Abstract
Coastal sediments are an important site for transient and long-term mercury (Hg) storage and they foster a geochemical environment optimal for Hg methylation. Therefore, efforts have been taken to constrain the role of sediments as a source of methylmercury (MeHg) to the estuarine water column. This study employed the Gust Microcosm Erosion Core system capable of quantifying particle removal from undisturbed cores under measurable shear stress conditions to assess particulate Hg and MeHg exchange between sediments and the water column. Samples were collected from organic rich and organic poor sediment types from the mid- and lower- Delaware Bay. It was found that bulk sediment samples from organic rich systems overpredict total Hg and MeHg release to the water column whereas organic poor sediments under-predict the exchange. In general, organic rich sediments in shallow environments have the most impact on surface particle dynamics. There is little evidence to suggest that MeHg formed in the sediments is released to the water column via particulate exchange, and therefore non-sedimentary sources likely control MeHg levels in this estuarine water column.
Keywords: Methylmercury, Mercury, Resuspension, Estuary
Introduction
Mercury (Hg) is a recognized ubiquitous contaminant in aquatic ecosystems. The Hg species of particular interest is methylmercury (MeHg) due to its propensity to bioaccumulate and potential to cause health problems for humans and wildlife at high exposure levels. For most people, exposure to MeHg is primarily from the consumption of seafood, and many of the species consumed inhabit coastal zones1. Historically, sediments have been focused on as the dominant source of MeHg to the coastal pelagic ecosystem as they are a site for transient and long-term Hg storage2,3, which elevates their Hg levels relative to other pools, and they foster the anaerobic environment preferred by methylating bacteria4. However, recent studies using stable isotopes suggest that dissolved MeHg flux rates from sediments may actually be quite low e.g.5. Futhermore, pelagic organisms and those feeding at the sediment surface appear to acquire most of their MeHg from atmospheric and terrestrial Hg sources, not sediments6–8. Forage fish MeHg levels have also been shown to have stronger relationships with suspended particle MeHg than that in sediments8,9 appearing to negate the sediment’s role as a source of MeHg to the pelagic food web.
The impact that sediments have on water column MeHg levels is often calculated based on the difference in Hg concentration between surface particulate/water and bulk sediment/porewater concentrations or quantified using flux core or resuspension experiments. The nuances associated with calculating dissolved MeHg fluxes from sediments have been investigated elsewhere e.g.10 and is not a focus of this research. Particulate Hg sediment inputs are less well studied. Balcom et al.11 compared suspended particulate and sediment Hg and MeHg, collected using bulk sampling techniques, across multiple estuaries and found in shallow, turbid systems bulk sediment samples were indicative of the suspended pool, but in deeper estuarine waters or systems with substantial tidal flushing they were not. Bed sediments and riverbanks tend to have low %MeHg so under high estuarine flow or in turbid systems suspended particulate %MeHg also tends to decrease12,11. Laboratory resuspension studies also showed that suspended particulate total Hg (HgT) in mesocosms exposed to tidal resuspension cycles better reflected the sediments (high Hg(II), low %MeHg) compared to non-resuspended chambers; the effect was attributed to the relatively higher %MeHg in plankton relative to resuspended sediment13.
While field studies capture a snapshot of the dynamics of a naturally mixed system, the bulk sampling approach masks the natural dynamics of the system14,15. For instance, typical sediment removal in coastal systems under naturally occurring shear stress conditions is a few mm16, which corresponds to depths at which Hg, especially MeHg, concentration profiles can change rapidly pending the depth of the redox layer. Thus, homogenizing over even the top centimeter of the sediment surface may misrepresent the particles actively exchanging between the sediments and water column over a typical tidal cycle. Mesocosm resuspension experiments depict the longer-term impact of resuspension but do not mimic the complex mixing regime of natural estuarine systems and require the destruction of the in-situ surface sediment profiles when constructing. Studies estimating the impact of natural sediment resuspension on Hg dynamics are lacking due to the limited methods able to measure particle resuspension using undisturbed cores under quantifiable shear stress conditions. Resuspension occurs when water moving across the sediment surface generates a shear stress at a critical strength for a given sediment type. Therefore, sandy sediments and organic rich sediments behave differently under similar shear stress conditions17. Natural resuspension events are influenced by the physical attributes of a coastal system including water depth, tidal currents, waves, and human activities such as dredging or trawling; each of which works at different temporal and spatial scales and have differential impact on resuspension depending on the event magnitude16. Sampling resuspendable material, however, is difficult and nearly impossible without proper sampling techniques. In this paper we used one approach, the Gust MicrocosmSystem, to examine the impact of sediment resuspension on water column Hg and MeHg in a typical temperate coastal ecosystem.
The Gust System has been previously used to quantify easily mobilized HgT within flocculated layers at the sediment water interface of two systems in Eastern Canada14. There, flocculated surface material had higher HgT concentrations, as well as higher levels for a suite of other metals, than the corresponding 0.5 cm bulk sediment samples. Their study and similar work by Kalnejais et al.18 focusing on other metals and pollutants strongly suggest that the resuspended particulate fraction provides a more accurate understanding of mobile contaminants from estuarine sediments. This study builds on these findings but with a focus on HgT and MeHg over low and high shear stress conditions. Our goal was to assess the robustness of a bulk sediment sample for determining the connectivity between surface sediments and suspended water column particulates by comparing results of the Gust Experiment to traditionally collected bulk samples. The Gust results were then used to assess the potential importance of resuspension in contributing particulate MeHg to the Delaware Bay water column.
Methods
Site
The measured cores were collected from shore by hand in the Delaware Bay (Fig. S1). Two sites were sampled, one upstream (UP) and one downstream (DWN) ~50km air distance apart, both on the Delaware side of the bay. Within each site two nearby subsites (within ~5 km) were selected based on characteristics of the environment, namely, one subsite was representative of a salt marsh and the other typical of a sandy beach. The sub-site denotation (high organic carbon, HOC; low organic carbon, LOC) is based upon the bulk sediment organic carbon characteristics of the site pairs. The upstream marsh (UP-HOC) sampling site was in the Duck Creek waterway system located within the Woodland Beach Wildlife Area. The beach site (UP-LOC) was downstream adjacent to the mouth of the saltmarsh system directly on the shore of the bay at Woodland Beach. The downstream marsh site (DWN-HOC) was located along an inlet of Cedar Creek towards Slaughter Creek, and the beach site (DWN-LOC) was downstream adjacent to the saltmarsh system on the shore of the bay at Slaughter Beach. The two downstream sub-sites were done in replicate for the erosion experiment. Overall, the upstream marsh site is a larger waterway and marsh system than that of the downstream marsh site (Fig. S1).
Resuspension set-up
The Gust Microcosm Erosion System was employed in July 2015. The Gust System allows for the characterization of resuspended particles from natural, undisturbed cores under variable shear stress conditions in a way not feasible using other methods 14 They consist of a rotating head that fits snugly onto a ten cm diameter core tube with water input and output connections (Fig. S2). The rotation rate of the shear plate within the head and the rate that water is pumped through the system is controlled by computer software. Together they generate a quantifiable uniform shear stress across the sediment surface. The shear stresses that were applied include 0.01, 0.05, 0.1, 0.2, 0.3, 0.45, and 0.57 Pa with the first step generally considered a flushing step to remove suspended particles in the bottom water. Each shear stress level was held constant for 20 minutes, which is the time it takes for all the erodible particles to be removed and the system to return to background levels. The flow-through water was collected from the site and the particles were allowed to gravimetrically settle out of solution before use. Water pumped out of the chamber with the eroded particles passed through a turbidity meter before collection in acid cleaned containers. During the experiment, two cores were eroded consecutively. One core was filtered for total suspended solids (TSS). The other core samples were filtered for MeHg, HgT (Millipore AQFA, quartz fiber filter), TSS, Carbon/ Nitrogen/ Sulfur (CNS), and chlorophyll a/phaeo pigment (chla/pha) analyses (Whatman™ GF/F glass fiber filters). The TSS data were used to calibrate the turbidity measurements taken continuously on the outflowing water 17. The erosion core data were not normalized to the particulate material that may have been present in the flow-through water. It is recognized that particles derived from the flow-through water could contribute to the particles collected at the out-flow of the erosion device, but by comparing the size fractionated surface water TSS (0.2–20 ¼M) to the erosion sample TSS it is likely that <12% of the resuspended material could be derived from the flow-through water (Table S1). Results from 19, where settling rates of different particle sizes were measured and were largely removed after 90 minutes, suggest that the percentage would be even less as particles were allowed to settle for >2.5 hours prior to starting the experiment.
Sample Collection
The upstream cores were collected by hand with push cores at the mid-rising tide, and the downstream cores were collected the following day during the falling tide near low tide. Attention was taken to not disturb the surface of the sediment upon collection. Benthic organisms such as crabs and snails were removed prior to eroding if present. No visible microbial or algal surface mat was present. The cores and site-water were brought to the Delaware Department of Natural Resources and Environmental Control Division of Water in Dover, DE where they were eroded the same day. Ancillary parameters including water column temperature and pH were measured at the site. Water column samples were collected at high tide and low tide in two fractions, >20¼m and 0.2–20¼m (0.2¼m Isopore polycarbonate filters and 20¼m Spectrum™ nylon filters), which were summed to determine the bulk. The samples were collected within days of the erosion experiment. The same particulate parameters were quantified as presented for the erosion experiment, and dissolved sample constituents included nutrients, DOC, dissolved HgT and MeHg which were also saved from each of the shear stress intervals. Bulk sediment samples were collected using 7.5 cm diameter push cores which were extruded, cut at 4cm, homogenized, and preserved by freezing.
Analysis
Particulate and dissolved MeHg samples were analyzed on a Tekran 2700 Automated Methylmercury Analysis System following standard techniques20. Briefly, particulate samples were digested in 4.5N nitric acid overnight, neutralized, and ethylated using sodium tetraethylborate followed by gas chromatography separation with cold vapor atomic fluorescence detection (Alfa Aesar CAS: 115–09–3, LOT: 1791821 spike recovery= 103±14%). Particulate HgT was analyzed on a Tekran 2600 by cold vapor atomic fluorescence spectrometry following EPA method 1631 refined by Hammerschmidt and Fitzgerald20. The particulate samples were digested in 4.5N nitric acid overnight, treated with BrCl for 16 hrs, followed by hydroxylamine hydrochloride and stannous chloride before analysis (J.T.Baker CAS: 7732–16–5, Batch No: 0000127949 spike recovery= 121±2%). Bulk sediment HgT was analyzed via a direct mercury analyzer, DMA-80, at Umeå University (ARC-CNRC MESS-3 CRM recovery= 101±2%). MeHg was extracted from the bulk sediment via aqueous distillation21 and analyzed on the Tekran 2700 as described above. Particulate carbon and nitrogen were quantified, non-acidified, using a Costech 4010 CNS elemental analyzer (USGS 40 and 41 L-glutamic acid CRM recovery= 102±11%, 109 ±18%, respectively). A subset of acidified filters that were analyzed suggest that the total C measured was dominantly organic (Standard error between non-acidified and acidified samples = 12±15%). Chla and pha were quantified using fluorescence techniques after 90% acetone extraction. Sediment and water column bulk organic matter content was based on percent loss on ignition (%LOI) when a known sample mass was burned at 550°C. A dry sediment density was also determined and used convert sediment mass removed during the erosion experiment to a depth term.
Results and Discussion
Site Description
The research location was the Delaware Bay as it is a typical temperate, urban estuary and because of our previous studies within this ecosystem (e.g.11‘22‘23). The Delaware Bay is a 210 km coastal plain estuary with a 36,570 km2 watershed. It is relatively shallow, on average 8 m, but has a dredged channel that extends to 45 m depth. The dominant freshwater input is the Delaware River. Stratification in the bay fluctuates; it is often characterized as weakly stratified but can be stratified during periods of high river discharge and well mixed during low river discharge which often occurs during summer months24. To simplify this study, but still understand HgT cycling under different scenarios, we sampled at an upstream site (UP) influenced by riverine inputs (salinity 8.1 ± 0.3 ppt) and a downstream site (DWN) with greater marine influence (salinity 28.4 ± 0.2 ppt). Within each site two subsites were selected as described above. A site map is shown in (supporting information Fig. S1). The marsh sites (UP-HOC & DWN-HOC) had organic rich sediments (HOC; 9.2 ± 0.8% LOI) and the beach sites (DWN-LOC & UP-LOC) organic poor (LOC; 1.2 ± 0.8% LOI). Overall, the HOC sediments were composed of silt and clay whereas the LOC sediments were composed largely of sand (>75%; Table S2). At UP-HOC, enhanced particle removal was initiated at a shear stress of 0.1 Pa marking that as its critical shear stress. No change in erodibility was observed at either UP-LOC or DWN-LOC so it was assumed that the critical shear stresses exceeded 0.55 Pa (the highest shear stress applied). Two cores were eroded from DWN-HOC, one of which was more erodible than the other. The first core, DWN-HOC 1, had higher removal at the 0.1 and 0.2 Pa shear stress intervals, but became stable after 0.3 Pa, whereas the second core, DWN-HOC 2, surpassed its critical shear strength at 0.45 Pa. The average depth eroded from the DWN-HOC cores was ~0.24 cm versus ~0.23 cm from the UP-HOC core. Depth eroded was ~0.04 cm in both the UP-LOC & DWN-LOC cores. As described by Law17, the %sand/silt/clay of sediments strongly dictates the behavior of the surface sediments when stress is applied. Sandy-silts are generally non-cohesive and winnow under stress releasing finer silts and clays to the surface water. This process causes a disproportionate %sand/silt/clay in the eroded particles relative to the sediment. When the fraction of clay exceeds ~7.5% the sediment surface becomes cohesive and the resuspended particulate grain sizes will be more proportionate to that found in the sediment. For this study, %sand/silt/clay was assessed only on the bulk sediment using gravimetric techniques (Table S2) which does not provide enough information to calculate size specific sediment mobility. However, it is noted that the site with the lowest %clay fraction (UP-LOC) was the least erodible, and erodibility increased with increasing %silt/clay in the sediment. Based on17, it is likely that the LOC sediments underwent sorting and the finer sediment fraction removed whereas a more proportionate size fraction, and furthermore Hg burden, from the HOC sites was likely removed. Since pollutants like Hg generally partition into the smaller size fraction, these dynamics are important to consider for pollutant cycling in estuarine systems14,17. It is also recognized that flocculated material resting at the sediment surface could be driving the particulate composition, especially at low shear stresses, and that this flocculated fraction is important for pollutant transport and bioavailability14,15,25.
Site bulk Hg concentrations
Particle settling in estuarine environments removes the majority of HgT from the water column before it enters the marine environment. Where it settles, and whether it is resuspended, depends on the physical dynamics of the system and the particle/ sediment properties.17 The main source of inorganic Hg to the Delaware Bay is from riverine inputs enhanced by industry in the upper portion of the estuary. In general, suspended particulate HgT concentrations decrease from the head to the mouth of the estuary with the sediments following a similar pattern (22 and references within). Due to formation/ degradation dynamics, MeHg does not have a clear source signal or downstream removal pattern like HgT, but instead concentrations are patchy with some elevation near the estuary mouth22. Besides sediment dissolved and particulate flux, potential non-sedimentary MeHg sources include watershed inputs, tidal advection, and in situ water column methylation.
The HgT concentrations in this study were similar to previous studies from the Delaware Bay22. The bulk HOC sediments had elevated HgT and MeHg at the upstream site relative to downstream, but the opposite was true at the LOC sites (Fig. 2, Table S2). Furthermore, the bulk sediment (0–4 cm) HOC cores were enriched in HgT and MeHg compared to their lower OC counterparts, significantly so for all except DWN-LOC (p<0.01; Fig. 2). %MeHg was higher at the HOC sites compared to the LOC sites for both locations, and the downstream sites had higher %MeHg than the upstream sites (Fig. 2). The suspended HgT pool had less variability between the HOC and LOC sites within and between locations compared to the bulk sediments and had a concentration between that measured in the HOC and LOC bulk sediments (Fig. 2). Suspended particulate MeHg was enriched in the downstream water columns relative to upstream resulting in greater %MeHg at the downstream sites (Fig. 2). The suspended particles had a higher %LOI than measured in the sediments (28–76% versus 1–11%; Fig. 3, Table S2) which resulted in lower ratios of Hg species to %LOI (i.e. Hg normalized to organic content) in suspended particles (Fig. 3). Chla in the suspended particles followed a similar pattern to the %LOI with the greatest values at UP-HOC (304 ug/g chla) and UP-LOC (267 ug/g chla), then DWN-HOC (214 ug/g chla) and DWN-LOC (167 ug/g chla; tidal details in Table S2). Overall, pha made up a smaller fraction of the suspended organic matter (Table S2). The differences between sediment and suspended characteristics suggests that there were substantial additional sources of OC to the water column particulate besides resuspension.
Figure 2.
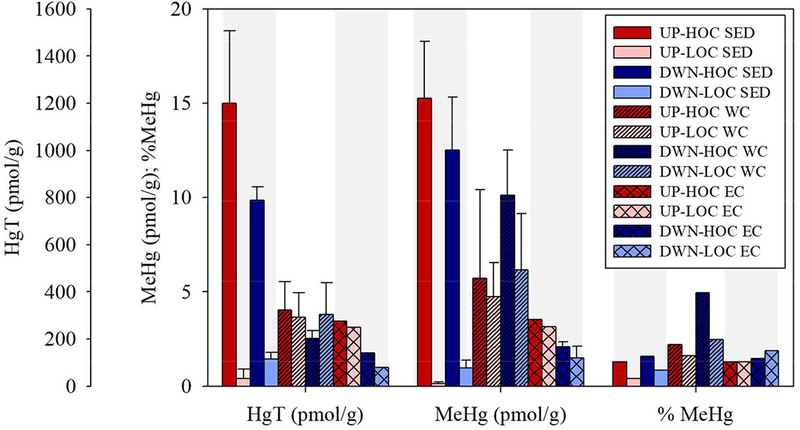
Comparison of total mercury (HgT), methylmercury (MeHg), and % MeHg from bulk sediment samples (SED; 0–4cm; solid bars), bulk water column particulates (WC; diagonally hatched bars), and particles collected from the erosion core experiment 0.01–0.2 Pa summed shear stresses (EC; cross-hatched bars). Error bars represent the standard deviation of field duplicates. DWN-HOC and DWN-LOC had erosion core duplicates. Water column samples are averaged over high tide and low tide. Analytical errors are given in Table S2 of SI. The sites are delineated by bar shade in order from upstream (UP) to downstream (DWN), high organic carbon (HOC, based on sediment type) and low organic carbon (LOC) (SI Fig. S1); UP-HOC is dark, UP-LOC is medium dark, DWN-HOC is medium light, and DWN-LOC is light.
Figure 3.
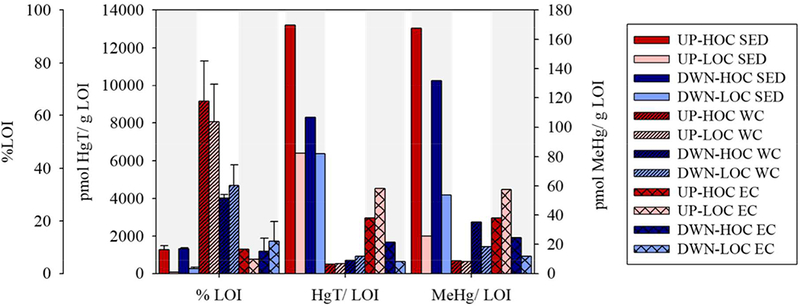
Organic matter measured as percent loss on ignition (%LOI) at 550°C and mercury (total, HgT; methylmercury, MeHg) normalized to %LOI in the bulk sediment (SED; 0–4cm; solid bars), bulk water column particulate averaged over high tide and low tide (WC; diagonally hatched bars), and particles collected from the erosion core experiment 0.01–0.2 Pa summed shear stresses (EC; cross-hatched bars). Error is detailed in the text and in SI Table S2. The sites are delineated by bar shade, as in Fig. 2, in order from upstream to downstream (SI Fig. S1) the same way as Fig. 2.
Assuming particulate HgT and MeHg cycle within the estuary dominantly bound to particulate organic matter (POM), we would expect a similar Hg/LOI ratio at each site if the Hg and POM were from similar sources. In the bulk sediment, the relative HgT/LOI (pmol/mg) was greatest at UP-HOC followed by DWN-HOC, with similar ratios at UP-LOC and DWN-LOC (Fig. 3, Table S2). The enrichment at HOC sites indicates either a HgT source to the marsh sediments that is not present in the beach sediment, or an alternative, less HgT rich C source to the beach sediment not present at the marsh sites. The further elevation of Hg at UP-HOC could be due to the greater % clay at that site relative to DWN-HOC (Table S2). The MeHg/LOI (pmol/mg) was also greatest at UP-HOC followed by DWN-HOC, then, unlike Hg-T/LOI, DWN-LOC was dissimilar to and greater than UP-LOC (Fig. 3, Table S2). This suggests that there is also a dissimilarity in sources, either MeHg, POM, or both, at the HOC sites relative to the LOC sites and furthermore at the downstream site relative to the upstream site. Due to the greater suspended solid %LOI in the water column (Fig. 3), the overall Hg/LOI ratios of the water column suspended solids were much lower than the sediments. The highest tidal average water column ratio was observed for DWN-LOC, then DWN-HOC, followed by UP-LOC and UP-HOC. In general, however, the values are quite similar between sites unlike the sediments which had a stronger C signal (Fig. 3). The similarity between suspended solids at the paired HOC/LOC sites suggests not only connectivity between the sites, but also begins to show the disconnect between surface water particles and the bulk composition of the sediment. This disconnect reflects the difference in composition of the material which is suspended compared to what is buried and points to the significant role of fine particles, clay and organic, that make up the majority of the HOC sediments and carry the burden of the Hg. The surface sediment-particle link is discussed further in the next section. Non-averaged tidal results are provided in Table S2.
Resuspended Hg and MeHg
The range of HgT and MeHg in the eroded particles at UP-HOC was significantly less than the bulk homogenized sediment fraction (p<0.01) but similar to what was measured in the water column (Fig. 1b, 2). The HgT and MeHg concentrations at UP-LOC were greater than what was measured in the sediments, but less than what was measured in the water column (Fig. 1b, 1c, 2). At DWN-HOC, the average sediment and water column HgT and MeHg concentrations were significantly greater than the concentration in the eroded particles (p<0.01). The DWN-LOC eroded particles were similar to the bulk sediment concentration, however, both HgT and MeHg concentrations were elevated in the water column (Fig. 1b, 1c, 2). The bulk sediment and water column particulate concentrations are discussed in the previous section. MeHg and HgT correlated in the eroded particles at both sites (R2=0.79, p<0.01 upstream; R2=0.91, p<0.01 downstream). Compared to each other, the eroded particles from paired sites were more similar than their respective bulk sediments. At the HOC sites, the particles eroded at high shear stress were enriched in HgT and MeHg per gram compared to those removed at lower shear stresses (Fig. 1b, 1c). At the LOC sites, HgT released per gram was consistent over increasing shear stress with a slight enrichment at low shear stresses in some cores. The %MeHg of the resuspended particles was similar over the course of the experiment in all cores, except the downstream site had slightly higher %MeHg at the lowest shear stresses increasing their averages (Fig. S3). The overall average %MeHg of the eroded particles at the upstream sites was 1.10 ± 0.27% and at the downstream sites 1.69 ± 0.78%, which is less than what was observed in the water column at the upstream site (1.91 ± 1.36%) and notably less than at the downstream site (3.71 ± 1.77%; Table S2). Summed over the low shear stress conditions (0.01–0.2Pa), the eroded particle %MeHg values were similar among the sites (Fig. 2). The eroded material %MeHg from the HOC cores was similar to the bulk sediment, but the LOC bulk was much lower (Fig. 1) indicating that the easily eroded material is dissimilar to the material that makes up the bulk LOC sediment. Again, there is little evidence that MeHg in the eroded particles is derived entirely from sediment production, as will be discussed further in later sections.
Figure 1.
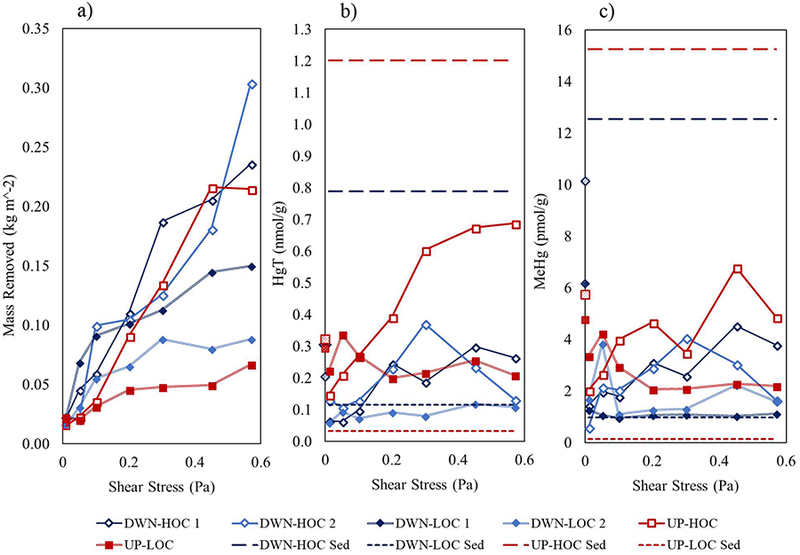
Resuspension results generated using the Gust Erosion Microcosm System from upstream (UP) and downstream (DWN) sites within the Delaware Bay. Close proximity subsites characteristic of a marsh system (high organic carbon, HOC) and beach system (low organic carbon, LOC) were sampled at each site. a) Depicts mass eroded normalized to core top area (kg/m2) at each shear stress interval for all cores; b) Depicts the eroded particle HgT normalized to mass (nmol/g) at each experimental shear stress interval; and c) Depicts the eroded particle MeHg normalized to mass (pmol/g) at each experimental shear stress interval. The upstream sites (UP) are red with square markers; UP-HOC are open squares and UP-LOC are filled squares. The downstream sites are blue with diamond markers; DWN-HOC are open and DWN-LOC are filled. Both DWN-HOC and DWN-LOC had duplicate erosion cores which are indicated as e.g. DWN-HOC 1 and DWN-HOC 2. Dashed lines in b) and c) indicate the bulk homogenized sediment values (0–4cm); long dashes are from organic rich, marsh sediments (DWN-HOC and UP-HOC) and short dashes from organic poor, beach sediments (UP-LOC and DWN-LOC). Water column high tide and low tide averaged particulate values are plotted on the y-axis, at 0 shear stress, and are patterned for differentiation. For figure clarity, sample replicate standard deviations are not shown in Fig. 1, and analytical errors are given in SI Table S2.
The resuspended material from the upstream sites had higher chla levels (UP-HOC= 82.3 ¼g/g; UP-LOC= 136 ¼g/g) than the downstream site (DWN-HOC= 30.3 ¼g/g, DWN-LOC = 81.3 ¼g/g), and the LOC sites had more chla than the HOC sites (Table S2). The water column had consistently higher levels of chla than the eroded particles. Overall, the chla and pha concentrations did not change with increasing shear stress, but there was a slight enrichment at the lower shear stress intervals at the HOC sites. %LOI in the resuspended particulate at all sites (11%) was close to that of the HOC bulk sediment (9%) and dissimilar to the LOC bulk sediment (1%; Fig. 3). Additionally, the average %LOI of the resuspended particles were more similar between the sub-sites compared to their respective bulk sediments, and much lower than the water column particulate (Fig. 3). In the following discussion, values reported were calculated from the 0.01–0.2 Pa shear stress samples, but average values from all shear stresses are given in Table S2. The HgT/LOI values (pmol/mg) from the eroded particles fell between their respective bulk sediment and water column values, except at DWN-LOC, whereas the MeHg/LOI (pmol/mg) varied by site ranging from reflecting the sediments to reflecting the suspended particulates (Fig. 3). HgT/LOI was greater at the upstream sites. UP-LOC more closely reflected the sediments whereas UP-HOC was more similar to the water column particles. The downstream sites resuspended material both reflected the water column, especially DWN-LOC (Fig. 3). MeHg/LOI in the resuspended material from UP-LOC and UP-HOC were similar to each other, but compared to the bulk pools, UP-LOC was greater than both the water column particulates and bulk sediment and UP-HOC fell between the water and sediment values. The DWN-HOC and DWN-LOC MeHg/LOI were nearly identical to their respective water column suspended particulates, and lower than the sediment. The intermediate Hg concentrations observed in the eroded particles exemplify the notion that a relatively clean or contaminated layer can exist above the surface bulk sediments that may be driving surface interaction but this is not recorded in the bulk sediment due to particle burial dynamics14. The impact of erosion on the suspended particulate composition is discussed in further detail below.
Relationship between Hg and organic matter turnover
In the previous section, differences in HgT and MeHg between the resuspended particles and the sediment and water column pools were presented. To further parse out what may be driving the similarities and differences between the three pools, pigment and carbon data from the eroded and suspended particulates were assessed assuming that Hg cycling is strongly influenced by organic matter (OM) cycling. These relationships are supported by strong correlations between MeHg and chla (upstream r=0.63, p=0.017, downstream r=−0.50, p=0.007), and MeHg and pha (upstream r=0.94, p< 0.001, downstream r=0.92, p<0.001). The highest fraction of pha relative to chla for the resuspendable material was found at DWN-HOC (17–51%) followed by UP-HOC (9–30%). The LOC sediments had lower % pha (<21%) with DWN-LOC 1 being <9% and UP-LOC <15%. Previous studies have found that bulk surface sediment pigments are typically >50% pha (e.g. Slater and Carton, 2009) suggesting that the resuspended material in this study had a higher percent of living or non-degraded material than typical bulk sediment but with the HOC resuspended materials approaching that value. Overall, the range in %pha was comparable to, but somewhat higher, than that of the water column (1–30%). Mesocosm resuspension experiments found that resuspension increased the %pha in the water column by a factor of two compared to nonresuspension mesocosms (56% versus 30%, respectively28). Using the mesocosm study for reference, the DWN-HOC %pha is the only site with a resuspension signal approaching that of the mesocosm resuspension chamber, but the higher level is not reciprocated in the water column. The results therefore suggest a higher living phytoplankton fraction in the water column dissimilar to that which has settled at these sites prior to the resuspension experiment, and dissimilar sources of planktonic carbon between the water column and surface sediment pool.
The higher % pha at the HOC sites suggests greater OM remineralization, and this is also reflected in the C/N data. The HOC resuspended material C/N ratios generally increased from ~5.8–9.1 to as high as 10.4 with increasing shear stress, but the C/N values for the LOC sites remained near Redfield ratios throughout the erosion experiment (5.7 ± 1.4; Fig. S4). The UP-LOC core was unique in that the C/N ratio decreased with increasing shear (6.7 to 4.8), excluding an outlier at 0.1 Pa of 11.2, which indicates that degraded organic material may be more easily resuspended and more similar to surface waters. The C/N ratio of suspended water column particulates was 6.6 at low tide supporting this idea. The DWN-LOC site also had similar C/N ratios between particles removed at the lower shear stress and the water column (5.1 and 4.8, respectively), but both the HOC sites had higher C/N in surface water particles than what was collected at the lowest shear stress (DWN-HOC=6.8, UP-HOC=6.6 vs 5.9 and 5.7, respectively). The average of the resuspended particles over tidal shear stress values was similar to what was measured in the water column (DWN-HOC=6.7, UP-HOC=6.6), but the average over all shear stresses was higher (DWN-HOC=8.0, UP-HOC=8.1). These relationships suggest OM remineralization in HOC sediments, and likely within the surface resuspendable layer, but that this is not as prevalent in LOC sediments. However, the remineralization could also be occurring within the water column during or prior to settling to the sediment surface.
The C/chla ratio (g C/g chla) can be used as an indicator for how much OM is phytoplankton under the assumption that the C/chla ratio for phytoplankton averages 40 in estuarine ecosystems but recognizing that this value varies with species and location29. The highest C/chla ratio was found at DWN-HOC (2101770, increasing with increasing shear stress) which concurs with that site having the highest % pha and C/N ratios. The lowest overall C/chla values were found at the LOC sites (DWN-LOC =250 ± 50, UP-LOC=170 ± 55, no trends with increasing shear stress). UP-HOC was more comparable to DWN-LOC, but like DWN-HOC, the C/chla ratio increased with increasing shear stress (170–600). In most cases, these values were elevated compared to the water column particulate (95–220), especially at high tide (<150). This indicates that the water column particulate had a larger fraction as plankton for all sites (1840%), while for the resuspended material the phytoplankton fraction was lower (<20% plankton). In all cases, the %OC in the water column particulate ranged from 1.5 to 5.6%, while it ranged from 1.4 to 3.0% for the resuspended particulate. These values again suggest an overall high inorganic fraction in both the resuspended particulate and the water column TSS.
Role of resuspension on suspended particle dynamics
The difference between what was measured in the water column versus what was resuspended during the erosion experiment indicates the contribution of resuspension to surface water particle composition, or particle cycling between surface waters and the local sediments. The following calculations represent the amount of Hg and pigments measured in the water column at high tide and low tide that come from sources other than resuspension under low shear stress (0.01–0.2 Pa; tidal range) and high shear stress (0.01–0.55 Pa; storm range) conditions. Low shear stress values are more representative of the conditions at the time of sampling. Values less than 10% provide the most confidence that sediment resuspension can account for the majority of the surface water particles, or there is connectivity between the sediments and water column, whereas higher values indicate that particles in the water column likely come from non-local sources or result from primary production. Negative numbers suggest that sediment resuspension over-predicts what was measured in the water column at the time of sampling. An overview of the calculations is reported in Table S3.
At DWN-HOC, MeHg had a strong, non-local signal (60–81%) whereas HgT had a resuspension signal (13–40%) which was stronger at high tide (−13–27%) and during high shear stress conditions (−13–11%). Sediment MeHg sources were slightly higher at high tide than low tide (within 10% of each other) and high shear stress conditions (within 5% of low shear). The lack of change in water column particulate composition at DWN-HOC under varying conditions further indicates that MeHg in the water column was not connected to the surface sediments at this site at the time of collection. The dichotomy between the HgT and MeHg source signals was also evident in the higher %MeHg measured in the water column at DWN-HOC compared to the sediment and resuspended pools (Fig. 2). Similar to MeHg, chla had a strong, non-resuspension signal (56–88%) with higher %’s at high tide by ~20%. The finding of higher water column chla and %MeHg is similar to what was observed in the non-resuspension chamber in the mesocosm experiments mentioned previously28. The pha signal differed in that resuspension at high tide overpredicted what was measured in the water column (<−150%), but other sources were important at low tide (56–64%) when the salt marsh would be draining into the coastal water. At DWN-LOC, MeHg was again dominantly from other sources (>75%) with no real trend between tides or shear stress conditions suggesting the same conclusion as for DWN-HOC. HgT had a stronger sediment signal at high tide (~52%) but was similar to MeHg in terms of external sources dominating at low tide (~75%) and were similar between shear conditions. The difference in site characteristics between DWN-LOC and DWN-HOC, namely one a beach and the other a marsh, likely explains the difference in source characteristics of HgT and may be a function of the cohesive properties of the HOC sediment as described above. The DWN-LOC chla data showed opposite tidal trends from DWN-HOC. Chla had a larger resuspension signal at high tide (~40–50%) compared to low tide (67–75%) and it was nearly the same between shear stress conditions. Pha from DWN-LOC 1 all had strong non-local signals, but greater at high (~93%, similar between shear) than low tide (44–66% between high and low shear). The DWN-LOC 2 core high tide signal was ~77% non-local but was <−40% at low tide.
Sediment signals were much stronger at the upstream site. MeHg had a strong resuspension signal at high tide for both UP-HOC and UP-LOC (−89–21%), but non-local sources were strong at low tide (47–56%) for both shear stress conditions. At UP-HOC, the importance of resuspension of HgT was high (−121–17%) except at the high tide, low shear condition (32%). Sediment sources of HgT were not as strong at UP-LOC at low tide (~33% for both shear stress conditions), but they were important at high tide (~−11% for both shear stress conditions). Chla at DWN-HOC had a strong non-local signal for both tides and shear conditions (61–74%; similar between tides ~10% higher at high shear), but strongly negative pha signals under all conditions (low shear: −650% at high tide, −35 at low tide; high shear: −810% at high tide, −63% at low tide). Chla at UP-LOC had a strong sediment signal at low tide (−2%), but not at high tide (64%) averaged over the shear conditions, which were within 7% of each other. Like UP-HOC, UP-LOC pha had a strong sediment signal (−41–0%) with lower signals at low tide by ~20% and high shear conditions by ~20%. The stronger signal for chla relative to pha is indicative of the production of phytoplankton in surface waters that die and settle to the sediment surface.
Particulate HgT and MeHg speciation and concentration is influenced by POM in estuarine water columns resulting in depositional sites such as HOC sediments being enriched in HgT and MeHg relative to their LOC counterparts. However, this study demonstrates that the material actively being cycled between the sediment surface and water column is different from the underlying bulk sediment with bulk HOC sediments generally over-predicting the resuspendable Hg load and LOC sediments underpredicting, therefore negating the use of bulk sediment characteristics to describe Hg exchange with surface waters. That said, HOC sediments have a higher propensity to be resuspended and therefore that sediment type likely plays a larger role in HgT and MeHg release into the water column under higher shear stress conditions. This result implies the importance of understanding sediment critical shear stress and Hg loading when determining potential particulate sediment fluxes to the water column. Site location is also important. For instance, at the upstream site where the channel is narrower, sediments tend to have a stronger impact on the water column composition relative to the more non-local signals observed at the downstream site which is near the widest part of the estuary (Fig. S1). Therefore, the stronger overall sediment signals at the upstream site could be due to it being a more turbid environment. Furthermore, particle associated bacterial activity has been shown to increase near the mouth of the Delaware Bay which supports the possibility for water column MeHg production30, and may explain the non-sedimentary source signal of MeHg in that region of the estuary. The calculations presented in this section and the lack of MeHg enrichment in the eroded particles (%MeHg; Fig. 2) both suggest that net methylation is not occurring within the eroded material nor is derived from methylation occurring deeper in the sediments even though the pigment data suggests OM degradation in the HOC sediments. Finally, the results of this work show that the lack of correlation found in previous studies between bulk sediment and water column particulate MeHg across estuaries is likely due to the misrepresentation of the fraction of sediment actively exchanging with surface water particles. Therefore, a bulk sediment measurement is an overall poor proxy for sediment derived MeHg to the water column. The results presented here demonstrate the necessity to better characterize the resuspendable fraction when assessing particulate Hg cycling through estuarine ecosystems.
Supplementary Material
ACKNOWLEDGMENT
This paper forms part of the dissertation research of EAS and uses the Gust Microcosm System of VIMS operated by GMM. We thank personnel in the Mason Lab for their help in field preparation/ sampling (Nashaat Mazrui, Prentiss Balcom, and Kathleen Gosnell) and sample analysis (Nashaat Mazrui and Sofi Jonsson). We would also like to thank the reviewers for their helpful comments. The research was funded by the National Science Foundation Graduate Research Fellowship Grant # DGE-1747453, the University of Connecticut Marine Science Pre-Doctoral Fellowship, and the Dartmouth SUPERFUND Program Grant # P42 ES007373. The authors declare no competing financial interest.
ABBREVIATIONS
- DWN-HOC
Downstream high organic carbon
- Chla
Chlorophyll a
- UP-HOC
Upstream high organic carbon
- Hg
Mercury
- HgT
Total mercury
- HOC
High organic carbon
- LOC
Low organic carbon
- %LOI
Percent loss on ignition
- MeHg
Methylmercury
- OM
Organic matter
- Pha
Phaeo phytin
- POM
particulate organic matter
- DWN-LOC
Downstream low organic carbon
- TSS
Total suspended solids
- UP-LOC
Upstream low organic carbon
Footnotes
Supporting Information. Figure S1. Map of site locations; Figure S2. Diagram of Gust Microcosm System; Figure S3. Calculated %MeHg from experiment and field samples; Figure S4. Calculated C/N ratios from experiment and field samples; Table S1. Assessment of the potential for TSS in Gust system flow-through water; Table S2. Detailed results of the experiment; Table S3. Table of calculation results regarding the impact of resuspension on particulate Hg concentrations in coastal waters.
Author Contributions
The manuscript was written with substantial contributions from all authors. All authors have given approval to the final version of the manuscript.
REFERENCES
- (1).Chen CY; Driscoll CT; Lambert KF; Mason RP; Rardin LR; Serrell N; Sunderland EM Marine Mercury Fate: From Sources to Seafood Consumers. Environ. Res 2012, 119, 1–2. [DOI] [PubMed] [Google Scholar]
- 2).Benoit JM; Gilmour CC; Mason RP; Riedel GS Behavior of Mercury in the Patuxent River Estuary. Biogeochem 40 1998, 249–265. [Google Scholar]
- 3).Conaway CH; Squire S; Mason RP; Flegal AR Mercury Speciation in the San Francisco Bay Estuary. Mar. Chem 2003, 80 (2–3), 199–225. [Google Scholar]
- 4).Compeau GC; Bartha R Methylation and Demethylation of Mercury under Controlled Redox, PH,and Salinity Conditions. Control. Redox, pH, Salin. Cond. Appl. Environ. Microbiol 1984, 48 (6), 1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Schartup AT; Balcom PH; Soerensen AL; Gosnell KJ; Calder RSD Freshwater Discharges Drive High Levels of Methylmercury in Arctic Marine Biota. PNAS 2015, 112 (38), 11789–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Jonsson S; Skyllberg U; Nilsson MB; Lundberg E; Andersson A; Björn E Differentiated Availability of Geochemical Mercury Pools Controls Methylmercury Levels in Estuatine Sediment and Biota. Nat. Commun 2014, 5:4624, DOI: 10.1038. [DOI] [PubMed] [Google Scholar]
- 7).Taylor VF; Bugge D; Jackson BP; Chen CY Pathways of CH 3 Hg and Hg Ingestion in Benthic Organisms: An Enriched Isotope Approach. Environ. Sci. Technol 2014, 48, 5058–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Chen CY; Borsuk ME; Bugge DM; Hollweg T; Balcom PH; Ward DM; Williams J; Mason RP Benthic and Pelagic Pathways of Methylmercury Bioaccumulation in Estuarine Food Webs of the Northeast United States. PLoS One 2014, 9 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Chen CY; Dionne M; Mayes BM; Ward DM; Sturup S; Jackson BP Mercury Bioavailability and Bioaccumulation in Estuarine Food Webs in the Gulf of Maine. Environ. Sci. Technol 2009, 43 (6), 1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shi X; Mason RP; Charette MA; Mazrui NM; Cai P Mercury Flux from Salt Marsh Sediments: Insights from a Comparison Between224Ra/228Th Disequilibrium and Core Incubation Methods. Geochim. Cosmochim. Acta 2018, 222, 569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Balcom PH; Schartup AT; Mason RP; Chen CY Sources of Water Column Methylmercury across Multiple Estuaries in the Northeast U.S. Mar. Chem 2015, 177, 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Lawson NM; Mason RP; Laporte J-M The Fate and Transport of Mercury, Methylmercury, and Other Trace Metals in Chesapeake Bay Tributaries. Water Res 2001, 35 (2), 501–515. [DOI] [PubMed] [Google Scholar]
- 13).Kim E-H; Mason RP; Porter ET; Soulen HL The Impact of Resuspension on Sediment Mercury Dynamics, and Methylmercury Production and Fate: A Mesocosm Study. Mar. Chem 2006, 102 (3–4), 300–315. [Google Scholar]
- 14).Milligan TG; Law BA Contaminants at the Sediment - Water Interface: Implications for Environmental Impact Assessment and E Ff Ects Monitoring. Environ. Sci. Technol 2013, 47, 5828–5834. [DOI] [PubMed] [Google Scholar]
- 15).Kalnejais LH; Martin WR; Signell RP; Bothner MH Role of Sediment Resuspension in the Remobilization of Particulate-Phase Metals from Coastal Sediments. Environ. Sci. Technol 2007, 41, 2282–2288. [DOI] [PubMed] [Google Scholar]
- 16).Schaaff E; Grenz C; Pinazo C; Lansard B Field and Laboratory Measurements of Sediment Erodibility: A Comparison. J. Sea Res 2006, 55 (1), 30–42. [Google Scholar]
- 17).Law BA; Hill PS; Milligan TG; Curran KJ; Wiberg PL; Wheatcroft RA Size Sorting of Fine-Grained Sediments during Erosion: Results from the Western Gulf of Lions. Cont. Shelf Res 2008, 28 (15), 1935–1946. [Google Scholar]
- 18).Kalnejais LH; Martin WR; Signell RP; Bothner MH Role of Sediment Resuspension in the Remobilization of Particulate-Phase Metals from Coastal Sediments. Environ. Sci. Technol 2007, 41, 2282–2288. [DOI] [PubMed] [Google Scholar]
- 19).Porter ET; Mason RP; Sanford LP Effects of Shear Stress and Hard Clams on Seston, Microphytobenthos, and Nitrogen Dynamics in Mesocosms with Tidal Resuspension. Mar. Ecol. Prog. Ser 2013, 479, 25–45. [Google Scholar]
- 20).Hammerschmidt CR; Fitzgerald WF Bioaccumulation and Trophic Transfer of Methylmercury in Long Island Sound. Arch. Environ. Contam. Toxicol 2006, 51 (3), 416–424. [DOI] [PubMed] [Google Scholar]
- 21).Hammerschmidt CR; Fitzgerald WF Formation of Artifact Methylmercury during Extraction from a Sediment Reference Material. Anal. Chem 2001, 73 (24), 5930–5936. [DOI] [PubMed] [Google Scholar]
- 22).Buckman K; Taylor V; Broadley H; Hocking D; Balcom P; Mason R; Nislow K; Chen C Methylmercury Bioaccumulation in an Urban Estuary: Delaware River, USA. Estuaries and Coasts 2017, 40 (5), 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Gosnell K; Balcom P; Ortiz V; Dimento B; Schartup A; Greene R Seasonal Cycling and Transport of Mercury and Methylmercury in the Turbidity Maximum of the Delaware Estuary. Aquat. Geochemistry 2015, 22 (4), 313–336. [Google Scholar]
- 24).Aristizábal M; Chant R Mechanisms Driving Stratification in Delaware Bay Estuary. Ocean Dyn 2014, 64 (11), 1615–1629. [Google Scholar]
- 25).Kach DJ; Ward JE The Role of Marine Aggregates in the Ingestion of Picoplankton-Size Particles by Suspension-Feeding Molluscs. Mar. Biol 2008, 153 (5), 797–805. [Google Scholar]
- 26).Luengen AC; Flegal AR Role of Phytoplankton in Mercury Cycling in the San Francisco Bay Estuary. Limnol. Oceanogr 2009, 54 (1), 23–40. [Google Scholar]
- 27).Slater MJ; Carton AG Effect of Sea Cucumber (Australostichopus Mollis) Grazing on Coastal Sediments Impacted by Mussel Farm Deposition. Mar. Pollut. Bull 2009, 58 (8), 1123–1129. [DOI] [PubMed] [Google Scholar]
- 28).Porter ET; Mason RP; Sanford LP Effect of Tidal Resuspension on Benthic-Pelagic Coupling in an Experimental Ecosystem Study. Mar. Ecol. Prog. Ser 2010, 413, 33–53. [Google Scholar]
- 29).Jakobsen HH; Markager S Carbon-to-Chlorophyll Ratio for Phytoplankton in Temperate Coastal Waters: Seasonal Patterns and Relationship to Nutrients. Limnol. Oceanogr 2016, 61 (5), 1853–1868. [Google Scholar]
- 30).Campbell BJ; Kirchman DL Bacterial Diversity, Community Structure and Potential Growth Rates along an Estuarine Salinity Gradient. ISME J. 2013, 7, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


