Abstract
Purpose of Review
Ketamine produces rapid (within hours) antidepressant actions, even in patients considered treatment resistant, and even shows promise for suicidal ideation. Here, we review current research on the molecular and cellular mechanisms of ketamine and other novel rapid-acting antidepressants, and briefly explore gender differences in the pathophysiology and treatment of MDD.
Recent Findings
Ketamine, an NMDA receptor antagonist, increases BDNF release and synaptic connectivity, opposing the deficits caused by chronic stress and depression. Efforts are focused on the development of novel rapid agents that produce similar synaptic and rapid antidepressant actions, but without the side effects of ketamine. The impact of gender on the response to ketamine and other rapid-acting antidepressants is in early stages of investigation.
Summary
The discovery that ketamine produces rapid therapeutic actions for depression and suicidal ideation represents a major breakthrough and much needed alternative to currently available medications. However, novel fast acting agents with fewer side effects are needed, as well as elucidation of the efficacy of these rapid-acting antidepressants for depression in women.
Keywords: Depression, Antidepressants, BDNF, Ketamine, Sex differences
Introduction
Major depressive disorder (MDD) is a chronic and debilitating neuropsychiatric illness that affects nearly 1/5 of the population and causes substantial social and economic consequences [1–4]. Depression affects nearly 300 million people worldwide, and rates of MDD are climbing, with an 18% increase in prevalence between 2005 and 2015 [5]. Traditionally, depression and closely related anxiety disorders have been treated using a combination of behavioral therapy and monoaminergic agents, notably, the selective serotonin reuptake inhibitors (SSRIs). However, one-third of patients will require a trial-and-error period before they find an appropriate treatment and another one-third of patients will not respond to multiple trials of antidepressants and are thus classified as having treatment resistant depression (TRD) [6]. Another serious limitation of SSRI medications is a time lag of weeks to months to be effective, and combined with the variable efficacy among MDD patients, pose significant constraints. In 2014 alone, suicide claimed the lives of more than 42,000 people, compared to 16,000 by homicide, and suicide rates are on the rise in the USA, with rates 24% higher in 2014 than 1999 [7, 8].
Depression is a recurrent and pervasive disease that can affect individuals throughout life. However, depression is approximately twofold more common in women than men. Although the exact biological cause for the observed differential diagnosis remains elusive, it is known that women suffer from specific forms of depression-related events during periods of hormonal fluctuations, namely puberty, peripartum periods, and menopause. However, prevalence of MDD in women is higher across the lifespan, independent of hormonal stage, suggesting other factors that place women at a higher risk [9]. Therefore, a thorough understanding of the underlying mechanisms driving the sex differences in depression is critical for developing better treatments.
Given the extensive personal and economic consequences and anticipated rise in rates of MDD, more efficacious and faster acting treatments are sorely needed. Current pharmacological treatments, while effective for some, are largely inadequate and are associated with undesirable side effects. One logical step towards the development of effectual treatments is to better understand the etiology of the disease. Much of the work has focused on deficits in monoamine neurotransmitter systems, including serotonin and norepinephrine, and is based largely on the discovery that drugs that block the metabolism or reuptake of monoamines have clinical efficacy for some [10]. However, the therapeutic limitations of these agents, combined with a lack of evidence to support a monoamine deficiency hypothesis, have led to new avenues of investigation.
The seminal findings showing rapid and sustained antidepressant effects of acute ketamine infusions has revolutionized the way the field understands both the pathophysiology of and potential treatments for depression [11, 12]. While the underlying etiology and pathophysiology of depression remain incomplete, clinical and basic research studies are beginning to provide evidence that depression is associated with atrophy of neurons in cortical and limbic brain regions that control mood and emotion [13, 14]. The discovery that antagonists of the N-methyl-D-aspartate (NMDA) receptor, notably ketamine, produce rapid improvement in depressive symptoms (within hours), even in TRD patients, has shifted efforts towards novel agents targeting the glutamatergic system. Importantly, rodent studies demonstrate that ketamine rapidly increases synaptic connections in the prefrontal cortex (PFC) and reverses the deficits caused by chronic stress [15, 16]. This pioneering work on ketamine has launched investigations into a variety of rapid agents that act at different NMDA sites or within the glutamate system. A number of non-glutamatergic drugs, such as the muscarinic acetylcholine inhibitor scopolamine, are also under investigation for their rapid antidepressant effects. However, ketamine also produces transient dissociative and psychotomimetic effects, and is a drug of abuse, limiting its wide spread use. The ultimate goal of drug development efforts is to identify rapid acting agents that reverse the stress-induced neuronal deficits caused by stress and depression, but with fewer side effects.
The purpose of this review is to present the fields current understanding of the pathophysiology of depression and the mechanisms and potential of rapid-acting antidepressants. Furthermore, we will discuss how sex differences impact our understanding of the pathophysiology and treatment of depression.
Pathophysiological Consequences of Stress and Depression
The adverse effects of MDD extend well beyond perceptible behavioral deficits, as decades of research have begun to elucidate the cellular and molecular changes that contribute to the underlying pathophysiology of depression and stress-related illnesses. Clinical and pre-clinical studies have focused on the neural circuits that are altered following prolonged bouts of stress and depression. Although not exclusive, the PFC, hippocampus, NAc, and amygdala are a few brain regions disrupted in depression. These studies have furthered our understanding of the pathophysiology of MDD and can help direct the rational design of novel antidepressants that correct the disrupted synaptic and circuit level alterations.
Stress and depression cause neuronal atrophy
MDD has been characterized by reduced blood flow and glucose metabolism, a proxy for neural activity, in the PFC, and is attributed to the episode-dependent reductions in volume in depressed patients [17, 18]. Furthermore, rodent models of stress and depression and post-mortem MDD studies report decreased synapse numbers in the PFC [16, 17]. Synapses are the key connections linking neurons, and reductions in synaptic number can decrease neural communication. Reduced activity and connectivity in the PFC is believed to underlie impairments in executive function observed in patients with MDD [14], and could also lead to loss of top down control of other brain regions, such as the amygdala and NAc that underlie anxiety, emotion, motivation, and reward.
Preclinical studies in rodent models demonstrate that chronic stress can result in symptoms of depression, including helplessness/despair and anhedonia, as seen in the forced-swim test (FST) and sucrose preference test (SPT), respectively [16]. The behavioral deficits of chronic stress are associated with reduced number and function of spine synapses in the medial PFC (mPFC) [16, 19–21]. Evidence of a direct link between PFC synapse number and behavior is provided by a recent report that an inhibitor of mammalian target of rapamycin complex 1 (mTORC1) signaling, REDD1 (regulated in development and DNA damage responses 1) that decreases synapse number causes helpless and anhedonic behavior in rodents in the absence of stress exposure [22]. Together, these human and rodent studies of PFC indicate that depression can be viewed as a mild neurodegenerative disease, characterized by neuronal atrophy and that treatments should target neural repair systems and reversal of the observed atrophy. Although depression should be considered a system-wide disorder, the PFC is an ideal target for treatments as it receives and sends substantial projections throughout the brain to both cortical and subcortical regions that have been implicated in depression.
Role of BDNF in Stress and Depression
Individual differences in stress resilience and susceptibility have been linked to the expression of brain-derived neurotrophic factor (BDNF) genetic polymorphisms, particularly the Val/Met polymorphism at codon 66 (Val66Met). When compared with Val/Val homozygotes, Met-allele carriers exhibit heightened levels of anxiety and depression [23]. BDNF Val/Met transgenic mice have provided additional insight into the role of BDNF in the development and treatment of depression. Rodents carrying the Met-allele show reduced spine and dendrite complexity in the hippocampus and PFC [24, 25], as well as diminished synaptic plasticity in the medial PFC and reduced dendritic secretion of BDNF in the hippocampus [26, 27]. The BDNF Met-allele blocks the processing and activity dependent release of mature BDNF, which are required for activity dependent synaptic plasticity and which could explain the deficit in synapse number in mice carrying the Met-allele. Additionally, preclinical studies further emphasize the importance of BNDF in treatment response as neither SSRIs nor ketamine are effective in Met/Met mice [25, 28]. Together, studies over the past decade have established support for a role of BDNF in the etiology and treatment of depression.
Excitation-Inhibition (E:I) Imbalance and MDD
The neocortex in mammals gives rise to and supports a diverse set of cognitive functions, making it a primary target of investigations in depression. Explorations into the connections and function of microcircuits in the neocortex are critical for understanding how the system works in both healthy and diseased states. In the PFC, glutamatergic and GABAergic signaling work in concert to control complex cognitive behaviors and executive control. Historically, pyramidal neurons have been the focus of studies targeting the PFC, but over the past decade, research has broadened to include the inhibitory interneurons and their extensive projections onto pyramidal cells. At the level of neurotransmitters and their receptors, individuals with MDD exhibit profound deficits in GABAergic signaling that is believed to be either a causal or a contributing factor in MDD. Inhibitory neurons maintain control over the activity of neighboring excitatory neurons through the release γ-aminobutyric acid (GABA), which binds to postsynaptic ionotropic GABAA receptors and increases the membrane Cl− permeability that hyperpolarizes neurons [29]. In patients with MDD, it has been reported that there are reduced concentrations of GABA [30–32], expression of GABAA receptors [33], and expression of glutamic acid decarboxylase (GAD), a key GABA synthetic enzyme [33, 34]. Accompanying these GABAergic deficits are disruptions in functional glutamatergic activity. Some MDD studies report a decrease in glutamatergic transmission [35] as well as reduced number and function of NMDA receptors [36], as well as deficits in the number of glutamate synapses.
Disruption to the E:I balance in the PFC is believed to underlie the regional hypotrophy and deficits in executive functioning that characterize MDD [14]. Human neuroimaging studies show reduced activity and volume in the PFC [17, 18], and both studies in human MDD post-mortem and pre-clinical models of stress and depression report reduced spine number and function [16, 17]. Prolonged stress and depression leads to atrophy and loss of function in the PFC, which is attributed to the adverse homeostatic-like reductions in both GABA [30–34, 37] and glutamate [35, 36, 38] as a result of prolonged stress and depression. Furthermore, rodents with reduced levels of GABAAR function show a depressive and anxiety-like phenotype that is reversed following ketamine administration [39•].
Human findings on the relationship between MDD and an E:I imbalance are supported by causal studies in rodents. Mice with reduced GABAAR function show a depressive and anxiety-like phenotype that is reversed following chronic treatment with an SSRI [40]. Ketamine also rapidly reverses the depressive-like phenotype observed in these mice, possibly due to its potentiation of inhibitory synapses in the mPFC [39•]. Furthermore, ketamine’s effects were stronger in the mice with GABA deficits compared to WT controls. These studies suggest that impaired GABA synaptic activity in the mPFC could underlie depression-related brain states and is a promising target for antidepressant treatments. Ultimately, the local circuit is becoming disorganized from within and disconnected from regional circuits involved in controlling mood.
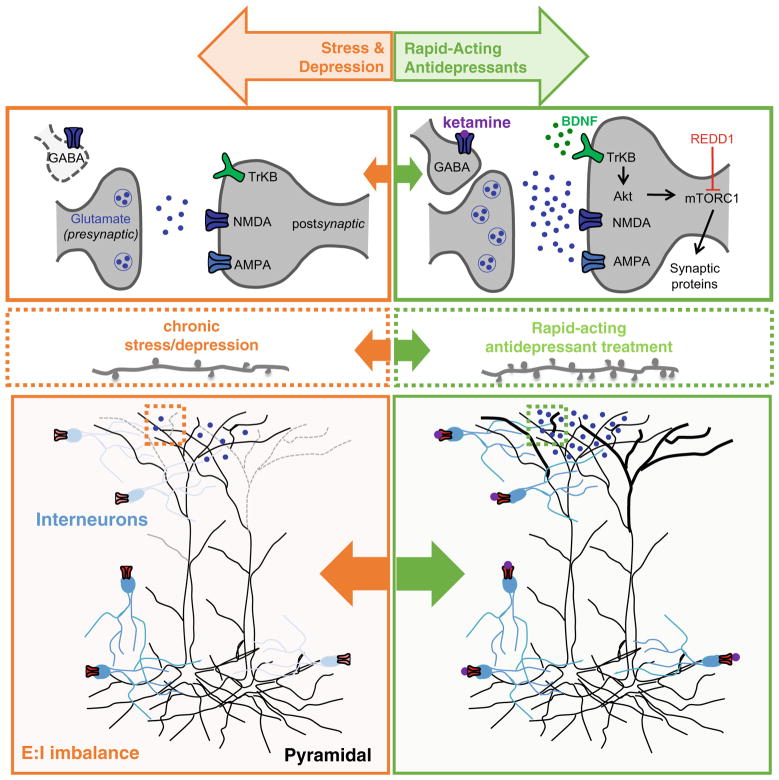
Collectively, studies investigating the pathophysiological consequences of stress and depression highlight deficits in neurotropic signaling and synaptic connectivity and suggest an overall imbalance in E:I activity in the PFC (Fig. 1). The significant limitations of currently available treatments combined with the accumulation of evidence suggesting that MDD is a neurodegenerative disease have driven the development of novel, faster-acting antidepressants that work to reverse these deficits.
Fig. 1.
Pathophysiological consequences of stress and depression and reversal following acute treatment with rapid-acting antidepressants. Chronic stress and depression result in neuronal atrophy and an E:I imbalance in the PFC. These deficits are reversed by rapid-acting antidepressants like ketamine. Low-dose ketamine triggers a rapid and transient burst of glutamate in the mPFC, possibly through disinhibition of pyramidal neurons, which then activates post-synaptic AMPARs. Activity-dependent release of BDNF and activation of TrkB receptors initiates an mTOR-dependent intracellular signaling cascade that regulates synaptogenesis
Rapid-acting antidepressants reverse stress-induced deficits
Ketamine, prototypical rapid acting antidepressant
Drug development rarely progresses uni-directionally from pre-clinical models up the chain to clinical practice. Instead, headway is recursive in nature, traversing back and forth from the basic sciences to clinical trials. The journey of ketamine is a perfect example of this process. As this review has already highlighted, ketamine has shown immense promise as a rapid-acting antidepressant, particularly helpful in treating TRD and suicidal ideation. A number of studies have shown that ketamine’s rapid antidepressant effects are dependent on the activation of AMPA receptors, the activity-dependent release of BDNF, and downstream activation of mTORC1 signaling [15, 41–43]. Other rapid-acting antidepressants (discussed below in more detail) have also been shown to require activation of these same pathways (Table 1).
Table 1.
Ketamine and other rapid-acting antidepressants require activation of similar pathways. Ketamine, the prototypical rapid-acting antidepressant, is AMPAR-, BDNF-, and mTOR-dependent and is effective in TRD. In comparison, SSRIs are slow acting, ineffective in TRD, and are not mTOR-dependent. The mechanism of action of other rapid-acting antidepressants are still ongoing, however they show activation of similar pathways to ketamine
| Rapid-acting? | TRD? | Side effect profile | AMPA-dependent? | BDNF-dependent? | mTOR-dependent? | |
|---|---|---|---|---|---|---|
| SSRIs | No [6] | No [6] | Sexual dysfunction, weight gain [6] | Not tested | Yes [24] | No [15] |
| Ketamine | Yes [11, 12, 15, 16, 41, 43] (rodent and clinical studies) | Yes [11, 12] (clinical trials) | Psychotomimetic, dissociative, abuse potential [11, 12] PPI and CPP effects in rodent studies | Yes [15, 41, 43] | Yes [42] | Yes [15] |
| GluN2B antagonists | Yes [15, 16, 41] (rodent studies only) | – | No effect in PPI or CPP in rodent studies [41] | Yes [15, 41] | Not tested | Yes [15] |
| (S)-ketamine | Yes [44, 45] (rodent and clinical studies) | Yes [44] (clinical trials) | Psychotomimetic, dissociative, abuse potential. [45] PPI effects in rodents | Yes [45] | Yes [45] | Yes [46] |
| (R)-ketamine | Yes [45, 47, 48] (rodent studies only) | – | No effect in PPI or CPP in rodent studies [45] | Yes [45, 48] | Yes [45] | No [46] |
| (2R,6R)-HNK | Yes [46] (rodent studies only) | – | No effect in PPI or CPP in rodent studies [49••] | Yes[49••] | Yes (Fukumoto et al., unpublished data) | Yes (Fukumoto et al., unpublished data) |
| Glyx-13 | Yes [50–54, 55•] (rodent studies and clinical trials) | In Phase III clinical trial [52] | No effect in PPI or CPP in rodent studies [55•] | Yes [51] | Yes [53] | Yes [52] |
| Scopolamine | Yes[56–61] (rodent and clinical studies) | – | Light headed, blurred vision, drowsy, dry mouth [56–58] | Yes [59] | Yes [60] | Yes [59] |
– = still unknown to clarify what the bars in the table mean
A major question in the field is how an NMDA receptor antagonist leads to a rapid increase in synapse number and function? Early studies reported that ketamine produces a transient increase in extracellular glutamate [62], and glutamate activation of AMPA receptors is required for the synaptic and behavioral actions of ketamine in rodent models [15]. Evidence that ketamine initially blocks tonic firing GABA neurons, leading to a burst of glutamate, supported a disinhibition hypothesis [13, 63, 64] (Fig. 1). This is supported by preliminary studies demonstrating that knockdown of GluN2B receptors on GABA, but not glutamate neurons in the mPFC blocks the antidepressant actions of ketamine (Gerhard et al., unpublished data). There is also evidence that ketamine acts directly on excitatory neurons, and further studies are underway to address this question [65].
Due to the psychotomimetic and dissociative properties of ketamine, some may find ketamine infusions intolerable. Furthermore, ketamine, known by many by its street name “Special K,” has potential for abuse following chronic use and potentially chronic treatment [66, 67]. To bypass these adverse side effects and achieve comparable efficacy, the field has begun to further explore ketamine’s actions.
Selective GluN2B Antagonists
A better understanding of the cellular mechanisms underlying ketamine can guide the development of more selective agents that lack side effects and are more effective by virtue of their selectivity. The effectiveness of ketamine has engendered a novel route of research for antidepressants towards effective and rapid-acting therapies rooted in modulating NMDAR-mediated glutamatergic transmission. Recently, studies have focused on agents that select for NMDA receptor subtypes [68].
GluN2B has been the focus of depression drug development studies following a clinical report that a selective GluN2B antagonist produces a relatively rapid antidepressant response. Preskorn and colleagues conducted a double-blind placebo-controlled clinical study of the GluN2B selective antagonist CP-101,606 (traxoprodil) and found antidepressant responses 5 days after drug administration, including in patients with TRD [69]. Furthermore, compared to ketamine, the psychotomimetic effects of CP-101,606 appear to be diminished, although not absent. Following these clinical findings, preclinical studies of another GluN2B antagonist, Ro-25-6891 have been conducted and compared with ketamine. Ro-25-6891 administration rapidly increases mTORC1 signaling within 1 h and elevates synaptic proteins in the PFC within 6 h after dosing [15]. Ro-25-6891 also produces antidepressant responses in the forced swim and novelty suppressed feeding tests. Another study reports that a single dose of Ro-25-6891 produces a rapid reversal of depressive-like behaviors resulting from chronic unpredictable stress, as seen in the SPT and novelty-suppressed feeding test (NSFT) [16]. However, a phase 2 clinical trial with another GluN2B antagonist, CERC-301 was negative, although there was a small effect at the higher dose tested (Cerecor press release), and further studies are needed to confirm the antidepressant efficacy of this and other GluN2B antagonists.
Antidepressant Efficacy of (R, S)-Ketamine Enantiomers and Metabolites
Despite the potential for abuse and toxicity, ketamine represents a compelling therapeutic agent as well as prototype for drug discovery, and clinical studies of ketamine are currently underway. This has sparked further investigation into the chemical makeup of ketamine in the hopes of discovering a more specific and efficacious treatment for the clinic. The parent compound (R, S)-ketamine is a racemic mixture of the (R)- and (S)-enantiomers. Due to its higher affinity for the NMDA receptor, (S)-ketamine has been considered the active isomer and thus the leading contender as a rapid-acting antidepressant [70]. In support of this hypothesis, a recent clinical study reported rapid-acting (within 2 h) antidepressant effects of (S)-ketamine compared to placebo [44]. However, it is important to note that a saline placebo was used instead of an active placebo like midazolam, thus treatment condition may not have been fully blinded. Furthermore, (S)-ketamine injections produce adverse effects similar to the racemic mixture. Nevertheless, (S)-ketamine, formulated as a nasal spray application, has fast-track designation for the treatment of depression and suicide ideation by the FDA.
Recent studies in rodents suggest that, contrary to original hypotheses, (R)-ketamine produces rapid-acting antidepressant effects that are comparable to (R, S)-ketamine but without side effect behaviors, including prepulse inhibition and conditioned place preference, measures of sensory gating, and drug abuse potential, respectively [45, 47, 49••]. A rodent study using repeated administration of corticosterone to induce depressive-like behavior found that acute injections of either (R, S)-ketamine or (R)-ketamine, but not (S)-ketamine, was sufficient to produce long-lasting antidepressant effects, as measured by the FST and tail suspension test (TST) 24 and 48 h after injection, respectively [48]. However, there has yet to be a comprehensive investigation of (R)-ketamine in clinical trials, studies that are critical for validating the efficacy and side effect profile of this enantiomer. Interestingly, a recent study found that (S)-ketamine’s antidepressant effects are mTOR-dependent whereas ERK signaling appears to be key to the antidepressant effects of (R)-ketamine [46]. The observed divergence in signaling pathways may explain the differential efficacy and side effect profiles reported in rodent studies.
The parent drug (R, S)-ketamine is metabolized into an array of metabolites, and the contribution of these individual metabolites to the antidepressant effects of ketamine has largely been unknown. New evidence suggests that an active me-tabolite of ketamine, (2R,6R)-hydroxynorketamine (HNK), exerts antidepressant-like effects in mice, but without side effect behaviors (i.e., no effect in prepulse inhibition or conditioned place preference [49••]. Similar to ketamine, preliminary research suggests that HNK’s effects are BDNF- and mTOR-dependent (Fukumoto et al., unpublished data). Furthermore, the study concludes that (2R,6R)-HNK does not act at any known NMDA sites and the binding site is currently unknown. Like (R)-ketamine, clinical trials are required to determine the efficacy and side effect profile of (2R,6R)-HNK as findings in rodent models do not always translate to the clinical setting.
Antidepressant Efficacy of GLYX-13 (Rapastinel)
The binding site for the co-agonist glycine is located on the GluN2B subunit, making it a unique target for the treatment of depression, since, as discussed, this subunit had been under investigation. One compound of note is GLYX-13 (Rapastinel), a tetrapeptide and functional glycine-like partial agonist, specifically at GluN2B containing NMDARs [50]. Recent preclinical work has explored antidepressant models and found that a single dose of GLYX-13 is sufficient to produce a rapid antidepressant response, including reversal of anhedonia resulting from chronic unpredictable stress exposure [51, 52]. Similar to ketamine, GLYX-13 increases spine synapse formation in the PFC, and its antidepressant effects are AMPAR-, BDNF-, and mTORC1-dependent [51, 53, 54]. However, unlike ketamine, GLYX-13 does not influence responding in prepulse inhibition or conditioned place preference, or 5-HT2A induced head-twitch response of impulsivity in a serial reaction time task [51].
Phase II trials in depressed patients have also demonstrated that GLYX-13 produces rapid antidepressant action, but without the dissociative and psychotomimetic effects of ketamine [55•]. Preliminary, but unpublished, research on NRX-1074 (Naurex, Inc.), report rapid and robust antidepressant effects following a single infusion [unpublished data available at: www.naurex.com/pipeline/nrx-1074]. Furthermore, an orally bioavailable analogue of GLYX-13 underwent Phase I trials, but data has yet to be published on the safety and tolerability of the drug candidate [ClinicalTrials.gov Identifier: NCT02366364].
Scopolamine, Acetylcholine Muscarinic Antagonist with Rapid Acting Antidepressant Actions
Non-glutamatergic agents have also come under investigation for their rapid acting antidepressant actions. Recent studies demonstrate that a single dose of scopolamine, a nonselective muscarinic acetylcholine (ACh) receptor (mAChR) antagonist, produces rapid antidepressant actions within days, not as fast as ketamine [56–58]. Like ketamine, scopolamine treatment results in a rapid and transient burst of glutamate in the mPFC and increases the number of spine synapses [59]. The antidepressant effects of scopolamine in rodent models have recently been shown to be mediated through M1-AChR, specifically on somatostatin interneurons in the mPFC, and are dependent on activity-dependent release of BDNF [60, 61]. These studies provide evidence that scopolamine, via blockade of M1-AChR on GABA interneurons increases glutamate through a disinhibition mechanism similar to ketamine blockade of GluN2B receptors on GABA interneurons.
Sexual Dimorphism in MDD
Most of the preclinical studies on ketamine and other rapid-acting agents have been performed in male rodents, and most of the clinical studies do not provide sex-specific effects of treatment response. While both males and female patients are diagnosed with MDD, females are twice as likely to suffer from depression [71], and it is well known that drugs can have differential efficacy in males and females [72, 73]. For example, a meta-analysis of 15 randomized, placebo-controlled trials on six different commonly prescribed SSRIs or SNRIs found that female MDD patients had significantly greater responses to SSRIs than men [74].
Over the years, great effort has gone towards identifying differences in neuroendocrine responses, circulating hormones, and cognitive control circuits to explain the sexual dimorphism observed in MDD [9, 75, 76]. However, MDD is a heterogeneous disease with a multifactorial etiology. Although there may be comparable and conserved deficits across individuals with MDD, the variability in the expression and experience of the disease is likely accounted for by the interplay of numerous factors. Thus, a better understanding of how all of the parts (genetics, hormones, circuitry, early life experiences) produce the whole (depression) is vital to developing more personalized, and therefore effective, treatments.
A recent study has started to explore differences in gene expression that could further explain the observed sexual dimorphism in MDD [77••]. The study reports on sex-specific disease-associated modules identified in postmortem brains of depressed male and female subjects. Not only does this study find sexual dimorphism in the levels of differential expressed genes in individuals with MDD compared to healthy controls, but the results also demonstrate sex-specific depression-associated modules. For example, expression of Dusp6, a dual specificity MAP kinase phosphatase, is decreased in the vmPFC of females while the transcription factor EMX1 was increased in the vmPFC in males. Further exploration of these interconnected hub genes in both clinical and rodent studies and a more thorough understanding of the development of these sex-specific modules in MDD will be of great value to the field [78].
These disease-associated and sex-specific modules for depression and the molecular circuits they give rise to may be instrumental in establishing the E:I imbalance. A number of studies report on deficits in GABA-mediated inhibition in MDD [32, 79–81]. While these deficits emerge in both sexes, down-regulation of somatostatin (SST), a marker of a GABA neuron subtype, is significantly greater in women with MDD [9]. Furthermore, a gene co-expression network analysis in healthy controls finds that SST and GAD67 are co-regulated by X-chromosome genetic polymorphisms, providing additional mechanistic insight into the observed sexual dimorphism in MDD.
Considering Sex When Treating Depression
While additional research is needed to unravel and understand how gender-differences effect the etiology and pathophysiology of MDD, ongoing pre-clinical and clinical studies are needed to further our understanding of treating these sex-differences. A clinical trial investigating the antidepressant efficacy of scopolamine found that both males and females show a rapid antidepressant response but females had a higher magnitude of response [58]. Furthermore, only the females showed an anti-anxiety response. To our knowledge, this is the only clinical trial using a rapid-acting antidepressant to have performed gender-specific analyses on antidepressant response. More studies like this are sorely needed to address the higher rates of depression in women.
Moving forward, it is important to perform these same studies and all future studies in women and female mice. A rodent study comparing male and female responses to ketamine found that female mice responded to lower doses of ketamine (3 mg/kg) than males and that following chronic mild stress females were more reactive to ketamine, yet the effects lasted longer in males [82•]. A recent study examined the pharmacokinetic profiles of ketamine and its metabolites in the brains of male and female mice and found that while levels of ketamine and norketamine were similar, females had threefold higher levels of the metabolite (2S,6S;2R,6R)-HNK, which may explain the observed enhanced responses to ketamine in females (Gerhard et al., unpublished data).
Male and female rats exposed to a chronic social isolation paradigm used to induce depressive-like symptoms exhibited conflicting effects to ketamine treatment (during estrous cycle for females) [83•]. While male rats showed both anhedonia and depressive-like symptoms (i.e., helplessness in FST and anhedonia in SPT) following 8 weeks of isolation that were reversed by a 5 mg/kg dose of ketamine, female rats only showed depression-like behaviors (not anhedonia), which were reversed by both the 2.5 and 5 mg/kg doses. Furthermore, although both male and female rats exhibited significant reductions in spine density and levels of synaptic proteins following chronic isolation, only male rats had a reversal in these deficits following ketamine treatment. This study went on to show that spine density is higher for females in the proestrous than the estrous stage. It has long been known that increased levels of estrogen, such as during the proestrous stage, increases apical dendritic spine density on CA1 pyramidal cells and that these fluctuations in estrogen levels may affect cognitive performance across hormonal change [84–86].
Another study reported that female rats also responded to lower doses of ketamine and also found that these effects were completely abolished in ovariectomized females but restored when supplemented with physiological levels of estrogen and progesterone [87]. Interestingly, a recent study looking at the long-term effects of ketamine abuse found that women, but not men, reported higher depression scores and showed increased functional connectivity between the subgenual anterior cingulate cortex and the dorsal medial PFC [88]. Collectively, these studies further highlight how gender differences may affect the development and possibly the treatment of depression.
A better understanding of the pathophysiology of depression in both males and females could help reveal novel and more therapeutic pharmaceutical targets. However, beyond understanding sex differences in the etiology, pathophysiology, and treatment of MDD, women suffering from depression present additional compounding variables for the treatment equation, including postpartum and peri-menopausal depression. Thus, further studies are needed to determine the safest and most effective treatments for not only men vs. women, but also women vs. pregnant women vs. women undergoing menopause.
Conclusions
The finding of ketamine’s rapid-acting antidepressant effects is possibly the most important discovery in depression research in the past 60 years. Despite ketamine’s efficacy, especially for TRD and suicidality [89], it also has negative or undesirable side effects and has the potential for abuse. Furthermore, for most patients, the antidepressant effects are short-lived following infusions. This necessitates further investigation into ketamine’s mechanism of action to guide the development of more selective treatments that lack these effects and are safe for chronic use.
We explore how neuronal atrophy and E:I imbalances inform our understanding of both the etiology and treatment of MDD, and then how rapid-acting agents help to repair the long-term sequelae of stress and depression. Although there are some discrepancies in the literature as to the exact neuro-chemical alterations in the mPFC accompanying depression, it is clear that disruptions are ongoing in the mPFC circuity, likely explained by the brain’s attempt to reach homeostasis amid the atrophy. Beyond reversing stress-induced behavioral deficits, the aim of rapid-acting antidepressants in rodent studies has been to reverse observed neuronal atrophy and increase release of BDNF, two hallmark indicators of depression and rapid-acting antidepressant efficacy. In addition to the parent compound ketamine, preliminary studies in rodents demonstrate the potential of ketamine’s enantomers, (R)- and (S)-ketamine, and more recently, one of ketamine’s metabolites, (2R,6R)-HNK. It will be exciting to see if the success of these agents in rodent models of depression translates to clinical efficacy. Beyond ketamine, other agents have been found to produce comparable rapid-acting antidepressant effects through mechanisms much the same to ketamine. However, Glyx-13 is hypothesized to be a glycine-like partial agonist whereas scopolamine achieves these ketamine-like effects by antagonizing AChR-M1.
Most of the field’s understanding of the underlying cellular and molecular changes following both conventional SSRI and rapid-acting antidepressant treatments is markedly driven by rodent studies in males and clinical studies that often fail to report male and female cohorts separately. Although clinical trials are often constrained by sample size, additional analyses exploring the effects of gender, and even age x gender, could greatly benefit preclinical studies and possibly have more immediate impacts in the clinic. Depression has a recurrent trajectory and is described as a neuroprogressive disease where with each recurring episode, patients experience increasing severity, reduced therapeutic response, and shorter remission period, thus identifying and successfully treating MDD during early onset is essential [90–92]. In modeling trajectories of relapse, a recent study found that female gender significantly increased the odds of membership in the relapse trajectory [93]. While sex is an important factor in determining the optimal treatment for an individual, it is not the only variable. Age, concurrent medications and illnesses, and pregnancy are only a few factors that accompany an MDD diagnosis and further confound our understanding of the pathophysiology of MDD and the best course of treatment. Despite these challenges, new rapid acting, highly efficacious agents like ketamine provide important alternatives for depression and suicide and mark a new era in the treatment of depression.
Footnotes
Compliance with Ethical Standards
Conflicts of Interest Dr. Duman reports grants from Allergan, Navitor, and Relmada, grants and personal fees from Janssen, Naurex, and Taisho.
Dr. Gerhard has nothing to disclose.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-Month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617. doi: 10.1001/archpsyc.62.6.617. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC. The costs of depression. Psychiatr Clin N Am. 2012;35(1):1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon GE. Social and economic burden of mood disorders. Biol Psychiatry. 2003;54(3):208–15. doi: 10.1016/s0006-3223(03)00420-7. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO | Depression [Internet] Who; 2017. Available from: http://www.who.int/mediacentre/factsheets/fs369/en/ [Google Scholar]
- 6.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 7.CDC. National Violent Death Reporting System [Internet] 2015 Available from: https://www.cdc.gov/violenceprevention/nvdrs/index.html.
- 8.Hedegaard H, Warner M, Curtin SC. Increase in suicide in the United States, 1999–2014. NCHS Data Brief. 2016;241:1–8. Available from: https://www.cdc.gov/nchs/data/databriefs/db241.pdf. [PubMed] [Google Scholar]
- 9.Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, et al. The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry. 2013 Sep;4 doi: 10.3389/fpsyt.2013.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Undurraga J, Baldessarini RJ. Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology. 2012;37(4):851–64. doi: 10.1038/npp.2011.306. Available from: http://www.nature.com/doifinder/10.1038/npp.2011.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman RM, Cappiello A, Anand A, Da O, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Soc Biol Psychiatry. 2000;47(4):351–4. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 12.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856. doi: 10.1001/archpsyc.63.8.856. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 13.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23042884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (80-) 2010;329(5994):959–64. doi: 10.1126/science.1190287. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69(8):754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18(9):1413–7. doi: 10.1038/nm.2886. Available from: http://www.nature.com/doifinder/10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77(3):285–94. doi: 10.1016/j.biopsych.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R-J, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105(1):359–64. doi: 10.1073/pnas.0706679105. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2224217&tool=pmcentrez&rendertype=abstract Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2224217&tool=pmcentrez&rendertype=abstract . . https://doi.org/10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–13. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota KT, Liu R-J, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014;20(5):531–5. doi: 10.1038/nm.3513. Available from: http://www.nature.com/doifinder/10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colzato LS, Van der Does AJW, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val66Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. 2011;36(10):1562–9. doi: 10.1016/j.psyneuen.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science (80-) 2006;314(5796):140–3. doi: 10.1126/science.1129663. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 27.Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32(7):2410–21. doi: 10.1523/JNEUROSCI.5205-11.2012. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, et al. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37(5):1297–304. doi: 10.1038/npp.2011.318. Available from: http://www.nature.com/doifinder/10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6(3):215–29. doi: 10.1038/nrn1625. Available from: http://www.nature.com/doifinder/10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 30.Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35(3):818–25. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Hasler G, van der Veen J, Tumonis T, Meyers N, Shen J, Drevets W. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–7. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 33.Guilloux J-P, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–42. doi: 10.1038/mp.2011.113. Available from: http://www.nature.com/doifinder/10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karolewicz B, Maciag D, O’Dwyer G, Stockmeier CA, Feyissa AM, Rajkowska G. Reduced level of glutamic acid decarboxyl-ase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol. 2010;13(4):411. doi: 10.1017/S1461145709990587. Available from: https://academic.oup.com/ijnp/article-lookup/doi/10.1017/S1461145709990587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niciu MJ, Ionescu DF, Richards EM, Zarate CA. Glutamate and its receptors in the pathophysiology and treatment of major depressive disorder. J Neural Transm. 2014;121(8):907–24. doi: 10.1007/s00702-013-1130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2009;33(1):70–5. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klumpers UMH, Veltman DJ, Drent ML, Boellaard R, Comans EFI, Meynen G, et al. Reduced parahippocampal and lateral temporal GABAA-[11C]flumazenil binding in major depression: preliminary results. Eur J Nucl Med Mol Imaging. 2010;37(3):565–74. doi: 10.1007/s00259-009-1292-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19890631. [DOI] [PubMed] [Google Scholar]
- 38.Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci. 2005;102(43):15653–8. doi: 10.1073/pnas.0507901102. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, et al. Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric Acidergic deficits and keta-mine treatment. Biol Psychiatry. 2016;80(6):457–68. doi: 10.1016/j.biopsych.2016.02.009. Study demonstrating how GABAergic deficits can alter glutamatergic synaptic transmission in the prefrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. γ-aminobutyric acid-type a receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68(6):512–20. doi: 10.1016/j.biopsych.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–52. doi: 10.1016/j.biopsych.2007.05.028. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17643398. [DOI] [PubMed] [Google Scholar]
- 42.Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18(1):pyu033. doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224(1):107–11. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 44.Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, et al. Intravenous Esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–31. doi: 10.1016/j.biopsych.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Yang C, Shirayama Y, Zhang J, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5(9):e632. doi: 10.1038/tp.2015.136. Available from: http://www.nature.com/doifinder/10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin–independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83(1):18–28. doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J, Hasimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361(1):9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 49••.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6. doi: 10.1038/nature17998. Available from: http://www.nature.com/doifinder/10.1038/nature17998 First studying demonstrating the rapid antidepressant potential of the ketamine metabolite (2R,6R)-hydroxynorketamine (HNK) in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Xl, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55(7):1238–50. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgdorf J, Zhang X, Nicholson KL, Balster RL, David Leander J, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38(5):729–42. doi: 10.1038/npp.2012.246. Available from: http://www.nature.com/doifinder/10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moskal JR, Burgdorf JS, Stanton PK, Kroes RA, Disterhoft JF, Burch RM, et al. The development of rapastinel (formerly GLYX-13); a rapid acting and long lasting antidepressant. Curr Neuropharmacol. 2017;15(1):47–56. doi: 10.2174/1570159X14666160321122703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology. 2016;111:242–52. doi: 10.1016/j.neuropharm.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu R-J, Duman C, Kato T, Hare B, Lopresto D, Bang E, et al. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology. 2017;42(6):1231–42. doi: 10.1038/npp.2016.202. Available from: http://www.nature.com/doifinder/10.1038/npp.2016.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Preskorn S, Macaluso M, Mehra DV, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21(2):140–9. doi: 10.1097/01.pra.0000462606.17725.93. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00131746-201503000-00006 Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00131746-201503000-00006 . . https://doi.org/10.1097/01.pra.0000462606.17725.93 Study demonstrating the rapid and sustained antidepressant effects of the novel rapid-acting agent GLYX-13 in a clinical population. [DOI] [PubMed] [Google Scholar]
- 56.Drevets WC, Zarate CA, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156–63. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine. Arch Gen Psychiatry. 2006;63(10):1121. doi: 10.1001/archpsyc.63.10.1121. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furey ML, Khanna A, Hoffman EM, Drevets WC. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479–88. doi: 10.1038/npp.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry. 2013;74(10):742–9. doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, et al. Activity-dependent brain-derived neurotrophic factor release is required for the rapid antidepressant actions of scopolamine. Biol Psychiatry. 2018;83(1):29–37. doi: 10.1016/j.biopsych.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126(7):2482–94. doi: 10.1172/JCI85033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–7. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49. doi: 10.1038/nm.4050. Available from: http://www.nature.com/doifinder/10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454–64. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 66.Freedman R. Further investigation of ketamine. Am J Psychiatr. 2016;173(8):761–2. doi: 10.1176/appi.ajp.2016.16050581. [DOI] [PubMed] [Google Scholar]
- 67.Newport DJ, Schatzberg AF, Nemeroff CB. Whither ketamine as an antidepressant: panacea or toxin? Depress Anxiety. 2016;33(8):685–8. doi: 10.1002/da.22535. Available from: http://doi.wiley.com/10.1002/da.22535. [DOI] [PubMed] [Google Scholar]
- 68.Jaso B, Niciu M, Iadarola N, Lally N, Richards E, Park M, et al. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr Neuropharmacol. 2016;15(1):57–70. doi: 10.2174/1570159X14666160321123221. http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-159X&volume=15&issue=1&spage=57 Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-159X&volume=15&issue=1&spage=57 . . https://doi.org/10.2174/1570159X14666160321123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28(6):631–7. doi: 10.1097/JCP.0b013e31818a6cea. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-200812000-00008 Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-200812000-00008 . . https://doi.org/10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 70.Domino EF. Taming the ketamine tiger. Anesthesiology. 2010;113(3):678–86. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 71.Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4(2):146–58. doi: 10.1016/S2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 72.Mazure CM, Jones DP. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health. 2015;15(1):94. doi: 10.1186/s12905-015-0251-9. Available from: http://bmcwomenshealth.biomedcentral.com/articles/10.1186/s12905-015-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–57. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25(4):318–24. doi: 10.1097/01.jcp.0000168879.03169.ce. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-200508000-00005 Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004714-200508000-00005 . . https://doi.org/10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- 75.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–19. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:16065. doi: 10.1038/nrdp.2016.65. Available from: http://www.nature.com/articles/nrdp201665. [DOI] [PubMed] [Google Scholar]
- 77••.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23(9):1102–11. doi: 10.1038/nm.4386. Available from: http://www.nature.com/doifinder/10.1038/nm.4386 A major strength of this study is the translational experiments performed in mice. Using a viral mediated strategy to either downregulate Dusp6 or overexpress EMX1 in female and male mice, respectively, the mice exhibited increased susceptibility to chronic variable stress (CVS) and partially reproduced the depressive behaviors seen with CVS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duman RS. Sex-specific disease-associated modules for depression. Nat Med. 2017;23(9):1015–7. doi: 10.1038/nm.4391. Available from: http://www.nature.com/doifinder/10.1038/nm.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–7. doi: 10.1038/nn2001. Available from: http://www.nature.com/doifinder/10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 80.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4(8):e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383–406. doi: 10.1038/mp.2010.120. Available from: http://www.nature.com/doifinder/10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 83•.Sarkar A, Kabbaj M. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry. 2016;80(6):448–56. doi: 10.1016/j.biopsych.2015.12.025. Study demonstrating sex differentiated responses to chronic social isolation and subsequent ketamine treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001;98(23):13391–5. doi: 10.1073/pnas.241507698. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=60881&tool=pmcentrez&rendertype=abstract Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=60881&tool=pmcentrez&rendertype=abstract . . https://doi.org/10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26(33):8517–22. doi: 10.1523/JNEUROSCI.5279-05.2006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16914677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith CC, Vedder LC, LLMM Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34S1:S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 88.Li C-SR, Zhang S, Hung C-C, Chen C-M, Duann J-R, Lin C-P, et al. Depression in chronic ketamine users: sex differences and neural bases. Psychiatry Res. 2017;269(April):1–8. doi: 10.1016/j.pscychresns.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.17040472. Available from: http://ajp.psychiatryonline.org/doi/10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed]
- 90.Sibille E, French B. Biological substrates underpinning diagnosis of major depression. Int J Neuropsychopharmacol. 2013;16(8):1893–909. doi: 10.1017/S1461145713000436. Available from: https://academic.oup.com/ijnp/article-lookup/doi/10.1017/S1461145713000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chekroud AM, Zotti RJ, Shehzad Z, Gueorguieva R, Johnson MK, Trivedi MH, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3(3):243–50. doi: 10.1016/S2215-0366(15)00471-X. [DOI] [PubMed] [Google Scholar]
- 92.Chekroud AM, Gueorguieva R, Krumholz HM, Trivedi MH, Krystal JH, McCarthy G. Reevaluating the efficacy and predictability of antidepressant treatments. JAMA Psychiatry. 2017;74(4):370. doi: 10.1001/jamapsychiatry.2017.0025. Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/jamapsychiatry.2017.0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gueorguieva R, Chekroud AM, Krystal JH. Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis. Lancet Psychiatry. 2017;4(3):230–7. doi: 10.1016/S2215-0366(17)30038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]