Summary
Around 20% of MG patients are ACHR antibody negative. In 2001, MuSK antibodies were identified as the cause of MG in 70% of these patients. MuSK antibodies are found in around 30–40% of ACHR negative MG patients and are associated with specific clinical phenotypes. One is a predominantly bulbar form with fewer ocular symptoms than in ACHR positive MG. Others show an isolated head drop or symptoms indistinguishable from ACHR positive MG. These patients usually respond well to immunosuppressive therapy but not as well to cholinesterase inhibitors. Other antibodies associated with MG including LRP4 are discussed.
Keywords: Myasthenia Gravis, MuSK, Cortactin, Agrin, LRP4, Rapsyn
Introduction
Acetylcholine receptor (ACHR) antibodies are found in approximately 80% of patients with myasthenia gravis (MG) leaving approximately 20% antibody negative (SNMG)1. It was suspected that SNMG patients had an autoimmune etiology since they responded to autoimmune therapy including plasma exchange2,3. In addition, passive transfer of disease to mice from the serum of seronegative patients2,4, the development of transient neonatal myasthenia in infants of seronegative myasthenic women5–7, and the binding of IgG from seronegative MG patients to muscle8 suggest an autoimmune etiology. In 2001, Hoch and colleagues9 discovered that antibodies to muscle specific tyrosine kinase (MuSK) were responsible for producing Myasthenia Gravis in about 70% of these SNMG patients. More recently antibodies to LRP4 (Low Density Lipoprotein Receptor-Related Protein 4)10–12 and agrin13,14 have been discovered in patients with MG. Other antibodies including titin15,16, cortactin17 and rapsyn18 are associated with MG. Defects of these proteins are associated with congenital myasthenic syndromes19–34. The functions of these newly identified target proteins differ from ACHR since they are responsible for the proper formation and maintenance of the neuromuscular junction rather than serving as ACHR. In this chapter, we will discuss the roles of these proteins and the known features of myasthenic patients who have antibodies to them.
When muscles are denervated there is a significant alteration at the neuromuscular junction including the expression of fetal ACHR remote from the neuromuscular junction and increased muscle sensitivity to acetylcholine. With reinnervation, neuromuscular junctions are reformed, the fetal ACHR are eliminated as normal sensitivity to acetylcholine returns. This indicates that muscles are innervated and receive signals from motor neuron terminals. In addition, denervated muscle attracts neighboring neurons to sprout and form new connections. Agrin, a heparin sulfate proteoglycan35, released by the axon terminal plays an important role in the development and maintenance of neuromuscular junctions. Activation of MuSK, anchored in skeletal muscle is responsible for the clustering of ACHR at the neuromuscular junction. While it is known that agrin is necessary for the activation of MuSK there is no direct interaction between agrin and MuSK. In the last decade, LRP436,37 was discovered to be the agrin receptor which is responsible for activating MuSK. Agrin signaling including MuSK and LRP4 have additional roles in inhibiting neurite outgrowth38, which may be responsible for the inhibition of sprouting. In addition, MuSK along with ColQ is responsible for anchoring acetylcholinesterase to the neuromuscular junction39–41.
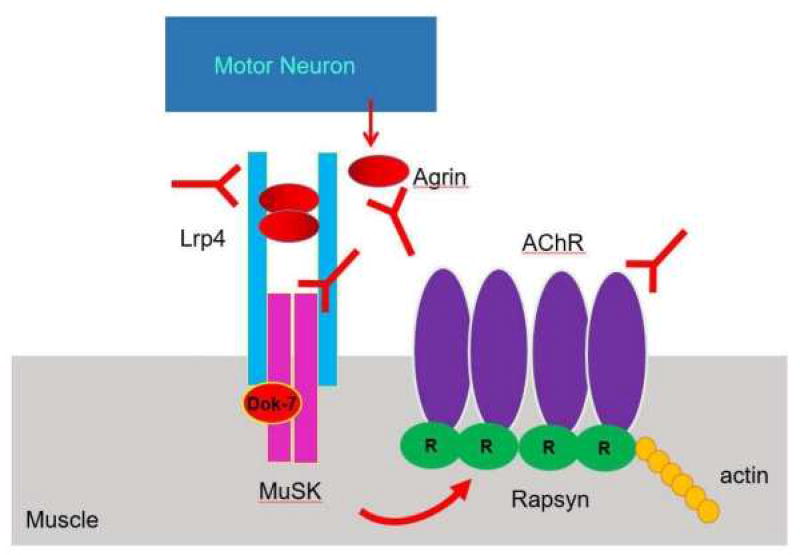
Figure 1 illustrates that interaction between the axon terminal and muscle. Agrin released by the axon terminal binds to LRP4 in muscles to activate MuSK. The formation of a agrin-LRP4 tetrameric complex (2 agrin molecules and 2 LRP4 molecules) is critical for MuSK activation42. DOK7 a muscle cytoplasmic protein is also involved in the activation of MuSK43–46. Activated MuSK interacts with Rapsyn, a scaffolding protein,47–52 causing the clustering of ACHR at the neuromuscular junction. Rapsyn binds all subunits of the ACHR51,53 and is bound to actin54. For further review of the molecular anatomy of the neuromuscular junction as we well as the electrophysiological properties, the reader is referred to Chapter 2.
Figure 1.
Structure of the Neuromuscular junction.
These elements of the neuromuscular junction are necessary for its proper development and maintenance. In addition to these actions, LRP4, agrin and MuSK act on the axonal terminal. Congenital defects of these proteins are linked to defects in neuromuscular transmission and will be discussed further in chapter 11 of this volume. While there are many proteins of the neuromuscular junction that might be susceptible to antibodies it is those that are exposed to the extracellular space namely agrin, LRP4, MuSK and ACHR, that are most likely to be vulnerable.
MuSK antibody-positive MG
MuSK was first described by Valenzuela et al. in 1995 and was localized to the post-junction region of the neuromuscular junction55. MuSK was shown to be involved in agrin signaling causing aggregation of acetylcholine receptors at the neuromuscular junction56,57. In 2001 Hoch et al9 described 17 cases of MuSK antibody-positive MG out of 24 SNMG patients. IgG from MuSK antibody-positive patients produce MG-like symptoms including muscle weakness and destruction of the neuromuscular junction when injected into mice58. Mice who developed anti-MuSK antibodies after being injected with rat MuSK acquire symptoms consistent with myasthenia gravis including weakness, a decrement to repetitive stimulation and reduced amplitude miniature end plate potentials59. MuSK antibodies produce wasting of muscles when injected in mice with predilection to the masseter and facial muscles60 which might explain why these muscles are more involved clinically. Around 7 to 10% of all MG patients have antibodies to MuSK. Initially, Hoch et al reported MuSK antibodies in 70% of AChR antibody negative cases9. However, subsequently most believe that this figure is closer to 30–40%61,62. Early studies indicate that MuSK antibody-positive MG patients have important clinical differences when compared to ACHR antibody-positive MG63,64. These patients have more bulbar, neck and respiratory symptoms along with less ocular symptoms. The patients are less responsive to cholinesterase inhibitors, but responded to plasma exchange and other immunosuppressant medications.
Based on 16 years of experience, clinicians now recognize some characteristic features of MuSK antibody-positive MG. They are more likely to be female than male65; blacks are more likely to have these antibodies compared to whites66–68. There is considerable variation of the incidence of MuSK antibody-positive MG depending on geographic location66,69–71. Anti-MuSK antibody positive patients have HLA-DRB1, DQB1, DQ5 and DR14 alleles72–74. Patients have marked bulbar weakness65 often having marked wasting and fasciculations of the tongue and must be distinguished from patients with bulbar ALS75,76. They often have facial, neck and respiratory weakness. Their ocular symptoms are less prominent than other MG patients. They are less likely to respond to anticholinesterase medication perhaps because of deficient binding of acetylcholinesterase to the neuromuscular junction77,78. "In addition to a case report (ref 79), our experience suggests that, while only 16% of MuSK MG respond to cholinesterase inhibitors, hypersensitivity or worsening of symptoms occurred in 20% of cases (ref 76). Others have also reported worsening symptoms in response to cholinesterase inhibitors in 5% of MuSK MG cases, while 57% improved (143)..
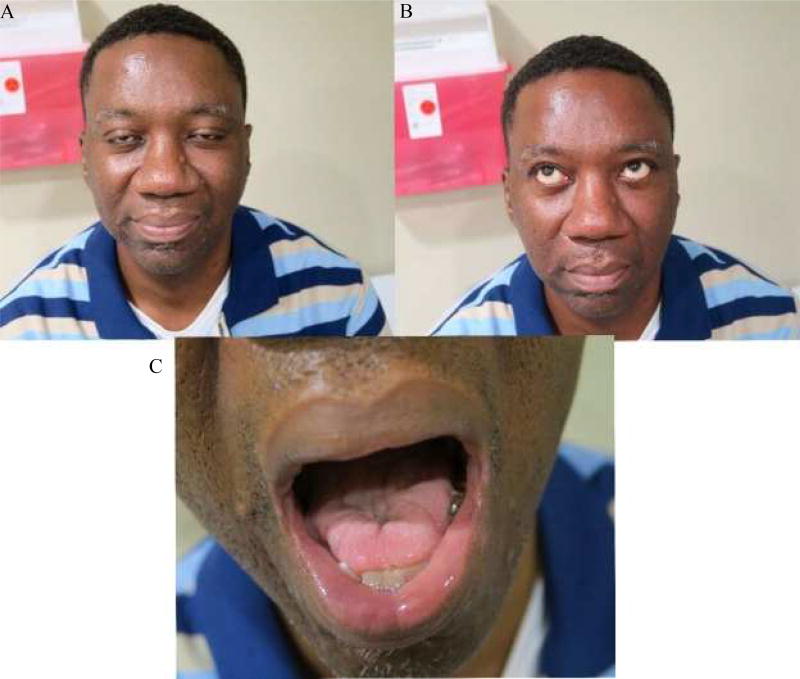
A patient of one of the authors (MHR) illustrates a typical case of MuSK antibody positive MG. He is a 50-year-old black man who was in his usual state until he developed a flu-like illness. He displayed dysarthria, swelling of tongue, facial weakness and bilateral ptosis, but without diplopia. He lost 50 pounds over a 3-month period. He noticed some weakness of his right shoulders, which became worse later in the day. Chest CT scan was normal. Pulmonary function tests showed mild restrictive airway disease. Pyridostigmine produced little improvement of his symptoms; however, prednisone improved his symptoms. On examination, the patient had moderate ptosis without fatigue, with 4/5 strength of his orbicularis oculi and 4+/5 strength of orbicularis oris (Figure 2a). He was unable to wrinkle his brow (figure 2b) and his voice was dysarthric and there was mild weakness of his uvula. His tongue exhibited moderate wasting, fasciculations and had mildly slowed rapid alternating movements (figure 2c). His neck and limbs were normal as was the remainder of his exam. He was Myasthenia Gravis Foundation of America (MGFA) Class80 IIIb at his worst. He was negative for ACHR and anti-striatal muscle antibodies but anti-MuSK antibodies were markedly elevated at 5.91 (< 0.03). Repetitive stimulation of his left facial nerve was normal, but needle exam revealed slight increased insertional activity of his orbicularis oculi with markedly diminished motor unit size with short duration motor units. Other muscles studied were normal. Stimulated SFEMG of the right orbicularis oculi was very abnormal with an average MCD of 260 µsec with 80% of the fibers having blocks.
Figure 2.
Patient with Anti-MuSK MG
While MuSK antibody-positive MG patients have less prominent ocular findings compared ACHR antibody-positive MG, cases of patients with pure ocular and predominantly ocular disease are described81–86. Cases of isolated neck weakness and wasting are described associated with anti-MuSK antibodies63,87–89. In addition, mutations of the MuSK protein have been associated with dropped head90. Vocal cord paralysis is seen in some patients with MuSK antibody-positive MG91–93. While most patients just have anti-MuSK antibodies, patients with antibodies to MuSK and ACHR94,95 and voltage gated calcium channels (VGCC)96,97 are described. Anti-MuSK antibody level is generally correlated with symptoms98. MuSK antibodies have crossed the placenta in pregnant women, producing neonatal myasthenia99–103. Guptill and Sanders described three different clinical presentations of MuSK antibody-positive MG104. Firstly, patients with predominantly bulbar and respiratory symptoms, secondly, patients indistinguishable from ACHR antibody-positive MG and thirdly, patients presenting with head drop as their most prominent symptom. The above described MuSK antibody-positive MG patient was an example of the first clinical presentation pattern. While thymic pathology including thymoma has been described in patients with MuSK antibody-positive MG105–107 this is far less common than in ACHR antibody-positive MG64,67,108–110. It is unknown if thymectomy is of any value in these patients63,78. In the Pasnoor et al case series, some patients with MuSK MG who had thymectomy did improve over time. Since several other therapies were also being administered, we cannot definitively say that thymectomy was the driver behind their improvement.76 There have been reports of patients who initially presented as ACHR antibody-positive and after thymectomies, developed MuSK antibody-positive MG95,111–114.
MuSK antibody-positive MG patients are more likely to have abnormal repetitive nerve studies in facial and proximal limb than in distal muscles64,115,116. Repetitive studies are less likely to be abnormal than in MuSK antibody-negative patients65,117. Single fiber EMG is usually abnormal in the facial muscles but less likely to be abnormal in the limbs68,117,118. EMG findings often show severe myopathic changes with reduced amplitude and duration without polyphasia in affected muscles115,119–121. Fibrillation potentials are reported more frequently than in ACHR antibody-positive MG115. In our patient, increased insertional activity was seen and only a few very small units were seen in the orbicularis oculi. Muscle atrophy particularly in the face and tongue midline atrophy are very prevalent in these patients104,122–124 but some series found tongue atrophy to be uncommon78,107,115. Muscle biopsies from MuSK antibody-positive MG patients have severe myopathic and mitochondrial abnormalities125,126. MRI studies have shown that facial muscle bulk is less in abnormal patients122,127. Following successful treatment, previously seen tongue atrophy improves in MuSK antibody-positive MG76,128. The increased atrophy preferentially seen in the facial muscles compared to limb muscles might be related to reduced levels of MuSK found in facial and respiratory muscles129,130. The type of immunoglobulins causing MuSK antibody-positive MG might play a role in the differences in the clinical course. Unlike ACHR antibody-positive MG where IgG1 and IgG3 antibodies cause complement activation, IgG4 antibodies are found in MuSK antibody-positive MG131–133. It is believed that IgG4 antibodies may block MuSK signaling by interfering with the binding of LRP4 to MuSK thereby destabilizing the neuromuscular junction131,132,134,135.
Treatment of MuSK antibody-positive MG differs somewhat from ACHR antibody-positive MG. MuSK antibody-positive MG patients tend to respond less to pyridostigmine and other cholinesterase inhibitors78,136,137. There have been reports of worsening of symptoms with pyridostigmine in MuSK antibody-positive MG79. These patients respond less often and have lower tolerance to cholinesterase inhibitors with more nicotinic and muscarinic side effects137. One explanation for this is that COLQ which binds acetylcholinesterase to the neuromuscular junction is also bound to MuSK and antibodies to MuSK might affect this connection132,138. These interactions might impair acetylcholinesterase activity at baseline in MuSK antibody-positive MG and further interference by cholinesterase inhibitors might produce worsening symptoms. In a mouse model of MuSK antibody-positive MG, 3,4 diaminopyridine has been shown to be efficacious139. In addition, 3,4 diaminopyridine which enhances the release of acetylcholine from the axonal terminal has been shown to be of benefit in MuSK antibody-positive MG patients140. This is due to 3,4 diaminopyridine increasing the amount of acetylcholine in the neuromuscular junction rather than increasing its duration of action as pyridostigmine would141. Generally, pyridostigmine should be tried at a low dose initially in MuSK antibody-positive MG patients but its actions need to be monitored since it is often not beneficial and could worsen symptoms. If not beneficial at 90 mg q4h, no further dose escalation should be considered. 3,4 diaminopyridine, if available, might be considered as an alternative treatment.
In most instances, patients respond to immunosuppressant therapy. Prednisone, azathioprine, mycophenolate, tacrolimus, methotrexate and cyclosporine have been tried with success in these patients64,78,115,125,142–144. Initial therapy would include prednisone combined with a steroid sparing immunosuppressant. Prednisone should be started at a dose of 40–60 mg a day and reduced to the lowest effective dose. In most instances, these patients respond similarly to patients with ACHR antibody-positive MG. For acute exacerbations of MuSK antibody-positive MG, IVIG and plasma exchange have been used, but IVIG is less efficacious than plasma exchange in most patients63,78,143,145. On occasion patients will respond to IVIG who have not responded well to plasma exchange but this is less common125,146,147. Patients with poorly responsive disease have responded well to rituximab 375 mg/m2, weekly for 4 weeks. Often the response to rituximab is better than what is seen in ACHR antibody-positive MG and can lead to long lasting remissions lasting 6 months to longer than a year. There is a report of a patient responding to high dose cyclophosphamide at 50mg/kg for 4 days who had failed other therapies including rituximab148. There have also been reports of patients going into spontaneous remission149.
In summary, while many cases of MuSK antibody-positive MG present with distinct symptoms consisting of bulbar, facial and respiratory weakness and may have a more severe course of the disease, other patients present with head drop or with symptoms typical of ACHR antibody-positive MG. In most MuSK antibody-positive MG patients, with proper immunosuppressant therapy, symptoms can be well controlled78.
LRP4 antibody-positive MG
After the discovery of MuSK antibody-positive MG, around 10 to 13% of patients with MG remain negative (i.e., for antibodies against ACHR and MuSK, thus double-negative MG or DNMG for short). Since the discovery of LRP436,37 it was felt that it would be a good target for autoimmune attack since much of this molecule is extracellular. Three groups simultaneously reported the presence of LRP4 antibodies in MG10–12. Shen et al showed that mice injected with LRP4 developed weakness, a decremental response to repetitive stimulation and produced LRP4 antibodies150. These antibodies reduced cell surface LRP4 level, agrin activation of MuSK and the aggregation of ACHR at the neuromuscular junction. Furthermore, these antibodies were predominantly IgG1 followed by IgG2 and IgG3 which are known to activate complement. Using mice that are genetically designed to delete the LRP4 gene when exposed to doxycycline, LRP4 has been shown to be essential to the preservation of the neuromuscular junction151. These mice develop weakness and neuromuscular transmission failure. LRP4 also has been shown to have retrograde effects on the axonal terminal producing presynaptic differentiation152,153. Since the discovery of LRP4 antibody-positive MG some data has been reported on these patients but there is only limited information about the characteristics of these patients.
The following case of one of the authors (MHR), illustrates a patient with LRP4 antibody-positive MG. The patient is a 49-year-old white female who developed shortness of breath, soft voice and generalized fatigue 2 years prior to her first visit. She complained of intermittent facial weakness and fatigue while chewing and periodic difficulty swallowing both liquids and solids. She had ptosis and diplopia. She fatigues, feeling that her weakness is worse in the afternoon. Her ACHR, MuSK and VGCC antibodies were negative. She had normal repetitive nerve and pulmonary function testing. She is unable to tolerate pyridostigmine because of gastrointestinal side effects. She had a gastric bypass in the past. She had bilateral ptosis with mild diplopia. She had mild weakness of her orbicularis oculi, orbicularis oris, masseter and sternocleidomastoid muscles. She had mild dysarthria. The remainder of her cranial nerves were normal. She had moderate neck weakness. She had mild proximal arm weakness. Needle exam of her right deltoid was normal. SFEMG of her orbicularis oculi was abnormal with 50 % of her fibers having increased jitter and 8% showing block. Left biceps muscle biopsy showed mild type II atrophy. Her LRP4 antibody level is 0.471 (<0.267). Her agrin antibody level is also elevated at 0.446 (<0.264). She is on prednisone, methotrexate and azathioprine and is uncertain of improvement. She responds very well to plasma exchange noting improvement of strength following every treatment. She recently received a course of rituximab but it is too early to know if she received benefit. When weakest she was MGFA class80 IIIb.
In most cases described in the literature LRP4 antibodies are more common in women than men154. The prevalence in DNMG has been as low as 2–4 % in Asian populations10,155 to as high as 53% in the study of Pevzner et al11. In the largest reported series, Zisimopoulou et al found a prevalence of 18.7 %154. Regional variation in prevalence has been reported154. Most cases are described as mild to moderate and ocular muscles are usually involved154–156. Despite this, some patients have more severe symptoms (class III-IV)10,11,154. Patients having myasthenic crises have been described (class V)10,11,154,157. Patients with antibodies for both LRP4 and ACHR or MuSK are more symptomatic and do less well than patients with only LRP4 antibodies154,156. One of our patients with LRP4 antibodies had VGCC antibodies and has features of both MG and Lambert Eaton Myasthenic syndrome. Currently, these patients generally present similarly to ACHR antibody-positive MG patients but are usually milder. Most patients respond to pyridostigmine, steroids and immunosuppressant medications like other MG patients. Thymus pathology is usually not present but has been described including thymomas154,155,158. While in most cases respiratory dysfunction is mild, respiratory failure has been described157.
LRP4 is an important signaling receptor in many other systems and is important for development. LRP4 antibodies have not been seen in control patients with other diseases except in 10 % of ALS patients159,160 and 2 cases of NMO12. Whether it is pathogenic in ALS is not currently known. Since LRP4 antibodies are present in approximately 13–18% of patients with DNMG and LRP4 antibodies have been shown to produce pathology in mice it could in the future be part of routine MG patient screening. Further research is needed to understand what features are seen in these patients and determine its prevalence.
Agrin and other antibodies in MG
Agrin is another potential target for antibodies that can produce MG. Four papers found agrin antibodies in MG patients13,14,156,161. In these studies, most of the patients are positive for other antibodies as well13,14,156,161. Four of the patients reported by Gasperi13, but none of the patients reported by Cossins161, Zhang14 or Cordts156 had antibodies to MuSK. In all four studies, agrin positive MG patients had antibodies to ACHR as well: 13 reported by Cossins161, 1 reported by Gasperi13, 5 reported by Zhang14 and 3 reported by Cordts156. In addition, one patient who was positive for agrin and MuSK antibodies also had antibodies to LRP413. There is a total of 14 patients reported who have antibodies only to agrin but clinical data was available for only 1 patient14,156,161. This patient was MGFA Class III and only partially responded to pyridostigmine and prednisone156. Based on this limited data the incidence and clinical characteristics of agrin antibody-positive MG patients are unknown. Further research is required before we know the role of agrin antibodies in MG. Perhaps the coexistence of these antibodies might have implications on the severity of the disease. Similar to LRP4 antibodies, agrin antibodies have been seen in ALS patients even though their role is unknown in this disease state159.
Titin, is a large structural protein found in muscle that extends the entire length of the sarcomere and has an important role in muscle elasticity. While this protein is very large, antibodies are directed against a small segment at the A/I band junction representing less than 1% of this protein. These antibodies have been found in patients with MG often in association with other pathogenic antibodies16. Titin antibodies are age related often being found in older patients with MG. These antibodies have been associated with thymoma in thymectomized patients16 and may have predictive value in determining which patients have thymomas. More recently, two studies looked at titin antibodies in seronegative MG15,156. Cordts et al156 found that 23% of seronegative MG had titin antibodies, while Stergiou et al15 found 18 % were positive. In both studies, patients positive for only titin antibodies have milder disease and are responsive to therapy. Patients with ACHR and titin antibodies have more severe disease and are more likely to have thymomas. Further research is needed to determine if this antibody is causative or just a bystander antibody.
Agius et al, describe Rapsyn antibodies in MG patients18. This was found in around 13% of the limited number of patients tested. However, these antibodies were non-specific and are of uncertain significance. Congenital defects in rapsyn are associated with congenital MG21,22,25. Antibodies to ColQ have been found in 6 patients with MG (4%) of which 4 patients had DNMG161. The significance of these antibodies is unknown.
Gallardo et al17, describes antibodies to cortactin in 19.7 % of 91 DNMG patients. These antibodies were found in only 4.8% of either ACHR antibody-positive or MuSK antibody-positive patients. None of these patients were positive for LRP4. These patients were predominantly women (68%) and most were less than 50 years old at disease onset. Twenty-two percent had ocular disease while the remainder had generalized disease with ocular predominance. One patient had a thymoma. Cortactin antibodies are also detected in 12.5% of patients with other autoimmune neurological diseases. In a study of 250 MG cases, most (201) had ACHR antibodies, 11 had MuSK antibodies while 28 had antibodies to cortactin162. Of those, only 9 were DNMG and most (19) had ACHR antibodies. The frequency of cortactin antibodies were significantly higher in the DNMG group (23.7%) compared to the ACHR positive group (9.5%). No patients in this study had antibodies to both cortactin and MuSK. The 9 DNMG cortactin antibody positive patients had either ocular MG or mild MG (MGFA grade IIA) and fewer bulbar symptoms. Of 17 patients with ocular DNMG, 23.5% had antibodies to cortactin. The role of these antibodies is still unknown and requires further study.
Conclusion
In summary, new antigenic targets have been discovered that might account for many of the ACHR antibody-negative MG patients. The best studied of these is Anti-MuSK antibody MG. In some instances, their distinctive phenotype might raise clinical suspicion for this form of the disease. Treatment of these patients is similar to that of ACHR antibody-positive MG with the notable exception that they are less responsive to cholinesterase inhibitors such as pyridostigmine. In addition, difficult to treat MuSK antibody-positive MG patients generally have an excellent response to rituximab. Anti-Striatal muscle antibodies seen in a third of MG cases are non-specific to MG and are not a reliable predictor of thymic pathology. While there is less experience with LRP4 antibody-positive MG, there is evidence that antibodies to LRP4 can cause neuromuscular defects. At this point, there are no specific phenotypes for this form of MG but it usually causes a milder form of the disease even though there are notable exceptions. Treatment for these patients is like that of ACHR antibody-positive patients. The role of agrin, titin and cortactin antibodies are currently unknown and more research is needed. These antibodies are associated with the disease but we do not know if they cause the disease or are an epiphenomenon. With more information about the role of these antibodies, we might be able to diagnose patient with MG earlier and design better therapies.
Key Points.
Approximately 20% of MG patients are ACHR negative. In some of these patients, antibodies to proteins responsible for the maintenance of the neuromuscular junction have been found. Antibodies associated with MG are directed against MuSK, LRP4, Agrin, Cortactin, Rapsyn and Titin.
MuSK antibodies are found in around 30–40% of DNMG patients. Three clinical presentation are associated with these antibodies. Firstly, a predominantly bulbar presentation with facial and tongue atrophy, often with few ocular symptoms. Secondly, presenting with head drop as the predominant symptom. Thirdly, presenting like ACHR positive MG.
MuSK positive patients generally respond well to immunosuppressive therapy but may not respond to pyridostigmine as well as ACHR positive MG patients. They respond well to rituximab often with a prolonged remission.
LRP4 antibodies are present in around 18% of DNMG patients. There is evidence that these antibodies are pathogenic as they cause\ MG in mice. We do not have enough clinical experience to identify specific clinical characteristics of these patients but most patients have milder disease.
The role of antibodies to agrin, rapsyn, cortactin in myasthenia gravis are not well defined at present and further research is needed.
Acknowledgments
Dr. Rivner is on the speaker’s bureau for Allergan and has received grants from Allergan, Alexion, Biogen, Cytokinetics, Elan, GlaxoSmithKline, Grifols, and UCB.
Dr. Dimachkie is on the speaker’s bureau or is a consultant for Alnylam, Baxalta, Catalyst, CSL-Behring, Mallinckrodt, Novartis and NuFactor. He has also received grants from Alexion, Biomarin, Catalyst, CSL-Behring, FDA/OPD, GSK, Grifols, MDA, NIH, Novartis, Sanofi & TMA.
Dr Barohn is on the speaker’s bureau for NuFactor, Grifols Therapeutics Inc., and Plan 365 Inc. He is on the advisory board for CSL Behring GmbH, and has received an honorarium from Option Care. He has received research grants from NIH, FDA/OOPD, NINDS, Novartis, Sanofi/Genzyme, Biomarin, IONIS, Teva, Cytocenetics, Eli Lilly, and PTC.
Dr. Mei has a patent with Augusta University on a LRP4 assay. He has received grants from the NIH and Veterans Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
Dr. Pasnoor: None
References
- 1.Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- 2.Evoli A, Batocchi AP, Lo Monaco M, et al. Clinical heterogeneity of seronegative myasthenia gravis. Neuromuscular disorders : NMD. 1996;6(3):155–161. doi: 10.1016/0960-8966(96)00009-0. [DOI] [PubMed] [Google Scholar]
- 3.Birmanns B, Brenner T, Abramsky O, Steiner I. Seronegative myasthenia gravis: clinical features, response to therapy and synthesis of acetylcholine receptor antibodies in vitro. Journal of the neurological sciences. 1991;102(2):184–189. doi: 10.1016/0022-510x(91)90067-h. [DOI] [PubMed] [Google Scholar]
- 4.Mossman S, Vincent A, Newsom-Davis J. Myasthenia gravis without acetylcholine-receptor antibody: a distinct disease entity. Lancet. 1986;1(8473):116–119. doi: 10.1016/s0140-6736(86)92259-2. [DOI] [PubMed] [Google Scholar]
- 5.Oteiza Orradre C, Navarro Serrano E, Rebage Moises V, Lopez Pison J, Valle Traid A, Marco Tello A. Seronegative transient neonatal myasthenia: report of a case. An Esp Pediatr. 1996;45(6):651–652. [PubMed] [Google Scholar]
- 6.Sisman J, Ceri A, Nafday SM. Seronegative neonatal myasthenia gravis in one of the twins. Indian Pediatr. 2004;41(9):938–940. [PubMed] [Google Scholar]
- 7.Townsel C, Keller R, Johnson K, Hussain N, Campbell WA. Seronegative Maternal Ocular Myasthenia Gravis and Delayed Transient Neonatal Myasthenia Gravis. AJP Rep. 2016;6(1):e133–136. doi: 10.1055/s-0036-1579624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaes F, Beeson D, Plested P, Lang B, Vincent A. IgG from "seronegative" myasthenia gravis patients binds to a muscle cell line, TE671, but not to human acetylcholine receptor. Annals of neurology. 2000;47(4):504–510. [PubMed] [Google Scholar]
- 9.Hoch WMJ, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. NAT MED. 2001;7(3):365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Annals of neurology. 2011;69(2):418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 11.Pevzner A, Schoser B, Peters K, et al. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. Journal of neurology. 2012;259(3):427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Tzartos JS, Belimezi M, et al. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Archives of neurology. 2012;69(4):445–451. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- 13.Gasperi C, Melms A, Schoser B, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology. 2014;82(22):1976–1983. doi: 10.1212/WNL.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, Shen C, Bealmear B, et al. Autoantibodies to agrin in myasthenia gravis patients. PloS one. 2014;9(3):e91816. doi: 10.1371/journal.pone.0091816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stergiou C, Lazaridis K, Zouvelou V, et al. Titin antibodies in "seronegative" myasthenia gravis--A new role for an old antigen. Journal of neuroimmunology. 2016;292:108–115. doi: 10.1016/j.jneuroim.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto AM, Gajdos P, Eymard B, et al. Anti-titin antibodies in myasthenia gravis: tight association with thymoma and heterogeneity of nonthymoma patients. Archives of neurology. 2001;58(6):885–890. doi: 10.1001/archneur.58.6.885. [DOI] [PubMed] [Google Scholar]
- 17.Gallardo E, Martinez-Hernandez E, Titulaer MJ, et al. Cortactin autoantibodies in myasthenia gravis. Autoimmunity reviews. 2014;13(10):1003–1007. doi: 10.1016/j.autrev.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Agius MA, Zhu S, Kirvan CA, et al. Rapsyn antibodies in myasthenia gravis. Annals of the New York Academy of Sciences. 1998;841:516–521. doi: 10.1111/j.1749-6632.1998.tb10972.x. [DOI] [PubMed] [Google Scholar]
- 19.Bogdanik LP, Burgess RW. A valid mouse model of AGRIN-associated congenital myasthenic syndrome. Human molecular genetics. 2011;20(23):4617–4633. doi: 10.1093/hmg/ddr396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huze C, Bauche S, Richard P, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. American journal of human genetics. 2009;85(2):155–167. doi: 10.1016/j.ajhg.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke G, Cossins J, Maxwell S, et al. Rapsyn mutations in hereditary myasthenia: distinct early- and late-onset phenotypes. Neurology. 2003;61(6):826–828. doi: 10.1212/01.wnl.0000085865.55513.ae. [DOI] [PubMed] [Google Scholar]
- 22.Dunne V, Maselli RA. Identification of pathogenic mutations in the human rapsyn gene. Journal of human genetics. 2003;48(4):204–207. doi: 10.1007/s10038-003-0005-7. [DOI] [PubMed] [Google Scholar]
- 23.Ioos C, Barois A, Richard P, Eymard B, Hantai D, Estournet-Mathiaud B. Congenital myasthenic syndrome due to rapsyn deficiency: three cases with arthrogryposis and bulbar symptoms. Neuropediatrics. 2004;35(4):246–249. doi: 10.1055/s-2004-820993. [DOI] [PubMed] [Google Scholar]
- 24.Maselli R, Dris H, Schnier J, Cockrell J, Wollmann R. Congenital myasthenic syndrome caused by two non-N88K rapsyn mutations. Clinical genetics. 2007;72(1):63–65. doi: 10.1111/j.1399-0004.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- 25.Maselli RA, Dunne V, Pascual-Pascual SI, et al. Rapsyn mutations in myasthenic syndrome due to impaired receptor clustering. Muscle & nerve. 2003;28(3):293–301. doi: 10.1002/mus.10433. [DOI] [PubMed] [Google Scholar]
- 26.Muller JS, Mildner G, Muller-Felber W, et al. Rapsyn N88K is a frequent cause of congenital myasthenic syndromes in European patients. Neurology. 2003;60(11):1805–1810. doi: 10.1212/01.wnl.0000072262.14931.80. [DOI] [PubMed] [Google Scholar]
- 27.Ohno K, Engel AG, Shen XM, et al. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. American journal of human genetics. 2002;70(4):875–885. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben Ammar A, Soltanzadeh P, Bauche S, et al. A mutation causes MuSK reduced sensitivity to agrin and congenital myasthenia. PloS one. 2013;8(1):e53826. doi: 10.1371/journal.pone.0053826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luan X, Tian W, Cao L. Limb-girdle congenital myasthenic syndrome in a Chinese family with novel mutations in MUSK gene and literature review. Clinical neurology and neurosurgery. 2016;150:41–45. doi: 10.1016/j.clineuro.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Maselli RA, Arredondo J, Cagney O, et al. Mutations in MUSK causing congenital myasthenic syndrome impair MuSK-Dok-7 interaction. Human molecular genetics. 2010;19(12):2370–2379. doi: 10.1093/hmg/ddq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selcen D, Ohkawara B, Shen XM, McEvoy K, Ohno K, Engel AG. Impaired Synaptic Development, Maintenance, and Neuromuscular Transmission in LRP4-Related Myasthenia. JAMA Neurol. 2015;72(8):889–896. doi: 10.1001/jamaneurol.2015.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maselli RA, Fernandez JM, Arredondo J, et al. LG2 agrin mutation causing severe congenital myasthenic syndrome mimics functional characteristics of non-neural (z-) agrin. Human genetics. 2012;131(7):1123–1135. doi: 10.1007/s00439-011-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicole S, Chaouch A, Torbergsen T, et al. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain. 2014;137(Pt 9):2429–2443. doi: 10.1093/brain/awu160. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawara B, Cabrera-Serrano M, Nakata T, et al. LRP4 third beta-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner. Human molecular genetics. 2014;23(7):1856–1868. doi: 10.1093/hmg/ddt578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsen G, Halfter W, Kroger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. The Journal of biological chemistry. 1995;270(7):3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- 36.Kim N, Stiegler AL, Cameron TO, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimitropoulou A, Bixby JL. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Molecular and cellular neurosciences. 2005;28(2):292–302. doi: 10.1016/j.mcn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Cartaud A, Strochlic L, Guerra M, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. The Journal of cell biology. 2004;165(4):505–515. doi: 10.1083/jcb.200307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karmouch J, Dobbertin A, Sigoillot S, Legay C. Developmental consequences of the ColQ/MuSK interactions. Chemico-biological interactions. 2013;203(1):287–291. doi: 10.1016/j.cbi.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Nakata T, Ito M, Azuma Y, et al. Mutations in the C-terminal domain of ColQ in endplate acetylcholinesterase deficiency compromise ColQ-MuSK interaction. Human mutation. 2013;34(7):997–1004. doi: 10.1002/humu.22325. [DOI] [PubMed] [Google Scholar]
- 42.Zong Y, Zhang B, Gu S, et al. Structural basis of agrin-LRP4-MuSK signaling. Genes & development. 2012;26(3):247–258. doi: 10.1101/gad.180885.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergamin E, Hallock PT, Burden SJ, Hubbard SR. The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Molecular cell. 2010;39(1):100–109. doi: 10.1016/j.molcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueta R, Tezuka T, Izawa Y, et al. The carboxyl-terminal region of Dok-7 plays a key, but not essential, role in activation of muscle-specific receptor kinase MuSK and neuromuscular synapse formation. Journal of biochemistry. 2017;161(3):269–277. doi: 10.1093/jb/mvw073. [DOI] [PubMed] [Google Scholar]
- 45.Inoue A, Setoguchi K, Matsubara Y, et al. Dok-7 activates the muscle receptor kinase MuSK and shapes synapse formation. Science signaling. 2009;2(59):ra7. doi: 10.1126/scisignal.2000113. [DOI] [PubMed] [Google Scholar]
- 46.Yamanashi Y, Higuch O, Beeson D. Dok-7/MuSK signaling and a congenital myasthenic syndrome. Acta myologica : myopathies and cardiomyopathies : official journal of the Mediterranean Society of Myology / edited by the Gaetano Conte Academy for the study of striated muscle diseases. 2008;27:25–29. [PMC free article] [PubMed] [Google Scholar]
- 47.Antolik C, Catino DH, Resneck WG, Bloch RJ. The tetratricopeptide repeat domains of rapsyn bind directly to cytoplasmic sequences of the muscle-specific kinase. Neuroscience. 2006;141(1):87–100. doi: 10.1016/j.neuroscience.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 48.Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18(4):623–635. doi: 10.1016/s0896-6273(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 49.Frail DE, McLaughlin LL, Mudd J, Merlie JP. Identification of the mouse muscle 43,000-dalton acetylcholine receptor-associated protein (RAPsyn) by cDNA cloning. The Journal of biological chemistry. 1988;263(30):15602–15607. [PubMed] [Google Scholar]
- 50.Gautam M, Noakes PG, Mudd J, et al. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377(6546):232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y, Rudell J, Ferns M. Rapsyn interacts with the muscle acetylcholine receptor via alpha-helical domains in the alpha, beta, and epsilon subunit intracellular loops. Neuroscience. 2009;163(1):222–232. doi: 10.1016/j.neuroscience.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuber B, Unwin N. Structure and superorganization of acetylcholine receptor-rapsyn complexes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):10622–10627. doi: 10.1073/pnas.1301277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burden SJ, DePalma RL, Gottesman GS. Crosslinking of proteins in acetylcholine receptor-rich membranes: association between the beta-subunit and the 43 kd subsynaptic protein. Cell. 1983;35(3 Pt 2):687–692. doi: 10.1016/0092-8674(83)90101-0. [DOI] [PubMed] [Google Scholar]
- 54.Bartoli M, Ramarao MK, Cohen JB. Interactions of the rapsyn RING-H2 domain with dystroglycan. The Journal of biological chemistry. 2001;276(27):24911–24917. doi: 10.1074/jbc.M103258200. [DOI] [PubMed] [Google Scholar]
- 55.Valenzuela DM, Stitt TN, DiStefano PS, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15(3):573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 56.DeChiara TM, Bowen DC, Valenzuela DM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85(4):501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 57.Glass DJ, Bowen DC, Stitt TN, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85(4):513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 58.Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Annals of neurology. 2008;63(6):782–789. doi: 10.1002/ana.21371. [DOI] [PubMed] [Google Scholar]
- 59.Jha S, Xu K, Maruta T, et al. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK) Journal of neuroimmunology. 2006;175(1–2):107–117. doi: 10.1016/j.jneuroim.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Benveniste O, Jacobson L, Farrugia ME, Clover L, Vincent A. MuSK antibody positive myasthenia gravis plasma modifies MURF-1 expression in C2C12 cultures and mouse muscle in vivo. Journal of neuroimmunology. 2005;170(1–2):41–48. doi: 10.1016/j.jneuroim.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Lee JY, Sung JJ, Cho JY, et al. MuSK antibody-positive, seronegative myasthenia gravis in Korea. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2006;13(3):353–355. doi: 10.1016/j.jocn.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 62.Chan KH, Lachance DH, Harper CM, Lennon VA. Frequency of seronegativity in adult-acquired generalized myasthenia gravis. Muscle & nerve. 2007;36(5):651–658. doi: 10.1002/mus.20854. [DOI] [PubMed] [Google Scholar]
- 63.Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60(12):1978–1980. doi: 10.1212/01.wnl.0000065882.63904.53. [DOI] [PubMed] [Google Scholar]
- 64.Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative mysthenia gravis. Brain. 2003;126:2304–2311. doi: 10.1093/brain/awg223. [DOI] [PubMed] [Google Scholar]
- 65.Padua L, Tonali P, Aprile I, Caliandro P, Bartoccioni E, Evoli A. Seronegative myasthenia gravis: comparison of neurophysiological picture in MuSK+ and MuSK− patients. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2006;13(3):273–276. doi: 10.1111/j.1468-1331.2006.01214.x. [DOI] [PubMed] [Google Scholar]
- 66.Hurst RL, Gooch CL. Muscle-Specific Receptor Tyrosine Kinase (MuSK) Myasthenia Gravis. Curr Neurol Neurosci Rep. 2016;16(7):61. doi: 10.1007/s11910-016-0668-z. [DOI] [PubMed] [Google Scholar]
- 67.Lavrnic D, Losen M, Vujic A, et al. The features of myasthenia gravis with autoantibodies to MuSK. Journal of neurology, neurosurgery, and psychiatry. 2005;76(8):1099–1102. doi: 10.1136/jnnp.2004.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2005;116(9):2065–2068. doi: 10.1016/j.clinph.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Huang YC, Yeh JH, Chiu HC, Chen WH. Clinical characteristics of MuSK antibody-positive myasthenia gravis in Taiwan. J Formos Med Assoc. 2008;107(7):572–575. doi: 10.1016/S0929-6646(08)60171-0. [DOI] [PubMed] [Google Scholar]
- 70.Yeh JH, Chen WH, Chiu HC, Vincent A. Low frequency of MuSK antibody in generalized seronegative myasthenia gravis among Chinese. Neurology. 2004;62(11):2131–2132. doi: 10.1212/01.wnl.0000128042.28877.c3. [DOI] [PubMed] [Google Scholar]
- 71.Kostera-Pruszczyk A, Kaminska A, Dutkiewicz M, et al. MuSK-positive myasthenia gravis is rare in the Polish population. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2008;15(7):720–724. doi: 10.1111/j.1468-1331.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- 72.Bartoccioni E, Scuderi F, Augugliaro A, et al. HLA class II allele analysis in MuSK-positive myasthenia gravis suggests a role for DQ5. Neurology. 2009;72(2):195–197. doi: 10.1212/01.wnl.0000339103.08830.86. [DOI] [PubMed] [Google Scholar]
- 73.Alahgholi-Hajibehzad M, Yilmaz V, Gulsen-Parman Y, et al. Association of HLA-DRB1 *14, -DRB1 *16 and -DQB1 *05 with MuSK-myasthenia gravis in patients from Turkey. Hum Immunol. 2013;74(12):1633–1635. doi: 10.1016/j.humimm.2013.08.271. [DOI] [PubMed] [Google Scholar]
- 74.Kanai T, Uzawa A, Kawaguchi N, et al. HLA-DRB1*14 and DQB1*05 are associated with Japanese anti-MuSK antibody-positive myasthenia gravis patients. Journal of the neurological sciences. 2016;363:116–118. doi: 10.1016/j.jns.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 75.Furuta N, Ishizawa K, Shibata M, et al. Anti-MuSK Antibody-positive Myasthenia Gravis Mimicking Amyotrophic Lateral Sclerosis. Internal medicine. 2015;54(19):2497–2501. doi: 10.2169/internalmedicine.54.4645. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi H, Kawaguchi N, Ito S, Nemoto Y, Hattori T, Kuwabara S. Is tongue atrophy reversible in anti-MuSK myasthenia gravis? Six-year observation. Journal of neurology, neurosurgery, and psychiatry. 2010;81(6):701–702. doi: 10.1136/jnnp.2009.171793. [DOI] [PubMed] [Google Scholar]
- 77.Ohno K, Ito M, Kawakami Y, Ohtsuka K. Collagen Q is a key player for developing rational therapy for congenital myasthenia and for dissecting the mechanisms of anti-MuSK myasthenia gravis. Journal of molecular neuroscience : MN. 2014;53(3):359–361. doi: 10.1007/s12031-013-0170-x. [DOI] [PubMed] [Google Scholar]
- 78.Pasnoor M, Wolfe GI, Nations S, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a U.S. experience. Muscle & nerve. 2010;41(3):370–374. doi: 10.1002/mus.21533. [DOI] [PubMed] [Google Scholar]
- 79.Punga AR, Flink R, Askmark H, Stalberg EV. Cholinergic neuromuscular hyperactivity in patients with myasthenia gravis seropositive for MuSK antibody. Muscle & nerve. 2006;34(1):111–115. doi: 10.1002/mus.20515. [DOI] [PubMed] [Google Scholar]
- 80.Jaretzki A, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: Recommendations for clinical research standards. Neurology. 2000;55(1):16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 81.Caress JB, Hunt CH, Batish SD. Anti-MuSK myasthenia gravis presenting with purely ocular findings. Archives of neurology. 2005;62(6):1002–1003. doi: 10.1001/archneur.62.6.1002. [DOI] [PubMed] [Google Scholar]
- 82.Bau V, Hanisch F, Hain B, Zierz S. Ocular involvement in MuSK antibody-positive myasthenia gravis. Klinische Monatsblatter fur Augenheilkunde. 2006;223(1):81–83. doi: 10.1055/s-2005-858629. [DOI] [PubMed] [Google Scholar]
- 83.Bennett DL, Mills KR, Riordan-Eva P, Barnes PR, Rose MR. Anti-MuSK antibodies in a case of ocular myasthenia gravis. Journal of neurology, neurosurgery, and psychiatry. 2006;77(4):564–565. doi: 10.1136/jnnp.2005.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan JW, Orrison WW. Ocular myasthenia: a rare presentation with MuSK antibody and bilateral extraocular muscle atrophy. Br J Ophthalmol. 2007;91(6):842–843. doi: 10.1136/bjo.2006.108498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hanisch F, Eger K, Zierz S. MuSK-antibody positive pure ocular myasthenia gravis. Journal of neurology. 2006;253(5):659–660. doi: 10.1007/s00415-005-0032-8. [DOI] [PubMed] [Google Scholar]
- 86.Hosaka A, Takuma H, Ohta K, Tamaoka A. An ocular form of myasthenia gravis with a high titer of anti-MuSK antibodies during a long-term follow-up. Internal medicine. 2012;51(21):3077–3079. doi: 10.2169/internalmedicine.51.8196. [DOI] [PubMed] [Google Scholar]
- 87.Casasnovas C, Povedano M, Jauma S, Montero J, Martinez-Matos JA. Musk-antibody positive myasthenia gravis presenting with isolated neck extensor weakness. Neuromuscular disorders : NMD. 2007;17(7):544–546. doi: 10.1016/j.nmd.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Okano T, Fujitake J, Suzuki K, et al. A case of anti-MuSK antibody-positive myasthenia gravis with dropped head as the initial presenting symptom. Rinsho shinkeigaku = Clinical neurology. 2006;46(7):496–500. [PubMed] [Google Scholar]
- 89.Spengos K, Vassilopoulou S, Papadimas G, et al. Dropped head syndrome as prominent clinical feature in MuSK-positive Myasthenia Gravis with thymus hyperplasia. Neuromuscular disorders : NMD. 2008;18(2):175–177. doi: 10.1016/j.nmd.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Giarrana ML, Joset P, Sticht H, et al. A severe congenital myasthenic syndrome with "dropped head" caused by novel MUSK mutations. Muscle & nerve. 2015;52(4):668–673. doi: 10.1002/mus.24687. [DOI] [PubMed] [Google Scholar]
- 91.Hara K, Mashima T, Matsuda A, et al. Vocal cord paralysis in myasthenia gravis with anti-MuSK antibodies. Neurology. 2007;68(8):621–622. doi: 10.1212/01.wnl.0000254617.15644.f4. [DOI] [PubMed] [Google Scholar]
- 92.Jimenez Caballero PE, Fermin Marrero JA, Trigo Bragado I, Casado Naranjo I. Vocal cord paralysis as a manifestation of myasthenia gravis with anti-MuSK antibodies. Neurologia. 2014;29(4):253–254. doi: 10.1016/j.nrl.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Sylva M, van der Kooi AJ, Grolman W. Dyspnoea due to vocal fold abduction paresis in anti-MuSK myasthenia gravis. Journal of neurology, neurosurgery, and psychiatry. 2008;79(9):1083–1084. doi: 10.1136/jnnp.2007.135319. [DOI] [PubMed] [Google Scholar]
- 94.Zouvelou V, Kyriazi S, Rentzos M, et al. Double-seropositive myasthenia gravis. Muscle & nerve. 2013;47(3):465–466. doi: 10.1002/mus.23645. [DOI] [PubMed] [Google Scholar]
- 95.Zouvelou V, Zisimopoulou P, Psimenou E, Matsigkou E, Stamboulis E, Tzartos SJ. AChR-myasthenia gravis switching to double-seropositive several years after the onset. Journal of neuroimmunology. 2013 doi: 10.1016/j.jneuroim.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 96.Basta I, Nikolic A, Losen M, et al. MuSK myasthenia gravis and Lambert-Eaton myasthenic syndrome in the same patient. Clinical neurology and neurosurgery. 2012;114(6):795–797. doi: 10.1016/j.clineuro.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 97.Diaz-Manera J, Rojas-Garcia R, Gallardo E, et al. Antibodies to AChR, MuSK and VGKC in a patient with myasthenia gravis and Morvan's syndrome. Nature clinical practice Neurology. 2007;3(7):405–410. doi: 10.1038/ncpneuro0526. [DOI] [PubMed] [Google Scholar]
- 98.Bartoccioni E, Scuderi F, Minicuci GM, Marino M, Ciaraffa F, Evoli A. Anti-MuSK antibodies: correlation with myasthenia gravis severity. Neurology. 2006;67(3):505–507. doi: 10.1212/01.wnl.0000228225.23349.5d. [DOI] [PubMed] [Google Scholar]
- 99.Behin A, Mayer M, Kassis-Makhoul B, et al. Severe neonatal myasthenia due to maternal anti-MuSK antibodies. Neuromuscular disorders : NMD. 2008;18(6):443–446. doi: 10.1016/j.nmd.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Kanzaki A, Motomura M. A pregnant patient with anti-MuSK antibody positive myasthenia gravis and her infant with transient neonatal myasthenia gravis. Rinsho shinkeigaku = Clinical neurology. 2011;51(3):188–191. doi: 10.5692/clinicalneurol.51.188. [DOI] [PubMed] [Google Scholar]
- 101.Murray EL, Kedar S, Vedanarayanan VV. Transmission of maternal muscle-specific tyrosine kinase (MuSK) to offspring: report of two cases. J Clin Neuromuscul Dis. 2010;12(2):76–79. doi: 10.1097/CND.0b013e3181f8a9aa. [DOI] [PubMed] [Google Scholar]
- 102.Niks EH, Verrips A, Semmekrot BA, et al. A transient neonatal myasthenic syndrome with anti-musk antibodies. Neurology. 2008;70(14):1215–1216. doi: 10.1212/01.wnl.0000307751.20968.f1. [DOI] [PubMed] [Google Scholar]
- 103.O'Carroll P, Bertorini TE, Jacob G, Mitchell CW, Graff J. Transient neonatal myasthenia gravis in a baby born to a mother with new-onset anti-MuSK-mediated myasthenia gravis. J Clin Neuromuscul Dis. 2009;11(2):69–71. doi: 10.1097/CND.0b013e3181a78280. [DOI] [PubMed] [Google Scholar]
- 104.Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Current opinion in neurology. 2010;23(5):530–535. doi: 10.1097/WCO.0b013e32833c0982. [DOI] [PubMed] [Google Scholar]
- 105.Saka E, Topcuoglu MA, Akkaya B, Galati A, Onal MZ, Vincent A. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;65(5):782–783. doi: 10.1212/wnl.65.5.782. author reply 782–783. [DOI] [PubMed] [Google Scholar]
- 106.Ito A, Sasaki R, Ii Y, Nakayama S, Motomura M, Tomimoto H. A case of thymoma-associated myasthenia gravis with anti-MuSK antibodies. Rinsho shinkeigaku = Clinical neurology. 2013;53(5):372–375. doi: 10.5692/clinicalneurol.53.372. [DOI] [PubMed] [Google Scholar]
- 107.Tsonis AI, Zisimopoulou P, Lazaridis K, et al. MuSK autoantibodies in myasthenia gravis detected by cell based assay--A multinational study. Journal of neuroimmunology. 2015;284:10–17. doi: 10.1016/j.jneuroim.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 108.Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;64(3):536–538. doi: 10.1212/01.WNL.0000150587.71497.B6. [DOI] [PubMed] [Google Scholar]
- 109.Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Annals of neurology. 2005;57(3):444–448. doi: 10.1002/ana.20386. [DOI] [PubMed] [Google Scholar]
- 110.Ponseti JM, Caritg N, Gamez J, Lopez-Cano M, Vilallonga R, Armengol M. A comparison of long-term post-thymectomy outcome of anti-AChR-positive, anti-AChR-negative and anti-MuSK-positive patients with non-thymomatous myasthenia gravis. Expert Opin Biol Ther. 2009;9(1):1–8. doi: 10.1517/14712590802588831. [DOI] [PubMed] [Google Scholar]
- 111.Kostera-Pruszczyk A, Kwiecinski H. Juvenile seropositive myasthenia gravis with anti-MuSK antibody after thymectomy. Journal of neurology. 2009;256(10):1780–1781. doi: 10.1007/s00415-009-5215-2. [DOI] [PubMed] [Google Scholar]
- 112.Saulat B, Maertens P, Hamilton WJ, Bassam BA. Anti-musk antibody after thymectomy in a previously seropositive myasthenic child. Neurology. 2007;69(8):803–804. doi: 10.1212/01.wnl.0000267694.45244.f4. [DOI] [PubMed] [Google Scholar]
- 113.Jordan B, Schilling S, Zierz S. Switch to double positive late onset MuSK myasthenia gravis following thymomectomy in paraneoplastic AChR antibody positive myasthenia gravis. Journal of neurology. 2016;263(1):174–176. doi: 10.1007/s00415-015-7982-2. [DOI] [PubMed] [Google Scholar]
- 114.Rajakulendran S, Viegas S, Spillane J, Howard RS. Clinically biphasic myasthenia gravis with both AChR and MuSK antibodies. Journal of neurology. 2012;259(12):2736–2739. doi: 10.1007/s00415-012-6661-9. [DOI] [PubMed] [Google Scholar]
- 115.Oh S. Muscle-Specific Receptor Tyrosine Kinase Antibody Positive Myasthenia Gravis Current Status. J Clin Neurol. 2009;5:53–64. doi: 10.3988/jcn.2009.5.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oh SJ, Hatanaka Y, Hemmi S, et al. Repetitive nerve stimulation of facial muscles in MuSK antibody-positive myasthenia gravis. Muscle & nerve. 2006;33(4):500–504. doi: 10.1002/mus.20498. [DOI] [PubMed] [Google Scholar]
- 117.Nikolic A, Basta I, Stojanovic VR, Stevic Z, Lavrnic D. Electrophysiological profile of the patients with MuSK positive myasthenia gravis. Neurological research. 2014;36(11):945–949. doi: 10.1179/1743132814Y.0000000387. [DOI] [PubMed] [Google Scholar]
- 118.Kuwabara S, Nemoto Y, Misawa S, Takahashi H, Kawaguchi N, Hattori T. Anti-MuSK-positive myasthenia gravis: neuromuscular transmission failure in facial and limb muscles. Acta neurologica Scandinavica. 2007;115(2):126–128. doi: 10.1111/j.1600-0404.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 119.Farrugia ME, Kennett RP, Hilton-Jones D, Newsom-Davis J, Vincent A. Quantitative EMG of facial muscles in myasthenia patients with MuSK antibodies. Clinical neurophysiology : official journal of the International Federation of Clinical. Neurophysiology. 2007;118(2):269–277. doi: 10.1016/j.clinph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Nikolic A, Basta I, Stojanovic VR, Stevic Z, Peric S, Lavrnic D. Myopathic changes detected by quantitative electromyography in patients with MuSK and AChR positive myasthenia gravis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016;27:126–129. doi: 10.1016/j.jocn.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 121.Nikolic AV, Bacic GG, Dakovic MZ, et al. Myopathy, muscle atrophy and tongue lipid composition in MuSK myasthenia gravis. Acta Neurol Belg. 2015;115(3):361–365. doi: 10.1007/s13760-014-0364-1. [DOI] [PubMed] [Google Scholar]
- 122.Farrugia ME, Robson MD, Clover L, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain. 2006;129(Pt 6):1481–1492. doi: 10.1093/brain/awl095. [DOI] [PubMed] [Google Scholar]
- 123.Ishii W, Matsuda M, Okamoto N, et al. Myasthenia gravis with anti-MuSK antibody, showing progressive muscular atrophy without blepharoptosis. Internal medicine. 2005;44(6):671–672. doi: 10.2169/internalmedicine.44.671. [DOI] [PubMed] [Google Scholar]
- 124.Moon SY, Lee SS, Hong YH. Muscle atrophy in muscle-specific tyrosine kinase (MuSK)-related myasthenia gravis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2011;18(9):1274–1275. doi: 10.1016/j.jocn.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 125.Cenacchi G, Papa V, Fanin M, Pegoraro E, Angelini C. Comparison of muscle ultrastructure in myasthenia gravis with anti-MuSK and anti-AChR antibodies. Journal of neurology. 2011;258(5):746–752. doi: 10.1007/s00415-010-5823-x. [DOI] [PubMed] [Google Scholar]
- 126.Martignago S, Fanin M, Albertini E, Pegoraro E, Angelini C. Muscle histopathology in myasthenia gravis with antibodies against MuSK and AChR. Neuropathology and applied neurobiology. 2009;35(1):103–110. doi: 10.1111/j.1365-2990.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 127.Zouvelou V, Rentzos M, Toulas P, Evdokimidis I. MRI evidence of early muscle atrophy in MuSK positive myasthenia gravis. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2011;21(3):303–305. doi: 10.1111/j.1552-6569.2009.00456.x. [DOI] [PubMed] [Google Scholar]
- 128.Kitamura E, Takiyama Y, Nakamura M, Iizuka T, Nishiyama K. Reversible tongue muscle atrophy accelerated by early initiation of immunotherapy in anti-MuSK myasthenia gravis: A case report. Journal of the neurological sciences. 2016;360:10–12. doi: 10.1016/j.jns.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 129.Punga AR, Lin S, Oliveri F, Meinen S, Ruegg MA. Muscle-selective synaptic disassembly and reorganization in MuSK antibody positive MG mice. Exp Neurol. 2011;230(2):207–217. doi: 10.1016/j.expneurol.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 130.Punga AR, Maj M, Lin S, Meinen S, Ruegg MA. MuSK levels differ between adult skeletal muscles and influence postsynaptic plasticity. The European journal of neuroscience. 2011;33(5):890–898. doi: 10.1111/j.1460-9568.2010.07569.x. [DOI] [PubMed] [Google Scholar]
- 131.Huijbers MG, Vink AF, Niks EH, et al. Longitudinal epitope mapping in MuSK myasthenia gravis: implications for disease severity. Journal of neuroimmunology. 2016;291:82–88. doi: 10.1016/j.jneuroim.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Mori S, Shigemoto K. Mechanisms associated with the pathogenicity of antibodies against muscle-specific kinase in myasthenia gravis. Autoimmunity reviews. 2013;12(9):912–917. doi: 10.1016/j.autrev.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 133.Niks EH, van Leeuwen Y, Leite MI, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. Journal of neuroimmunology. 2008;195(1–2):151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 134.Huijbers MG, Zhang W, Klooster R, et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20783–20788. doi: 10.1073/pnas.1313944110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1–3 can disperse preformed agrin-independent AChR clusters. PloS one. 2013;8(11):e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hatanaka Y, Hemmi S, Morgan MB, et al. Nonresponsiveness to anticholinesterase agents in patients with MuSK-antibody-positive MG. Neurology. 2005;65(9):1508–1509. doi: 10.1212/01.wnl.0000183145.91579.74. [DOI] [PubMed] [Google Scholar]
- 137.Shin HY, Park HJ, Lee HE, Choi YC, Kim SM. Clinical and Electrophysiologic Responses to Acetylcholinesterase Inhibitors in MuSK-Antibody-Positive Myasthenia Gravis: Evidence for Cholinergic Neuromuscular Hyperactivity. J Clin Neurol. 2014;10(2):119–124. doi: 10.3988/jcn.2014.10.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Plomp JJ, Huijbers MG, van der Maarel SM, Verschuuren JJ. Pathogenic IgG4 subclass autoantibodies in MuSK myasthenia gravis. Annals of the New York Academy of Sciences. 2012;1275:114–122. doi: 10.1111/j.1749-6632.2012.06808.x. [DOI] [PubMed] [Google Scholar]
- 139.Mori S, Kishi M, Kubo S, et al. 3,4-Diaminopyridine improves neuromuscular transmission in a MuSK antibody-induced mouse model of myasthenia gravis. Journal of neuroimmunology. 2012;245(1–2):75–78. doi: 10.1016/j.jneuroim.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 140.Evoli A, Alboini PE, Damato V, Iorio R. 3,4-Diaminopyridine may improve myasthenia gravis with MuSK antibodies. Neurology. 2016;86(11):1070–1071. doi: 10.1212/WNL.0000000000002466. [DOI] [PubMed] [Google Scholar]
- 141.Morsch M, Reddel SW, Ghazanfari N, Toyka KV, Phillips WD. Pyridostigmine but not 3,4-diaminopyridine exacerbates ACh receptor loss and myasthenia induced in mice by muscle-specific kinase autoantibody. The Journal of physiology. 2013;591(10):2747–2762. doi: 10.1113/jphysiol.2013.251827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El-Salem K, Yassin A, Al-Hayk K, Yahya S, Al-Shorafat D, Dahbour SS. Treatment of MuSK-Associated Myasthenia Gravis. Curr Treat Options Neurol. 2014;16(4):283. doi: 10.1007/s11940-014-0283-8. [DOI] [PubMed] [Google Scholar]
- 143.Evoli A, Bianchi MR, Riso R, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Annals of the New York Academy of Sciences. 2008;1132:76–83. doi: 10.1196/annals.1405.012. [DOI] [PubMed] [Google Scholar]
- 144.Deymeer F, Gungor-Tuncer O, Yilmaz V, et al. Clinical comparison of anti-MuSK- vs anti-AChR-positive and seronegative myasthenia gravis. Neurology. 2007;68(8):609–611. doi: 10.1212/01.wnl.0000254620.45529.97. [DOI] [PubMed] [Google Scholar]
- 145.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle & nerve. 2011;44(1):36–40. doi: 10.1002/mus.22006. [DOI] [PubMed] [Google Scholar]
- 146.Shibata-Hamaguchi A, Samuraki M, Furui E, et al. Long-term effect of intravenous immunoglobulin on anti-MuSK antibody-positive myasthenia gravis. Acta neurologica Scandinavica. 2007;116(6):406–408. doi: 10.1111/j.1600-0404.2007.00877.x. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi H, Kawaguchi N, Nemoto Y, Hattori T. High-dose intravenous immunoglobulin for the treatment of MuSK antibody-positive seronegative myasthenia gravis. Journal of the neurological sciences. 2006;247(2):239–241. doi: 10.1016/j.jns.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 148.Lin PT, Martin BA, Weinacker AB, So YT. High-dose cyclophosphamide in refractory myasthenia gravis with MuSK antibodies. Muscle & nerve. 2006;33(3):433–435. doi: 10.1002/mus.20411. [DOI] [PubMed] [Google Scholar]
- 149.Bouwyn JP, Magnier P, Bedat-Mill AL, Ahtoy P, Maltete D, Lefaucheur R. Anti-MuSK myasthenia gravis with prolonged remission. Neuromuscular disorders : NMD. 2016;26(7):453–454. doi: 10.1016/j.nmd.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 150.Shen C, Lu Y, Zhang B, et al. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. The Journal of clinical investigation. 2013;123(12):5190–5202. doi: 10.1172/JCI66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Barik A, Lu Y, Sathyamurthy A, et al. LRP4 Is Critical for Neuromuscular Junction Maintenance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(42):13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yumoto N, Kim N, Burden SJ. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature. 2012;489(7416):438–442. doi: 10.1038/nature11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu H, Lu Y, Shen C, et al. Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation. Neuron. 2012;75(1):94–107. doi: 10.1016/j.neuron.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zisimopoulou P, Evangelakou P, Tzartos J, et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. Journal of autoimmunity. 2014;52:139–145. doi: 10.1016/j.jaut.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 155.Li Y, Zhang Y, Cai G, et al. Anti-LRP4 autoantibodies in Chinese patients with myasthenia gravis. Muscle & nerve. 2017 doi: 10.1002/mus.25591. [DOI] [PubMed] [Google Scholar]
- 156.Cordts I, Bodart N, Hartmann K, et al. Screening for lipoprotein receptor-related protein 4-, agrin-, and titin-antibodies and exploring the autoimmune spectrum in myasthenia gravis. Journal of neurology. 2017 doi: 10.1007/s00415-017-8514-z. [DOI] [PubMed] [Google Scholar]
- 157.Beck G, Yabumoto T, Baba K, et al. Double Seronegative Myasthenia Gravis with Anti-LRP4 Antibodies Presenting with Dropped Head and Acute Respiratory Insufficiency. Internal medicine. 2016;55(22):3361–3363. doi: 10.2169/internalmedicine.55.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marino M, Scuderi F, Samengo D, et al. Flow Cytofluorimetric Analysis of Anti-LRP4 (LDL Receptor-Related Protein 4) Autoantibodies in Italian Patients with Myasthenia Gravis. PloS one. 2015;10(8):e0135378. doi: 10.1371/journal.pone.0135378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rivner MH, Liu S, Quarles B, et al. Agrin and low-density lipoprotein-related receptor protein 4 antibodies in amyotrophic lateral sclerosis patients. Muscle & nerve. 2017;55(3):430–432. doi: 10.1002/mus.25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tzartos JS, Zisimopoulou P, Rentzos M, et al. LRP4 antibodies in serum and CSF from amyotrophic lateral sclerosis patients. Ann Clin Transl Neurol. 2014;1(2):80–87. doi: 10.1002/acn3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cossins J, Belaya K, Zoltowska K, et al. The search for new antigenic targets in myasthenia gravis. Annals of the New York Academy of Sciences. 2012;1275:123–128. doi: 10.1111/j.1749-6632.2012.06833.x. [DOI] [PubMed] [Google Scholar]
- 162.Cortes-Vicente E, Gallardo E, Martinez MA, et al. Clinical Characteristics of Patients With Double-Seronegative Myasthenia Gravis and Antibodies to Cortactin. JAMA Neurol. 2016;73(9):1099–1104. doi: 10.1001/jamaneurol.2016.2032. [DOI] [PubMed] [Google Scholar]