Abstract
This pilot study evaluated associations between carotid wall echogenicity, cardiovascular disease (CVD) risk factors, and three markers of smoking heaviness in a cohort of active smokers.
Common carotid artery (CCA) grayscale median (GSM) values were measured from sonographic images. Univariable correlations and exploratory multivariable models were used to determine associations between CCA GSM, CVD risk factors, and measures of smoking heaviness.
CCA GSM was measured in 162 smokers and was correlated inversely with cigarettes smoked/day (r=−0.16, p=0.048), pack-years (r=−0.204, p=0.009), CVD risk factors such as age, male sex, waist circumference, and low-density lipoprotein cholesterol (all p≤0.03) and positively with high-density lipoprotein cholesterol (p<0.001). Associations between CCA GSM and smoking heaviness markers were not statistically significant after adjustment for traditional risk factors.
The results from this pilot study demonstrate the feasibility of measuring the GSM value of the CCA far wall and its association with measures of smoking heaviness and traditional CVD risk factors among current smokers.
Keywords: Carotid sonography, common carotid artery, grayscale median value
1. Introduction
Atherosclerosis is a chronic systemic disease that begins early in life with injury to the arterial lumen resulting in inflammation, increased lipid accumulation, and hyperplasia of medial smooth muscle fibers1–4. Progression of atherosclerotic vascular disease is dependent upon genetic and environmental risk factors, such as hyperlipidemia, hypertension, and cigarette smoking2, 4, 5.
Smoking is a powerful risk factor for cardiovascular disease (CVD) that is associated with an increased risk for coronary heart disease and stroke6, 7–9. Smoking promotes atherogenesis and increased CVD risk by several mechanisms that contribute to arterial injury, plaque formation, and plaque vulnerability8, 9. Imaging markers indicative of late stage atherosclerosis, such as increased coronary artery calcification8, carotid artery plaque formation, and carotid wall intima-media thickness (IMT)10 have been associated with smoking. Inflammation is an important mechanism for increased CVD risk among smokers. Atherosclerotic plaques of smokers have a greater content of inflammatory cells than observed in non-smokers11. Smokers have increased macrophage recruitment to the arterial wall11, increased leukocyte counts, and higher levels of C-reactive protein and fibrinogen8, 12.
It has been suggested that early atherosclerotic changes in arterial composition (i.e., increased lipid accumulation and inflammatory changes) can be seen with gray-scale sonography, evidenced as a more hypoechoic or echolucent intima-media complex that will be manifest prior to changes in wall thickness1–3. Thus, measuring carotid artery wall echogenicity may prove to be an early in vivo marker for assessing atherosclerosis risk and monitoring treatment1, 2.
Carotid wall echogenicity is a novel, sensitive predictor of CVD risk that is related to but independent of carotid IMT3, 13, 14. To our knowledge, the relationship between smoking heaviness (cigarettes smoked/day and pack-years) and common carotid artery wall echogenicity has not been explored previously in a smoking cohort. Others15 have demonstrated a non-linear, V-shaped relationship between carotid plaque echogenicity (but not arterial wall echogenicity) and current smoking. The advantage to looking at arterial wall echogenicity versus plaque echogenicity is that it can assess all individuals instead of being limited to only evaluating individuals with plaque present3. Understanding the relationships among carotid wall echogenicity, smoking heaviness, and other CVD risk factors may suggest mechanisms for understanding the effects of smoking on arterial injury, how it interacts with other risk factors, and to assess the arterial health of smokers. A pilot study was conducted to assess the relationship among CCA GSM, three markers of smoking heaviness, and other CVD risk factors in current smokers.
2. Materials and Methods
2.1 Participants
This research used baseline, pre-treatment data from smokers who participated in a randomized, double-blind, placebo-controlled smoking cessation trial16. The institutional review board at the University of Wisconsin School of Medicine and Public Health approved of this study and all participants provided written informed consent. Major inclusion and exclusion criteria were based on participation in the clinical trial and included age ≥18 years old, current smoking of ≥10 cigarettes/day for the previous 6 months, expired carbon monoxide (CO) level of >9 ppm, and stated motivation to try to quit smoking. Details of other exclusion criteria are described in the primary analysis of this cohort16.
Participants were 162 smokers from Madison, Wisconsin, who were selected based on having images acquired with the same ultrasound equipment preset (e.g. CV preset, linear grayscale map [map L] maximum dynamic range 70 dB, baseline lab values available and carotid intima media measurements).
These images were used to assess common carotid artery (CCA) gray scale median (GSM), a measure of carotid wall echogenicity. Participants provided a fasting blood sample to assess total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, glucose, high-sensitivity C-reactive protein (hsCRP), and leukocyte count using standard techniques. Measures of smoking heaviness were cigarettes smoked/day, pack-years (cigarettes smoked/day*years smoking), and exhaled carbon monoxide (CO). Tobacco dependence was assessed with the Fagerstrom Test for Cigarette Dependence (FTCD)17.
2.2 Carotid Ultrasonography and Measurement of CCA GSM
Digital images of the far wall of the right CCA were acquired as described previously that were performed on the same diagnostic ultrasound imaging system (CV 70, Siemens Medical Solutions, Mountain View, CA), same linear transducer L10-5 and using the same imaging preset18. Prior to the start of the study and throughout the study, quality assurance measures were performed on a Gammex Small Parts (Grey Scale) Phantom (404GS-LE 0.7) (Gammex, a Sun Nuclear Company, Melbourne, FL, USA). Quality assurance measures were performed to assess and confirm lateral and axial resolution as well as vertical and horizontal calibration measurements (see figure 1 A-D, demonstrating phantom measurements made on the CV70 system as part of our routine QA program).
Figure 1.
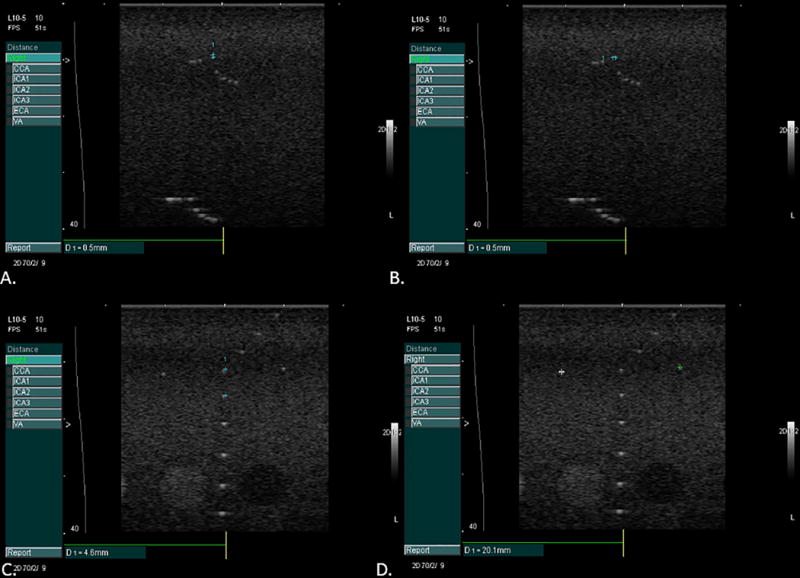
Phantom images taken for quality assurance to measure axial (panel A) and lateral (panel B) resolution and to confirm calibration in the vertical (panel C) and horizontal planes (panel D).
After acquisition, images were transferred to a DICOM server (Freeland Systems LLC, Alpharetta, GA) at a core ultrasound laboratory. CCA GSM was measured using dedicated plaque texture analysis software (LifeQ Medical, Cyprus). All images were digitized into bitmap images. Normalization was performed utilizing the software normalization module with blood assigned a gray scale value of 0 and the adventitia a gray scale value of 190 and then were standardized to a standard pixel density of 20.00 per millimeter19–22.
After images were normalized and standardized, the carotid arterial wall was segmented for analysis. The distal common carotid artery was identified by locating the carotid bulb and then placing an online ruler tool (Microsoft Windows Ruler) scaled to 1.0 cm adjacent to the proximal edge of the carotid bulb and on the leading edge of the arterial wall adventitia. The arterial wall was segmented for grayscale analysis by manually tracing the intima-media complex of the far wall for a distance of 1.0 cm (figure 2). The cursor was placed on the intima-blood interface of the far wall and the leading edge of the adventitia to define the segmented arterial wall region of interest. LifeQ software was used to crop the segmented area and analyzed the grayscale characteristics. The measure we were interested for this study is the grayscale median value. The grayscale median (GSM) value was used instead of the mean value because the grayscale values are skewed (see figure 3). In the “features extraction module” the cropped segmented arterial wall is demonstrated as well as the colorized version depicting the range of grayscale values (see figure 4). Once the trace was completed, all data and images were automatically saved digitally and uploaded into a secure study database for statistical analysis.
Figure 2.
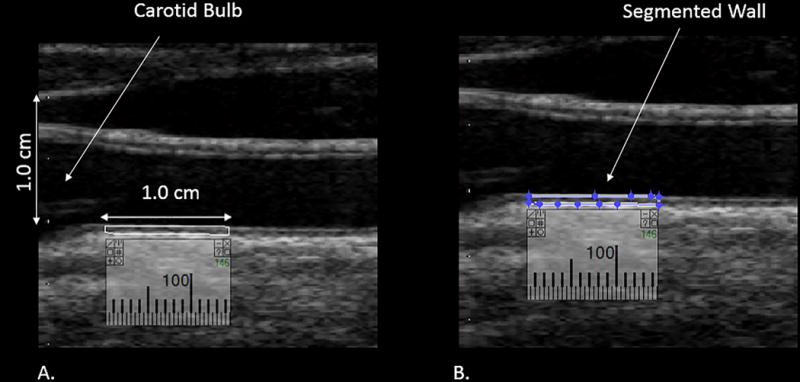
Distal 1.0 cm of the common carotid artery measured to obtain the GSM value. Panel A demonstrates the calibration of 1.0 cm and the segment of the wall to be measured. Panel B demonstrates segmentation of the distal 1.0 cm of the CCA for GSM measurement.
Figure 3.
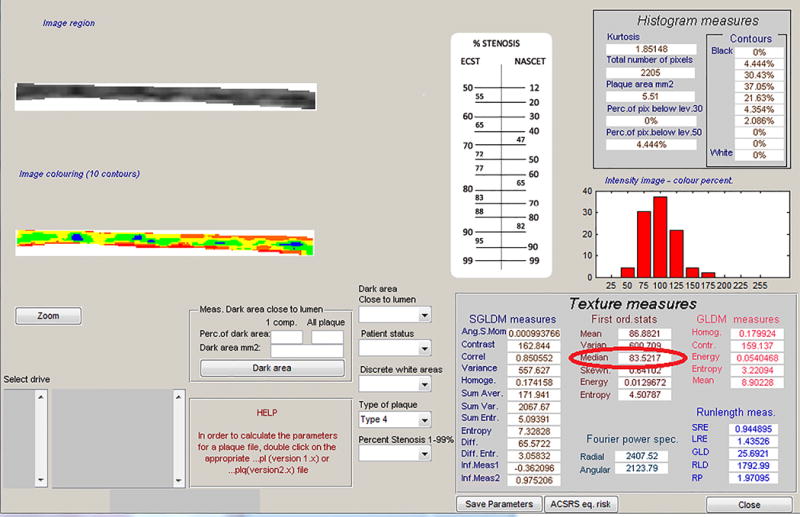
Output from the software demonstrating the measurement of the GSM (red circle).
Figure 4.
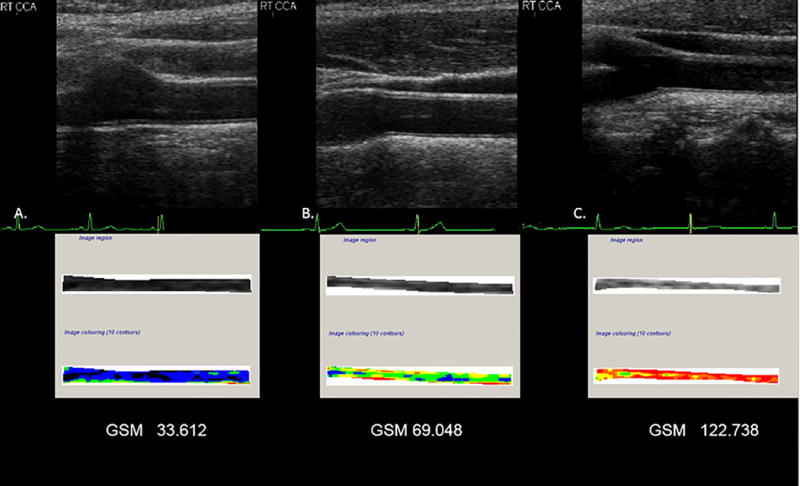
Sonographic image and corresponding grayscale analysis of the arterial wall. Panel A, demonstrates the normalized and standardized B-mode image and associated tracing of the far wall of a common carotid artery for GSM. This artery is more echolucent. Panel B, demonstrates the normalized and standardized B-mode image and associated tracing of the far wall of a common carotid artery for GSM. This artery is representative of the mean GSM value of the study cohort. Panel C, demonstrates the normalized and standardized B-mode image and associated tracing of the far wall of a carotid artery for GSM. This artery is less echolucent. Image colorization (10 contours) depicts the far wall of a common carotid artery based on the local GSM value. Values of 0-25 units are displayed as black, 26-50 as blue, 51-75 as green, 76-100 as yellow, 101-125 as orange, and 126-255 as red40.
Intra-reader measurement reproducibility for far wall CCA GSM measurements was excellent (r=0.99 for 26 blinded paired readings, mean [standard deviation] absolute delta = 1.95 (1.22). The intra-class correlation coefficient was 0.99. Inter-reader measurement reproducibility also was excellent (r=0.98 for blinded paired readings, absolute delta = 2.29 [1.78] units with an intra-class correlation coefficient of 0.97).
2.3 Statistical Analysis
Statistical analyses were performed using SPSS (USA IBM Corporation. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corporation; 2013). Descriptive statistics were computed and sex differences in GSM values were examined using Student’s t-test. Pearson correlations were used to examine the relations between CCA GSM and smoking markers and CVD risk factors. We then conducted multivariable linear regressions with CCA GSM as the dependent variable for each smoking heaviness marker, adjusting for age, sex, and race (coded as white vs. non-white). Finally, we conducted multivariable linear regression to examine the impact of non-smoking CVD risk factors (systolic blood pressure, total cholesterol, HDL-C, waist circumference, hsCRP, hemoglobin A1c, and use of antihypertensive and lipid-lowering medications) on CCA GSM. Because this was a pilot study based on image availability, no a priori power analysis was performed.
3. Results
3.1 Participant Characteristics (Table 1)
Table 1.
Baseline Participant (n=162) Characteristics
| Value* | |
|---|---|
| Common carotid artery gray scale median (unitless) | 69.1 (18.5) |
| Age (years) | 44.9 (11.4) |
| Male (N, %) | 62 (38.3%) |
| Female (N, %) | 100 (61.7%) |
| Race (N, %) | |
| White | 152 (93.8%) |
| Black | 6 (3.7%) |
| Asian/Pacific Islander | 1 (0.6%) |
| Other | 3 (1.9%) |
| Smoking markers | |
| Cigarettes smoked/day | 20.7 (7.5) |
| Cigarette smoking (pack-years) | 27.5 (17.4) |
| Carbon monoxide (ppm) | 26.4 (13.3) |
| Fagerstrom Test of Cigarette Dependence | 5.2 (2.1) |
| Systolic blood pressure (mmHg) | 119.2 (14.1) |
| Antihypertensive medication use (N, %) | 11 (6.8%) |
| Total cholesterol (mg/dL) | 184.0 (34.4) |
| Low-density lipoprotein cholesterol (mg/dL) | 116.2 (29.0) |
| High-density lipoprotein cholesterol (mg/dL) | 46.2 (13.6) |
| Lipid-Lowering medication use (N, %) | 9 (5.6%) |
| Waist circumference (cm) | 95.4 (15.5) |
| Body-mass index (kg/m2) | 28.7 (6.2) |
| Leukocyte count (/1000 cells) | 7.6 (2.2) |
| C-Reactive Protein (mg/L) | 2.6 (3.0) |
All values are means (standard deviations) unless otherwise noted.
Participants had a mean age of 44.9 (11.4 standard deviation, SD) years old and 61.7% were females. They smoked 20.7 (7.5 SD) cigarettes/day and reported 27.5 (17.4 SD) pack-years of smoking. Their CO was 26.4 (13.3 SD) ppm and their FTCD score was 5.2 (2.1 SD). These measures provide a description of the smoking burden on participants in this pilot study. Compared to definitions previously used to characterize an individual as a “light smoker” (<15 cigarettes/day) our participants smoked more cigarettes per day and would be considered “moderate” smokers23. Exhaled CO measures previously have been reported as 3.6 (2.15) ppm in healthy non-smokers, 5.2 (3.4) ppm passive smokers and 17.13 (8.5) ppm healthy smokers24. A FTCD score of 0-3 represent an individual with low dependence on nicotine, a score of 4-6 is associated with moderate dependence on nicotine, a score of 7-10 is considered to be highly dependent on nicotine17,24, 25. For White and Black Americans, a waist circumference greater than 35 inches for women and greater than 40 inches for men is indicative of high risk26.
3.2 Correlates of CCA GSM
The mean right CCA GSM was 69.1 (18.5) units. Mean CCA GSM was lower in men than in women (63.0 (16.5) versus 72.9 (18.8), t=−3.40, p=0.001). CCA GSM was correlated inversely with cigarettes/day (r=−0.16, p=0.048) and pack-years (r=−0.20, p=0.01) but not with CO or FTCD score (all p>0.05). CCA GSM was inversely associated with CVD risk factors as expected: age (r=−0.26, p=0.001), waist circumference (r=−0.38, p<0.001), hemoglobin A1C (r=−0.18, p=0.02), total cholesterol (r=−0.17, p=0.03), and systolic blood pressure (r=−0.16, p=0.047). HDL-C was positively correlated with CCA GSM (r=0.33, p<0.001). Leukocyte count, hsCRP, and heart rate were not correlated significantly with CCA GSM (all p>0.05).
3.3 Multivariable Models of CCA GSM
Covariate models are used to determine if participant characteristics such as age and sex are confounders (variables which, if not controlled for, may affect the relationship being studied)27. Multivariable models are used to assess relationships between a number of independent variables (in this study, CVD risk factors, measures of smoking heaviness) and an outcome, or dependent variable (in this study gray scale median value)28.
In the covariate-only model, age (β= −0.22, p=0.005) and sex (β=0.23, p=.004) were significant predictors of CCA GSM, but race was not (β= −0.002, p=0.98). After adjusting for age, sex and race, none of the smoking heaviness markers predicted CCA GSM with statistical significance: cigarettes smoked/day, (β= −0.08, p=0.29), pack-years of smoking (β= −0.04, p=0.68), and CO (β= 0.06, p=0.41).
In the CVD risk factor multivariable model, we initially included systolic blood pressure, total cholesterol, HDL-C, waist circumference, hsCRP, hemoglobin A1C, and use of antihypertensive and lipid-lowering medications. Using a backward model building procedure, the final model (adjusted R2=22.2%), adjusted for age (β=−0.20, p=0.01), sex (β=0.09, p=0.29) and race (β=0.01, p=0.89), and included total cholesterol (β=−0.16, p=0.04), HDL-C (β=0.21, p=0.01), and waist circumference (β=−0.20, p=0.03) as the statistically significant predictors of CCA GSM.
4. Discussion
In this pilot study, we demonstrated the feasibility of measuring GSM and findings differences related to smoking burden in a cohort of active smokers. We observed inverse univariate associations between CCA GSM, a measure of carotid wall echogenicity, and two markers of smoking heaviness. Lower GSM values were associated with more cigarettes smoked per day and more years of smoking (pack-years). However, after adjusting for age, sex, and race/ethnicity, these markers of smoking heaviness no longer were associated independently with CCA GSM. Age, waist circumference and HDL-C were the most consistent CVD risk factors associated with carotid wall echogenicity, supporting findings inprevious studies that GSM values are associated with CVD risk factors (such as age, dyslipidemia, smoking, etc.)3, 13, 14, 29. In this pilot study, the multivariable models were underpowered; however, the effects were in the correct direction although the sizes were not very large relative to those of sex and age.
Pack-years, a marker of smoking heaviness, was a univariate associate with CCA GSM, but this association was no longer present in multivariable models that included age, likely because age and pack-years are collinear. Since pack-years will increase with age, it is difficult to determine the independent influences of age and pack-years in multi-variable models30, especially with our relatively small sample size and wide range of ages. However, we also observed that CCA GSM values decreased with the number of cigarettes smoked per day, indicating that smoking may influence the echogenicity of the carotid arterial wall, and that a larger sample size is needed to more thoroughly evaluate the independent contributions of smoking heaviness parameters and other CVD risk factors.
The GSM value is a measure of ultrasound echogenicity (brightness/darkness) of the carotid arterial wall3, 13, 14, 29, 31. B-mode ultrasound interacts with tissues and the reflected strength of the signal is indicative of tissue composition32. Therefore, it is believed that the shades of gray are representative of the tissue present in the arterial wall3, 13, 14, 33. Based on histopathological examination, lipids and hemorrhage appear as darker shades of gray (hypoechoic) and fibrous and calcific tissue as brighter shades of gray and white (hyperechoic) when imaged with ultrasound20, 34, 35. Inflammation has been associated with both diffuse hypoechogenicity36 and focal (discrete) white areas without acoustic shadowing19, 20. Abnormal levels of CVD risk factors are associated with altered arterial wall tissue composition (i.e., higher lipid content) and have different patterns of echogenicity measured by the GSM value3, 13, 14, 29. Lower CCA GSM values have been associated with CVD risk factors (older age, higher body-mass index, lower HDL-C, higher LDL-C, oxidative stress, inflammation3, 13 and increased risk for all-cause and CVD mortality14).
In a cross-sectional study of HIV-infected and non-infected women, current smoking was associated paradoxically with higher rather than lower CCA GSM33. This differs from our finding of lower GSM values being associated with markers of smoking heaviness, however, all participants in our study were smokers, so there are no comparisons to non-smokers. Furthermore, our study examined relationships to markers of smoking heaviness (cigarettes smoked /day and pack-years).
This is the first study, which we are aware of, to report associations of smoking heaviness (cigarettes smoked/day and pack-years) with CCA echogenicity in a smoking cohort. However, these relations may have less impact on CCA echogenicity than gender and age. We also identified relationships between increasing age, total cholesterol, waist circumference and low HDL-C with CCA GSM among active smokers, suggesting that being an overweight and dyslipidemic smoker may contribute to carotid wall injury. Indeed, secretion of adipocytokines from adipose tissue adversely influences cellular components of the arterial wall and has been postulated to be associated with echolucent plaques,37–39 and thus could also be associated with carotid wall echogenicity, smoking heaviness and central adiposity.
4.1 Limitations
This was a relatively small pilot study, a cross-sectional analysis restricted to current smokers intending to make a quit attempt. The novel associations we identified need to be reproduced in a larger, more racially diverse sample of smokers, and carotid GSM measures and their associates among smokers need to be compared to data from non-smokers and former smokers. Measuring non-traditional CVD risk markers, including markers of oxidant stress and additional inflammatory markers also may shed light on the pathobiology of arterial wall injury among smokers.
These images were acquired with the same preset (controlling for transducer, dynamic range, and grayscale map), however sonographers were allowed to optimize the time-gain compensation controls (TGC) and the overall gain. Ideally with this software, the TGC curve should be set to compensate for image depth, but have the potentiometers (i.e. slide pods) at the same vertical setting in the arterial lumen19, 21, 22, 40, 41. Since this was not controlled for we are not sure what effect this may have on the results of this study.
5. Conclusions
This pilot study demonstrated the feasibility of measuring the GSM of the CCA far wall and preliminary associations with measures of smoking heaviness and traditional CVD risk factors. The independent associations identified in this study need to be explored in a larger cohort, longitudinally, and with additional biomarkers to determine the mechanisms underlying arterial injury with active smoking and how they change with cessation.
Acknowledgments
Funding: This research was supported by grant 5R01HL109031 from the National Heart, Lung, and Blood Institute and grant K05CA139871 from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention.
Footnotes
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT01116986?term=NCT01116986&rank=1
Unique Identifier: NCT01553084
Conflicts of Interest Statement
C. Mitchell: Davies Publishing, Inc, authorship for two echocardiography textbooks, currently under review, may have future royalties. Elsevier, Wolters-Kluwer, author textbook chapters, may have future royalties.
M.E. Piper: None.
C.E. Korcarz: None.
K. Hansen: None.
J. Weber: None.
M.C. Fiore: None.
T.B. Baker: None.
J.H. Stein: Wisconsin Alumni Research Foundation-patent related to carotid wall thickness and vascular age.
References
- 1.Loizou CP, Pantziaris M, Pattichis MS, Kyriacou E, Pattichis CS. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput Med Imag Graph. 2009;33:317–324. doi: 10.1016/j.compmedimag.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Ellis SM, Sidhu PS. Granularity of the carotid artery intima-medial layer: reproducibility of quantification by a computer-based program. Br J Radiol. 2000;37:595–600. doi: 10.1259/bjr.73.870.10911781. [DOI] [PubMed] [Google Scholar]
- 3.Peters SA, Lind L, Palmer MK, Grobbee DE, Crouse JR, 3rd, O’Leary DH, Evans GW, Raichlen J, Bots ML, den Ruijter HM. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: the METEOR study. Journal of internal medicine. 2012;272:257–66. doi: 10.1111/j.1365-2796.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 4.Lusis AJ. Genetics of Atherosclerosis. Trends Genet. 2012;28:267–275. doi: 10.1016/j.tig.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Meer IM, del Sol AI, Hak AE, Bots ML, Hofman A, Witteman JCM. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree The Rotterdam Study. Stroke. 2003;34:2374–2379. doi: 10.1161/01.STR.0000088643.07108.19. [DOI] [PubMed] [Google Scholar]
- 6.Baldassare D, Castelnuovo S, Frigerio B, Amato M, Werba JP, DeJong A, Ravani AL, Tremoli E, Sirtori CR. Effects of timing and extent of smoking, type of cigarettes, and concomitant risk factors on the association between smoking and subclinical atherosclerosis. Stroke. 2009;40:1991–1998. doi: 10.1161/STROKEAHA.108.543413. [DOI] [PubMed] [Google Scholar]
- 7.Mast H, Thompson JL, Lin IF, Hofmeister C, Hartmann A, Marx P, Mohr JP, Sacco RL. Cigarette smoking as a determinant of high-grade carotid artery stenosis in Hispanic, black, and white patients with stroke or transient ischemic attack. Stroke. 1998;29:908–12. doi: 10.1161/01.str.29.5.908. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–10. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumagai S, Amano T, Takashima H, Waseda K, Kurita A, Ando H, Maeda K, Ito Y, Ishii H, Hayashi M, Yoshikawa D, Suzuki S, Tanaka A, Matsubara T, Murohara T. Impact of cigarette smoking on coronary plaque composition. Coronary artery disease. 2015;26:60–5. doi: 10.1097/MCA.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 10.Kweon S, Lee Y, Shin M, Coi J, Rhee J, Choi S, Ryu S, Kim B, Nam S, Jeong S, Park K. Effects of cumulative smoking exposure and duration of smoking cessation on carotid artery structure. Circ J. 2012;76:2041–2047. doi: 10.1253/circj.cj-11-1353. [DOI] [PubMed] [Google Scholar]
- 11.Kangavari S, Matetzky S, Shah PK, Yano J, Chyu KY, Fishbein MC, Cerek B. Smoking increases inflammation and metalloproteinase expression in human carotid atherosclerotic plaques. J Cardiovasc Pharmacol Therapeut. 2004;9:291–298. doi: 10.1177/107424840400900410. [DOI] [PubMed] [Google Scholar]
- 12.Asthana A, Johnson HM, Piper ME, MC F, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. Am Heart J. 2010;160:458–463. doi: 10.1016/j.ahj.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson J, Sundstrom J, Gustavsson T, Hulthe J, Elmgren A, Zilmer K, Zilmer M, Lind L. Echogenicity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis. 2009;204:612–618. doi: 10.1016/j.atherosclerosis.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Wohlin M, Sundstrom J, Andren B, Larsson A, Lind L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis. 2009;205:486–91. doi: 10.1016/j.atherosclerosis.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Yang D, Iyer S, Gardener H, Della-Morte D, Crisby M, Dong C, Cheung K, Mora-McLaughlin C, Wright CB, Elkind MS, Sacco RL, Rundek T. Cigarette Smoking and Carotid Plaque Echodensity in the Northern Manhattan Study. Cerebrovasc Dis. 2015;40:136–143. doi: 10.1159/000434761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of general psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 18.Johnson HM, Piper ME, Jorenby D, Fiore MC, Baker TB, Stein JH. Risk factors for subclinical carotid atherosclerosis among current smokers. Prev Cardiol. 2010;13:166–171. doi: 10.1111/j.1751-7141.2010.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Doré CJ, Morris TP, Naylor R, Abbott AL. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486–1496. doi: 10.1016/j.jvs.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell CC, Stein JH, Cook TD, Salamat S, Wang X, Varghese T, Jackson DC, Sandoval Garcia C, Wilbrand SM, Dempsey RJ. Histopathologic Validation of Grayscale Carotid Plaque Characteristics Related to Plaque Vulnerability. Ultrasound in Med & Biol. 2017;43:129–137. doi: 10.1016/j.ultrasmedbio.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin M, Kyriacou E, pattichis C, Bond D, Kakkos S, Sabetai M, Geroulakos G, Georgiou N, Dore C, Nicolaides A. Juxtaluminal hypoechoic area in ultrasonic images of carotid plaques and hemispheric symptoms. J Vasc Surg. 2010;52:69–76. doi: 10.1016/j.jvs.2010.02.265. [DOI] [PubMed] [Google Scholar]
- 22.Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Satetai MM, Tegos T, Makris GC, Thomas DJ, Geroulakos G. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg. 2013;57:609–618. doi: 10.1016/j.jvs.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: A review. Circulation. 2010;121:1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deveci SE, Deveci F, Acik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respiratory Medicine. 2004;98:551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Fagerstrom K. The Fagerstrom Test for Nicotine Dependence asa predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1467–1473. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 26.National Heart Lung and Blood Institute. Aim for a healthy weight. Available online at: https://wwwnhlbinihgov/health/educational/lose_wt/riskhtm. Accessed 10-17-2017.
- 27.Van Belle G, Fisher LD, Heagerty PJ, Lumley T. biostatistics: A Methodology for hte Health Sciences. 2nd. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2004. Association and Prediction: Multiple regression analysis and linear models with multiple predictor variables. [Google Scholar]
- 28.Hildago B, Goodman M. Multivariate or Multivariable Regression? American journal of public health. 2013;103:39–40. doi: 10.2105/AJPH.2012.300897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lind L, Andersson J, Ronn M, Gustavsson T. The echogenicity of the intima-media complex in the common carotid artery is closely related to the echogenicity in plaques. Atherosclerosis. 2007;195:411–414. doi: 10.1016/j.atherosclerosis.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (US), National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health (US) How tobacco smoke causes disease: The biology and behavioral basis for smoking - attributable disease: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2010. [PubMed] [Google Scholar]
- 31.Lind L, Peters SAE, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR, O’Leary DH, Evans GW, Raichlen JS, Bots ML. Effect of rosuvastatin on the echolucency of the common carotid intima-media in low-risk individuals: The METEOR Trial. J Am Soc Echocardiogr. 2012;25:1120–1127.e1. doi: 10.1016/j.echo.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Lal BK, Hobson RW, Pappas PJ, Kubicka R, Hameed M, Chakhtura EY, Jamil Z, Padberg FT, Haser PB, Duran WN. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg. 2002;35:1210–1217. doi: 10.1067/mva.2002.122888. [DOI] [PubMed] [Google Scholar]
- 33.Jung M, Parrinello CM, Xue X, Mack WJ, Anastos K, Lazar JM, Selzer RH, Chircore AM, Plankey M, Tien P, Cohen MA, Gange SJ, Hodis HN, Kaplan RC. Echolucency of the carotid artery intima-media complex and intima-media thickness have different cardiovascular risk factor relationships: The Women’s Interagency HIV Study. J Am Heart Assoc. 2015;4:e001405. doi: 10.1161/JAHA.114.001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tegos TJ, Sohail M, Sabetai MM, Robless P, Akbar N, Pare G, Stansby G, Nicolaides AN. Echomorphologic and histopathologic characteristics of unstable carotid plaques. Am J Neuroradiol. 2000;21:1937–44. [PMC free article] [PubMed] [Google Scholar]
- 35.El-Barghouty NM, Levine T, Ladva S, Flanagan A, Nicolaides A. Histological verification of computerised carotid plaque charaterisation. Eur J Vasc Endovasc Surg. 1996;11:414–416. doi: 10.1016/s1078-5884(96)80172-9. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt WA, Kraft HE, Vorpahl K, Volker L, Grominica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med. 1997;337:1336–1342. doi: 10.1056/NEJM199711063371902. [DOI] [PubMed] [Google Scholar]
- 37.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M, Nakamura T, Shimomura I, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor -induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 38.Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, Arita Y, Kihara S, Matsuzawa Y. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Internal Medicine. 1999;38:202–206. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- 39.Irie Y, Katakami N, Kaneto H, Takahara M, Sakamoto K, Kosugi K, Shimomura I. The risk factors associated with ultrasonic tissue characterizaton of carotid plaque in type 2 diabetic patients. Journal of Diabetes and Its Complications. 2014;28:523–527. doi: 10.1016/j.jdiacomp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 40.LifeQMedical V. Carotid Plaque Texture Analysis Research Software for Ultrasonic Arterial Wall and Atherosclerotic Plaques Measurements. Operation Manual Version 4.5. 2013:1–45. [Google Scholar]
- 41.Griffin M, Kyriacou E, Kakkos SK, Beach KW, Nicolaides A. Image normalization, plaque typing, and texture feature extraction. In: Nicolaides A, Beach KW, Kyriacou E, Pattichis CS, editors. Ultrasound and carotid bifurction atherosclerosis. London: Springer; 2012. p. 194. [Google Scholar]


