Abstract
Alcohol consumption remains one of the predominant causes of liver disease and liver-related death worldwide. Intriguingly, dysregulation of the gut barrier is a key factor promoting the pathogenesis of alcoholic liver disease (ALD). A functional gut barrier, which consists of a mucus layer, an intact epithelial monolayer and mucosal immune cells, supports nutrient absorption and prevents bacterial penetration. Compromised gut barrier function is associated with the progression of ALD. Indeed, alcohol consumption disrupts the gut barrier, increases gut permeability, and induces bacterial translocation both in ALD patients and in experimental models with ALD. Moreover, alcohol consumption also causes enteric dysbiosis with both numerical and proportional perturbations. Here, we review and discuss mechanisms of alcohol-induced gut barrier dysfunction to better understand the contribution of the gut-liver axis to the pathogenesis of ALD. Unfortunately, there is no effectual Food and Drug Administration-approved treatment for any stage of ALD. Therefore, we conclude with a discussion of potential strategies aimed at restoring the gut barrier in ALD. The principle behind antibiotics, prebiotics, probiotics and fecal microbiota transplants is to restore microbial symbiosis and subsequently gut barrier function. Nutrient-based treatments, such as dietary supplementation with zinc, niacin or fatty acids, have been shown to regulate tight junction expression, reduce intestinal inflammation, and prevent endotoxemia as well as liver injury caused by alcohol in experimental settings. Interestingly, saturated fatty acids may also directly control the gut microbiome. In summary, clinical and experimental studies highlight the significance and efficacy of the gut barrier in treating ALD.
Keywords: Alcoholic liver disease (ALD), Gut barrier, Gut hyperpermeability, Dietary intervention, Microbiota treatment
1. Introduction
Long-term excessive alcohol consumption causes liver disease, namely alcoholic liver disease (ALD). ALD is one of the major causes of liver-related mortality worldwide. Alcohol-related deaths account for up to 48% of liver-related deaths in the United States,1 whereas it has been estimated that 60%–80% of liver-related deaths in Europe are due to excessive alcohol consumption.2 Unfortunately, effective therapies for ALD are currently unavailable. The major obstacle is the limited understanding of the pathogenesis of ALD. The spectrum of ALD involves three progressive stages: alcoholic steatosis (fatty liver), hepatitis and cirrhosis.1,3 Alcoholic steatosis, characterized by macrovesicular and/or microvesicular lipid droplet accumulation in hepatocytes, is the earliest manifestation of ALD and is generally reversible with abstinence. Alcoholic hepatitis features inflammation and necrosis in the liver, which encompasses a spectrum of severity ranging from asymptomatic dysregulation of biochemistries to fulminant liver failure. Alcoholic cirrhosis, the most advanced form of ALD, refers to the replacement of functional liver tissue with nonfunctional fibrotic tissue and regenerative nodules, and may result in clinical manifestations of portal hypertension and liver failure. It is noteworthy that not all heavy drinkers develop alcoholic hepatitis, and the disease can occur in people who drink only moderately. Indeed, approximately 90%–95% of people who consume large quantities of alcohol develop steatosis, of which only 10%–40% eventually develop liver fibrosis.4 Despite the correlation between the per capita alcohol consumption and mortality rates from hepatic cirrhosis,5 there is little evidence suggesting that alcohol itself is able to cause permanent damage to the liver. Thus, factors other than alcohol intake influence the development and progression of ALD.
Early studies in ALD identified increased levels of lipopolysaccharide (LPS), a bacterial endotoxin, in the plasma of both ALD patients and experimental animal models of ALD (namely endotoxemia).6–9 Experimentally-induced endotoxemia indicates that it is a key factor in the development of ALD.10,11 In addition to LPS, increasing evidence from recent studies supports an emerging role for bacterial pathogen-associated molecular patterns (PAMPs) in ALD.12–14 Clinical studies have shown that only alcoholics with gut leakiness develop liver injury,15 suggesting a pivotal role for gut-derived toxins and the gut barrier in ALD. This review focuses on the gut barrier and gut-derived PAMPs in the pathogenesis of ALD. Potential therapeutic approaches targeting the gut for the treatment of ALD will also be discussed.
2. Gut barrier
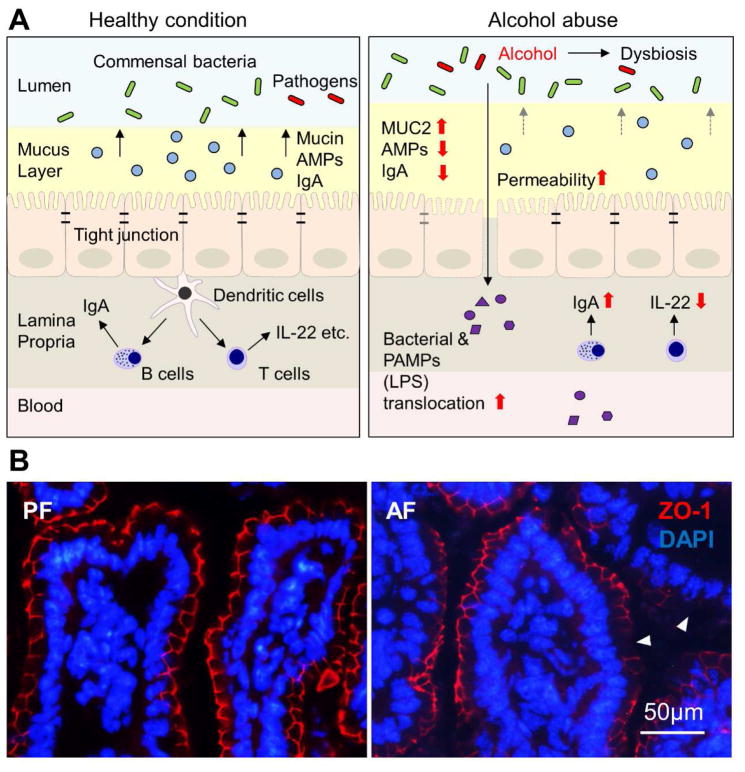
The primary function of the gastrointestinal tract is to digest food and absorb nutrients. It has the largest surface that the body exposes to the external environment, which puts the gastrointestinal tract at risk from exogenous pathogenic microorganisms such as bacteria, fungi and viruses.16–18 Therefore, another essential function of the gastrointestinal tract is to act as a barrier preventing the invasion of the circulation by microorganisms. The gut barrier is a multi-layer system, and has both physical and immune defense functions. It consists of three major components, including mucus, epithelial cells and immune cells (illustrated in Fig. 1A).19–21 While the mucus and epithelial cells act basically as physical barriers, all three layers contribute to the immune barrier function.
Fig. 1. Alcohol-induced gut barrier dysfunction.
(A) Schematic diagrams of the gut barrier under healthy and alcohol-intoxicated conditions. (B) Immunofluorescence of ileal tight junction protein ZO-1. Arrowheads indicate dissociated ZO-1. Abbreviations: AMP, antimicrobial peptide; LPS, lipopolysaccharide; PF, pair-fed; AF, alcohol-fed; ZO-1, zonula occludens-1; DAPI, 4′,6-diamidino-2-phenylindole.
2.1. Mucus layer
The first line of defense is the stratified mucus layer, which together with the glycocalyx of the epithelial cells, provides a protective spacer against physical and chemical injury caused by ingested food, microbes and microbial products.20,21 The organization of the mucus system varies markedly along the gastrointestinal tract; the small intestine has a single unattached mucus layer which limits bacteria reaching the intestinal epithelium, whereas the stomach and colon has a two-layered mucus, with inner and outer layers. The inner colonic mucus layer is dense and firmly attached to the epithelial cells, and does not allow bacterial penetration. The outer colonic mucus layer is loose and unattached, and it is the natural habitat of commensal bacteria.22
Mucus is mainly produced and secreted by goblet cells.23 The major structural component of the intestinal mucus is mucins, which are large highly glycosylated glycoproteins. The two major types of mucins can be functionally distinguished; transmembrane mucins and secreted mucins.24,25 Transmembrane mucins, including MUC1, MUC3, MUC4, MUC11–13, MUC15–17, MUC20 and MUC21, have a single membrane-spanning domain and are essential components of the glycocalyx of the mucosal surface, and are involved in intracellular signaling.24 Secreted mucins, especially gel-forming mucins, make up the skeleton of the mucus layer. In the small intestine and colon, the mucus is built of the gel-forming MUC2 mucin, compared with MUC5AC mucin in the stomach.26,27 Mucins can be considered a two-edged sword, as their normal function protects against unwanted substances and microorganism penetration while malfunction of mucins may be a causal factor in disease.28 In addition to MUC2 secretion, the intestinal goblet cells also secrete a number of other mucus components, including trefoil factor peptide 3 (TFF3), resistin-like molecule β (RELMβ), Fc-γ binding protein (FCGBP), zymogen granule protein 16 (ZG16) and calcium-activated chloride channel regulator 1 (CLCA1),20,23 all of which contribute to a highly viscous extracellular layer.
To maintain intestinal homeostasis between the host and microorganisms, a variety of biomolecules are produced and released into the mucus layer by cells other than goblet cells. A subset of these biomolecules, antibacterial peptides (AMPs), are particularly important. Intestinal AMPs are secreted by both Paneth cells and enterocytes. Paneth cells are exocrine cells located at the base of crypts of Lieberkühn, which secrete various AMPs, including lysozyme, C-type lectins, secretory phospholipase A2 (sPLA2), angiogenin 4 (Ang4) and α-defensins (HD5 and HD6 in humans and cryptdins in mice).29–31 The enterocytes do not merely provide a passive barrier function but also contribute actively by secreting AMPs.32 The major AMPs secreted by villus enterocytes include β-defensins (hBD-1, 2, 3, 4), cathelicidins (LL-37 in humans and cathelin-related antimicrobial peptide in mice) and regenerating islet-derived protein 3 β (Reg3β) and Reg3γ. These AMPs generate an antibacterial gradient in the mucus layer, and prevent microorganisms from penetrating to the epithelial cell surface.
2.2. Intestinal epithelial cells
Epithelial cells provide both a physical and immune defense barrier in the intestine. This selectively permeable barrier prohibits passage of microorganisms and toxins while permitting transport of nutrients and water.33,34 The paracellular permeability of the intestinal epithelium is controlled by an apical junction complex, composed of tight junctions, adherens junctions and desmosomes, in an apical to basal orientation. The tight junction is located at the most apical position of epithelial cells, and forms the actual seal between adjacent cells.34,35 It is the principal determinant of intestinal epithelial permeability. Adherens junctions and desmosomes provide the adhesive forces necessary for maintenance of cell-cell interactions, and prevent mechanical disruption of the epithelium.36,37 The tight junction is composed of transmembrane proteins, including occludin, claudins, junctional adhesion molecule and tricellulin, and cytoplasmic scaffold proteins, such as zonula occludens (ZO-1, ZO-2 and ZO-3).34,38 The most crucial transmembrane proteins are claudins, which define tight junction permeability. Claudins are categorized into barrier forming (claudin-1, -3, -4, -5 -8, -9, -11 and -14) and channel pore forming (claudin-2, -7, -12 and -15) subtypes. The barrier forming claudins decrease, whereas the channel pore forming claudins increase, the paracellular permeability.39 The function of occludin has not been fully elucidated thus far. Knockdown of intestinal occludin in mice increased the gut permeability to macromolecules.40 Furthermore, mice deficient in occludin develop chronic inflammation and hyperplasia, which suggests that the function of occludin is more complex than originally thought.41 ZO proteins regulate tight junction assembly and maintenance through anchoring occludin or claudins to the cytoskeleton. Disruption of the integrity of the tight junction results in dysfunction of the gut barrier and the diffusion of macromolecules from the intestinal lumen into the blood.
In addition to the barrier functions of intestinal epithelial cells and the substances they secrete as described above, the intestinal epithelial cells also perform immune surveillance and send signals to the mucosal immune system by producing cytokines, such as interleukin (IL)-1β, IL-6, IL-18, tumor necrosis factor (TNF)-α, and chemokines, including CXC-motif chemokine ligand (CXCL) 8, CXCL10, CC-motif chemokine ligand (CCL) 2, CCL6, CCL20 and CCL25.19,42,43 The primary role of the cytokines/chemokines produced is to induce immune cell migration and promote innate and adaptive immunity. Notably, a subset of chemokines, including intestinal epithelium-derived CCL6 (human homologs CCL14 and CCL15) display antimicrobial properties.44–47
2.3. Mucosal immune cells
The intestine possesses an integrated mucosal immune system. Gut-associated lymphoid tissue (GALT) is a prominent part of the mucosal-associated lymphoid tissue, and contains up to 70% of the immune cells of the whole human body.48 The GALT harbors diverse immune cells, including dendritic cells, T and B lymphocytes, plasma cells, innate lymphoid cells (ILCs), macrophages and neutrophils.49–51 While residential macrophages are responsible for phagocytosing bacteria diffused into the lamina propria,49 ILCs protect the mucosa against bacterial invasion by secreting cytokines.52–54 Of special importance are dendritic cells, which play a key role in shaping the intestinal immune response through their ability to orchestrate protective immunity and immune tolerance.55 T and B lymphocytes which reside in the lamina propria are major effector cells in adaptive immune responses that are induced and directed by dendritic cells. T cells respond to signals from the gut lumen and initiate immune responses.56,57 B cells, especially IgA-producing plasma cells, contribute to the protection of the gut barrier.58,59 Of note, immune cells also play a role in regulation of the intestinal barrier function by secreting cytokines. For example, IL-22 produced by type 3 ILCs and CD4+ T cells has been shown to stimulate AMP secretion from the intestinal epithelial cells and upregulate epithelial tight junction proteins.60,61 IL-10 secreted by regulatory T cells (Tregs) and macrophages promotes mucosal wound healing and enhances gut barrier function.62–64
3. Gut barrier dysfunction and bacterial translocation in ALD
3.1. Alcohol-induced gut hyperpermeability
Gut permeability is a term describing the control of the passage of macromolecules through the epithelium into systemic circulation.65 Macromolecules in the gut lumen are not able to penetrate into the blood when the gut barrier functions normally. However, under disease conditions, the gut barrier function is impaired or disrupted, which leads to uncontrolled passage of macromolecules. Multiple methods have been used to assess gut permeability, including in vivo assay of macromolecules penetrating from the gut to the blood, ex vivo assay of macromolecules passing through the mucosa of intestinal explants, and morphological and biochemical analysis of tight junction structure and/or proteins.66–68 The in vivo gut permeability assay in humans is usually conducted by oral administration of a pair of molecules, an indigestible large molecule (i.e., lactulose) that penetrates through the mucosa only when the gut barrier is impaired and a small molecule (i.e., mannitol) that crosses the mucosa freely regardless of gut barrier function. The degree of gut permeability is reflected by the ratio of urinary excretion of these two molecules.
Alcohol-induced gut hyperpermeability has been well documented in both clinical and experimental studies.69–73 In fact, the gut appears to be the first site of injury upon alcohol intoxication.11,73 Patients with ALD showed a significant increase in gut permeability to a variety of permeability markers, such as lactulose/mannitol, polyethylene glycol (PEG) and 51Cr-ethylenediaminetetraacetic acid (51Cr-EDTA). In a clinical study, alcoholics with chronic liver disease demonstrated a marked increase in lactulose absorption as well as in urinary lactulose/mannitol ratio compared with alcoholics with no liver disease and nonalcoholics with liver disease.15 Another clinical study tested gut permeability to PEGs in patients with ALD, and found that urinary levels of PEGs and plasma endotoxins are significantly higher in patients with ALD than those in healthy controls.74 Moreover, alcohol-induced gut hyperpermeability seems to be a persistent effect in ALD patients; elevated plasma 51Cr-EDTA could be detected in alcoholics with liver cirrhosis even after 2 weeks of abstinence, whereas it was more transient in healthy subjects and in alcoholics without cirrhosis.75
As summarized in Fig. 1A, mechanistic studies suggest that alcohol breaches intestinal integrity at multiple levels. Acute alcohol intoxication may cause histopathological alterations, such as loss of the epithelial cells at the top of intestinal villi, both in vivo and in vitro.76–78 Chronic alcohol exposure has been reported to reduce the distribution of tight junction proteins without significantly affecting intestinal histopathology in mice.70 Colon biopsies from patients with ALD exhibited a reduction of ZO-1 protein levels in comparison with normal controls.79 Animal studies demonstrated that ileal tight junction proteins, such as occludin and ZO-1 (Fig. 1B), are reduced in mice chronically fed alcohol.70 A recent study showed that deficiency of occludin exacerbates alcohol-induced gut barrier dysfunction and liver damage in mice,80 which provides direct evidence that disassembly/depletion of tight junction proteins is likely an important mechanism underlying alcohol-induced gut permeability increase. Alcohol promotes the disruption of intestinal tight junctions through multiple pathways, including induction of oxidative stress,81 elevation of microRNAs,15,79 perturbation of circadian rhythm,82 and malnutrition.70,83,84 Our group has reported that chronic alcohol exposure induces intestinal oxidative stress, which leads to zinc deficiency;70 and that zinc deficiency may sensitize alcohol-induced disassembly of tight junctions through inactivating hepatocyte nuclear factor 4α.85 Of note, alcohol metabolites, rather than alcohol itself, are believed to be more responsible for the deleterious effects caused by alcohol. Acetaldehyde, a major toxic metabolite of alcohol, is accumulated in the intestine after alcohol exposure,83,84 and has been shown to reduce tight junctions and promote leakiness of Caco-2 cells.86,87
A growing body of evidence has revealed that alcohol also affects the gut mucus layer and immune cells in addition to the epithelial junctions. The expression of MUC2 was decreased in the ileum of mice fed-alcohol for 8 weeks.88 Recovery of the alcohol-reduced Akkermansia muciniphila population, a mucin-degrading bacterium that resides in the mucus layer, enhanced mucus thickness, and ameliorated experimental ALD.89 However, another study showed that knockout of MUC2 ameliorates ALD in mice through adaptively upregulated Reg3β and Reg3γ.90 This controversy necessitates further investigation into the function of MUC2 and its role in the pathogenesis of ALD. Several studies have reported that alcoholics have increased levels of IgA.91 A recent study further showed that alcohol causes increased IgA levels in tissue homogenates and decreased IgA levels in the intestinal contents, which suggests impaired secretion of IgA.92 Loss of IgA is not sufficient to promote the development of ALD in mice due to various compensations, such as increased levels of intestinal and plasma IgM.93 Antimicrobial peptides, Reg3β and Reg3γ, are suppressed in the small intestine of mice after alcohol exposure.94,95 Moreover, alcohol damages intestinal stem cells.96 As stem cells are pivotal in intestinal cell proliferation and differentiation, dysfunction of intestinal stem cells may represent a key mechanism for alcohol-induced long-lasting damage.
3.2. Bacterial translocation in the pathogenesis of ALD
Bacterial translocation is the invasion of viable intestinal bacteria or microbial products through the gut mucosa to extraintestinal sites, such as the mesenteric lymph nodes, liver, spleen, and bloodstream. Translocation of these pathogens to the liver elicits an inflammatory cascade, oxidative stress, and consequently liver damage. One of the most well studied phenomena is endotoxemia in ALD. Elevated blood LPS levels are found both in ALD patients and animal models of ALD.6–9 It has been reported that endotoxemia and gut barrier dysfunction are early events prior to the development of ALD,73 and persist through to advanced stages of alcoholic cirrhosis.97 The blood endotoxin levels correlate well with the severity of ALD and TNF-α levels,98 and are higher in alcoholic cirrhosis compared with other stages of ALD.97 Our previous work showed that orally administered LPS can be detected in the plasma of alcohol-intoxicated mice but not in the control mice,76 which provides direct evidence indicating that alcohol increases gut permeability to endotoxin. Gut permeability assays have shown that alcohol intoxication increases permeability of the duodenum,99 ileum,70 proximal colon100,101 and distal colon101 to macromolecules.
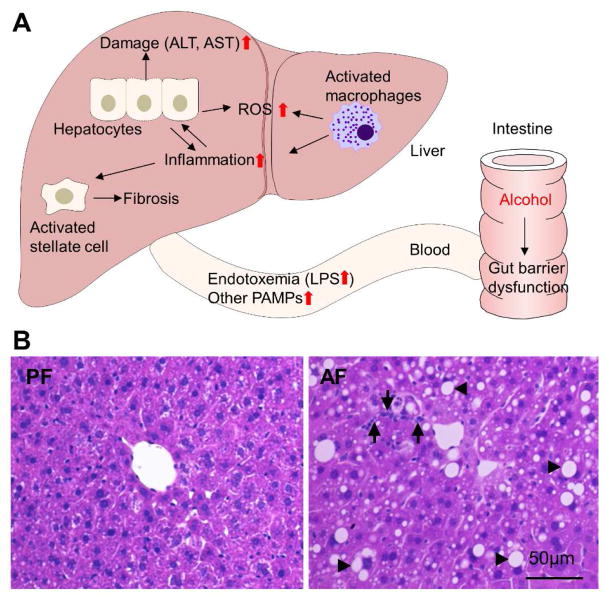
LPS in the systemic circulation activates hepatic Kupffer cells via Toll-like receptor 4 (TLR4) to produce inflammatory cytokines and chemokines which, in turn, attract neutrophils and monocytes to the liver.102 Engagement of TLR4 signaling in the liver has been repeatedly reported in both clinical and experimental studies of ALD. TLR4 is essential for the progression of alcohol-induced hepatic steatosis, inflammation and fibrosis.103 Mice deficient in TLR4, CD14 or LPS binding protein that have a perturbed LPS-receptor complex are protected from alcohol-induced liver injury.104–107 Moreover, the plasma endotoxin levels are comparable between TLR4 knockout and wild type mice,105 which suggests that TLR4 signaling is not involved in the modulation of gut permeability. Fig. 2 summarizes the process of alcohol-induced bacterial translocation in the pathogenesis of ALD at the gut-liver axis.
Fig. 2. Gut-liver axis in the development of alcoholic liver disease.
(A) Schematic diagram of pathological alterations at the gut-liver axis after alcohol consumption. (B) Hematoxylin and eosin staining of the liver. Alcohol causes hepatic lipid accumulation (arrowheads) and inflammatory cell infiltration (arrows). Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; ROS, reactive oxygen species; PAMP, pathogen-associated molecular pattern.; PF, pair-fed; AF, alcohol-fed.
In addition to LPS, multiple microbial products can also translocate from the intestine to other organs after alcohol intoxication, and play a critical role in ALD progression. Bacterial DNA was elevated in the plasma of ALD patients.108 Acute alcohol binge drinking resulted in increased bacterial 16S ribosomal DNA in correlation with serum LPS levels in healthy human volunteers.109 Bacterial DNA is recognized by TLR9 and sensitizes to LPS-induced liver injury.110 Peptidoglycan, a component of gram-positive bacteria, is detected in human ALD patients,111 and injected peptidoglycan deteriorated liver injury and inflammation in alcohol-fed mice.13
3.3. Intestinal dysbiosis and ALD
The intestinal commensal bacteria plays a major role in regulating the host immune response and maintaining the integrity of the gut mucosa.112 Alcohol decreases short chain fatty acids (SCFAs) and branched chain amino acids, which are a source of nutrition for microbes, in the gastrointestinal tract.84 Thus alcohol may directly and/or indirectly alter the composition of gut microbiota. Indeed, quantitative (bacterial overgrowth) and qualitative changes of the gut microbiome have been reported in ALD.94,113 This review will only focus on the pathological relationship between intestinal dysbiosis and the onset of ALD. Detailed information regarding the alcohol-perturbed microbiome has been discussed in-depth in other reviews.114–116 First, microbial-derived acetaldehyde may represent a mechanism of how microbiota participate in the development of ALD. As mentioned, acetaldehyde is known to disrupt the gut barrier through disassembling tight junctions.86,87 Overgrowth of bacteria affects intestinal acetaldehyde levels which, in turn, elevates intestinal permeability.117 Oral administration of metronidazole to rats led to high levels of acetaldehyde in the intestinal lumen by increasing aerobic bacteria and reducing anaerobic bacteria in the intestine.118 Treatment with the antibiotic ciprofloxacin reduced colonic microbiota and prevented acetaldehyde accumulation.119 Second, alcohol induces the expansion of bacteria that may augment bacterial translocation under disease conditions. The Proteobacteria phylum includes Gram-negative bacteria, most of which are regarded as opportunistic pathogens, and is a major source of LPS. A proportional increase of Proteobacteria has been reported in ALD patients with liver cirrhosis as well as in mice chronically fed alcohol,120,121 which suggests a causal link between alcoholic dysbiosis and endotoxemia as well as hepatic inflammation. Third, intestinal microbiota may directly mediate the development of ALD. A recent study showed that mice harboring intestinal microbiota from alcoholic hepatitis patients develop more severe liver inflammation, greater gut permeability, and higher bacterial translocation.122 Another study reported that alcohol increased intestinal Enterococcus spp. and translocation of Enterococcus spp. led to hepatic inflammation and hepatocyte death.123 In addition to the aspects mentioned above, fungi, another part of the intestinal microbiome, may also mediate the pathogenesis of ALD. It has recently been reported that chronic alcohol consumption increased gut fungal populations in mice, and the subsequent translocation of fungal β-glucan induced liver inflammation.124 These studies demonstrate that dysbiosis contributes to the development of alcoholic hepatitis and necessitates further mechanistic investigation.
4. Targeting the leaking gut to treat ALD
Efforts exploring potential ALD therapies have ongoing for decades, and one of the major focuses with promising findings is on sealing the leaky gut. As emphasized above, the pathophysiology of ALD is clearly linked with alcohol-induced gut barrier dysfunction. Indeed, animal studies demonstrated that neutralizing circulating endotoxin abrogates the endotoxin signaling cascade, and thereby attenuates alcohol-induced hepatic cytokine production, inflammatory cell infiltration and liver damage.125 In contrast, knockout of either Reg3β or Reg3γ enhances bacterial translocation and promotes ALD progression in mice.95 Therefore, further discussion on potential ALD therapies targeting alcohol-induced gut barrier dysfunction is merited. The discussion will focus on microbiota-based and nutrient-based treatments.
4.1. Microbiota-based treatments
4.1.1. Antibiotics
Experimental and pre-clinical studies indicate that treatment with antibiotics reduces Gram-negative bacteria and prevents ALD.126–128 In a preliminary study involving a small number of ALD patients, administration of antibiotics (norfloxacin and neomycin) led to an improvement of the Child-Pugh score after three and six months of treatment.129 However, due to the fear of antibiotic resistance and possible hepatic side effects, further studies investigating antibiotic treatment in patients with ALD are lacking. Rifaximin, a nonabsorbable antibiotic with broad spectrum antimicrobial activity, represents an alternative in treating ALD. Indeed, rifaximin is found to reduce endotoxemia resulting from intestinal decontamination and improves not only the prognosis of patients but also cirrhosis-related thrombocytopenia.130–132
4.1.2. Prebiotics
In contrast to antibiotics, which kill or inhibit the growth of bacteria, the concept of using prebiotics and/or probiotics is to restore gut microbiota symbiosis, and warrants study as a potential treatment for ALD. Prebiotics are indigestible dietary polysaccharides that can only be digested by commensal microbiota, such as Bifidobacteria and Lactobacilli, to promote the growth of a subset of gut microbiota.133 Dietary supplementation with oats mitigated alcohol-induced liver damage by improving gut permeability and reducing endotoxemia in rats.134 In a separate study utilizing mice, administration of prebiotic fructooligosaccharides restored the host antimicrobial peptide Reg3γ, reduced bacterial overgrowth, and ameliorated steatohepatitis caused by alcohol.94 Intake of lactulose by cirrhotic patients was reported to be effective in treating subclinical hepatic encephalopathy.135 However, studies that explore the efficacy of prebiotics in ALD patients are somewhat limited.
4.1.3. Probiotics
Probiotics are live bacteria that are benign for the host, especially in regard to gut homeostasis. Studies of probiotic administration to ALD patients or rodents with ALD suggest that probiotics may improve the prognosis of ALD (summarized in Table 1). The first report was the study by Nanji et al.,136 which showed that Lactobacillus GG supplementation reduced alcohol-induced endotoxemia and liver injury in rats. Since then, a number of probiotics, mainly Lactobacillus spp. and Bifidobacteria spp., have been tested in the context of ALD. A short-term 5-day therapy with Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 to ALD patients lowered plasma alanine aminotransferase and aspartate aminotransferase levels, restored the gut microbiota, and improved ALD compared with patients treated with standard therapy (abstinence plus vitamins) alone.137 Intake of Lactobacillus subtilis and Streptococcus faecium for 7 days reduced LPS levels and the severity of liver damage in alcoholic hepatitis patients.138 In another study, Lactobacillus casei Shirota administration three times daily for 4 weeks reestablished microbiota balance and restored neutrophil phagocytic capacity in alcoholic cirrhotic patients.139 In addition, a long-term study administering the probiotic VSL#3 for up to three months showed significantly reduced oxidative stress and cytokine production and improved liver function in patients with ALD.140
Table 1.
Studies exploring the protective effects of probiotics/prebiotics against alcoholic liver disease.
| Probiotic/Prebiotic | Subjects | Duration of treatment | Outcome | Reference | Year |
|---|---|---|---|---|---|
| Probiotics | |||||
| Lactobacillus rhamnosus GG | Male Wistar rats | 1 month | Probiotic feeding reduced alcohol-induced endotoxemia and liver injury. | Nanji et al.136 | 1994 |
| A mixture containing 450 billion bacteria (VSL #3) | Alcoholic cirrhosis patients | 3 months | Treatment of probiotic lowered plasma levels of cytokines and oxidative stress parameters. | Loguercio et al.140 | 2005 |
| L. casei Shirota | Alcoholic cirrhosis patients | 4 weeks | Probiotic supplementation restored neutrophil phagocytic capacity. | Stadlbauer et al.139 | 2008 |
| Heat-killed L. brevis SBC8803 | C57BL/6N mice | 35 days | L. brevis SBC8803 ameliorated alcohol- induced liver injury and fatty liver. | Segawa et al.182 | 2008 |
| Bifidobacterium bifidum and L. plantarum 8PA3 | Male Russian adults | 5 days | Patients treated with probiotics had significantly lower ALT and AST activity, and restored gut microbiota compared with patients treated with standard therapy alone. | Kirpich et al.137 | 2008 |
| L. rhamnosus GG | Male Sprague-Dawley rats | 10 weeks | L. rhamnosus GG reduced alcohol-induced gut leakiness and attenuated alcohol-induced oxidative stress and inflammation both in the intestine and liver. | Forsyth et al.72 | 2009 |
| L. rhamnosus GG | Male C57BL/6N mice | Last 2 weeks of the 8-week feeding | L. rhamnosus GG supplementation reduced alcohol-induced endotoxemia and hepatic steatosis. | Wang et al.141,183 | 2011, 2013 |
| L. paracasei | Male Fischer 344 rats | 10 weeks | L. paracasei altered the fatty acid composition of the plasma and liver. | Komatsuzaki et al.184 | 2012 |
| L. rhamnosus GG culture supernatant | Male C57BL/6N mice | 5 days | Bacteria-free L. rhamnosus GG culture supernatant ameliorated acute alcohol-induced gut leakiness and liver injury. | Wang et al.185 | 2012 |
| Male Sprague-Dawley rats | Up to 8 weeks | Probiotic administration reduced plasma elevated-endotoxin levels caused by alcohol and altered gut microbiota. | Zhang et al.186 | 2012 | |
| Live or heat-killed VSL #3 | Male rats | Up to 12 hours | VSL #3 administration reduced plasma endotoxin levels and cytokine production caused by alcohol exposure. | Chang et al.144 | 2013 |
| Heat-killed L. casei MYL01 | HepG2 cells | 20 hours | L. casei MYL01 modulated proinflammatory cytokine production. | Chiu et al.187 | 2014 |
| Escherichia coli Nissle 1917 secreting pyrroloquinoline quinone | Male Foster rats | 10 weeks | Probiotic treatment ameliorated alcohol- induced oxidative damage and hyperlipidemia in rats | Singh et al.188 | 2014 |
| Lactobacillus subtilis/Streptococcus faecium | Patients with alcoholic hepatitis | 7 days | Oral supplementation with L. subtilis/S. faecium reduced E. coli in stool, and decreased the levels of LPS and TNF-α. | Han et al.138 | 2015 |
| L. rhamnosus GG | C57BL/6 mice | 11 days | L. rhamnosus GG upregulated ileal tight junction proteins, decreased E. coli protein in the liver, and balanced Treg and Th17 cells in peripheral blood. | Chen et al.143 | 2016 |
| Prebiotics | |||||
| L. rhamnosus GG or oats | Male Sprague-Dawley rats | 10 weeks | Supplementation with L. rhamnosus GG or oats prevented alcohol-induced altered colonic mucosa-associated microbiota composition in rats. | Mutlu et al.189 | 2009 |
| Fructooligosaccharides | Male C57BL/6J mice | 3 weeks | Administration of fructooligosaccharides to alcohol-fed mice reduced bacterial overgrowth and ameliorated alcoholic steatohepatitis through partially restoring the host antimicrobial protein Reg3γ. | Yan et al.94 | 2011 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; LPS, lipopolysaccharide; TNF-α, tumour necrosis factor-alpha; Treg cell, regulatory T cell; Th 17 cell, T-helper 17 cell.
Several potential mechanisms through which probiotics improve ALD symptoms have been proposed. Administration of probiotics can enhance liver function by reducing endotoxin levels, decreasing oxidative damage,140 and improving immune response to enteric pathogens.139 Lactobacillus GG supplementation in mice has been shown to increase intestinal tight junction expression, prevent gut leakiness, balance Treg and T-helper (Th) 17 cells, and attenuate hepatic TNF-α production as well as oxidative stress.72,141–143 Notably, the beneficial effects of probiotics are achieved not only by live bacteria, but also by heat-inactivated bacteria or bacteria culture supernatant.144 Importantly, a study employing 16S ribosome RNA sequencing revealed that Lactobacillus GG not only reduced bacterial overgrowth in alcohol-fed mice, but also prevented alcohol-induced expansion of the Proteobacteria and Actinobacteria phyla, which implicates the capacity of Lactobacillus GG in orchestrating gut microbiota symbiosis.121 A recent study reported that Akkermansia muciniphila, a Gram-negative commensal bacterium, is diminished both in mice and humans with ALD, and oral supplementation of A. muciniphila promotes intestinal barrier integrity and ameliorates experimental ALD.89
Synbiotics, combinations containing both probiotics and prebiotics, represent another promising microbiota-based treatment for ALD. A recent study reported that Lactobacillus plantarum and a prebiotic for Lactobacillus plantarum, epigallocatechin gallate, synergistically reversed alcohol-induced endotoxemia and liver injury in rats.145 Further pre-clinical and mechanistic investigations are needed to confirm the potency of synbiotics in ALD treatment.
4.1.4. Fecal microbiota transplant (FMT)
FMT is the process of transplantation of fecal material, which contains bacteria from a healthy individual, to a recipient.146 It is an effective therapeutic approach addressing various conditions characterized by microbiota dysbiosis, such as ulcerative colitis.147 The mechanism of FMT may involve the establishment of beneficial bacterial strains and production of antimicrobial components.146 The first attempt utilizing FMT in treating ALD has just been published recently. In the study, feces from alcohol-resistant donor mice were transplanted to alcohol-sensitive recipient mice three times a week for three weeks, which resulted in prevention of alcohol-induced gut dyshomeostasis and hepatic steatohepatitis.148
4.2. Nutrient-based treatments
Alcohol abuse is often associated with malnutrition.149,150 Dietary supplementation is therefore a potent strategy for treating ALD. Related studies have shown that nutrient-based treatments not only improve the function of the liver per se, but also impact on the gut-liver axis through regulating the gut barrier function.
4.2.1. Zinc
Zinc is the second most abundant trace element in the body.151 It plays a vital role in maintaining physiological processes, such as metabolism, signaling transduction, and cell growth and differentiation.152 Hypozincemia (low serum zinc levels) and reduction of hepatic zinc levels have long been observed in patients with ALD, and serum zinc levels correlate positively with the severity of liver damage.153,154 In a mouse model of ALD, acquired zinc deficiency occurs as early as after two weeks of alcohol feeding.155 Our group has reported that in addition to serum and hepatic zinc decreases, alcohol-induced zinc deficiency could also be detected in the intestine, especially in the distal small intestine or ileum, which exacerbates gut barrier dysfunction caused by alcohol.70 We also found that dietary zinc deficiency exaggerates alcohol-induced endotoxemia and liver damage.69 These observations suggest an important role of zinc in regulating gut barrier function, and the potency and necessity of zinc supplementation in treating ALD.
Zinc supplementation has been shown to tighten the leaky gut under a variety of disease conditions. Patients with Crohn’s disease showed improved gut permeability after oral zinc sulfate supplementation.156 Evaluation of the tight junction ultrastucture by electron microscopy demonstrated that dietary zinc supplementation in rats with colitis reduced the number of opened tight junction complexes in the colon epithelium.157 The effect of zinc treatment on alcohol-induced leaky gut has been tested in both acute and chronic rodent models of ALD. In an acute model of alcohol intoxication, mice were treated with three doses of ZnSO4 at 5 mg elemental zinc/kg in a 12-h interval prior to an oral dose of alcohol. Alcohol intoxication elevated plasma endotoxin levels and caused pathological liver changes, which were abrogated by zinc pretreatment.76 Dietary zinc supplementation also attenuated alcohol-increased ileal permeability, restored the distribution of tight junction proteins, and mitigated hepatic endotoxin signaling in rats.158 Therefore, zinc prevention of increased intestinal permeability contributes to the beneficial effects of zinc on alcohol-induced liver damage.
4.2.2. Niacin
Niacin, also known as nicotinic acid, is a naturally occurring B3 vitamin. A well-established role for niacin is as a broad-spectrum hypolipidemic drug.159,160 It also exhibits potent antioxidant and anti-inflammatory properties.161,162 Niacin is the precursor of nicotinamide adenine dinucleotide (NAD+), which plays a crucial role in energy metabolism, including ethanol/acetaldehyde clearance. Chronic alcohol abuse causes niacin deficiency in human, termed pellagra,163 which may further worsen alcohol metabolism-perturbed redox imbalance. Supplementation with niacin has been reported to attenuate hepatic oxidative stress and reverse indices of ALD in mice.161 Studies by our group showed that niacin increases hepatic fatty acid oxidation and decreases de novo lipogenesis in rats.164 Dietary supplementation with niacin also reversed alcoholic endotoxemia, and upregulated the expression of tight junction proteins, especially claudins.83 Interestingly, niacin significantly lowered endotoxin and acetaldehyde levels in the intestinal lumen, which indicates a direct impact of niacin on gut microbiota.83
An intimate interrelationship between niacin and zinc has been suggested for decades. Zinc participates in the regulation of niacin metabolism,165 and zinc deficiency is associated with increased histidine oxidation and consequently pellagra,166 whereas zinc supplementation is capable of increasing NAD+ concentrations.165 In contrast, niacin may enhance zinc absorption and utilization.167 Therefore, a possible synergistic effect of niacin and zinc should be taken into account when interpreting data generated by supplementation of either alone, and a combination of niacin and zinc may represent another possible therapy in ALD.
4.2.3. Fatty acids
Fatty acids differ in length of carbon chain (e.g., short, medium and long chain fatty acids) and degree of saturation (e.g., saturated, monounsaturated and polyunsaturated fatty acids). There are several lines of evidence suggesting that different types of dietary fatty acids may mitigate alcohol-induced intestinal barrier dysfunction, endotoxemia and liver injury.
Short-chain fatty acids (SCFAs) are fatty acids with fewer than six carbon atoms, and are produced by bacterial fermentation.168 They are the main source of energy for colonic cells. Our group has reported that alcohol exposure dramatically decreased intestinal luminal levels of all SCFAs, except for acetic acid, in rats fed alcohol for eight weeks.84 Supplementation with butyrate (tributyrin) mitigated alcohol-induced gut barrier disruption and liver injury both in acute and chronic alcohol-intoxicated mice.169,170
The fatty acids in medium chain triglycerides (MCTs) are mainly saturated with carbon chain lengths ranging from 6–12. Studies have demonstrated that dietary MCTs improved alcohol-induced liver histological changes through multiple mechanisms involving both the liver and the intestine.88,171–173 Kirpich and colleagues examined the therapeutic intervention against ALD using a diet enriched in saturated fats (MCTs: beef tallow) in mice. They reported that the saturated fat-enriched diet improves intestinal tight junction expression,173 alleviates intestinal inflammation,88 enhances MUC2 expression,88 and modulates gut microbiome and metabolome in mice intoxicated by alcohol.172 In a separate study using rats, a MCT-enriched diet normalized alcohol-reduced intestinal tight junction proteins, occludin and ZO-1, prevented endotoxemia, and alleviated hepatic LPS signaling.174
In humans and mice, alcohol abuse decreases the capacity of the microbiome to synthesize saturated long chain fatty acids (LCFAs), and reduces the proportion of Lactobacillus, a strain of bacteria known to metabolize saturated LCFAs.175 Administration of saturated LCFAs to alcohol-fed mice increased Lactobacillus spp., enhanced gut barrier function, and reduced intestinal inflammation and liver injury. These findings indicate that alterations in bacterial metabolism contribute to the pathogenesis of ALD.175 The protective roles of saturated LCFAs in the intestine may also involve stimulating release of gut hormones, including glucagon-like peptide (GLP)-1 and GLP-2,176 regulating the production of MUC2 by goblet cells,177 and enhancing antimicrobial activity.178 Future studies are required to explore whether these mechanisms are involved in saturated LCFAs-mediated protection against ALD.
Although dietary interventions are the focus of the present review, other methods of targeting the leaky gut in ALD should not be ignored. For example, several reports have described the protective role of IL-22 in treating ALD in rodents.179–181 In a mouse model of alcohol plus burn injury, alcohol augmented burn-induced IL-22 reduction and gut permeability increase; administration of IL-22 prevented these deleterious effects.179 The same group further demonstrated that the IL-22 induced protection is through signal transducer and transcription activator 3-mediated upregulation of AMPs expression and reduction of gut Enterobacteriaceae.181
5. Conclusion
The significance of the gut-liver axis in the pathogenesis of ALD has been widely accepted, although the mechanisms remain largely unknown. Based on the observation that increased gut permeability is a leading cause of alcohol-induced endotoxemia and liver damage, modulation of the gut barrier seems to be a promising strategy. Investigations targeting the gut barrier have been conducted in pre-clinical and clinical settings to understand the relationships between alcohol, the gut barrier and liver damage. The long-term clinical benefits and safety of these treatments should be evaluated in a clinical setting involving ALD patients. Recent studies suggest that gut commensal bacteria play a pivotal role in shaping both the host immune response and the integrity of the gut barrier. Exploration of specific microbial strains that contribute or even control the progression of alcohol-disrupted barrier function as well as the underlying mechanism is challenging, but could provide potential new avenues of research for a better understanding of ALD progression.
Acknowledgments
This work was supported by the USA National Institutes of Health (R01AA020212 and R01AA018844).
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowe IA. Lessons from epidemiology: the burden of liver disease. Dig Dis. 2017;35:304–309. doi: 10.1159/000456580. [DOI] [PubMed] [Google Scholar]
- 3.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 4.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt W, Popham RE. Alcohol consumption and ischemic heart disease: some evidence from population studies. Br J Addict. 1981;76:407–417. doi: 10.1111/j.1360-0443.1981.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 7.Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 8.Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841–849. [PMC free article] [PubMed] [Google Scholar]
- 9.Thurman RG, Bradford BU, Iimuro Y, et al. Role of Kupffer cells, endotoxin and free radicals in hepatotoxicity due to prolonged alcohol consumption: studies in female and male rats. J Nutr. 1997;127:903S–906S. doi: 10.1093/jn/127.5.903S. [DOI] [PubMed] [Google Scholar]
- 10.Fujita T. Endotoxemia in alcoholic liver disease. Hepatology. 2009;50:1319. doi: 10.1002/hep.23234. [DOI] [PubMed] [Google Scholar]
- 11.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massonnet B, Delwail A, Ayrault JM, Chagneau-Derrode C, Lecron JC, Silvain C. Increased immunoglobulin A in alcoholic liver cirrhosis: exploring the response of B cells to Toll-like receptor 9 activation. Clin Exp Immunol. 2009;158:115–124. doi: 10.1111/j.1365-2249.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustot T, Lemmers A, Moreno C, et al. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- 14.Stadlbauer V, Mookerjee RP, Wright GA, et al. Role of Toll-like receptors 2, 4, and 9 in mediating neutrophil dysfunction in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G15–G22. doi: 10.1152/ajpgi.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 16.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 17.König J, Wells J, Cani PD, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017;49:e338. doi: 10.1038/emm.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelaseyed T, Bergström JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8–20. doi: 10.1111/imr.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurashima Y, Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 2017;35:119–147. doi: 10.1146/annurev-immunol-051116-052424. [DOI] [PubMed] [Google Scholar]
- 22.Hansson GC. Role of mucus layers in gut infection and inflammation. Curr Opin Microbiol. 2012;15:57–62. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 25.Corfield AP. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 27.Nordman H, Davies JR, Lindell G, de Bolós C, Real F, Carlstedt I. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem J. 2002;364:191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behera SK, Praharaj AB, Dehury B, Negi S. Exploring the role and diversity of mucins in health and disease with special insight into non-communicable diseases. Glycoconj J. 2015;32:575–613. doi: 10.1007/s10719-015-9606-6. [DOI] [PubMed] [Google Scholar]
- 29.Wehkamp J, Chu H, Shen B, et al. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Lett. 2006;580:5344–5350. doi: 10.1016/j.febslet.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 30.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 31.Ayabe T, Ashida T, Kohgo Y, Kono T. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 2004;12:394–398. doi: 10.1016/j.tim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Wassing GM, Bergman P, Lindbom L, van der Does AM. Complexity of antimicrobial peptide regulation during pathogen-host interactions. Int J Antimicrob Agents. 2015;45:447–454. doi: 10.1016/j.ijantimicag.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 33.France MM, Turner JR. The mucosal barrier at a glance. J Cell Sci. 2017;130:307–314. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 38.Wells JM, Brummer RJ, Derrien M, et al. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Sadi R, Khatib K, Guo S, Ye D, Youssef M, Ma T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol. 2002;16:241–246. doi: 10.1155/2002/941087. [DOI] [PubMed] [Google Scholar]
- 43.Kagnoff MF. The intestinal epithelium is an integral component of a communications network. J Clin Invest. 2014;124:2841–2843. doi: 10.1172/JCI75225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotarsky K, Sitnik KM, Stenstad H, et al. A novel role for constitutively expressed epithelial-derived chemokines as antibacterial peptides in the intestinal mucosa. Mucosal immunol. 2010;3:40–48. doi: 10.1038/mi.2009.115. [DOI] [PubMed] [Google Scholar]
- 45.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 46.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 47.Eliasson M, Egesten A. Antibacterial chemokines--actors in both innate and adaptive immunity. Contrib Microbiol. 2008;15:101–117. doi: 10.1159/000136317. [DOI] [PubMed] [Google Scholar]
- 48.Vighi G, Marcucci F, Sensi L, Di Cara G, Frati F. Allergy and the gastrointestinal system. Clin Exp Immunol. 2008;153(Suppl 1):3–6. doi: 10.1111/j.1365-2249.2008.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall CHT, Campbell EL, Colgan SP. Neutrophils as components of mucosal homeostasis. Cell Mol Gastroenterol Hepatol. 2017;4:329–437. doi: 10.1016/j.jcmgh.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14:143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 52.Moro K, Koyasu S. Innate lymphoid cells, possible interaction with microbiota. Semin Immunopathol. 2015;37:27–37. doi: 10.1007/s00281-014-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17:765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 54.McKenzie AN, Spits H, Eberl G. Innate lymphoid cells in inflammation and immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 56.Lui JB, Devarajan P, Teplicki SA, Chen Z. Cross-differentiation from the CD8 lineage to CD4 T cells in the gut-associated microenvironment with a nonessential role of microbiota. Cell Rep. 2015;10:574–585. doi: 10.1016/j.celrep.2014.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muñoz M, Heimesaat MM, Danker K, et al. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009;206:3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann N Y Acad Sci. 2012;1247:97–116. doi: 10.1111/j.1749-6632.2011.06378.x. [DOI] [PubMed] [Google Scholar]
- 59.Carasi P, Racedo SM, Jacquot C, Romanin DE, Serradell MA, Urdaci MC. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J Immunol Res. 2015;2015:361604. doi: 10.1155/2015/361604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34:122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quiros M, Nishio H, Neumann PA, et al. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest. 2017;127:3510–3520. doi: 10.1172/JCI90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Yang H, Nose K, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008;294:G139–G347. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 65.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. 2015;421:44–53. doi: 10.1016/j.jim.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sequeira IR, Lentle RG, Kruger MC, Hurst RD. Assessment of the effect of intestinal permeability probes (lactulose and mannitol) and other liquids on digesta residence times in various segments of the gut Determined by wireless motility capsule: a randomised controlled trial. PloS One. 2015;10:e0143690. doi: 10.1371/journal.pone.0143690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra A, Makharia GK. Techniques of functional and motility test: how to perform and interpret intestinal permeability. J Neurogastroenterol Motil. 2012;18:443–447. doi: 10.5056/jnm.2012.18.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PloS One. 2013;8:e76522. doi: 10.1371/journal.pone.0076522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Enomoto N, Takei Y, Hirose M, et al. Thalidomide prevents alcoholic liver injury in rats through suppression of Kupffer cell sensitization and TNF-alpha production. Gastroenterology. 2002;123:291–300. doi: 10.1053/gast.2002.34161. [DOI] [PubMed] [Google Scholar]
- 72.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–172. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keshavarzian A, Farhadi A, Forsyth CB, et al. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 75.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 76.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- 77.Croft DN. Cell turnover and loss and the gastric mucosal barrier. Am J Dig Dis. 1977;22:383–386. doi: 10.1007/BF01072198. [DOI] [PubMed] [Google Scholar]
- 78.Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol Clin Exp Res. 2014;38:2217–2224. doi: 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang Y, Banan A, Forsyth CB, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 80.Mir H, Meena AS, Chaudhry KK, et al. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim Biophys Acta. 2016;1860:765–774. doi: 10.1016/j.bbagen.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- 82.Forsyth CB, Voigt RM, Shaikh M, et al. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhong W, Li Q, Zhang W, Sun Q, Sun X, Zhou Z. Modulation of intestinal barrier and bacterial endotoxin production contributes to the beneficial effect of nicotinic acid on alcohol-Iinduced endotoxemia and hepatic inflammation in rats. Biomolecules. 2015;5:2643–2658. doi: 10.3390/biom5042643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie G, Zhong W, Zheng X, et al. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12:3297–3306. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong W, Zhao Y, McClain CJ, Kang YJ, Zhou Z. Inactivation of hepatocyte nuclear factor-4{alpha} mediates alcohol-induced downregulation of intestinal tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2010;299:G643–G651. doi: 10.1152/ajpgi.00515.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1356–G1364. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol. 2008;447:171–183. doi: 10.1007/978-1-59745-242-7_13. [DOI] [PubMed] [Google Scholar]
- 88.Kirpich IA, Feng W, Wang Y, et al. Ethanol and dietary unsaturated fat (corn oil/linoleic acid enriched) cause intestinal inflammation and impaired intestinal barrier defense in mice chronically fed alcohol. Alcohol. 2013;47:257–264. doi: 10.1016/j.alcohol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2017 doi: 10.1136/gutjnl-2016-313432. pii:gutjnl-2016-313432. [DOI] [PubMed]
- 90.Hartmann P, Chen P, Wang HJ, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boullier S, Tanguy M, Kadaoui KA, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 92.López MC. Chronic alcohol consumption regulates the expression of poly immunoglobulin receptor (pIgR) and secretory IgA in the gut. Toxicol Appl Pharmacol. 2017;333:84–91. doi: 10.1016/j.taap.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Inamine T, Yang AM, Wang L, Lee KC, Llorente C, Schnabl B. Genetic loss of immunoglobulin A does not influence development of alcoholic steatohepatitis in mice. Alcohol Clin Exp Res. 2016;40:2604–2613. doi: 10.1111/acer.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Fouts DE, Stärkel P, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe. 2016;19:227–239. doi: 10.1016/j.chom.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu R, Voigt RM, Zhang Y, et al. Alcohol injury damages intestinal stem cells. Alcohol Clin Exp Res. 2017;41:727–734. doi: 10.1111/acer.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fujimoto M, Uemura M, Nakatani Y, et al. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 99.Sommansson A, Saudi WS, Nylander O, Sjöblom M. Melatonin inhibits alcohol-induced increases in duodenal mucosal permeability in rats in vivo. Am J Physiol Gastrointest Liver Physiol. 2013;305:G95–G105. doi: 10.1152/ajpgi.00074.2013. [DOI] [PubMed] [Google Scholar]
- 100.Summa KC, Voigt RM, Forsyth CB, et al. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PloS One. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chaudhry KK, Samak G, Shukla PK, et al. ALDH2 deficiency promotes ethanol-induced gut barrier dysfunction and fatty liver in mice. Alcohol Clin Exp Res. 2015;39:1465–1475. doi: 10.1111/acer.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Enomoto N, Ikejima K, Bradford BU, et al. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15(Suppl):D20–D25. doi: 10.1046/j.1440-1746.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 105.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 106.Uesugi T, Froh M, Arteel GE, et al. Role of lipopolysaccharide-binding protein in early alcohol-induced liver injury in mice. J Immunol. 2002;168:2963–2969. doi: 10.4049/jimmunol.168.6.2963. [DOI] [PubMed] [Google Scholar]
- 107.Yin M, Bradford BU, Wheeler MD, et al. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 108.Francés R, Benlloch S, Zapater P, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484–491. doi: 10.1002/hep.20055. [DOI] [PubMed] [Google Scholar]
- 109.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PloS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romics L, Jr, Dolganiuc A, Kodys K, et al. Selective priming to Toll-like receptor 4 (TLR4), not TLR2, ligands by P. acnes involves up-regulation of MD-2 in mice. Hepatology. 2004;40:555–564. doi: 10.1002/hep.20350. [DOI] [PubMed] [Google Scholar]
- 111.Leclercq S, De Saeger C, Delzenne N, de Timary P, Stärkel P. Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 2014;76:725–733. doi: 10.1016/j.biopsych.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 112.Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146:1477–1488. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gabbard SL, Lacy BE, Levine GM, Crowell MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Dig Dis Sci. 2014;59:638–644. doi: 10.1007/s10620-013-2960-y. [DOI] [PubMed] [Google Scholar]
- 114.Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhong W, Zhou Z. Alterations of the gut microbiome and metabolome in alcoholic liver disease. World J Gastrointest Pathophysiol. 2014;5:514–522. doi: 10.4291/wjgp.v5.i4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Engen PA, Green SJ, Voigt RM, Forsyth CB, Keshavarzian A. The gastrointestinal microbiome: alcohol effects on the composition of intestinal microbiota. Alcohol Res. 2015;37:223–236. [PMC free article] [PubMed] [Google Scholar]
- 117.Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986;90:103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- 118.Tillonen J, Väkeväinen S, Salaspuro V, et al. Metronidazole increases intracolonic but not peripheral blood acetaldehyde in chronic ethanol-treated rats. Alcohol Clin Exp Res. 2000;24:570–575. [PubMed] [Google Scholar]
- 119.Visapää JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161–1164. [PubMed] [Google Scholar]
- 120.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bull-Otterson L, Feng W, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 123.Llorente C, Jepsen P, Inamine T, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun. 2017;8:837. doi: 10.1038/s41467-017-00796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang AM, Inamine T, Hochrath K, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017;127:2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137–1146. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 127.Lu ZQ, Li MF, Qiu QM, et al. Effect of antimicrobial agents on the toll-like receptors and inflammatory cytokines in liver tissue of the alcohol-induced liver disease in rats with Vibrio vulnificus sepsis. Chin Med J (Engl) 2009;122:1910–1916. [PubMed] [Google Scholar]
- 128.Albillos A, de Hera Ad AL, Reyes E, et al. Tumour necrosis factor-alpha expression by activated monocytes and altered T-cell homeostasis in ascitic alcoholic cirrhosis: amelioration with norfloxacin. J Hepatol. 2004;40:624–631. doi: 10.1016/j.jhep.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 129.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251–1255. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- 130.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 131.Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin improves thrombocytopenia in patients with alcoholic cirrhosis in association with reduction of endotoxaemia. Liver Int. 2012;32:467–475. doi: 10.1111/j.1478-3231.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 132.Kalambokis GN, Mouzaki A, Rodi M, et al. Rifaximin improves systemic hemodynamics and renal function in patients with alcohol-related cirrhosis and ascites. Clin Gastroenterol Hepatol. 2012;10:815–818. doi: 10.1016/j.cgh.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 133.Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 134.Keshavarzian A, Choudhary S, Holmes EW, et al. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- 135.Watanabe A, Sakai T, Sato S, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26:1410–1414. doi: 10.1053/jhep.1997.v26.pm0009397979. [DOI] [PubMed] [Google Scholar]
- 136.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 137.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Han SH, Suk KT, Kim DJ, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27:1300–1306. doi: 10.1097/MEG.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 139.Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 140.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Liu Y, Kirpich I, et al. Lactobacillus rhamnosus GG reduces hepatic TNFα production and inflammation in chronic alcohol-induced liver injury. J Nutr Biochem. 2013;24:1609–1615. doi: 10.1016/j.jnutbio.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhao H, Zhao C, Dong Y, et al. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234:194–200. doi: 10.1016/j.toxlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 143.Chen RC, Xu LM, Du SJ, et al. Lactobacillus rhamnosus GG supernatant promotes intestinal barrier function, balances Treg and TH17 cells and ameliorates hepatic injury in a mouse model of chronic-binge alcohol feeding. Toxicol Lett. 2016;241:103–110. doi: 10.1016/j.toxlet.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 144.Chang B, Sang L, Wang Y, Tong J, Zhang D, Wang B. The protective effect of VSL#3 on intestinal permeability in a rat model of alcoholic intestinal injury. BMC Gastroenterol. 2013;13:151. doi: 10.1186/1471-230X-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rishi P, Arora S, Kaur UJ, Chopra K, Kaur IP. Better management of alcohol liver disease using a ‘microstructured synbox’ system comprising L. plantarum and EGCG. PloS One. 2017;12:e0168459. doi: 10.1371/journal.pone.0168459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borody TJ, Campbell J. Fecal microbiota transplantation: techniques, applications, and issues. Gastroenterol Clin North Am. 2012;41:781–803. doi: 10.1016/j.gtc.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 147.Tian Z, Liu J, Liao M, et al. Beneficial Effects of Fecal Microbiota Transplantation on Ulcerative Colitis in Mice. Dig Dis Sci. 2016;61:2262–2271. doi: 10.1007/s10620-016-4060-2. [DOI] [PubMed] [Google Scholar]
- 148.Ferrere G, Wrzosek L, Cailleux F, et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017;66:806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 149.Ross LJ, Wilson M, Banks M, Rezannah F, Daglish M. Prevalence of malnutrition and nutritional risk factors in patients undergoing alcohol and drug treatment. Nutrition. 2012;28:738–743. doi: 10.1016/j.nut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 150.Lieber CS. Alcohol, malnutrition and liver disease. J Fla Med Assoc. 1979;66:463–465. [PubMed] [Google Scholar]
- 151.Zhou Z. Zinc and alcoholic liver disease. Dig Dis. 2010;28:745–750. doi: 10.1159/000324282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Krebs NE, Hambidge KM. Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals. 2001;14:397–412. doi: 10.1023/a:1012942409274. [DOI] [PubMed] [Google Scholar]
- 153.Goode HF, Kelleher J, Walker BE. Relation between zinc status and hepatic functional reserve in patients with liver disease. Gut. 1990;31:694–697. doi: 10.1136/gut.31.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodríguez-Moreno F, González-Reimers E, Santolaria-Fernández F, et al. Zinc, copper, manganese, and iron in chronic alcoholic liver disease. Alcohol. 1997;14:39–44. doi: 10.1016/s0741-8329(96)00103-6. [DOI] [PubMed] [Google Scholar]
- 155.Sun Q, Li Q, Zhong W, et al. Dysregulation of hepatic zinc transporters in a mouse model of alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2014;307:G313–G322. doi: 10.1152/ajpgi.00081.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sturniolo GC, Di Leo V, Ferronato A, D’Odorico A, D’Incà R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 157.Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- 158.Zhong W, Li Q, Sun Q, et al. Preventing gut leakiness and endotoxemia contributes to the protective effect of zinc on alcohol-induced steatohepatitis in rats. J Nutr. 2015;145:2690–2698. doi: 10.3945/jn.115.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol. 2008;101:20B–26B. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 160.Karpe F, Frayn KN. The nicotinic acid receptor--a new mechanism for an old drug. Lancet. 2004;363:1892–1894. doi: 10.1016/S0140-6736(04)16359-9. [DOI] [PubMed] [Google Scholar]
- 161.Dou X, Shen C, Wang Z, Li S, Zhang X, Song Z. Protection of nicotinic acid against oxidative stress-induced cell death in hepatocytes contributes to its beneficial effect on alcohol-induced liver injury in mice. J Nutr Biochem. 2013;24:1520–1528. doi: 10.1016/j.jnutbio.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Godin AM, Ferreira WC, Rocha LT, et al. Nicotinic acid induces antinociceptive and anti-inflammatory effects in different experimental models. Pharmacol Biochem Behav. 2012;101:493–498. doi: 10.1016/j.pbb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 163.Badawy AA. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–250. doi: 10.1093/alcalc/agu010. [DOI] [PubMed] [Google Scholar]
- 164.Li Q, Xie G, Zhang W, et al. Dietary nicotinic acid supplementation ameliorates chronic alcohol-induced fatty liver in rats. Alcohol Clin Exp Res. 2014;38:1982–1992. doi: 10.1111/acer.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mocchegiani E, Malavolta M, Muti E, et al. Zinc, metallothioneins and longevity: interrelationships with niacin and selenium. Curr Pharm Des. 2008;14:2719–2732. doi: 10.2174/138161208786264188. [DOI] [PubMed] [Google Scholar]
- 166.Hsu JM, Rubenstein B. Effect of zinc deficiency on histidine metabolism in rats. J Nutr. 1982;112:461–467. doi: 10.1093/jn/112.3.461. [DOI] [PubMed] [Google Scholar]
- 167.Agte VV, Paknikar KM, Chiplonkar SA. Effect of nicotinic acid on zinc and iron metabolism. Biometals. 1997;10:271–276. doi: 10.1023/a:1018368231716. [DOI] [PubMed] [Google Scholar]
- 168.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 169.Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res. 2014;38:1489–1501. doi: 10.1111/acer.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol. 2017;32:1587–1597. doi: 10.1111/jgh.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:638–644. [PubMed] [Google Scholar]
- 172.Kirpich IA, Petrosino J, Ajami N, et al. Saturated and unsaturated dietary fats differentially modulate ethanol-induced changes in gut microbiome and metabolome in a mouse model of alcoholic liver disease. Am J Pathol. 2016;186:765–776. doi: 10.1016/j.ajpath.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kirpich IA, Feng W, Wang Y, et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhong W, Li Q, Xie G, et al. Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats. Am J Physiol Gastrointest Liver Physiol. 2013;305:G919–G932. doi: 10.1152/ajpgi.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen P, Torralba M, Tan J, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015;148:203–214e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voortman T, Hendriks HF, Witkamp RF, Wortelboer HM. Effects of long- and short-chain fatty acids on the release of gastrointestinal hormones using an ex vivo porcine intestinal tissue model. J Agric Food Chem. 2012;60:9035–9042. doi: 10.1021/jf2045697. [DOI] [PubMed] [Google Scholar]
- 177.Benoit B, Bruno J, Kayal F, et al. Saturated and unsaturated fatty acids differently Modulate colonic goblet cells in vitro and in rat pups. J Nutr. 2015;145:1754–1762. doi: 10.3945/jn.115.211441. [DOI] [PubMed] [Google Scholar]
- 178.Galbraith H, Miller TB, Paton AM, Thompson JK. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1971;34:803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 179.Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock. 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hammer AM, Morris NL, Cannon AR, et al. Interleukin-22 prevents microbial dysbiosis and promotes intestinal barrier regeneration following acute injury. Shock. 2017;48:657–665. doi: 10.1097/SHK.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Segawa S, Wakita Y, Hirata H, Watari J. Oral administration of heat-killed Lactobacillus brevis SBC8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed C57BL/6N mice. Int J Food Microbiol. 2008;128:371–377. doi: 10.1016/j.ijfoodmicro.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 183.Wang Y, Kirpich I, Liu Y, et al. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866–2875. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Komatsuzaki N, Shima J. Effects of live Lactobacillus paracasei on plasma lipid concentration in rats fed an ethanol-containing diet. Biosci Biotechnol Biochem. 2012;76:232–237. doi: 10.1271/bbb.110390. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang B, Lu XL, Song YH, Shi HT, Li J, Geng Y. Changes in the intestinal microenvironment during development of alcoholic fatty liver disease and related effects of probiotic therapy. Zhonghua gan zang bing za zhi. 2012;20:848–852. doi: 10.3760/cma.j.issn.1007-3418.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 187.Chiu YH, Tsai JJ, Lin SL, Lin MY. Lactobacillus casei MYL01 modulates the proinflammatory state induced by ethanol in an in vitro model. J Dairy Sci. 2014;97:2009–2016. doi: 10.3168/jds.2013-7514. [DOI] [PubMed] [Google Scholar]
- 188.Singh AK, Pandey SK, Naresh Kumar G. Pyrroloquinoline quinone-secreting probiotic Escherichia coli Nissle 1917 ameliorates ethanol-induced oxidative damage and hyperlipidemia in rats. Alcohol Clin Exp Res. 2014;38:2127–2137. doi: 10.1111/acer.12456. [DOI] [PubMed] [Google Scholar]
- 189.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]