a) PSNR and (b) SSIM Results (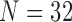 ) for the Input Stack (Baseline) and PVR Variants With Fixed (FS) and Multi-Scale (MS) Variants of Square Patches and Superpixels. All Mean Differences of PVR Against Baseline are Statistically Significant (p <0.05). Names of All Statistically Significantly Different PVR Variants are Stated in Bold.
) for the Input Stack (Baseline) and PVR Variants With Fixed (FS) and Multi-Scale (MS) Variants of Square Patches and Superpixels. All Mean Differences of PVR Against Baseline are Statistically Significant (p <0.05). Names of All Statistically Significantly Different PVR Variants are Stated in Bold.
| (a) | ||||||
|---|---|---|---|---|---|---|
| Baseline | Brain | Placenta | Uterus | |||
| 16.97 ± 3.77 | 19.95 ± 4.40 | 19.29 ± 3.82 | ||||
| Square Patches | Superpixels | Square Patches | Superpixels | Square Patches | Superpixels | |
| FS | 26.70 ± 1.45 | 26.60 ± 1.58 | 31.07 ± 1.35 | 31.00 ± 1.46 | 27.17 ± 1.43 | 27.35 ± 1.36 |
| MS | 27.03 ± 1.38 | 26.35 ± 1.54 | 30.85 ± 1.50 | 29.62 ± 1.30 | 26.87 ± 1.31 | 26.40 ± 1.24 |
The evaluation of the reconstruction quality of a whole 3D image into a single-valued metric may not properly reflect the performance differences, as it is based on averaging values of all the pixels of all the input stacks. Furthermore, Table I indicates significant differences between variants of PVR but these differences have only minimal qualitative effect on reconstruction accuracy. Therefore, Fig. 12 evaluates the reconstruction quality of PVR additionally using dissimilarity heat maps based on the measured DSSIM (see Sec. IV-A.3). This approach allows further qualitative evaluation and allows for uncertainty visualization of PVR reconstructions.
Fig. 12.
A sample 2D cutting plane through a motion-corrupted fetal brain (a) and placenta (f), after PVR using square patches with  and
and  (b) and (g). The DSSIM heat map for a baseline before reconstruction (c) and (h), and after PVR (d) and (i). The average DSSIM of the fetal brain equals 0.497 (c) and 0.248 (d), while for the placenta equals to 0.491 (h) and 0.214 in (i).
(b) and (g). The DSSIM heat map for a baseline before reconstruction (c) and (h), and after PVR (d) and (i). The average DSSIM of the fetal brain equals 0.497 (c) and 0.248 (d), while for the placenta equals to 0.491 (h) and 0.214 in (i).
3). Fetal Brain Reconstruction at Late Gestation:
Examination of the brain is one of the primary uses for fetal MRI. While PVR’s primary aim is to reconstruct large fields of view and non-rigid motion, we also compare how PVR performs under the conditions under which SVR is usually applied (accurate delineation of a brain mask followed by rigid full slice to volume registration). This experiment evaluates if PVR can efficiently substitute SVR in pipelines where only the reconstruction of a single, masked rigidly moving organ is required, i.e., for compensation of pure bulk motion.
To compare PVR’s performance, we collected a second dataset comprising of ten mature fetuses (GA > 33 weeks) with little motion, expert brain segmentation, and validated state-of-the-art SVR reconstructions [25]. PVR is applied only in the delineated brain area and compared to clinical SVR reconstructions in terms of PSNR, SSIM, CC and computational overhead. In contrast to [35], where only a small area of the brain was evaluated, we evaluate the full brain mask in the current work. We chose patch parameters similar to [35] (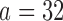 ,
, 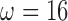 ), which performed best for square patches according to the evaluation in [35] and used the multi-scale superpixel approach presented in this paper. Figure 14 shows that PVR provides similar reconstruction and motion correction quality for the brain as would be the case if a tight expert mask [20] for a region of interest would have been used for SVR. However, the computational overhead of processing patches is always larger than if SVR is used. In this experiment SVR is considered to be the baseline processing 100% of the available pixels. The required runtime of any PVR variant will always be longer than if SVR is used for bulk motion compensation of a defined region of interest.
), which performed best for square patches according to the evaluation in [35] and used the multi-scale superpixel approach presented in this paper. Figure 14 shows that PVR provides similar reconstruction and motion correction quality for the brain as would be the case if a tight expert mask [20] for a region of interest would have been used for SVR. However, the computational overhead of processing patches is always larger than if SVR is used. In this experiment SVR is considered to be the baseline processing 100% of the available pixels. The required runtime of any PVR variant will always be longer than if SVR is used for bulk motion compensation of a defined region of interest.
Fig. 14.
Comparison of the best performing PVR parameters from [35] for square patches and multi-scale superpixels with SVR for motion compensation of the brain in mature (GA > 33 weeks) fetuses with little motion. PVR’s image quality is similar to the small-area SVR method currently used in the clinical practice. The necessary patch overlap for PVR requires more computation, which results generally in longer runtime for any PVR variant.
4). Multi-Scale Reconstruction:
We evaluate different scale parameters for the multi-scale superpixel reconstruction to further assess the influence of the number of scales on the reconstruction quality. Similar to the phantom brain experiment, we reconstruct a brain image from 3-stacks deformed with skewing angle 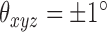 . We use the ground truth image for evaluating quantitatively each reconstructed image with five different scales as shown in Table II. Multi-scale (MS) reconstruction is configured to increase the scale of the patch by a factor of
. We use the ground truth image for evaluating quantitatively each reconstructed image with five different scales as shown in Table II. Multi-scale (MS) reconstruction is configured to increase the scale of the patch by a factor of 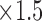 , four times starting from
, four times starting from 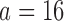 to 40 (2-iteration per each scale). Fixed-scale (FS) reconstruction is configured to fix the size of the patch for every reconstruction iteration. Figure 13 and Table II show that MS-PVR achieves similar performance as FS-PVR, while larger scale patches result in a lower number of input patches and overhead pixels.
to 40 (2-iteration per each scale). Fixed-scale (FS) reconstruction is configured to fix the size of the patch for every reconstruction iteration. Figure 13 and Table II show that MS-PVR achieves similar performance as FS-PVR, while larger scale patches result in a lower number of input patches and overhead pixels.
TABLE II. Comparison Between Fixed-Scale (FS) and Multi-Scale (MS) Superpixels Reconstruction Using 3 Phantom Brain Input Images Deformed With Skewing Angle 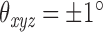 .
.
| FS-16 | FS-24 | FS-32 | FS-40 | FS-48 | MS-16 | |
|---|---|---|---|---|---|---|
| PSNR | 17.1 | 17.3 | 17.6 | 17.9 | 17.2 | 17.3 |
| SSIM | 0.441 | 0.473 | 0.464 | 0.435 | 0.391 | 0.420 |
| CC | 0.682 | 0.691 | 0.715 | 0.721 | 0.659 | 0.678 |
Fig. 13.
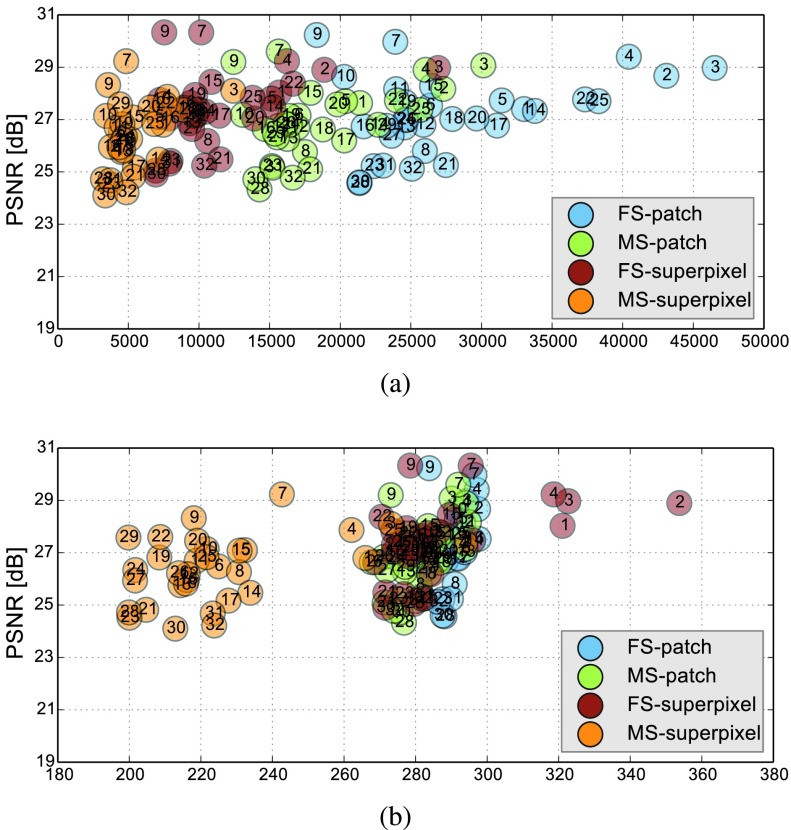
(a) Number of generated patches and (b) necessary additional overhead pixels (%) of the different PVR variants versus their reconstruction PSNR quality of the whole uterus (see Table I-a). Optimal results are found in the upper left corner of the plots, i.e., high reconstruction quality and low computational overhead. The subject number is highlighted inside each circle marker. Multi-scale superpixels (MS-superpixel) achieve similar reconstruction quality to fixed-size (FS-patch), multi-scale (MS-patch) square patches while clustering in the area of minimal computational overhead.
B. Local Deformations
1). Landmarks:
The results for each landmark are summarized in Table III. The inter-observer error is 0.92±0.41 mm. The average distance between all landmarks in PVR reconstructions and the mean landmark location in SVR reconstructions between the two observers is 1.13 ± 0.87 mm. In order to provide an indication of the scale of rigid motion being addressed in the fetal brain landmark images, the estimated displacement distance of all 3D landmarks during imaging is measured by calculating the distance between each annotated landmark and its inverse-transformed point from the correspondent slice of an input stack, assuming rigid slice motion for SVR. The maximum of the calculated displacements equals to 79.85 mm. The total average equals to 13.58 ± 15.23 mm for all landmarks, see Table III. The 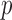 -values from a paired T-test show that the difference between the error introduced between observers and PVR can be considered to be not statistically significant (
-values from a paired T-test show that the difference between the error introduced between observers and PVR can be considered to be not statistically significant (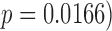 . Overall, this evaluation demonstrates that PVR does not introduce notable structural distortion compared to rigid SVR.
. Overall, this evaluation demonstrates that PVR does not introduce notable structural distortion compared to rigid SVR.
TABLE III. Motion in Terms of the Distance the 3D Landmarks Have Been Estimated (SVR) to Have Been Displaced During Imaging, and the Displacement of These Landmarks Between PVR and Clinically Validated SVR Reconstructions.
| Landmarks | Slice-to-volume motion [mm] | Inter-observer error [mm] | PVR-SVR distance [mm] |
 -value -value |
|---|---|---|---|---|
| Left center of the eye | 15.10 ± 15.90 | 0.95 ± 0.29 | 1.00 ± 0.43 | 0.7983 |
| Right center of the eye | 11.87 ± 11.01 | 0.88 ± 0.40 | 0.96 ± 0.41 | 0.6381 |
| Genu of corpus callosum outer aspect | 13.86 ± 15.16 | 0.69 ± 0.36 | 0.83 ± 0.24 | 0.7670 |
| Splenium of corpus callosum outer aspect | 13.02 ± 15.06 | 1.08 ± 0.56 | 1.11 ± 0.75 | 0.8901 |
| Left Frontal horn of lateral ventricle | 14.93 ± 16.83 | 1.03 ± 0.40 | 1.35 ± 1.00 | 0.3296 |
| Right Frontal horn of lateral ventricle | 17.04 ± 18.83 | 0.90 ± 0.22 | 1.25 ± 0.89 | 0.2511 |
| Left occipital horn of lateral ventricle | 13.40 ± 14.59 | 1.04 ± 0.34 | 0.98 ± 0.48 | 0.7483 |
| Right occipital horn of lateral ventricle | 11.41 ± 13.20 | 1.05 ± 0.38 | 1.05 ± 0.56 | 0.9906 |
| Left temporal horn of lateral ventricle | 12.89 ± 15.88 | 0.69 ± 0.36 | 1.28 ± 1.43 | 0.2278 |
| Right temporal horn of lateral ventricle | 11.83 ± 12.76 | 0.80 ± 0.46 | 1.47 ± 1.33 | 0.1396 |
| All landmarks | 13.58 ± 15.23 | 0.92 ± 0.41 | 1.13 ± 0.87 | 0.0166 |
2). Displacement:
The mean and maximum displacement of 9 reconstructed images are calculated for SVR and PVR with different parameters. The results in Table IV shows that PVR with patch size of 64 achieves the smallest mean error, while this error increases with the decrease of the patch size.
TABLE IV. Mean and Maximum Displacement Error in mm Resulting From Non-Rigid Registration Between Different Reconstructions and the Motion-Free Adult Brain Phantom Ground Truth.
| Displacement | SVR | PVR-Superpixel 16 | PVR-Patch 64 | PVR-Patch 32 | PVR-Patch 16 |
|---|---|---|---|---|---|
| Mean | 1.83 ± 1.55 | 1.70 ± 1.10 | 1.58 ± 1.03 | 1.67 ± 1.07 | 2.40 ± 1.71 |
| Max | 4.91 ± 4.26 | 4.86 ± 3.54 | 4.87 ± 3.92 | 5.02 ± 3.92 | 5.72 ± 4.09 |
C. Performance Analysis
We further evaluate the computational performance of each PVR variant. Measuring the overall runtime is not meaningful because this would be highly machine specific and would include data transfer overhead and non optimized functions. The runtime varied between 2000–4000s on our testing machines, depending on the system configuration. Instead we analyze the computational overhead introduced by PVR in comparison to SVR. The overhead can be measured by counting the number of processed patches and the number of additionally processed voxels. We compare these values to the achieved reconstruction quality in Fig. 13. Multi-scale superpixels show significantly better performance than other PVR variants and introduce only minimal necessary overhead while gaining similar image quality to more naïve PVR variants. Multi-scale superpixels are potentially five times faster than other variants.
VI. Discussion
PVR surpasses reconstruction performance of the state-of-the-art SVR method for cases with considerable non-rigid deformations and inconsistent bulk motion. We have evaluated different variants of PVR using fixed-size and multi-size square patches and fixed-size and multi-size superpixels. ANOVA analysis has shown significant differences between these approaches for different areas of the uterus. However, evaluation of motion compensation methods is difficult especially due to the lack of ground truth in fetal MRI. Mapping the reconstruction quality of a whole 3D volume into a single-valued metric may not properly reflect qualitative differences, if based on averaging all measured values of all input stacks. Therefore, we have additionally performed extensive qualitative analyses and present examples and evaluation based on structural dissimilarity (DSSIM) heat maps.
While an SVR reconstruction is constrained by using whole orthogonal slices assuming rigidity, PVR may introduce geometric deformations due to the relaxation of the rigidity assumption to smaller patches instead of whole slices. For this reason, we evaluated local deformations by selecting anatomical landmarks from the fetal head and compare PVR with SVR, which shows that the difference is not statistically significant for individual landmarks. We also employ dense, non-rigid registration to further validate the geometric accuracy of the reconstruction. Reconstructed images from SVR and PVR with different variants are non-rigidly aligned to a motion-free brain phantom. The mean and maximum displacements of the deformations are then calculated to measure the accuracy. Both experiments evaluate local deformations, although the latter may include errors introduced by the imperfect non-rigid registration process. Maximum displacement errors are mainly observed in feature-poor regions like the amniotic fluid, where automatic non-rigid registration does not perform well. Despite showing strong evidence that PVR works well for the whole uterus, it should be noted that our experiments to assess the reconstruction quality on brain anatomy do not necessarily extend to the achieved reconstruction accuracy of other areas (e.g. moving limbs). Focused validation of individual regions will be required for other, domain specific applications.
In addition to reconstruction and motion correction of the whole uterus, we have demonstrated that the proposed method works for multiple births cases with multiple fetal instances present in the volumetric image. These cases are more likely to have complications and to undergo MRI during pregnancy but would require extensive manual effort to be successfully reconstructed with state-of-the-art methods.
Although our method is able to reconstruct the whole uterus automatically, small parts like limbs that move rapidly between the acquisition of individual slices are more difficult to recover. This is especially problematic for very young fetuses that have more space to move inside the womb. In cases of rapid limb movements (>2 cm between individual slices) PVR is not able to find structural consensus between overlapping patches and blurry image regions will be reconstructed. This is a general problem of automatic intensity-based optimization methods and methods that are able to understand the semantic content of each patch will likely be required for future improvements.
PVR introduces a considerable computational overhead to the reconstruction stage of fetal MR image processing pipelines. We have evaluated the amount of necessary additional redundant information to give a general idea about the expected runtime of different PVR variants. Patches based on multi-scale superpixels are significantly more efficient than a naïve implementation of overlapping square patches, while maintaining a similar reconstruction accuracy. Quantitatively, square patches perform slightly better for the brain, which is most likely due to the rigid nature of the enclosing skull. Superpixel-based patches achieve better results for regions that are likely affected by non-rigid movements like the placenta and the whole uterus.
VII. Conclusion
We have introduced and evaluated the concept of patch-to-volume reconstruction (PVR) in order to compensate non-rigid motion artifacts from fetal MRI scans without requiring a defined region of interest. PVR splits the 3D input image into overlapping square patches and superpixels and employs automatic EM-based outlier rejection to find consistent data.
Our method is able to automatically reconstruct whole collections of motion corrupted stacks without the need for image segmentation and manual identification of rigid regions. We have shown that PVR can reconstruct the whole uterus, selected fetal organs, and secondary, non-rigidly moving pregnancy structures such as the placenta.
PVR’s reconstruction quality and computational performance has been evaluated quantitatively and qualitatively on an adult phantom T2-weighted brain with synthetic non-rigid motion artifacts, as well as on the whole uterus from motion corrupted fetal MRI data including fetal brain, placenta and cases with multiple births.
Acknowledgment
The authors thank the volunteers, the radiographers J. Allsop and M. Fox, MITK [51], IRTK (CC public license from IXICO Ltd.). Data access only in line with the informed consent of the participants, subject to approval by the project ethics board and under a formal Data Sharing Agreement. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding Statement
This work was supported in part by the NIHR Biomedical Research Centre, Wellcome Trust/EPSRC under Award IEH 102431/NS/A000025/1, ERC under Grant 319456, the NVIDIA (GPU donations), and EPSRC EP/N024494/1. The work of A. Alansary was supported by an Imperial College President’s Ph.D. Scholarship.
Footnotes
References
- [1].Mansfield P. and Maudsley A. A., “Planar spin imaging by NMR,” J. Magn. Reson., vol. 27, no. 1, pp. 101–119, 1977. [Google Scholar]
- [2].Ertl-Wagner B., Lienemann A., Strauss A., and Reiser M. F., “Fetal magnetic resonance imaging: Indications, technique, anatomical considerations and a review of fetal abnormalities,” Eur. Radiol., vol. 12, no. 8, pp. 1931–1940, 2002. [DOI] [PubMed] [Google Scholar]
- [3].Levine D.et al. , “Fetal fast MR imaging: Reproducibility, technical quality, and conspicuity of anatomy,” Radiology, vol. 206, no. 2, pp. 549–554, 1998. [DOI] [PubMed] [Google Scholar]
- [4].Huppert B., Brandt K., Ramin K., and King B., “Single-shot fast spin-echo MR imaging of the fetus: A pictorial essay,” Radiographics, vol. 19, no. 1, p. 10, 1999. [DOI] [PubMed] [Google Scholar]
- [5].Garel C., “Imaging the fetus: When does MRI really help?” Pediatric Radiol., vol. 38, pp. 467–470, Jun. 2008. [DOI] [PubMed] [Google Scholar]
- [6].Malamateniou C.et al. , “Motion-compensation techniques in neonatal and fetal MR imaging,” Amer. J. Neuroradiol., vol. 34, no. 6, pp. 1124–1136, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Glenn O. A. and Barkovich A. J., “Magnetic resonance imaging of the fetal brain and spine: An increasingly important tool in prenatal diagnosis, part 1,” Amer. J. Neuroradiol., vol. 27, no. 8, pp. 1604–1611, 2006. [PMC free article] [PubMed] [Google Scholar]
- [8].Linduska N.et al. , “Placental pathologies in fetal MRI with pathohistological correlation,” Placenta, vol. 30, no. 6, pp. 555–559, 2009. [DOI] [PubMed] [Google Scholar]
- [9].Zamora I. J.et al. , “Fetal MRI lung volumes are predictive of perinatal outcomes in fetuses with congenital lung masses,” J. Pediatric Surg., vol. 49, no. 6, pp. 853–858, 2014. [DOI] [PubMed] [Google Scholar]
- [10].Pierro A., Kiely E. M., and Spitz L., “Classification and clinical evaluation,” Seminars Pediatric Surg., vol. 24, no. 5, pp. 207–211, 2015. [DOI] [PubMed] [Google Scholar]
- [11].Frates M. C., Kumar A. J., Benson C. B., Ward V. L., and Tempany C. M., “Fetal anomalies: Comparison of MR imaging and US for diagnosis,” Radiology, vol. 232, no. 2, pp. 398–404, 2004. [DOI] [PubMed] [Google Scholar]
- [12].Patenaude Y.et al. , “The use of magnetic resonance imaging in the obstetric patient,” J. Obstetrics Gynaecol. Canada, vol. 36, no. 4, pp. 349–363, 2014. [DOI] [PubMed] [Google Scholar]
- [13].Cannie M. M.et al. , “Potential heating effect in the gravid uterus by using 3-T MR imaging protocols: Experimental study in miniature pigs,” Radiology, vol. 279, no. 3, pp. 754–761, 2015. [DOI] [PubMed] [Google Scholar]
- [14].Levine D., “Fetal magnetic resonance imaging,” J. Maternal-Fetal Neonatal Med., vol. 15, no. 2, pp. 85–94, Feb. 2004. [DOI] [PubMed] [Google Scholar]
- [15].Park S. C., Park M. K., and Kang M. G., “Super-resolution image reconstruction: A technical overview,” IEEE Signal Process. Mag., vol. 20, no. 3, pp. 21–36, May 2003. [Google Scholar]
- [16].Rousseau F.et al. , “Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images,” Acad. Radiol., vol. 13, no. 9, pp. 1072–1081, 2006. [DOI] [PubMed] [Google Scholar]
- [17].Kim K., Habas P. A., Rousseau F., Glenn O. A., Barkovich A. J., and Studholme C., “Intersection based motion correction of multislice MRI for 3-D in utero fetal brain image formation,” IEEE Trans. Med. Imag., vol. 29, no. 1, pp. 146–158, Jan. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gholipour A., Estroff J. A., and Warfield S. K., “Robust super-resolution volume reconstruction from slice acquisitions: Application to fetal brain MRI,” IEEE Trans. Med. Imag., vol. 29, no. 10, pp. 1739–1758, Oct. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kuklisova-Murgasova M., Quaghebeur G., Rutherford M. A., Hajnal J. V., and Schnabel J. A., “Reconstruction of fetal brain MRI with intensity matching and complete outlier removal,” Med. Image Anal., vol. 16, no. 8, pp. 1550–1560, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keraudren K.et al. , “Automated fetal brain segmentation from 2D MRI slices for motion correction,” NeuroImage, vol. 101, pp. 633–643, Nov. 2014. [DOI] [PubMed] [Google Scholar]
- [21].Alansary A.et al. , “Automatic brain localization in fetal MRI using superpixel graphs,” in Machine Learning Meets Medical Imaging. Cham, Switzerland: Springer, 2015, pp. 13–22. [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-319-27929-9_2 [Google Scholar]
- [22].Tourbier S.et al. , “Automated template-based brain localization and extraction for fetal brain MRI reconstruction,” NeuroImage, vol. 155, pp. 460–472, Jul. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kainz B.et al. , “Motion corrected 3D reconstruction of the fetal thorax from prenatal MRI,” in Medical Image Computing and Computer-Assisted Intervention—MICCAI. Cham, Switzerland: Springer, 2014, pp. 284–291. [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-319-10470-6_36 [DOI] [PubMed] [Google Scholar]
- [24].Lloyd D. F. A.et al. , “Three-dimensional modelling of the fetal vasculature from prenatal MRI using motion corrected slice-to-volume registration,” in Proc. Int. Soc. Magn. Reson. Med., vol. 24 Sep. 2016, p. O413. [Google Scholar]
- [25].Kainz B., et al. , “Fast volume reconstruction from motion corrupted stacks of 2D slices,” IEEE Trans. Med. Imag., vol. 34, no. 9, pp. 1901–1913, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Routledge E., Malamateniou C., and Rutherford M., “Can MR imaging of the placenta demonstrate a distinct placental phenotype in normal and abnormal pregnancies,” Bachelor Sci. Degree Imag. Sci., King’s College London, Univ. London, London, U.K., Tech. Rep., Apr. 2015. [Google Scholar]
- [27].Salafia C., Misra D., Yampolsky M., Charles A., and Miller R., “Allometric metabolic scaling and fetal and placental weight,” Placenta, vol. 30, no. 4, pp. 355–360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Keraudren K.et al. , “Automated localization of fetal organs in MRI using random forests with steerable features,” in Medical Image Computing and Computer-Assisted Intervention—MICCAI (Lecture Notes in Computer Science), vol. 9351 Cham, Switzerland: Springer, 2015, pp. 620–627. [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-319-24574-4_74 [Google Scholar]
- [29].Alansary A.et al. , “Fast fully automatic segmentation of the human placenta from motion corrupted MRI,” in Proc. 19th Int. Conf. Med. Image Comput. Comput.-Assist. Intervention—MICCAI, Athens, Greece, Oct. 2016 [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-319-46723-8_68 [Google Scholar]
- [30].Fei B., Duerk J. L., Boll D. T., Lewin J. S., and Wilson D. L., “Slice-to-volume registration and its potential application to interventional MRI-guided radio-frequency thermal ablation of prostate cancer,” IEEE Trans. Med. Imag., vol. 22, no. 4, pp. 515–525, Apr. 2003. [DOI] [PubMed] [Google Scholar]
- [31].Jiang S., Xue H., Glover A., Rutherford M., Rueckert D., and Hajnal J. V., “MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): Application to fetal, neonatal, and adult brain studies,” IEEE Trans. Med. Imag., vol. 26, no. 7, pp. 967–980, Jul. 2007. [DOI] [PubMed] [Google Scholar]
- [32].Rousseau F., Kim K., Studholme C., Koob M., and Dietemann J.-L., “On super-resolution for fetal brain MRI,” in Medical Image Computing and Computer-Assisted Intervention—MICCAI. Berlin, Germany: Springer; 2010, pp. 355–362. [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-642-15745-5_44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rousseau F.et al. , “BTK: An open-source toolkit for fetal brain MR image processing,” Comput. Methods Programs Biomed., vol. 109, no. 1, pp. 65–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tourbier S., Bresson X., Hagmann P., Thiran J.-P., Meuli R., and Cuadra M. B., “An efficient total variation algorithm for super-resolution in fetal brain MRI with adaptive regularization,” NeuroImage, vol. 118, pp. 584–597, Sep. 2015. [DOI] [PubMed] [Google Scholar]
- [35].Kainz B.et al. , “Flexible reconstruction and correction of unpredictable motion from stacks of 2D images,” in Medical Image Computing and Computer-Assisted Intervention—MICCAI (Lecture Notes in Computer Science), vol. 9350 Cham, Switzerland: Springer, 2015, pp. 555–562. [Online]. Available: https://link.springer.com/chapter/10.1007/978-3-319-24571-3_66 [Google Scholar]
- [36].Little J. A., Hill D. L. G., and Hawkes D. J., “Deformations incorporating rigid structures [medical imaging],” in Proc. IEEE Workshop Math. Methods Biomed. Image Anal., Jun. 1996, pp. 104–113. [Google Scholar]
- [37].Commowick O.et al. , “An efficient locally affine framework for the smooth registration of anatomical structures,” Med. Image Anal., vol. 12, no. 4, pp. 427–441, 2008. [DOI] [PubMed] [Google Scholar]
- [38].Arsigny V., Commowick O., Ayache N., and Pennec X., “A fast and log-Euclidean polyaffine framework for locally linear registration,” J. Math. Imag. Vis., vol. 33, no. 2, pp. 222–238, 2009. [Google Scholar]
- [39].Achanta R., Shaji A., Smith K., Lucchi A., Fua P., and Süsstrunk S., “SLIC superpixels compared to state-of-the-art superpixel methods,” IEEE Trans. Pattern Anal. Mach. Intell., vol. 34, no. 11, pp. 2274–2282, Nov. 2012. [DOI] [PubMed] [Google Scholar]
- [40].Felzenszwalb P. F. and Huttenlocher D. P., “Efficient graph-based image segmentation,” Int. J. Comput. Vis., vol. 59, no. 2, pp. 167–181, Sep. 2004. [Google Scholar]
- [41].Vedaldi A. and Soatto S., “Quick shift and kernel methods for mode seeking,” in Proc. ECCV, 2008, pp. 705–718. [Google Scholar]
- [42].Goldberg D., “What every computer scientist should know about floating-point arithmetic,” ACM Comput. Surv., vol. 23, no. 1, pp. 5–48, Mar. 1991. [Google Scholar]
- [43].Penney G. P., Weese J., Little J. A., Desmedt P., Hill D. L. G., and Hawkes D. J., “A comparison of similarity measures for use in 2-D-3-D medical image registration,” IEEE Trans. Med. Imag., vol. 17, no. 4, pp. 586–595, Aug. 1998. [DOI] [PubMed] [Google Scholar]
- [44].Charbonnier P., Blanc-Feraud L., Aubert G., and Barlaud M., “Deterministic edge-preserving regularization in computed imaging,” IEEE Trans. Image Process., vol. 6, no. 2, pp. 298–311, Feb. 1997. [DOI] [PubMed] [Google Scholar]
- [45].Nickolls J., Buck I., Garland M., and Skadron K., “Scalable parallel programming with CUDA,” Queue, vol. 6, no. 2, pp. 40–53, Mar. 2008. [Google Scholar]
- [46].Sanders J. and Kandrot E., CUDA by Example: An Introduction to General-Purpose GPU Programming, 1st ed. Boston, MA, USA: Addison-Wesley, 2010. [Google Scholar]
- [47].Steinberger M., Kainz B., Kerbl B., Hauswiesner S., Kenzel M., and Schmalstieg D., “Softshell: Dynamic scheduling on GPUs,” ACM Trans. Graph., vol. 31, no. 6, pp. 161:1–161:11, Nov. 2012. [Google Scholar]
- [48].Cocosco C. A., Kollokian V., Kwan R. K.-S., Pike G. B., and Evans A. C., “BrainWeb: Online interface to a 3D MRI simulated brain database,” in Proc. 3rd Int. Conf. Funct. Mapping Human Brain, vol. 5 Copenhagen, Denmark, May 1997, p. S425. [Google Scholar]
- [49].Wang Z., Bovik A. C., Sheikh H. R., and Simoncelli E. P., “Image quality assessment: From error visibility to structural similarity,” IEEE Trans. Image Process., vol. 13, no. 4, pp. 600–612, Apr. 2004. [DOI] [PubMed] [Google Scholar]
- [50].Rueckert D., Sonoda L. I., Hayes C., Hill D. L. G., Leach M. O., and Hawkes D. J., “Nonrigid registration using free-form deformations: Application to breast MR images,” IEEE Trans. Med. Imag., vol. 18, no. 8, pp. 712–721, Aug. 1999. [DOI] [PubMed] [Google Scholar]
- [51].Wolf I.et al. , “The medical imaging interaction toolkit,” Med. Image Anal., vol. 9, no. 6, pp. 594–604, 2005. [DOI] [PubMed] [Google Scholar]