Abstract
Measures (e.g. δ15N, δ13C, %C, %N and C:N) derived from animal tissues are commonly used to estimate diets and trophic interactions. Since tissue samples are often exposed to air or kept chilled in ice over a short-term during sample preparation, they may degrade. Herein, we hypothesize that tissue decomposition will cause changes in these measures. In this study, we kept marine fish, crustacean and mollusc tissues in air or ice over 120 h (5 days). We found that tissue decomposition in air enriched δ15N (range 0.6‰ to 1.3‰) and δ13C (0.2‰ to 0.4‰), decreased %N (0.47 to 3.43 percentage points from staring values of ~13%) and %C (4.53 to 8.29 percentage points from starting values of ~43%), and subsequently increased C:N ratio (0.14 to 0.75). In air, while such changes to δ13C were relatively minor and therefore likely tolerable, changes in δ15N, %N, %C and C:N ratio should be interpreted with caution. Ice effectively reduced the extent to which decomposition enriched δ15N (≤ 0.4‰) and δ13C (≤ 0.2‰), and eliminated decomposition in C:N ratio, %N and %C. In our second experiment, for fish tissues in either air or ice over 120 h, we observed no effects of decomposition on relationships between lipid content, C:N ratio, and Δδ13C (change in δ13C after lipid removal), which are employed to correct δ13C for samples containing lipid. We also confirmed that lipid in tissues caused large errors when estimating δ13C (mean ± standard error = -1.8‰ ± 0.1‰, range -0.6‰ to -3.8‰), and showed both lipid extraction and mathematical correction performed equally well to correct for lipids when estimating δ13C. We, therefore, recommend that specimens of marine animals should be kept in ice during sample preparation for a short-term, as it is an effective means for minimizing changes of the stable isotope measures in their tissue.
Introduction
Stable isotope ratios of nitrogen (15N/14N relative to atmospheric nitrogen, termed δ15N) and carbon (13C/12C relative to Vienna PeeDee Belemnite, termed δ13C) are widely used within aquatic and terrestrial ecological research for dietary reconstruction of consumers [1] and determining trophic structure of communities [2–4]. Derived ecological conclusions are dependent upon the accuracy of δ15N and δ13C estimates. Research has accordingly investigated potential sources of error when estimating δ15N and δ13C, including error associated with methods of sample preservation [5–8] and sample preparation [9,10]. Studies have shown that in some instances, such error is significant. For instance, Tarroux et al. [11] showed that the presence of lipid within tissues affected estimates of δ13C and subsequently biased estimates of resource contributions to consumers when using dietary mixing models. Post [12] showed the use of inappropriate δ15N discrimination values (the change in δ15N from resource to consumer) introduced error into trophic structure estimates of food chain length, while Perkins et al. [10] proved that selection of different tissue types during sample preparation could lead to such inappropriate δ15N discrimination values. Therefore, error in estimates of δ15N and δ13C introduced during sample preservation and preparation in many instances must be avoided or accounted for, though this is easily achieved once such error has been documented and is widely understood. As chemical preservation [13,14] and in a limited number of instances even freezing [7,15,16] may confound the fidelity of stable isotope values, many studies opt to keep samples unpreserved or in/on ice until advanced sample preparation is undertaken. Currently, however, we have limited empirical evidence to assess if and how tissue decomposition may affect δ15N and δ13C within unpreserved animal tissues in the short term (e.g. < 1 week).
Decomposition of samples has been better investigated for plants and algae, showing complex and variable effects [17,18]. Conversely, studies on animals have been of restricted scope. Payo-Payo et al. [19] and Burrows et al. [20] opportunistically sampled stranded cetaceans and turtles (n = 1 to 3) of unknown time of death, observing little change in either δ15N and δ13C over 62 or 14 days, respectively. Scarce studies employing greater experimental control have shown mixed results. Observations of a terrestrial invertebrate (Drosophila; [13]) and marine vertebrates (seal, shark, trout; [21]) have shown either no change or limited change (δ15N enriched by 0.4‰; δ13C depleted by 0.4‰ to 0.8‰) over a short-term (≤ 10 days). Variability amongst results and differences in experimental conditions across studies necessitates that further research is needed to determine if there exists consistent and generalisable patterns in how tissue decomposition affects stable isotope measures, or if decomposition effects are largely species- or tissue-specific. Given that tissues undergo both biological breakdown from microorganisms and self-breakdown with cessation of metabolism following death, we expect the chemical composition of tissue samples to change, either through loss of their constituents, change in relative proportion of different constituents, or formation of new constituents through cellular breakdown or deposited microorganism residues [18, 22, 23]. Thus, empirical evidence is required to inform researchers about the extent to which tissue decomposition may alter δ15N and δ13C in the short-term, for a broader range of species using fresh specimens.
In addition to δ15N and δ13C, ratios of percentage carbon (%C) to nitrogen (%N) (termed C:N ratio) of animal tissues provide a proxy of lipid to protein content and so can be informative of both lipid content and diet [24,25]. To date, empirical studies have barely examined potential tissue decomposition effects on C:N ratios of tissues following sampling (but see [18,21]). Decomposition is likely to proceed by physical breakdown and loss of tissues’ structural components, including nitrogen and carbon, so any decomposition would likely infer a change in C:N ratio (unless %C and %N degrade at rates that maintain this ratio). However, it is still unclear how tissue losses of nitrogen and carbon correspond to the heavier or lighter isotopes of each element. Losses in %N and %C may be associated with enriched δ15N and δ13C, respectively, provided that microbial or self-breakdown processes preferentially involve liberation of lighter metabolically more-active 14N and 12C from tissue, respectively [21].
Importantly, C:N ratios are also widely used to provide an a posteriori mathematical correction for δ13C values [26]. This is because tissues used for stable isotope analysis (SIA) often contain lipid, which is naturally depleted in δ13C [27]. This is a problem as lipid content varies between species, individuals, and tissues within individuals, and thus disproportionately affects estimates of δ13C in some samples, so needs to be accounted for [10]. However, lipid extraction prior to SIA can be costly and laborious, and may affect δ15N values [9,11,26]. Fortunately, the removal of lipids in a subset of samples can allow for the use of a derived equation of the relationship between C:N ratio and Δδ13C (the change in δ13C following removal of lipids) to provide mathematical correction of remaining samples simply based on their C:N ratios. The relationship between C:N ratio and Δδ13C follows two other important relationships between lipid content (%) and Δδ13C, and between C:N ratio and lipid content. The mathematical correction is extensively used, with a main paper reporting this procedure [26] currently cited >1000 times. Therefore, it is desirable to understand if and how tissue decomposition may affect the application of this mathematical correction. Decomposition may ultimately affect estimates of δ13C in two ways; either through using degraded tissues to derive the correction equation itself, or through using ‘fresh’ tissues to derive the equation but its subsequent application to correct δ13C for degraded tissue samples. In either instance, the use of a mathematical correction to estimate δ13C maybe robust to tissue decomposition unless decomposition, which includes microbial processes, adds or depletes compounds at a sufficiently high mass to alter relationships between C:N ratio, Δδ13C and lipid content of a tissue sample (i.e. tissue C:N ratio not matched by lipid content). In many instances, other approaches are used to estimate δ13C, including SIA only (where no attempt is made to correct / control for lipids in tissues), or lipid extraction prior to SIA. It would therefore be insightful to contrast how tissue decomposition may differentially affect estimates of δ13C made through either SIA only, lipid extraction prior to SIA, or mathematical correction after SIA.
In this study, we investigated the potential effects of tissue decomposition on multiple tissue measures in selected marine organisms over time. We conducted two separate experiments to answer three questions. Experiment 1 –Q1. How does tissue decomposition affect δ15N, δ13C, %N, %C and C:N ratio? Experiment 2 –Q2. Does tissue decomposition affect tissue lipid content or relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C? and Q3. Are alternative methodologies for estimating δ13C (SIA; lipid extraction then SIA; SIA then mathematical correction) differentially affected by tissue decomposition?
Materials and methods
Ethics statement
Freshly killed fishes, crustaceans and molluscs were brought from Aberdeen wholesale fish market, Hong Kong (coordinates: 22.248020, 114.150744). We were able to easily observe animals being killed in the market (i.e. removed from their tanks and left to expire), and so purchased animals for which we knew precise time of death. All individuals were inspected to ensure no visible damage or signs of poor health.
Overview
Here we describe two separate experiments. The first experiment provides a simple test of how decomposition affects common tissue measures. The second experiment tests if decomposition affects a widely applied methodological procedure that is used a posteriori to correct δ13C values against error caused by lipid content.
Experiment 1 ― Q1. How does tissue decomposition affect δ15N, δ13C, %N, %C and C:N ratio?
Sample preparation
Individuals of two fish species (grouper Cephalopholis boenak; rabbitfish Siganus canaliculatus), two crustacean species (mantis shrimp Miyakea nepa; crab Portunus sanguinoletus) and two mollusc species (gastropod Babylonia areolata; bivalve Paphia amabilis) were assigned randomly to air and ice treatments (n = 4 per species per treatment). The air treatment quantified the extent of natural decomposition, while the ice treatment evaluated the effectiveness of using an ice covering to reduce any decomposition effects. Repeated tissue sampling per individual was undertaken at 0, 30, 60, 90 and 120 h. Repeated measures within individuals were used because the results of a pilot experiment suggested that large between-individual variations in derived response variables might mask decomposition effects. Fish and mantis shrimp remained whole, with dorsal muscle and abdominal muscle, respectively, sampled at each time point. Due to difficulties of separating tissues because of decomposition / drying over time, crabs and molluscs were completely dissected fresh (Time = 0 h), with claw and leg muscle (crab), foot muscle (gastropod), or foot and adductor muscle (bivalve) pooled per individual on a petri dish from which subsequent samples were taken over time. Both air and ice treatments provided aerobic conditions. ‘Air’ treatment samples were kept in open trays within the laboratory (mean ± SD across experiment 1 and 2: temperature = 20.91°C ± 0.81°C, n = 10; humidity = 69.82% ± 3.22%, n = 10) while ‘ice’ treatment samples were kept in open freezer bags placed within ice (0.08°C ± 0.86°C, n = 10; humidity = 50.65% ± 2.10%, n = 10). Collected samples were immediately freeze dried for >48 h, homogenized and stored under dry conditions. For each of the freeze dried samples, 1.00mg ± 0.10mg dried tissue was carefully weighed and enclosed in a tin capsule and analysed for δ15N, δ13C, %N, and %C using stable isotope ratio mass spectrometry (EuroVector, model EA3028) at the Stable Isotope Laboratory of the Science Faculty, University of Hong Kong. For each sample, C:N ratio was subsequently calculated by dividing %C by %N. Further details can be found in S1 File.
Lipid correction
Following Post et al. [26], we applied an a posteriori mathematical correction to all estimates of δ13C for fish and crustacean samples to provide lipid free estimates of δ13C. Corrections were based on derived equations of relationships between C:N ratio and the difference in δ13C with and without lipid (Table A in S1 File). Thus, for fish and crustaceans, we assessed tissue decomposition over time in air and ice upon δ13C from tissues with lipid (termed δ13C +L) and for values of δ13C that were mathematically corrected to account for lipids (termed δ13C -L). It was not possible to derive such relationships for mollusc tissues, so for molluscs we only investigated effects of decomposition on uncorrected δ13C (δ13C +L) and interpreted the results with caution.
Data analysis
All statistical analyses were performed in the open source software R 3.4.0 [28] using lmer in lme4 [29]. We used a separate analysis per species per response variable. Per species, we used a linear mixed-model (LMM) to test for an interaction between the fixed effects of time (continuous variable) and treatment (2 level factor: air and ice), separately, on δ15N, δ13C +L, δ13C -L, %N, %C and C:N ratio. We included the random effect individual in each model to account for non-independence of repeated measures over time per individual. Significance of an interaction effect was determined using analysis of deviance between models with and without the interaction included. Interactions were dropped when they were not significant, and main effects were similarly tested by analysis of deviance between models with and without each term. For LMM, determination of model fit to the data was calculated with pseudo-R squared values [30] using the R package MuMIn [31], with estimates for the variance explained by model fixed effects reported with the prefix R2GLMM(m) and the combined fixed and random effects as R2GLMM(c). For all analyses, normality of residuals was checked visually using Q-Q plots, and homogeneity of variances checked visually using plots of residuals against each variable.
Experiment 2
Questions 2 and 3 are both tested using a common dataset derived from the experimental design below.
Sample preparation
In contrast to experiment 1, experiment 2 used only fish. This was because a pilot experiment suggested individual fish (c.f. mollusc and crustaceans) provided ample tissue for multiple lipid quantifications, while different fish species provided a broad range of lipid contents against which to test the effects of tissue decomposition. Using six species of fishes (seabream Acanthopagrus latus; grouper Epinephelus lanceolatus (hybrid); pomfret Pampus argenteus; scat Scatophagus argus; rabbitfish Siganus canaliculatus and mullet Valamugil cunnesius), one individual per species was assigned to each the air and ice treatment. For all individuals, repeated tissue samples (dorsal muscle) were collected at 0, 60 and 120 h. Thus, each level of treatment and time (Air 0h, A 60h, A 120h, Ice 0h, I 60h, I 120h) had a sample size of six (one sample per the single individual of each of the six fish species). Fish were kept in air or ice as described for Experiment 1 prior to sampling, where upon tissues were immediately freeze dried, homogenised and stored under dry conditions. For each sample we quantified lipid content, C:N ratio and Δδ13C. Δδ13C was calculated as the difference in δ13C between one sub-sample that underwent stable isotope analysis (SIA) and a second sub-sample that underwent lipid extraction and then SIA. Therefore, subsequent relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C were examined at each level of treatment and time. Note, it is appropriate to test these relationships across individuals of different species (c.f. across individuals within species) to maximise range of lipid content [26].
Lipid extraction & quantification
Samples for each individual per time and treatment underwent lipid extraction and quantification as described by Post et al. [26]. Briefly, 0.5g ± 0.0001g of homogenised dried tissue was placed in a 30ml boiling-tube to which 8ml of chloroform and 8ml of methanol were added, forming a 50:50 methanol-chloroform solution. Boiling tubes were heated in a water bath at 61°C for 40 min, allowed to cool to air temperature, and refilled to 25ml with the addition of chloroform. The mixture was poured through a No. 1 Whatman filter paper into a 250ml separatory funnel. 10ml of 0.9% saline solution was added to the separatory funnel, and the whole mixture shaken vigorously and allowed to settle and separate for 45min. The bottom methanol-chloroform fraction containing the lipid was drained into a pre-weighed aluminium dish and warmed on a hot-plate at 70°C until no liquid remained. The dish was cooled to air temperature, re-weighed to the nearest 0.0001g, and the mass of lipid calculated as the difference between post and pre tray weight, and expressed as a percentage of the original 0.5g sample mass, termed ‘lipid content’. The residual sediment on the filter paper was air dried and collected for use as the lipid extracted sample for SIA.
Stable isotope analysis
All samples were analysed for δ15N, δ13C, %N, %C and C:N ratio using stable isotope ratio mass spectrometry as described for Experiment 1.
Data Analysis ― Q2
Does tissue decomposition affect tissue lipid content or relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C? Firstly, we used a LMM to test if time, treatment (2 level factor: air and ice) and their interaction affected lipid content of fish tissues, and included the random effect individual to account for non-independence of repeated measures over time per individual fish. Next, for three known relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C, separately, we investigated how each relationship was affected by tissue decomposition in air or ice. To simplify the analyses, we combined treatment and time to produce a single variable termed combined treatment (6 level factor: Air 0h, A 60h, A 120h, Ice 0h, I 60h, I 120h). Using separate LMMs, we tested if (i) Δδ13C was affected by lipid content, combined treatment and their interaction, (ii) lipid content was affected by C:N ratio, combined treatment and their interaction, and (iii) Δδ13C was affected by C:N ratio, combined treatment and their interaction. The random effect individual was included in each model to account for non-independence of repeat measures over time per individual fish. Model selection and estimates of model fit were conducted for all analyses as previously described for Experiment 1.
Q3. Are alternative methodologies for estimating δ13C differentially affected by tissue decomposition?
Finally, we tested how tissue decomposition may affect δ13C values for samples prepared using different methodological procedures. At each sampling event (0, 60 and 120 h) per individual fish, we derived three measures of δ13C: from a sample that underwent SIA (termed uncorrected), a sample that underwent SIA and then a posteriori mathematical correction (termed mathematically corrected), and a sample that underwent lipid extraction and then SIA (termed lipid extracted). Mathematical correction was achieved using samples’ C:N ratios and equation 3 in Table A in S1 File. Given potentially larger between-individual than within-individual variation in derived δ13C values, to aid direct comparison of δ13C values derived across individuals over time and from multiple methodological procedures, we firstly standardised all values of δ13C. Within each individual fish, standardisation was achieved by subtracting all sampled values of δ13C from a ‘baseline δ13C’ which was taken as each fish’s value of lipid extracted δ13C at 0 h. Lipid extracted values were deemed to be the most accurate estimates of a lipid-free ‘true’ δ13C value, while using 0 h tissues ensured no decomposition had occurred. The difference between sample δ13C and baseline δ13C is hereafter termed standardised δ13C. Lipid extracted values of δ13C at 0 h were, therefore, not included in subsequent analysis, given all other values of δ13C were standardised against them. As previously, to simplify the analysis, we combined treatment (2 level factor: air or ice) and time to produce a single variable termed combined treatment (6 level factor for uncorrected and mathematically corrected samples: Air 0h, A 60h, A 120h, Ice 0h, I 60h, I 120h; but a 4 level factor for lipid extracted samples: A 60h, A 120h, I 60h, I 120h). Using a separate analysis for each methodological procedure, a LMM tested if standardised δ13C was affected by the main effect combined treatment, while the random effect individual was included in each model to account for non-independence of repeat measures over time per individual fish. Model selection and estimates of model fit were conducted as previously described for Experiment 1. Following a significant result, post-hoc analysis was used to determine differences between levels of factors using a P-value adjusted Tukey method (to account for multiple testing) in the R package lsmeans [32]. For all analyses, homogeneity of variances and normality of model residuals were checked as described in Experiment 1. All statistical analyses in questions 2 and 3 were performed in the open source software R 3.4.0 [28] using lme4 [29].
Results
Experiment 1
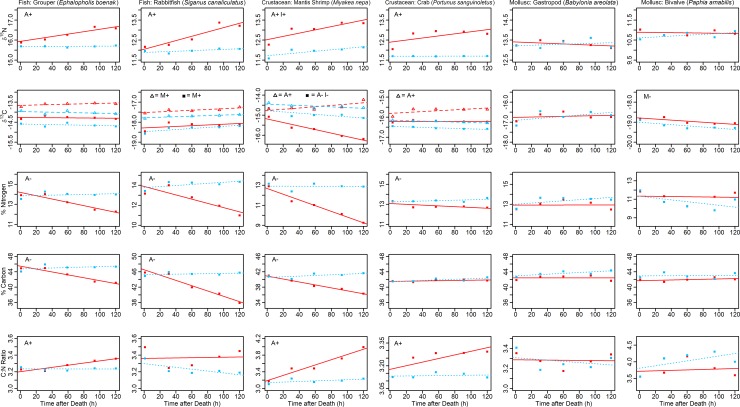
Q1. How does tissue decomposition affect δ15N, δ13C, %N, %C and C:N ratio? Across all species and response variables, 60% of analyses (18 of 30; Fig 1) showed a significant change over time. Tissue decomposition frequently altered tissue values in air (18 of 30) but infrequently in ice (3 of 30). Decomposition caused δ15N and δ13C to become enriched, caused decreases in both %N and %C, and subsequently increased C:N ratio (inferring decreases in %N were proportionally larger than decreases in %C). Broadly consistent patterns of change were observed for fish and crustacean tissues, which contrasted with the detection of only one significant change in mollusc tissues (1 in 10 analyses). Given the large non-directional variances seen within individual mollusc tissues regardless of the treatment (Fig A in S1 File), this suggested that within individual variability may have masked smaller effects of decomposition. Excluding molluscs, we observed a significant effect of tissue decomposition on the five measured parameters in 85% of cases (17 of 20; Fig 1). Notable results are presented per tissue measure below, with further details in S1 File.
Fig 1. Change in δ15N, δ13C, %N, %C and C:N ratio over 120 h for fish, crustacean and mollusc tissues kept in air (solid red lines) or ice (dotted blue lines).
Slopes are modelled relationships, and data represent mean values (n = 4). Error bars are not included as they are not appropriate here; between-individual variation was excluded from models using repeated measures within individuals and accounted for in analyses through use of a random effect ‘individual’. For δ13C, all species use δ13C +L (square symbols with either solid red or dotted blue lines), while we also modelled δ13C -L for fish and crustaceans (open triangle symbols with either red or blue dashed lines). δ13C -L values were obtained by applying a mathematical correction (Table A in S1 File) to δ13C +L data. Significant effects of tissue decomposition over time upon tissue measures are denoted for air (A) and ice (I) treatments where interactions indicated differences between treatments, or (M) when determined by a significant main effect indicating tissue decomposition that did not differ between treatments. + /—indicates the direction of effect. For fish and crustacean δ13C, symbol type with letter indicates significant relationships for either δ13C -L (open triangles) or δ13C +L (squares).
δ15N. For fish and crustacean analyses, significant interactions indicated that time affected δ15N but that this was dependent upon treatment (Grouper: χ2(1) = 17.91, P < 0.001, R2GLMM(m) = 0.37; Rabbitfish: χ2(1) = 20.10, P < 0.001, R2GLMM(m) = 0.63; Mantis Shrimp: χ2(1) = 6.88, P < 0.01, R2GLMM(m) = 0.68; Crab: χ2(1) = 7.74, P < 0.01, R2GLMM(m) = 0.66; Fig 1; Table B in S1 File). For these species, tissues kept in air enriched in δ15N between 0.6‰ to 1.3‰ over 120 h (Fig 1; Table B in S1 File). Conversely, δ15N of tissues kept in ice did not change over time, except for Mantis Shrimp, which were modestly enriched in δ15N by 0.4‰ over 120 h (Fig 1; Table B in S1 File).
δ13C. Using δ13C -L data, we observed that decomposition over time caused δ13C to become significantly enriched in air (Rabbitfish, Mantis Shrimp and Crab) and in ice (Rabbitfish only), though this change was small: 0.2‰ to 0.4‰ over 120 h (Fig 1; Table C in S1 File). For Rabbitfish, this was determined as a main effect of time (χ2(1) = 9.07, P < 0.01, R2GLMM(m) = 0.02; Table C in S1 File) indicating δ13C was enriched in both air and ice, while for Mantis Shrimp and Crab, significant interactions provided evidence of change in δ13C over time that was dependent upon treatment (Mantis Shrimp: χ2(1) = 8.80, P < 0.01, R2GLMM(m) = 0.13; Crab: χ2(1) = 9.88, P < 0.01, R2GLMM(m) = 0.59; Table C in S1 File). Conversely, for molluscs we only had δ13C +L data, and we observed decomposition over time caused δ13C to become significantly depleted in both air and ice (Bivalve only) by 0.3‰ over 120 h (main effect of time: χ2(1) = 16.40, P < 0.001, R2GLMM(m) = 0.13; Fig 1; Table D in S1 File). However, we suspected this result was likely due to lipid affecting Bivalve δ13C values. This was supported by the crustacean results, which showed tissue decomposition in air depleted δ13C (Mantis Shrimp) or had no effect (Crab) when using δ13C +L data (Fig 1; Table D in S1 File) but enriched δ13C when using δ13C -L data (Fig 1; Table C in S1 File). Thus, these conflicting results clearly demonstrate the need to account for lipids to accurately estimate δ13C and thus, that only our δ13C results based on δ13C -L data are reliable.
%N. For fish and crustacean analyses, significant interactions indicated that time affected %N but that this was dependent upon treatment (Grouper: χ2(1) = 16.17, P < 0.001, R2GLMM(m) = 0.59; Rabbitfish: χ2(1) = 23.38, P < 0.001, R2GLMM(m) = 0.71; Mantis Shrimp: χ2(1) = 29.30, P < 0.01, R2GLMM(m) = 0.78; Crab: χ2(1) = 7.07, P < 0.01, R2GLMM(m) = 0.52; Fig 1; Table E is S1 File). For these species, tissues kept in air showed decreases in %N ranging from 0.47 to 3.43 percentage points over 120 h (c.f. starting values at 0 h of ~13%), while %N of tissues kept in ice did not change over time (Fig 1; Table E in S1 File).
%C. For fish and Mantis Shrimp analyses, significant interactions indicated that time affected %C but that this was dependent upon treatment (Grouper: χ2(1) = 11.20, P < 0.001, R2GLMM(m) = 0.48; Rabbitfish: χ2(1) = 42.33, P < 0.001, R2GLMM(m) = 0.85; Mantis Shrimp: χ2(1) = 25.92, P < 0.001, R2GLMM(m) = 0.66; Fig 1; Table F in S1 File). For these species, tissues kept in air showed decreases in %C ranging from 4.53 to 8.29 percentage points over 120 h (c.f. starting values at 0 h of ~43%), while %C of tissues kept in ice did not change over time (Fig 1; Table F in S1 File).
C:N ratio. For Grouper and crustacean analyses, significant interactions indicated that time affected C:N ratios but that this was dependent upon treatment (Grouper: χ2(1) = 5.64, P < 0.05, R2GLMM(m) = 0.30; Mantis Shrimp: χ2(1) = 17.95, P < 0.001, R2GLMM(m) = 0.71; Crab: χ2(1) = 11.16, P < 0.001, R2GLMM(m) = 0.68; Fig 1; Table G in S1 File). For these species, in each instance, tissues kept in air showed increases in C:N ratios ranging from 0.14 to 0.75 over 120 h, while tissues kept in ice did not change (Fig 1; Table G in S1 File). Increases in C:N ratio suggested greater proportional deceases in %N than %C when compared to their respective starting values at 0 h, despite absolute decreases in percentage points (reported above) being greater in %C than %N. The magnitude of changes in C:N ratios should be compared with a mean C:N ratio across all species at 0 h of 3.31 ± 0.22 SD.
Experiment 2 ― Q2. Does tissue decomposition affect tissue lipid content or relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C?
Lipid content
Across all samples, lipid content ranged between 3.27% and 29.20% (Mean ±SD = 14.55% ± 7.39). Analysis revealed lipid content of fish tissues was not affected by the interaction (χ2(1) = 3.33, P = 0.07) or main effects of time (χ2(1) = 1.34, P = 0.25) and treatment (χ2(1) = 1.93, P = 0.16) (Table H in S1 File). Thus, observed changes in lipid content with time (between 0 and 120 h) were inconsistent between individuals and across species. Variation in lipid content across repeated measures within individuals ranged between 2.97% to 12.14%.
Relationships between C:N ratio, lipid content and Δδ13C
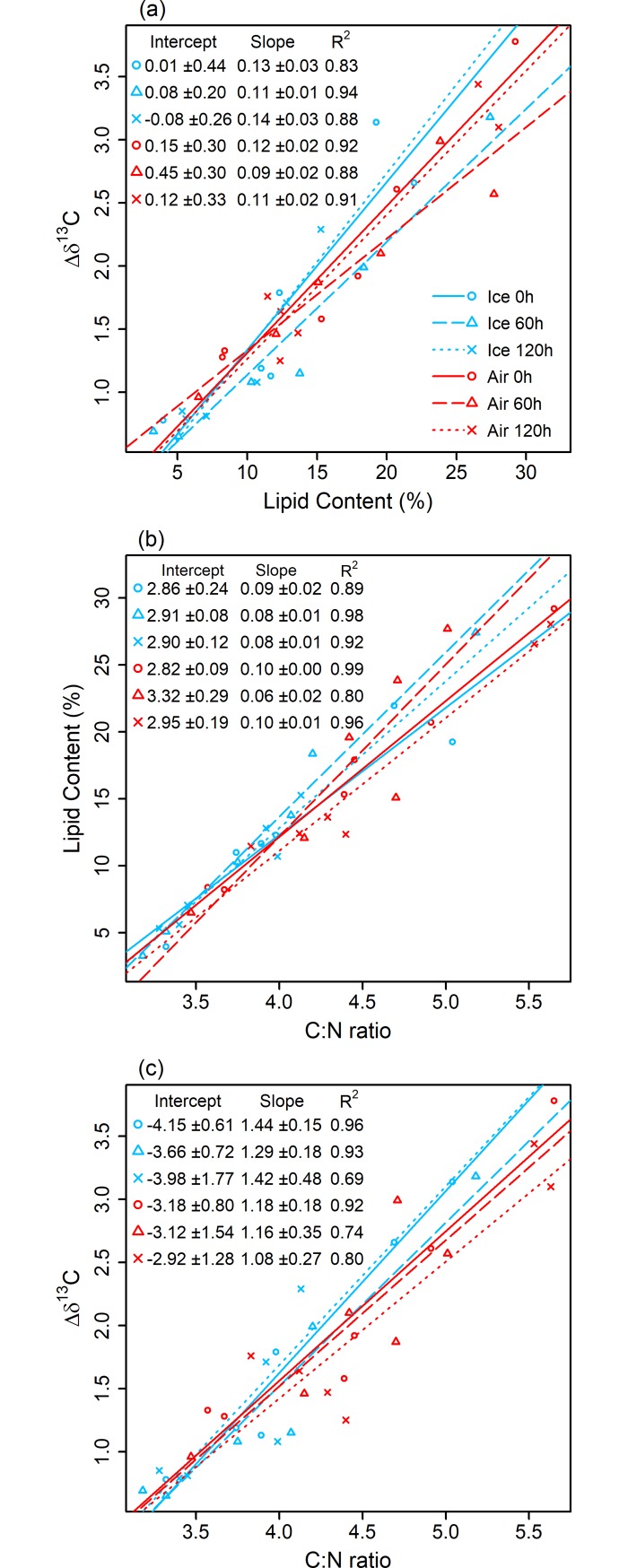
LMMs tested if relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C were significantly different across six levels of the composite variable ‘combined treatment’ (6 level factor: Air 0h, A 60h, A 120h, Ice 0h, I 60h, I 120h). Per each relationship in (i), (ii) and (iii), combined treatment was not significant either as an interaction (χ2(5) = 7.72, P = 0.17; χ2(5) = 10.19, P = 0.07; χ2(5) = 8.40, P = 0.14, respectively) or main effect (χ2(5) = 10.30, P = 0.07; χ2(5) = 7.57, P = 0.18; χ2(5) = 7.44, P = 0.19, respectively). Thus, our analyses did not detect any evidence for tissue decomposition affecting significant relationships between lipid content and Δδ13C (χ2(1) = 55.84, P < 0.001, R2GLMM(m) = 0.88, intercept ± SE = 0.26 ± 0.13, slope ± SE = 0.10 ± 0.01), C:N ratio and lipid content (χ2(1) = 63.51, P < 0.001, R2GLMM(m) = 0.92, intercept = -28.63 ± 2.37, slope = 10.27 ± 0.56), C:N ratio and Δδ13C (χ2(1) = 61.40, P < 0.001, R2GLMM(m) = 0.84, intercept = -2.79 ± 0.36, slope = 1.09 ± 0.08). Though no significant differences were detected between slopes and intercepts of levels of combined treatment, we report the individual combined treatment levels for relationships (i), (ii) and (iii) in Fig 2.
Fig 2.
Significant relationships between (a) lipid content and Δδ13C (b) C:N ratio and lipid content and, (c) C:N ratio and Δδ13C of fish muscle tissues were not affected by exposure to air or ice over 120 h. Per (a), (b) and (c), modelled slopes and raw data for each exposure time (0, 60 and 120 h) in air (red lines) and ice (blue lines) are shown, although they do not significantly differ from one another. Model estimates of slopes, intercepts and model variance explained (R2) are also given.
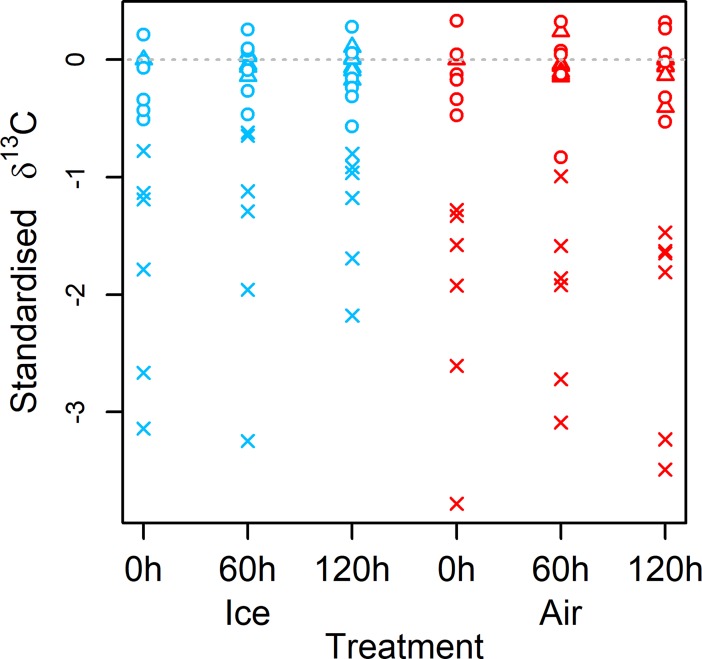
Q3. Are alternative methodologies for estimating δ13C differentially affected by tissue decomposition? Separate analyses per methodological procedure using LMMs showed that tissue decomposition over time in either air or ice did not affect estimates of standardised δ13C (the difference between sample δ13C and baseline δ13C) derived from samples that were uncorrected (χ2(5) = 9.41, P = 0.09), mathematically corrected (χ2(5) = 3.90, P = 0.56) or lipid extracted (χ2(3) = 2.07, P = 0.56). Subsequently, all data was pooled, and using a LMM we observed that methodological procedure (3 level factor: uncorrected, mathematically corrected, lipid extracted) significantly affected standardised δ13C (χ2(2) = 134.59, P < 0.001, R2GLMM(m) = 0.69). Post-hoc analysis revealed that standardised δ13C of uncorrected samples was significantly lower than mathematically corrected (difference ± SE = 1.7‰ ± 0.1, t.ratio = 16.07, df = 84, P < 0.001) and lipid extracted (difference ± SE = 1.8‰ ± 0.1, t.ratio = 14.92, df = 84, P < 0.001), while mathematically corrected and lipid extracted samples did not differ (difference ± SE = 0.1‰ ± 0.1, t.ratio = 0.55, df = 84, P = 0.85) (Fig 3). Thus, lipid extraction and mathematical correction both produced estimates of δ13C that approximated a δ13C baseline, while the use of uncorrected samples (where lipid content was not removed or accounted for) resulted in δ13C estimates that were between 0.6‰ and 3.8‰ depleted compared to the baseline value of δ13C.
Fig 3. Standardised δ13C of fish muscle tissue was not affected by tissue decomposition over 120 h in either air or ice, but was dependent upon methodological procedure.
Standardised δ13C of a sample was the difference in the sample’s δ13C from a lipid extracted value of δ13C at 0 h, termed baseline δ13C. Baseline δ13C is indicated as a dashed grey line at y = 0. Standardised δ13C of lipid extracted (triangles) and a posteriori mathematically corrected (circles) samples did not differ from one another and were typically small (range -0.8‰ to 0.3‰). By contrast, standardised δ13C of uncorrected samples (crosses) were significantly depleted (range -0.6‰ to -3.8‰) compared to lipid extracted and mathematically corrected samples.
Discussion
Overview
In our first experiment (question 1), over 120 h we observed changes in tissues of selected marine animals due to decomposition in air, but only rarely in ice. The latter was effective at minimizing tissue decomposition over this period. Decomposition of tissues in air caused δ15N and δ13C to become enriched, and caused proportionally greater decreases in %N relative to decreases in %C, subsequently increasing C:N ratio. Given that step-wise enrichment in δ15N from source to consumer is used to calculate consumer trophic levels and food chain length, so the largest changes in δ15N were equivalent to half a trophic level, while C:N ratios (when expressed as a proportion of C:N ratio at 0 h) showed notable increases up to 23%. Thus, alteration of δ15N and C:N ratio by decomposition was non-trivial and has the potential to affect subsequently derived results and conclusions, inferring decomposed tissue values should be interpreted with caution. In contrast, changes in δ13C were minor, suggesting it is likely to be acceptable to use δ13C signatures derived from decomposed (~120 h) samples if necessary.
Our second experiment (question 2) demonstrated that decomposition did not affect the use of relationships between C:N ratio, lipid content and Δδ13C to derive accurate estimates of δ13C within fish tissues, inferring that any effects of tissue decomposition remained proportional across these measures. Therefore, if a researcher was faced with samples that had been exposed to air for up to 120 h, our findings suggest that a sub-set of those samples could be subjected to lipid extraction and quantification and used to derive an accurate mathematical correction to account for the effects of lipid on δ13C. Additionally, our second experiment (question 3) clearly demonstrated that lipids caused δ13C to be significantly depleted (0.6‰ to 3.8‰), constituting error for isotope-based studies. This result puts into context the minor effects of tissue decomposition compared with the important need to account for lipids in samples to attain accurate δ13C values. Notably, both lipid extraction and mathematical correction performed equally well to correct for lipids when estimating δ13C.
Currently, freezing or drying are recommended as effective means to preserve stable isotope signatures of samples in the long-term [5,7,13], despite changes to signatures in a few instances following freezing [7,15,16]. From a practical perspective, our results demonstrate the effectiveness of ice as an important ‘bridge’ for preserving tissue measures such as stable isotope signatures and C:N ratios in the short-term, which may be necessary when pre-sorting and identifying large numbers of samples prior to long-term freezing / drying.
Experiment 1
Q1. How does tissue decomposition affect δ15N, δ13C, %N, %C and C:N ratio? Results of studies examining various derived measures of nitrogen and carbon in tissues subjected to decomposition have shown mixed results, suggesting highly species and context specific changes [17,18,21,33–35]. A general understanding of decomposition processes affecting tissues measures is, therefore, still emerging.
Our results suggest that decomposition of tissues proceeds through physical loss of nitrogen and carbon, observed as decreases in both %N and %C (Fig 1), in agreement with previous studies examining early- and late-stages of animal and early-stages of plant decomposition which showed net loss of nitrogen and/or carbon mass from marine vertebrates [21] and seagrasses and mangroves [33,35]. Our first experiment demonstrated such losses can be notable; when expressed as percentage change from the starting value at 0 h, losses after 120 h in air ranged between 3.53% and 26.58% for %N (note the absolute decreases in %N were 0.47 to 3.43 percentage points) and between 11.22% and 18.10% for %C (note the absolute decreases in %C were 4.53 to 8.29 percentage points). The shift to higher C:N ratios over time in air observed in our study is likely attributable to a greater proportional loss of %N than %C (Fig 1), despite percentage point losses of %C tending to be greater than %N. When expressed as a proportion of C:N ratio at 0 h, such changes were again considerable, being between 4.56% and 23.70% (note the absolute increases in C:N ratio were 0.14 to 0.75). Increases in C:N ratio over time were linear, inferring decomposition began immediately and greater changes would be expected after the 120 h period our experiment covered.
To date, studies using experimental decomposition of animal tissues to examine changes in nitrogen and carbon content have been scarce. Significant decreases in %N and %C (percentage points; N: 0.47 to 3.43; C: 4.53 to 8.29) of fish and crustacean muscle tissues over 5 days as observed in our study, are concurrent with smaller significant decreases (percentage points; N: 0.4 to 1.9; C: 3.3 to 7.3) within fish and shark muscle tissue over 256 days [21]. Conversely, observed significant increases in C:N ratio in our study (0.14 to 0.75) contrast with observations of no change for fish, shark and seal muscle tissue over 256 days [21]. Determination of mechanisms underlying tissue decomposition is beyond the scope of this study, but speculatively, the larger changes in %N and %C content, and change in C:N ratios, observed in a shorter-time period in our study, maybe reflective of different tissue sampling techniques between studies. For many species, we decomposed entire animals from which we re-sampled at each time point, whereas Yurkowski et al. [21] report dissecting all samples prior to the start of their experiment. In the latter study, smaller samples that were isolated from surrounding body tissue may have dried relatively more quickly so limiting change in tissue qualities. Indeed, we noted that odour associated with decomposition was reduced as specimens became increasing dry at later stages of our experiment, suggesting decay is associated with aridity, concurrent with findings in Yurkowski et al. [21].
Relative losses of carbon to nitrogen, or crudely lipid to protein, are dependent upon environmental context and specific to micro-organism activity based partly on available amino acids in substrate tissues [18,36]. Greater utilisation of nitrogen from the source tissue by micro-organisms is predicted when tissue C:N ratios are lower [37], with animal tissues having lower C:N ratios relative to plant tissues [38]. Enriched δ15N with decomposition of animal tissues observed in our study, and concurrent with previous work [13,21] may therefore be due to preferential loss from tissues of lighter 14N representing more easily degradable compounds altered and/or assimilated by microbial activity. This result contrasts with previous studies on degrading plant materials that observed depleted δ15N [18,33] or no change [33,35]. Higher C:N ratios in plant than animal tissues [38] may provide a more limited nitrogen pool where micro-organism driven processes may strongly influence nitrogen content (even increasing it through immobilization of environmental nitrogen) [39], thus potentially influencing δ15N, and whereby carbon losses may be greater relative to nitrogen as observed by decreases in C:N ratio with decomposition [18,37,39].
Importantly, the magnitude to which δ15N enriched in our study was up to 1.3‰ in air over 120 h (Fig 1, Table B in S1 File), though again the relationship was linear such that change could be greater over longer periods (Yurkowski et al. [21] observed δ15N of marine vertebrates enriched up to 2.2‰ over 256 days). Given that trophic discrimination of δ15N (the change in δ15N from resource to consumer) enriches per trophic transfer in the region of 2.5‰ to 3.4‰ [12,40,41], the largest changes we observed (~1.3‰) due to tissue decomposition may be equivalent to half a trophic level. Short-term decomposition in animal tissues has been scarcely documented; concurrent with our findings, decomposition of skin from Orca exposed in air at 20°C for 14 days also showed enriched δ15N of 6.4‰ [20], while trout muscle enriched in air by 0.4‰ over 8 days [21].
Our results also showed tissue decomposition enriched δ13C, after accounting for lipids (δ13C -L). Enriched δ13C with decomposition may be due to preferential loss from tissues of lighter 12C representing more easily degradable compounds, via microbial use or direct oxidation [17,35]. The magnitude of change in air was limited to 0.4‰ over 120 h (Fig 1, Table C in S1 File). Tarroux et al. [11] showed error in δ13C of ~2‰ caused spurious dietary estimates using mixing models, suggesting that decomposition error as we observed would likely introduce only minor bias into dietary analyses. Concurrent with our findings, decomposition of skin from Orca exposed in air at 20°C for 14 days enriched in δ13C by 1‰ [20], although other marine vertebrates have shown variable results, with cetacean and turtle tissues showing no changes over 62 days [19] and fish, shark and seal being enriched or depleted ≤ 0.8‰ after 256 days [21]. Elsewhere, studies have shown highly species-specific changes in δ13C for marine algae and plants, with no change [33,35] or either enriched or depleted δ13C of around 1‰ [18,34]. Therefore, further research is required to determine the mechanisms underlying such species- or tissue-specific differences in decomposition changes in δ13C.
Experiment 2
Q2. Does tissue decomposition affect tissue lipid content or relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C? As shown in this study, fish tissue lipid content was not affected by decomposition in either air or ice (Table H in S1 File). Non-directional changes in lipid content were observed within individuals (mean within-individual variance: 6.15% ± 2.71% SD), suggesting that within tissue variation was greater than any effects of tissue decomposition. Such within-individual changes in lipid content, either due to natural tissue lipid variation or undetected decomposition, remained proportional to both C:N ratio and Δδ13C; exposure to air or ice over 120 h did not affect linear relationships between (i) lipid content and Δδ13C, (ii) C:N ratio and lipid content, and (iii) C:N ratio and Δδ13C (Fig 2). Furthermore, for all three linear relationships, tissue decomposition did not affect the quantity of data variance explained by the relationships (range of R2 across time in air and ice remained high for all relationships: 0.69 to 0.99, Fig 2). Therefore, linear relationships between C:N ratio, lipid content and Δδ13C derived from tissues exposed in air for 120 h would provide accurate mathematically corrected estimates of δ13C. However, we advise the precautionary use of ice to ensure preservation of samples whenever possible. Notably, the range of C:N ratios in the present study (~ 3 to 6) ranged comparably to those of a key study reporting this mathematical correction method (~ 3 to 7; [26]), representing lipid content ranges from ~ 3% to 30%. Thus, our study provided a relevant test of the robustness of this widely applied a posteriori mathematical correction to the potential effects of tissue decomposition.
Q3. Are alternative methodologies for estimating δ13C (SIA; lipid extraction then SIA; SIA then mathematical correction) differentially affected by tissue decomposition? Our study additionally compared potential decomposition effects on three different methods of estimating δ13C (SIA; a posteriori mathematical correction of SIA values; samples subjected to lipid extraction prior to SIA). Tissue decomposition in air or ice over 120 h did not affect estimates derived from each method (Fig 3). Our first experiment demonstrated that δ13C derived from SIA, or from a posteriori mathematical correction of SIA values, may change over time in response to tissue decomposition (Fig 1, Tables C and D in S1 File). However, such change was typically small (≤ 0.4‰), while not all species showed change. Therefore, there was no expectation that δ13C derived from SIA or a posteriori mathematical correction in experiment 2 would necessarily show effects of tissue decomposition, and so the results of our experiments 1 and 2 are concurrent.
A further finding of this analysis was that δ13C values derived from different methodological procedures differed significantly. Compared with a lipid-free baseline δ13C value at 0 h, uncorrected SIA δ13C values were significantly depleted in δ13C (mean value ± SE = -1.8‰ ± 0.1) which contrasted to either mathematically corrected (-0.1‰ ± 0.0) or lipid extracted samples (0.0‰ ± 0.0) which were not (Fig 3). This is likely attributable to lipid contained within the uncorrected samples. Lipid is naturally depleted in δ13C [27] and its content varies between tissues and individuals [10]. Most ecological studies using isotopic data aim to derive feeding relationships or food web structure using assumed step-wise enrichment in δ13C from source to consumer, so the inclusion of lipid in estimates of δ13C may constitute error. It has been shown that error of ~2‰ in δ13C of sources or consumers may cause spurious conclusions when estimating diets using mixing models [11]. Therefore, our use of a lipid-free baseline at 0 h, and the near exact matching of that baseline by other lipid extracted samples (at 60 or 120 h) and mathematically corrected samples (which had not had lipids removed, at 0, 60 and 120 h), highlights the effectiveness of these techniques to derive lipid-free estimates of δ13C. It also demonstrates the potentially large errors in δ13C when not correcting for lipid (Fig 3). The need to account for lipids in samples when estimating δ13C has been previously highlighted [10,11,26], yet many studies still do not account for lipids or report doing so. Explicit demonstration of the large discrepancies in δ13C estimates for tissues when failing to account for lipids is thus a further important aspect of this present study, as we demonstrated that the decomposition error on δ13C (as shown in experiment 1) was much smaller when compared with error caused by lipids.
Conclusions
Firstly, our results show that tissue decomposition over the short-term in air can cause notable changes to measures of δ15N, %N, %C and C:N ratio (but less so δ13C), but that storing specimens in ice is effective in mitigating such changes to levels that are likely to have little impact upon results and conclusions of these parameters. Secondly, our results suggest that short-term tissue decomposition in air or ice has a negligible influence on the relationships between lipid content, C:N ratio, and Δδ13C in marine fishes. Nonetheless, we advise that ice should be used for short-term sample preservation to ensure that the relationship between C:N ratio and Δδ13C can be accurately applied as a mathematical correction to estimates of δ13C.
Supporting information
(DOCX)
(XLSX)
Acknowledgments
This research was substantially funded by a Research Grants Council of the Government of the Hong Kong Special Administrative Region (HKSAR) via a Collaborative Research Fund (CRF Project No. HKU5/CRF/12G) to KMYL, and partially supported by a Seed Fund for Basic Research (Project Code: 201611159133) by the University of Hong Kong (HKU) to KMYL and MJP. MJP thanks HKU for partially providing his postdoctoral research fellowship via the PDF/RAP matching fund scheme. The authors thank Helen Leung, and staff of the Stable Isotope Ratio Mass Spectrometry Laboratory of HKU for their technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was substantially funded by a Research Grants Council of the Government of the Hong Kong Special Administrative Region (HKSAR) via a Collaborative Research Fund (CRF Project No. HKU5/CRF/12G) to KMYL, and partially supported by a Seed Fund for Basic Research (Project Code: 201611159133) by the University of Hong Kong (HKU) to KMYL and MJP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, et al. (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835 [Google Scholar]
- 2.Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602 10.1111/j.1365-2656.2011.01806.x [DOI] [PubMed] [Google Scholar]
- 3.Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, et al. (2011) Applying stable isotopes to examine food–web structure: an overview of analytical tools. Biol Rev Cam Philos Soc 87:545–562 [DOI] [PubMed] [Google Scholar]
- 4.Perkins MJ, McDonald RA, van Veen FJF, Kelly SD, Rees G, Bearhop S (2014) Application of nitrogen and carbon stable isotopes (δ15N and δ13C) to quantify food chain length and trophic structure. PLoS ONE. 9(3): e93281 10.1371/journal.pone.0093281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson KA, Gibbs HL, Gloutney ML (1997) Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can J Zool 75:1720–1723 [Google Scholar]
- 6.Dannheim J, Struck U, Brey T (2007) Does sample bulk freezing affect stable isotope ratios of infaunal macrozoobenthos? J Exp Mar Biol Ecol 351:37–41 [Google Scholar]
- 7.Barrow LM, Bjorndal KA, Reich KJ (2008) Effects of preservation method on stable carbon and nitrogen isotope values. Physiol Biochem Zool 81:688–693 10.1086/588172 [DOI] [PubMed] [Google Scholar]
- 8.Lau DCP, Leung KMY, Dudgeon D (2012) Preservation effects on C/N ratios and stable isotope signatures of freshwater fishes and benthic macroinvertebrates. Limnol Oceanogr Methods 10:75–89 [Google Scholar]
- 9.Mateo MA, Serrano O, Serrano L, Michener RH (2008) Effects of sample preparation on stable isotope ratios of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157:105–115 10.1007/s00442-008-1052-8 [DOI] [PubMed] [Google Scholar]
- 10.Perkins MJ, McDonald RA, van Veen FJF, Kelly SD, Rees G, Bearhop S (2013) Important impacts of tissue selection and lipid extraction on ecological parameters derived from stable isotope ratios. Methods Ecol Evol 4:944–953 [Google Scholar]
- 11.Tarroux A, Ehrich D, Lecomte N, Jardine TD, Bety J, Berteaux D (2010) Sensitivity of stable isotope mixing models to variation in isotopic ratios: evaluating consequences of lipid extraction. Methods Ecol Evol 1:231–241 [Google Scholar]
- 12.Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718 [Google Scholar]
- 13.Ponsard S, Amlou M (1999) Effects of several preservation methods on the isotopic content of Drosophila samples. Comptes Rendus de l'Académie des Sciences-Series III-Sciences de la Vie 322:35–41 [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Bergonzoni I, Vidal N, Wang B, Ning D, Liu Z, Jeppesen E, et al. (2015) General validation of formalin-preserved fish samples in food web studies using stable isotopes. Methods Ecol Evol 6: 307–314 [Google Scholar]
- 15.Feuchtmayr H, Grey J (2003) Effect of preparation and preservation procedures on carbon and nitrogen stable isotope determinations from zooplankton. Rapid Commun Mass Spectrom 17:2605–2610 10.1002/rcm.1227 [DOI] [PubMed] [Google Scholar]
- 16.Syvaranta J, Martino A, Kopp D, Cereghino R, Santoul F (2011) Freezing and chemical preservatives alter the stable isotope values of carbon and nitrogen of the Asiatic clam (Corbicula fluminea). Hydrobiologia 658:383–388 [Google Scholar]
- 17.Fernandez I, Mahieu N, Cadisch G (2003) Carbon isotopic fractionation during decomposition of plant materials of different quality. Global Biogeochem Cycles 17:1075–1085 [Google Scholar]
- 18.Hill JM, McQuaid CD (2009) Variability in the fractionation of stable isotopes during decomposition of two intertidal red algae. Estuar Coast Shelf Sci 82:397–405 [Google Scholar]
- 19.Payo-Payo A, Ruiz B, Cardona L, Borrell A (2013) Effect of tissue decomposition on stable isotope signatures of striped dolphins Stenella coeruleoalba and loggerhead sea turtles Caretta caretta. Aquatic Biol 18:141–147 [Google Scholar]
- 20.Burrows DG, Reichert WL, Hanson MB (2014) Effects of decomposition and storage conditions on the δ13C and δ15N isotope values of killer whale (Orcinus orca) skin and blubber tissues. Mar Mam Sci 30:747–762 [Google Scholar]
- 21.Yurkowski DJ, Hussey AJ, Hussey NE, Fisk AT (2017) Effects of decomposition on carbon and nitrogen stable isotope values of muscle tissues of varying lipid content from three aquatic vertebrate species. Rapid Commun Mass Spectrom 31:389–395 10.1002/rcm.7802 [DOI] [PubMed] [Google Scholar]
- 22.Balzer A, Gleixner G, Grupe G, Schmidt HL, Schramm S, Turban-Just S (1997) In vitro decomposition of bone collagen by soil bacteria: the implications for stable isotope analysis in archaeometry. Archaeometry 39:415–429 [Google Scholar]
- 23.Passi S, Cataudella S, Tiano L, Littarru GP (2005) Dynamics of lipid oxidation and antioxidant depletion in Mediterranean fish stored at different temperatures. BioFactors 25:241–254 [DOI] [PubMed] [Google Scholar]
- 24.Fagan WF, Siemann E, Mitter C, Denno RF, Huberty AF, Woods HA, et al. (2002) Nitrogen in insects: Implications for trophic complexity and species diversification. Am Nat 160:784–802 10.1086/343879 [DOI] [PubMed] [Google Scholar]
- 25.Wilder SM, Norris M, Lee RW, Raubenheimer D, Simpson SJ (2013) Arthropod food webs become increasingly lipid-limited at higher trophic levels. Ecol Lett 16:895–902 10.1111/ele.12116 [DOI] [PubMed] [Google Scholar]
- 26.Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analysis. Oecologia 152:179–189 10.1007/s00442-006-0630-x [DOI] [PubMed] [Google Scholar]
- 27.DeNiro MJ, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263 [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- 29.Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48 [Google Scholar]
- 30.Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142 [Google Scholar]
- 31.Barton K (2013) MuMIn: multi-model inference, R package version 1.9.13.
- 32.Lenth RV (2016) Least-squares means. The R Package lsmeans. J. Stat. Softw. 69:1–33 [Google Scholar]
- 33.Zieman JC, Macko SA, Mills AL (1984) Role of seagrasses and mangroves in estuarine food webs: temporal and spatial changes in stable isotope composition and amino acid content during decomposition. Bull Mar Sci 35:380–392 [Google Scholar]
- 34.Fenton GE, Ritz DA (1988) Changes in carbon and hydrogen stable isotope ratios of macroalgae and seagrass during decomposition. Estuar Coast Shelf Sci 26:429–436 [Google Scholar]
- 35.Machas R, Santos R, Peterson B (2006) Elemental and stable isotope composition of Zostera noltii (Horneman) leaves during the early phases of decay in a temperate mesotidal lagoon. Estuar Coast Shelf Sci 66:21–29 [Google Scholar]
- 36.Macko SA, Estep MLF (1984) Microbial alteration of stable isotope nitrogen and carbon isotopic compositions of organic matter. Org Geochem 6:787–790 [Google Scholar]
- 37.Norderhaug KM, Fredriksen S, Nygaard K (2003) Trophic importance of Laminaria hyperborea to kelp forest consumers and the importance of bacterial decomposition to food quality. Mar Ecol Prog Ser 255:135–144 [Google Scholar]
- 38.Denno RF, Fagan WF (2003) Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology 84:2522–2531 [DOI] [PubMed] [Google Scholar]
- 39.Woitchik AF, Ohowa B, Kazungu JM, Rao RG, Goeyens L, Dehairs F (1997) Nitrogen enrichment during decomposition of mangrove leaf litter in an east African coastal lagoon (Kenya): Relative importance of biological nitrogen fixation. Biogeochemistry 39:15–35 [Google Scholar]
- 40.Vanderklift MA, Ponsard S (2003) Sources of variation in consumer–diet d15Nenrichment: a meta–analysis. Oecologia 136:169–182 10.1007/s00442-003-1270-z [DOI] [PubMed] [Google Scholar]
- 41.Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (d15N and d13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.