Abstract
In 1992, Brose et al. showed that the synaptic vesicle (SV) protein p65/synaptotagmin (syt) 1 binds - in a mutually dependent manner - Ca2+ and anionic phospholipids, prompting the idea that it functions as a Ca2+ sensor for exocytosis. These findings now define two key aspects of excitation-secretion coupling.
Keywords: exocytosis, membrane fusion, neurotransmitter, SNARE
In 1973, Ricardo Miledi published a lone author paper entitled: “Transmitter release induced by injection of calcium ions into nerve terminals” [1]. This elegant paper rigorously established the Ca2+ hypothesis for neurotransmitter release by demonstrating that Ca2+ was necessary and sufficient for triggering exocytosis in nerve terminals. Two key questions emerged: what is the Ca2+ sensor(s) that mediates the process? And what are the immediate consequences of Ca2+ binding to this protein? In other words, how – biophysically – could Ca2+ binding trigger a synaptic vesicle (SV) to fuse with the plasma membrane?
The molecular part of this story began in 1981, when Matthew et al. generated monoclonal antibodies against isolated synaptic junctions, which contain both pre- and post-synaptic elements. This effort resulted in the characterization of two antibodies that specifically recognized a 65 kDa SV and large dense core vesicle protein that the authors named p65. At the time, SVs could already be purified in large amounts, and the systematic cloning of all constituent proteins was underway. In 1990, Perin et al. cloned a cDNA encoding p65, and eventually renamed the protein synaptotagmin (syt) 1 [2]. The sequence of syt1 revealed a striking clue regarding a potential function: the presence of tandem C2-domains, conserved ~135-residue motifs found in protein kinase C isoforms that are activated by phosphatidylserine and – notably – Ca2+. This hinted at the possibility of p65/syt1 acting as a Ca2+ sensor, but does this protein actually bind Ca2+?
In 1992, Reinhard Jahn’s laboratory directly addressed this question when a graduate student, Nils Brose, conducted a ground-breaking experiment: he immunopurified syt1 from brain tissue and used a number of rigorous, independent methods to determine whether it sensed Ca2+ [3]. The use of native protein was crucial, as the original cDNA encoding syt1 harbored a point mutation (G374D) in its second C2-domain (C2B) that abolished function [4]. Brose et al. observed that syt1 bound multiple Ca2+ ions in the presence of acidic phospholipids. Tantalizingly, the estimated stoichiometry was ~4 Ca2+ ions per molecule of syt1. This was provocative because the Hill coefficient for the Ca2+ dependence of neurotransmitter release from nerve terminals is often observed to be ~4, a value that represents a lower bound for the number of Ca2+ ions that cooperate to trigger exocytosis.
One year after the Brose et al. paper was published, Littleton et al. [5] reported the first measurements of evoked synaptic transmission in syt1 nulls, using the Drosophila neuromuscular junction as a model system. These experiments revealed that syt1 was essential for rapid, robust, evoked neurotransmitter release. Similar observations were subsequently made in other model systems, and structure-function analyses ensued. These efforts were guided by structural biology, pioneered by Sutton et al. [6], who solved the X-ray crystal structure of the first C2-domain of syt1 (C2A), providing key insights into how these domains bind Ca2+ ions. Subsequent studies have suggested that syt1 C2A binds either two or three Ca2+ ions, while C2B binds two [7], consistent with the findings of Brose et al. (1992).
The next step was to determine whether the Ca2+-binding activity of syt1 was crucial for fast release. Ca2+-binding is largely mediated by the negatively charged side chains of a conserved set of aspartic acid residues, and these residues have been targeted via mutagenesis (Fig. 1). However, it is difficult to predict, a priori, the effect of disrupting individual Ca2+ binding sites. For example, if Ca2+ binds in an ordered and cooperative manner, disruption of the first Ca2+ binding site could impair binding to subsequent sites, but not visa-versa. Alternatively, substitution of these residues could potentially mimic the Ca2+-bound state, resulting in a gain-of-function. These issues have been partially addressed via rescue experiments using mice and fruit flies lacking syt1. It is now clearly established that substitution of individual acidic Ca2+ ligands in the second C2-domain (C2B), abolish the ability of syt1 to trigger fusion in response to Ca2+ [8]. These mutations also endowed syt1 with potent dominant negative activity when expressed in wild type neurons [8]; the inability of these mutants to support release precludes determination of a Hill slope.
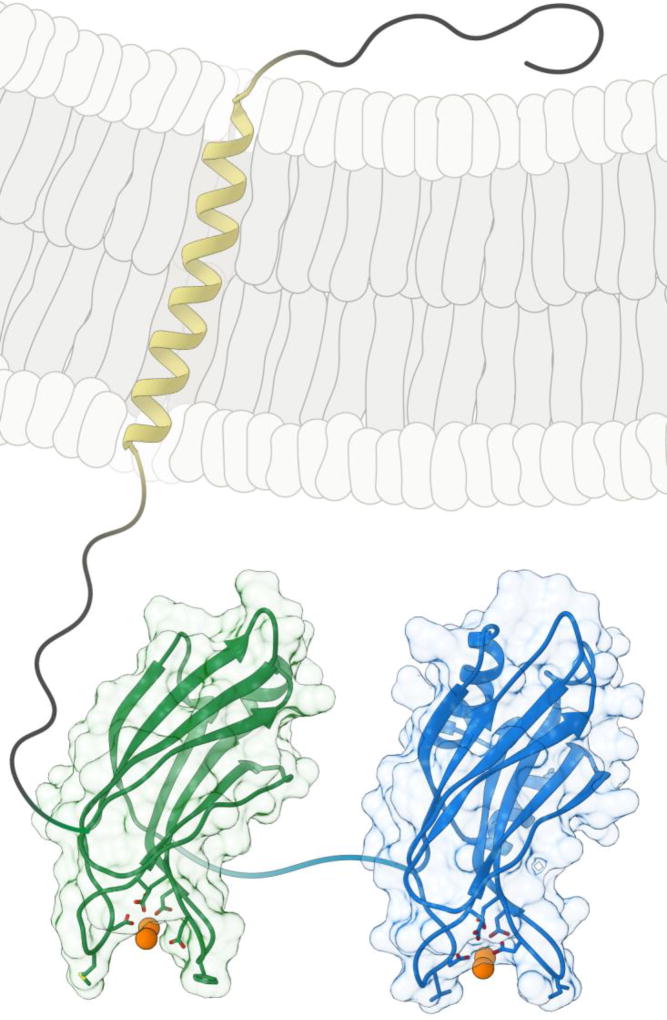
Figure 1.
Structure of syt 1. The C2A (green) and C2B (blue) domains were generated using PDB files from ref. [6] and [7], respectively. The aspartic acid side chains that are responsible for coordinating Ca2+, and the hydrophobic side chains (at the distal tips of the Ca2+ binding loops) that penetrate the target membrane, are rendered in wireframe. Ca2+ ions are represented as orange spheres; all linkers, as well as the transmembrane helix (yellow), were added using a drawing program.
A consensus regarding the effects of neutralizing acidic Ca2+ ligands in C2A has not yet been reached, with reports ranging from: 1) a gain of function and a concomitant decrease in Hill slope in that appeared to partially mimic the Ca2+ -bound state [4], 2) no effect at all on single evoked responses [9], 3) a loss of function [10]. While it remains unclear whether C2A must bind Ca2+ to regulate release, the presence of this domain is essential for syt1 to function in vivo [11]. Clearly, we are long way off from ‘mapping’ individual Ca2+ binding sites of syt1 to the Hill coefficient of synaptic release. But, given current findings, it seems likely that the observed cooperatively may result from a more distributed action of Ca2+, e.g. across more than one copy of syt1, to govern release.
In short, the evidence is now overwhelming that syt1 serves as a Ca2+ sensor for rapid, synchronous, SV release [12]. But how does it trigger release? Initial insights into this question again stem from Brose et al. (1992), who also showed that syt1 binds Ca2+ poorly in the absence of acidic phospholipids, so interactions with phospholipid head-groups appear to be essential for syt1 to sense relevant changes in [Ca2+]i. Later, by scanning the surface of the C2-domains with fluorescent probes, it was discovered that the distal tips of the Ca2+ binding loops themselves are actually inserted into the target (plasma) membrane upon Ca2+ binding (Fig. 1 and 2; ref. [12][13]). These interactions are extremely rapid and reversible and may serve to transiently juxtapose the bilayers that are destined to fuse, bringing them into proximity to facilitate SNARE catalyzed fusion [14]. The membrane penetration loops of Ca2+ •syt1 might also directly drive structural changes in the target membrane, such as localized bending or buckling, to lower the energy barrier for fusion [15] (Fig. 2). So, while structure-function experiments revealed that Ca2+ •syt1-membrane interactions constitute an essential step in Ca2+ -triggered SV exocytosis in living neurons, the precise effect of syt1 on bilayers - to accelerate fusion - remains somewhat elusive.
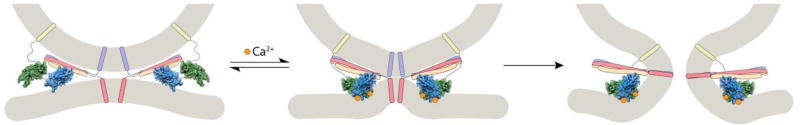
Figure 2.
Model for the action of Ca2+•syt 1 during exocytosis. At rest, vesicles are docked; upon stimulation, Ca2+ (orange spheres) enters and binds to syt1 (C2A rendered in green; C2B rendered in blue; the transmembrane domain is yellow). Ca2+·syt1 rapidly penetrates the target membrane to accelerate SNARE (shown in purple, red, and peach) catalyzed membrane fusion [12, 15].
Syt1 itself does not catalyze lipid mixing and membrane fusion itself. Rather, the pairing of vesicular and target membrane SNAREs into trans complexes serves to pull bilayers together to drive fusion; Ca2+ •syt1 accelerates these fusion reactions so that they occur on rapid time scales, in a manner that is precisely synchronized with increases in [Ca2+]i. Syt1 physically interacts with SNAREs, and in the presence of acidic lipids, Ca2+•syt1 can drive the assembly of stable SNARE complexes in vitro [12]. These observations provide an appealing connection between the Ca2+ sensor for exocytosis and the core of the membrane fusion machinery. However, findings concerning correlations between the ability of syt1 mutants to bind SNAREs and to drive release have been mixed, and this remains an open question.
In summary, the work of Brose et al. (1992) represents the first study to show that syt1 binds Ca2+ and negatively charged phospholipids. Twenty-six years later, these robust and reproducible observations have come to define two of the key steps in Ca2+-triggered exocytosis – Ca2+-sensing, and the ensuing rapid fusion of SVs with the plasma membrane.
Acknowledgments
The author thanks M.M. Bradberry for the artwork shown in Fig. 1 and Fig. 2. Structure and surface renderings were generated using UCSF Chimera. This work was supported by grants from the National Institute of Health (MH061876 and NS097362 to E.R.C.). E.R.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973;183(1073):421–5. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- 2.Perin MS, et al. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345(6272):260–3. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 3.Brose N, et al. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256(5059):1021–5. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 4.Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron. 2003;39(2):299–308. doi: 10.1016/s0896-6273(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 5.Littleton JT, et al. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74(6):1125–34. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 6.Sutton RB, et al. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80(6):929–38. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez I, et al. Three-dimensional structure of the synaptotagmin 1 C2B-domain. Synaptotagmin 1 as a phospholipid binding machine. Neuron. 2001;32(6):1057–69. doi: 10.1016/s0896-6273(01)00548-7. [DOI] [PubMed] [Google Scholar]
- 8.Mackler JM, et al. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418(6895):340–4. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Chacon R, et al. Structure/function analysis of Ca2+ binding to the C2A domain of synaptotagmin 1. J Neurosci. 2002;22(19):8438–46. doi: 10.1523/JNEUROSCI.22-19-08438.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin OH, et al. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A. 2009;106(38):16469–74. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J, et al. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J Neurosci. 2013;33(1):187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu Rev Biochem. 2008;77:615–41. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 13.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem. 1998;273(22):13995–4001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 14.Davis AF, et al. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24(2):363–76. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 15.Hui E, et al. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138(4):709–21. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]