Abstract
Pain is highly prevalent among people living with HIV (PLHIV). Although the association between stigma and pain among stigmatized individuals has been well-established in the non- HIV chronic pain literature, little is known about the association between stigma and pain among PLHIV and the mechanisms that underlie this association. The present study examined the indirect effect of HIV stigma and pain via anxiety sensitivity (fear of anxiety symptoms). The sample included 97 PLHIV (60.2% male, Mage = 48.40, SD = 7.75). Results indicated significant and medium-sized indirect effects of HIV stigma on pain severity, pain interference, and psychological inflexibility in pain via anxiety sensitivity. Alternative models did not yield significant indirect effects. The results suggest anxiety sensitivity may explain the association between stigma and pain among PLHIV. These findings provide novel empirical insight into the nature of stigma-pain relation among PLHIV and could be used to guide pain-based intervention development for this population.
Keywords: anxiety sensitivity, HIV, pain interference, psychological inflexibility, stigma
Pain is highly common among people living with HIV (PLHIV) (Cox & Rice, 2008; Parker, Stein, & Jelsma, 2014). PLHIV report pain at a 54% point prevalence and 83% 3-month past prevalence period (Parker et al., 2014). Such pain is often severe and interfering; on average, PLHIV report moderate to severe levels of pain intensity and moderate levels of pain interference (Parker et al., 2014). The source of pain among PLHIV is heterogeneous, occurring because of HIV-related conditions (e.g., neuropathy, osteonecrosis), immune suppression and subsequent opportunistic infection, and side effects of antiretroviral treatment (Hewitt et al., 1997; Merlin et al., 2014). Pain also may be due to non-HIV factors (Merlin et al., 2014). Currently, there is strikingly little known about the role of psychological factors in the experience of pain among PLHIV.
Stigma may be one psychological factor that is related to pain among PLHIV. Stigma is an attribute (or commonly labeled a “mark”) of social devaluation (Goffman, 2009); stigma is common among PLHIV (Herek, Capitanio, & Widaman, 2002; Skelton, 2006). Among PLHIV, stigma is associated with more depressive and anxiety symptoms (Kang, Rapkin, & DeAlmeida, 2006; Vanable, Carey, Blair, & Littlewood, 2006), more HIV-related symptoms, and poorer antiviral medication adherence (Langebeek et al., 2014; Vanable et al., 2006). There is limited evidence of the association between stigma and pain among PLHIV. However, indirect evidence from the non-HIV chronic pain literature suggests stigma and social invalidation are associated with more maladaptive pain responses, including pain, catastrophizing, and physical disability (Kool, Van Middendorp, Lumley, Bijlsma, & Geenen, 2012; Waugh, Byrne, & Nicholas, 2014). When stigma identity is concealed, it may be related to greater pain severity (Uysal & Lu, 2011). Other research has found PLHIV with multiple stigmatized identities (e.g., intravenous drug abuser, black HIV-positive persons) tend to report greater pain than those who do not possess additional stigmatized identities (Aouizerat et al., 2010; Del Borgo et al., 2001; Dobalian, Tsao, & Duncan, 2004; Hansen et al., 2011; Martin, Pehrsson, Österberg, Sönnerborg, & Hansson, 1999; Richardson et al., 2009). These data collectively suggest stigma may have a relation with pain among marginalized individuals. Yet, it is presently unclear if stigma is related to the experience of pain among PLHIV.
In addition to the association between stigma and pain among PLHIV, there is a need to examine possible factors that may explain this putative association. One such factor is anxiety sensitivity, defined as the fear of arousal-related sensations, arising from beliefs that sensations would lead to adverse consequences (Reiss & McNally, 1985). Non-HIV studies indicate anxiety sensitivity is related to selective attention bias for somatic threat-related information (Keogh, Dillon, Georgiou, & Hunt, 2001). Individuals with higher levels of anxiety sensitivity also are more likely to catastrophically misinterpret the physiological sensations of anxiety in a pain-provoking situation (Curzik & Jokic-Begic, 2011). Indeed, anxiety sensitivity is associated with a lower pain threshold, greater sensory pain (Keogh & Birkby, 1999; Keogh & Cochrane, 2002), greater pain catastrophizing (Asmundson & Taylor, 1996; Esteve & Camacho, 2008; Roelofs, Peters, McCracken, & Vlaeyen, 2003; Wong et al., 2014), and more pain-related disability (Esteve, Ramírez-Maestre, & López-Martínez, 2012; Keogh, Book, Thomas, Giddins, & Eccleston, 2010; Zvolensky et al., 2017).
Although research focused on anxiety sensitivity and pain among PLHIV is lacking, one study found anxiety sensitivity is related to the intensity of bodily symptoms and to distress experienced by PLHIV (Gonzalez, Zvolensky, Grover, & Parent, 2012). Experimental studies have found that the experience of social rejection is related to more bodily sensations among PLHIV (Iffland, Sansen, Catani, & Neuner, 2014; Kelly, McDonald, & Rushby, 2012). PLHIV with greater anxiety sensitivity may be more likely to misinterpret the arousal-related sensation as threatening or catastrophize their pain. Thus, PLHIV with higher levels of anxiety sensitivity may experience more severe pain, more pain interference, and more maladaptive pain-related cognitions. From this theoretical perspective, the next formative research step is to evaluate whether anxiety sensitivity explains the association between stigma and pain among PLHIV.
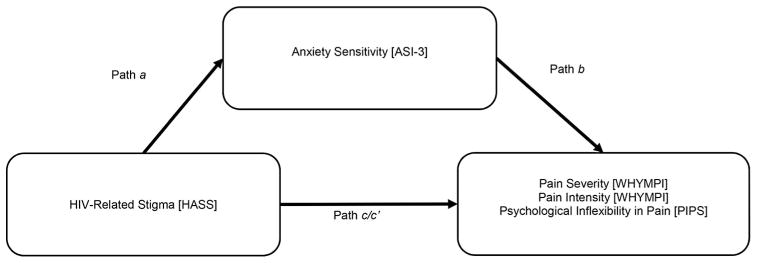
Together, the present study evaluated the hypothesis that, among PLHIV, stigma would be significantly associated with pain severity, pain interference, and psychological inflexibility in pain, and that anxiety sensitivity would explain the association between stigma and pain-related dependent measures (see Figure 1). It also hypothesized that these associations would be evident after controlling for the following covariates: racial/ethnic minority status, gender, sexual orientation, time since HIV diagnosis, and negative affectivity (NA) (tendency to experience negative mood), which have been known to be related to pain severity in past research (Fillingim, King, Ribeiro-Dasilva, Rahim-Williams, & Riley, 2009; Gedney & Logan, 2007; Lawson et al., 2015; Meints, Miller, & Hirsh, 2016; Vigil, Rowell, & Lutz, 2014).
Figure 1.
Proposed model examining the indirect association of HIV stigma via anxiety sensitivity in relation to pain severity, pain interference, and psychological inflexibility in pain.
Method
Participants
Participants in the present study included 97 adults with a self-reported diagnosis of HIV/AIDS (60.8% male, Mage = 48.2, SD = 7.7). Slightly less than half (43.3%) reported an AIDS diagnosis. Participants reported an average CD4T-cell count of 682.8 (SD = 986.7). The sample was diverse racial/ethnically, with 34.0% identifying as White, 55.7% as Black/Non-Hispanic, 5.2% as Black/Hispanic, 3.1% as Hispanic, and 2.1% as “Mixed/Other.” Less than half (48.4%) reported partial college or more with 35.1% completing high school and 16.5% reporting less than high school education. Most of the sample (81.4%) was unemployed with only 2.1% reporting full-time employment, 14.4% part-time employment, and 2.1% living as a dependent/spouse. Approximately half (49.5%) were single/never married with 18.6% married/living with someone, 23.7% divorced, 6.2% separated, and 2.1% widowed.
Measures
MINI International Neuropsychiatric Interview (MINI; Lecrubier et al., 1997). The MINI is a diagnostic interview used to assess DSM-IV disorders. The MINI has been successfully utilized in prior studies of PLHIV (e.g., Breuer et al., 2014) and has sound psychometric properties (see Lecrubier et al., 1997). In the current study, 12.5% of MINI diagnostic interviews were checked for reliability by a trained doctoral-level rater with no discrepancies noted. The observed rate of psychological disorders is presented in Table 1.
Table 1.
Current psychological disorders.
| Diagnosis | N (%) |
|---|---|
| Generalized anxiety disorder | 30 (30.9%) |
| Major depressive disorder | 28 (28.9%) |
| Agoraphobia | 23 (23.7%) |
| Other substance use disorders | 21 (21.6%) |
| Panic disorder | 21 (21.6%) |
| Dysthymia | 17 (17.5%) |
| Post-traumatic stress disorder | 15 (15.5%) |
| Alcohol use disorders | 14 (14.4%) |
| Social phobia | 10 (10.3%) |
| Obsessive compulsive disorder | 4 (4.1%) |
| Mania/hypomania | 3 (3.1%) |
Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). The PANAS is a self-report measure of positive and negative affectivity. Each of the 20 items (e.g., “disinterested”) is rated on a Likert-type scale from 1 (very slightly or not at all) to 5 (extremely) in terms of how the respondent generally feels. Items comprise two scales: positive affectivity (PA) and NA. Past studies indicate good psychometric properties for the PANAS among PLHIV (Gonzalez, Zvolensky, Parent, Grover, & Hickey, 2012). In the current sample, NA was used to indicate the generalized tendency to experience negative emotions (α = .86).
HIV/AIDS Stigma Scale (HASS; Bunn, Solomon, Miller, & Forehand, 2007). The HASS is a self-report assessment of lifetime HIV/AIDS related stigma (e.g., “I worry that people may judge me when they learn I have HIV/AIDS”). The HASS is comprised of 32 items on a Likert-type scale ranging from 1 (strongly disagree) to 4 (strongly agree). The HASS has demonstrated strong psychometric properties in past work among PLHIV (Bunn et al., 2007). In the current study, internal consistency for the HASS was excellent (α = .96).
Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007). The ASI-3 is an 18-item self-report measure of anxiety sensitivity. Items are rated on a Likert-type scale from 0 (very little) to 4 (very much) and summed to a total score. The ASI-3 maintains strong psychometric properties (Taylor et al., 2007) and has demonstrated excellent internal consistency among PLHIV (Brandt, Gonzalez, Grover, & Zvolensky, 2012). In the current sample, internal consistency was excellent for the ASI-3 total scale (α = .95).
The West Haven-Yale Multidimensional Pain Inventory (WHYMPI; Kerns, Turk, & Rudy, 1985). The WHYMPI is a well-established measure assessing various aspects of pain. The current study utilized the pain severity (3 items; e.g., “On average, how severe has your pain been during the last week?”) and pain interference (11 items; e.g., “In general, how much does your pain interfere with your day-to-day activities?”) subscales of the WHYMPI. Items are rated on various Likert-type scales from 0 (no interference) to 6 (extreme interference). The WHYMPI has demonstrated good reliability and validity among samples of chronic pain (Kerns et al., 1985) and has been successfully used among PLHIV (Barry et al., 2011). In the current sample, internal consistency was excellent for both the pain severity (α = .93) and pain interference (α = .96).
Psychological Inflexibility in Pain Scale (PIPS; Wicksell, Lekander, Sorjonen, & Olsson, 2010; Wicksell, Renöfält, Olsson, Bond, & Melin, 2008). The PIPS is a 12-item self-report measure of psychological inflexibility in relation to pain, and it has two components (i.e., cognitive fusion and avoidance). Sample items are: “It is important to understand what causes my pain (cognitive fusion)” and “Because of my pain, I no longer plan for the future (avoidance),” they are rated on a Likert-type scale from 1 (never true) to 7 (always true) with higher scores indicating more inflexibility. The PIPS has demonstrated good psychometric properties including internal consistency, preliminary test-retest reliability, and strong relations with pain and disability-related constructs (Wicksell et al., 2010). In the current study, internal consistency was excellent (overall: α = .92; cognitive fusion: α = .82; avoidance: α = .92).
Procedure
Data for the current study was taken from a larger project on medication adherence (Brandt et al., 2016). Eligible participants were between the ages of 18 and 65, had a diagnosis of HIV/AIDS, and the ability to provide informed written consent. Participants were excluded from the study if they were unable to provide informed consent, could not answer questions accurately due to illiteracy, or could not show up to their scheduled appointments. Interested individuals responded to flyers posted at local HIV/AIDS service organizations and contacted research staff. Potential participants were screened for eligibility via phone, and if deemed eligible, were scheduled for a baseline appointment. Upon completion of the appointment, participants were compensated with a $20 gift card. The University of Houston’s Institutional Review Board (IRB) approved study procedures.
Data analytic plan
Statistical analyses were conducted using the PROCESS macro for SPSS version 20 (Hayes, 2012), which calculates the indirect effect of a predictor (X) on an outcome (Y) via a mediator (West & Aiken, 1997). Specifically, the indirect effect (“path a*b”) is calculated as the product of the “a path” (the regression weight of X in predicting M, controlling for covariates) multiplied by the “b path” (the regression weight of M predicting Y, controlling for effects of X and covariates). Bootstrapping with 10,000 resamples was performed to obtain 95% confidence intervals (CI) around the “a*b path.” The indirect effect of HIV-related stigma via anxiety sensitivity was examined in relation to (a) pain severity, (b) pain interference, and (c) psychological inflexibility in pain. Effect size (κ2) was estimated for the indirect effect (Preacher & Kelley, 2011). Models were adjusted for participant racial/ethnic minority status, gender, sexual orientation, time since HIV diagnosis, and negative affectivity. Alternative models were also run, replacing the X and M variables (i.e., testing the indirect effect of anxiety sensitivity via HIV-related stigma) in relation to each dependent measure (Judd & Kenny, 2010). Finally, planned exploratory post-hoc tests evaluated subfactors of psychological inflexibility in pain (i.e., avoidance, fusion) as outcomes to test specificity of psychological inflexibility findings.
Results
Descriptive statistics
Bivariate correlations are presented in Table 2. HIV stigma was positively correlated with anxiety sensitivity (r = .37, p < .001) as well as pain severity (r = .24, p = .018), pain interference (r = .33, p < .001), and psychological inflexibility in pain (r = .39, p < .001). Anxiety sensitivity was positively associated with pain severity (r = .35, p < .001), pain interference (r = .42, p < .001), and psychological inflexibility in pain (r = .59, p < .001).
Table 2.
Means, standard deviations, internal consistencies, and bivariate correlations among variables.
| Variable | Mean/n (SD/%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Minority status | 64 (66.0%) | 1 | ||||||||||
| 2. Gender | 59 (60.8%) | −.13 | 1 | |||||||||
| 3. Sexual orientation | 51 (52.6%) | .23* | −.55** | 1 | ||||||||
| 4. Years since diagnosis | 16.8 (8.3) | −.05 | .13 | −.27** | 1 | |||||||
| 5. PANAS-NA | 24.1 (9.0) | −.10 | −.15 | .08 | −.19 | 1 | ||||||
| 6. HASS-Total | 77.5 (20.5) | −.05 | −.10 | .14 | −.21* | .37** | 1 | |||||
| 7. ASI-3 | 27.0 (18.3) | −.09 | −.08 | .02 | −.14 | .55** | .37** | 1 | ||||
| 8. WHYMPI-Severity | 2.3 (2.0) | −.04 | −.25* | .14 | .08 | .34** | .24* | .35** | 1 | |||
| 9. WHYMPI-Interference | 2.4 (1.9) | −.03 | −.26** | .15 | .05 | .37** | .33** | .42** | .87** | 1 | ||
| 10. PIPS-Total | 48.6 (17.7) | −.15 | −.06 | .06 | −.11 | .62** | .39** | .59** | .33** | .38** | 1 | |
| 11. PIPS-Avoidance | 29.1 (13.0) | −.13 | −.10 | .11 | −.11 | .65** | .39** | .66** | .35** | .40** | .96** | 1 |
| 12. PIPS-Fusion | 19.5 (6.4) | −.14 | .04 | −.05 | −.09 | .39** | .27** | .28** | .21* | .24* | .81** | .61** |
Note: Minority status (1 = racial/ethnic minority, 0 = not a racial/ethnic minority); gender (1 = male, 0 = not male); sexual orientation (1 = heterosexual, 0 = not heterosexual); time since diagnosis = years since HIV diagnosis; HASS = HIV/AIDS Stigma Scale; ASI-3 = Anxiety Sensitivity Index-3; WHYMPI = West Haven Yale Multidimensional Pain Index; severity = WHYMPI-Pain Severity Subscale; interference = WHYMPI-Pain Interference Subscale; PIPS = Psychological Inflexibility in Pain Scale; avoidance = PIPS-Avoidance Subscale; fusion = PIPS-Fusion Subscale Numbers across header correspond with variables numbered 1–12.
p < .05;
p < .01.
Test of the indirect effect
In predicting pain severity, there was a nonsignificant total effect of HIV-related stigma (B = .01, SE = .01, p = .163; Table 3). However, the indirect effect of HIV-related stigma via anxiety sensitivity was significant (B = .004, SE = .003, 95% CI [.0001, .01]; completely standardized point estimate = .04), and of medium size (κ2 = .10). There was no significant direct effect of HIV-related stigma after accounting for the indirect effect (B = .01, SE = .01, p = .317).
Table 3.
Unstandardized coefficients.
| Y | Model | B | SE | t | p | LLCI | ULCI |
|---|---|---|---|---|---|---|---|
| 1 | HASS → ASI-3 (a) | .17 | .08 | 2.02 | .047 | .002 | .34 |
| ASI-3 → WHYMPI-Severity (b) | .02 | .01 | 1.87 | .065 | −.002 | .05 | |
| HASS → WHYMPI-Severity (c) | .01 | .01 | 1.41 | .163 | −.01 | .03 | |
| HASS → WHYMPI-(c′) | .01 | .01 | 1.01 | .317 | −.01 | .03 | |
| HASS → ASI-3 → WHYMPI-Severity (a*b) | .004 | .003 | .0001 | .01 | |||
| 2 | ASI-3 → WHYMPI-Interference (b) | .03 | .01 | 2.58 | .012 | .01 | .05 |
| HASS → WHYMPI-Interference (c) | .02 | .01 | 2.33 | .022 | .003 | .04 | |
| HASS → WHYMPI-Interference (c′) | .01 | .01 | 1.82 | .072 | −.002 | .04 | |
| HASS → ASI-3 → WHYMPI-Interference (a*b) | .01 | .003 | .0004 | .01 | |||
| 3 | ASI-3 → PIPS-Total (b) | .32 | .09 | 3.51 | <.001 | .14 | .49 |
| HASS → PIPS-Total (c) | .16 | .08 | 2.09 | .039 | .01 | .31 | |
| HASS → PIPS-Total (c′) | .11 | .07 | 1.44 | .153 | −.04 | .25 | |
| HASS → ASI-3 → PIPS-Total (a*b) | .05 | .03 | .01 | .14 | |||
| 4 | ASI-3 → PIPS-Avoidance (b) | .29 | .06 | 4.86 | <.001 | .17 | .41 |
| HASS → PIPS-Avoidance (c) | .11 | .05 | 2.09 | .040 | .01 | .22 | |
| HASS → PIPS-Avoidance (c′) | .06 | .05 | 1.27 | .206 | −.04 | .16 | |
| HASS → ASI-3 → PIPS-Avoidance (a*b) | .05 | .03 | .01 | .11 | |||
| 5 | ASI-3 → PIPS-Fusion (b) | .02 | .04 | 0.50 | .615 | −.06 | .10 |
| HASS → PIPS-Fusion (c) | .05 | .03 | 1.41 | .161 | −.02 | .11 | |
| HASS → PIPS-Fusion (c′) | .04 | .03 | 1.27 | .206 | −.02 | .11 | |
| HASS → ASI-3 → PIPS-Fusion (a*b) | .004 | .01 | −.01 | .02 |
Note: a = effect of X on M; b = effect of M on Yi; c = total effect of X on Yi; c′ = direct effect of X on Yi controlling for M; HASS (HIV/AIDS Stigma Scale) is the predictor in all models; ASI-3 (Anxiety Sensitivity Index-3) is the explanatory variable; WHYMPI (West Haven Yale Multidimensional Pain Inventory) pain severity subscale is the outcome in Model 1; WHYMPI Pain Interference is the outcome in Model 2; PIPS (Psychological Inflexibility in Pain) total score is the outcome in model 3. Post-hoc tests evaluated PIPS Avoidance and Fusion subscales as outcomes in models 4 and 5, respectively. LLCI = lower bound of confidence interval; ULCI = upper bound; → = affects.
The total effect of HIV-related stigma on pain interference was significant (B = .02, SE = .01, p = .022). There was a significant indirect effect of HIV- related stigma via anxiety sensitivity (B = .01, SE = .003, 95% CI [.0004, .01]; completely standardized point estimate = .05), which was of medium effect size (κ2 = .13). After accounting for the indirect effect, there was no significant direct effect of HIV-related (B = .01, SE = .01, p = .072).
Likewise, in relation to psychological inflexibility in pain, there was a significant total effect of HIV-related stigma (B = .16, SE = .08, p = .039). After accounting for the indirect effect, there was no direct effect of HIV-related stigma via anxiety sensitivity (B = .05, SE = .03, 95% CI [.01, .14]; completely standardized point estimate = .07), which was of medium effect size (κ2 = .20). There was no direct effect of HIV-related stigma on psychological inflexibility in pain after accounting for the indirect effect (B = .11, SE = .07, p = .153).
Competing models
The comparison models testing the indirect effect of anxiety sensitivity via HIV-related stigma yielded nonsignificant indirect effects on pain severity (B = .003, SE = .003, 95% CI [−.002, .01]), pain interference (B = .004, SE = .003, 95% CI [−.0001, .01]), and psychological inflexibility in pain (B = .03, SE = .02, 95% CI [−.003, .10]), respectively.
Post-hoc tests
When examining the two subscales of psychological inflexibility in pain as outcomes, there was a significant indirect effect of HIV-related stigma via anxiety sensitivity in relation to the avoidance subscale (B = .05, SE = .03, 95% CI [.01, .11]; completely standardized point estimate = .10), which was medium-to-large size (κ2 = .23), but not the fusion subscale (B = .004, SE = .01, 95% CI [−.01, .02]; completely standardized point estimate = .01).
Discussion
The present study examined the association between stigma and pain in a sample of PLHIV. As hypothesized, HIV stigma was associated with pain severity, pain interference, and psychological inflexibility in pain. The indirect role of anxiety sensitivity in the association between HIV stigma and pain was also examined, and as expected, there were significant indirect effects of HIV stigma on pain severity, pain interference, and psychological inflexibility in pain via anxiety sensitivity. The indirect effects were medium-sized (κ2s ranged from .10 to .20), and evident over and beyond the variance accounted for by the covariates (racial/ethnic minority status, gender, sexual orientation, time since HIV diagnosis, and negative affectivity). Due to the cross-sectional nature of the data, competing models were run to examine potential alternative indirect effects. All competing models yielded nonsignificant indirect effects, providing convergent evidence to support the direction of hypothesized models (i.e., HIV stigma via anxiety sensitivity in relation to pain).
The findings in the present study align with the theoretical perspective that anxiety sensitivity may underlie the association between stigma and pain among PLHIV. Past work suggests social rejection is associated with physical pain (Eisenberger & Lieberman, 2004, 2005). Frequent and chronic encounters with stigma may theoretically increase anxiety sensitivity, resulting in an increased probability of arousal-related sensations misinterpreted as threatening. Such misinterpretations may, in turn, be related to the experience of more severe pain, more pain interference, and higher tendency to engage in behaviors that lead to avoidance of pain and related distress. Interestingly, post-hoc evaluation of subfacets of psychological inflexibility in pain (cognitive fusion and avoidance) revealed a significant indirect effect of HIV stigma via anxiety sensitivity in relation to avoidance, but not cognitive fusion. These findings suggest that the present anxiety sensitivity-based explanatory model is more relevant to avoidance (not engaging in activities due to pain) than cognitive fusion (beliefs about having to control pain to live a valued life) in terms of the studied pain-based dependent measures. These findings are consistent with past non-HIV work that has linked anxiety sensitivity strongly to avoidance behavior for somatic perturbation (Zvolensky & Forsyth, 2002).
The present study may be used to advance the development of interventions for PLHIV suffering from pain. For example, the results may suggest interventions that reduce anxiety sensitivity among stigmatized PLHIV, which may be helpful in alleviating pain symptoms and suffering. Among non-HIV samples, meta-analyses and empirical studies indicate reduced anxiety sensitivity is associated with more pain tolerance/threshold, less pain-related disability, less pain catastrophizing, and less fearful appraisals of pain (Asmundson & Taylor, 1996; Esteve & Camacho, 2008; Esteve et al., 2012; Keogh & Birkby, 1999; Keogh et al., 2010; Keogh & Cochrane, 2002; Ocañez, Kathryn McHugh, & Otto, 2010; Roelofs et al., 2003; Wong et al., 2014). Although there is no such work among PLHIV, a recent study shows an intervention that targets anxiety sensitivity as being effective in reducing pain-related anxiety and pain-related disability in a non-HIV sample (Sharpe et al., 2012). Future study may extend this research and explore the clinical utility of anxiety sensitivity reduction intervention in alleviating pain among PLHIV.
The present study has several limitations. First, while the rejection of competing models may provide greater confidence to the hypothesized directional associations among examined variables, causal inferences cannot be made because of the cross-sectional design of the study. Future research should examine the proposed model with a longitudinal design to explicate the temporal effects. Second, although the sample was ethnically diverse, it was primarily older male adults. To increase the generalizability of findings in this study, future research may benefit from replicating and extending this work to more heterogeneous samples of PLHIV. Previous studies show that PLHIV with stigmatized identities in addition to HIV status (e.g., intravenous drug abuser, black persons) tend to experience more pain than others who do not have further stigmatized intersectionalities (Aouizerat et al., 2010; Dobalian et al., 2004; Hansen et al., 2011; Martin et al., 1999; Richardson et al., 2009). These findings suggest the effect of stigma on pain may have different manifestations among PLHIV with multiple stigmatized identities; multiple stigmatized identities may also have different implications on PLHIV’s levels of anxiety sensitivity. Third, due to the monomethod measurement approach, method variance may influence the current findings. Thus, future work should consider multimethod assessment approaches to index the primary constructs. Fourth, our study documented that the pain PLHIV experience, regardless of its cause, existed in the presence of stigma and anxiety sensitivity. However, the lack of information on the cause of pain (e.g., neuropathic, nociceptive) in our sample may limit our understanding of the experience among PLHIV. Future studies should take the sources into consideration, and examine their potential moderating roles. Finally, while negative affect was measured and controlled in analysis, depression was highly prevalent among PLHIV (Ciesla & Roberts, 2001). It is possible that the pain PLHIV experience may be a somatic expression of depression and/or other mood disorders (Evans et al., 1998), and that depression may moderate the associations among HIV stigma, anxiety sensitivity, and pain experience. To tease apart the actual experience of pain and the somatic experience of depression, future study should include a measure of depressive symptoms, and have the levels of depressive symptoms controlled in analysis.
In summary, the present study represents the first empirical evaluation of the association between HIV stigma and an array of clinical pain processes among a sample of PLHIV. Findings indicated an indirect association between HIV stigma and pain-related outcomes via anxiety sensitivity. If replicated, future research may benefit by exploring the clinical utility of addressing stigma and anxiety sensitivity to reduce the pain burden among PLHIV.
Acknowledgments
Funding
This work is supported by 1F31MH099922-01A1 and an endowment awarded to Dr. Zvolensky.
Footnotes
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Aouizerat BE, Miaskowski CA, Gay C, Portillo CJ, Coggins T, Davis H, Lee KA. Risk factors and symptoms associated with pain in HIV-infected adults. Journal of the Association of Nurses in AIDS Care. 2010;21(2):125–133. doi: 10.1016/j.jana.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Taylor S. Role of anxiety sensitivity in pain-related fear and avoidance. Journal of Behavioral Medicine. 1996;19(6):577–586. doi: 10.1007/BF01904905. [DOI] [PubMed] [Google Scholar]
- Barry DT, Goulet JL, Kerns RK, Becker WC, Gordon AJ, Justice AC, Fiellin DA. Nonmedical use of prescription opioids and pain in veterans with and without HIV. Pain. 2011;152(5):1133–1138. doi: 10.1016/j.pain.2011.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CP, Bakhshaie J, Jardin C, Lemaire C, Kauffman BY, Sharp C, Zvolensky MJ. The moderating effect of smoking status on the relation between anxiety sensitivity and sexual compulsivity and suicidality among persons living with HIV/AIDS. International Journal of Behavioral Medicine. 2016;24(1):92–100. doi: 10.1007/s12529-016-9568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CP, Gonzalez A, Grover KW, Zvolensky MJ. The relation between emotional dysregulation and anxiety and depressive symptoms, pain-related anxiety, and HIV-symptom distress among adults with HIV/AIDS. Journal of Psychopathology and Behavioral Assessment. 2012;35(2):197–204. doi: 10.1007/s10862-012-9329-y. [DOI] [Google Scholar]
- Breuer E, Stoloff K, Myer L, Seedat S, Stein DJ, Joska JA. The validity of the Substance Abuse and Mental Illness Symptom Screener (SAMISS) in people living with HIV/AIDS in primary HIV care in Cape Town, South Africa. AIDS and Behavior. 2014;18(6):1133–1141. doi: 10.1007/s10461-014-0698-y. [DOI] [PubMed] [Google Scholar]
- Bunn JY, Solomon SE, Miller C, Forehand R. Measurement of stigma in people with HIV: A reexamination of the HIV Stigma Scale. AIDS Education and Prevention: Official Publication of the International Society for AIDS Education. 2007;19(3):198–208. doi: 10.1521/aeap.2007.19.3.198. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cox S, Rice A. HIV and AIDS. In: Wilson P, Watson PJ, Haythornthwaite JA, Jensen T, editors. Clinical pain management: Chronic pain. 2. London: Hodder and Stoughton; 2008. pp. 352–361. [Google Scholar]
- Curzik D, Jokic-Begic N. Anxiety sensitivity and anxiety as correlates of expected, experienced and recalled labor pain. Journal of Psychosomatic Obstetrics & Gynecology. 2011;32(4):198–203. doi: 10.3109/0167482X.2011.626093. [DOI] [PubMed] [Google Scholar]
- Del Borgo C, Izzi I, Chiarotti F, Del Forno A, Moscati AM, Cornacchione E, Fantoni M. Multidimensional aspects of pain in HIV-infected individuals. AIDS Patient Care and STDs. 2001;15(2):95–102. doi: 10.1089/108729101300003690. [DOI] [PubMed] [Google Scholar]
- Dobalian A, Tsao JC, Duncan RP. Pain and the use of outpatient services among persons with HIV: Results from a nationally representative survey. Medical Care. 2004;42(2):129–138. doi: 10.1097/01.mlr.0000108744.45327.d4. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why it hurts to be left out: The neurocognitive overlap between physical and social pain. In: Williams KD, Forgas JP, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York, NY: Psychology Press; 2005. pp. 109–127. [Google Scholar]
- Esteve MR, Camacho L. Anxiety sensitivity, body vigilance and fear of pain. Behaviour Research and Therapy. 2008;46(6):715–727. doi: 10.1016/j.brat.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Esteve R, Ramírez-Maestre C, López-Martínez AE. Experiential avoidance and anxiety sensitivity as dispositional variables and their relationship to the adjustment to chronic pain. European Journal of Pain. 2012;16(5):718–726. doi: 10.1002/j.1532-2149.2011.00035.x. [DOI] [PubMed] [Google Scholar]
- Evans S, Ferrando S, Sewell M, Goggin K, Fishman B, Rabkin J. Pain and depression in HIV illness. Psychosomatics. 1998;39(6):528–535. doi: 10.1016/S0033-3182(98)71285-X. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: A review of recent clinical and experimental findings. The Journal of Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedney JJ, Logan H. Perceived control and negative affect predict expected and experienced acute clinical pain: A structural modeling analysis. The Clinical Journal of Pain. 2007;23(1):35–44. doi: 10.1097/01.ajp.0000210940.04182.a3. [DOI] [PubMed] [Google Scholar]
- Goffman E. Stigma: Notes on the management of spoiled identity. New York, NY: Simon and Schuster; 2009. [Google Scholar]
- Gonzalez A, Zvolensky MJ, Grover KW, Parent J. The role of anxiety sensitivity and mindful attention in anxiety and worry about bodily sensations among adults living with HIV/AIDS. Behavior Therapy. 2012;43(4):768–778. doi: 10.1016/j.beth.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Zvolensky MJ, Parent J, Grover KW, Hickey M. HIV symptom distress and anxiety sensitivity in relation to panic, social anxiety, and depression symptoms among HIV-positive adults. AIDS Patient Care and STDs. 2012;26(3):156–164. doi: 10.1089/apc.2011.0309. [DOI] [PubMed] [Google Scholar]
- Hansen L, Penko J, Guzman D, Bangsberg DR, Miaskowski C, Kushel MB. Aberrant behaviors with prescription opioids and problem drug use history in a community-based cohort of HIV-infected individuals. Journal of pain and symptom management. 2011;42(6):893–902. doi: 10.1016/j.jpainsymman.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Process: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 http://imaging.mrc-cbu.cam.ac.uk/statswiki/FAQ/SobelTest.
- Herek GM, Capitanio JP, Widaman KF. HIV-related stigma and knowledge in the United States: Prevalence and trends, 1991–1999. American Journal of Public Health. 2002;92(3):371–377. doi: 10.2105/AJPH.92.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70(2):117–123. doi: 10.1016/S0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- Iffland B, Sansen LM, Catani C, Neuner F. Rapid heartbeat, but dry palms: Reactions of heart rate and skin conductance levels to social rejection. Frontiers in Psychology. 2014;5:956. doi: 10.3389/fpsyg.2014.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA. Data analysis in social psychology: Recent and recurring issues. 2010 Retrieved from http://onlinelibrary.wiley.com/doi/10.1002/9780470561119.socpsy001004/full.
- Kang E, Rapkin BD, DeAlmeida C. Are psychological consequences of stigma enduring or transitory? A longitudinal study of HIV stigma and distress among Asians and Pacific Islanders living with HIV illness. AIDS Patient Care and STDs. 2006;20(10):712–723. doi: 10.1089/apc.2006.20.712. [DOI] [PubMed] [Google Scholar]
- Kelly M, McDonald S, Rushby J. All alone with sweaty palms—physiological arousal and ostracism. International Journal of Psychophysiology. 2012;83(3):309–314. doi: 10.1016/j.ijpsycho.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Keogh E, Birkby J. The effect of anxiety sensitivity and gender on the experience of pain. Cognition & Emotion. 1999;13(6):813–829. doi: 10.1080/026999399379096. [DOI] [Google Scholar]
- Keogh E, Book K, Thomas J, Giddins G, Eccleston C. Predicting pain and disability in patients with hand fractures: Comparing pain anxiety, anxiety sensitivity and pain catastrophizing. European Journal of Pain. 2010;14(4):446–451. doi: 10.1016/j.ejpain.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Keogh E, Cochrane M. Anxiety sensitivity, cognitive biases, and the experience of pain. The Journal of Pain. 2002;3(4):320–329. doi: 10.1054/jpai.2002.125182. [DOI] [PubMed] [Google Scholar]
- Keogh E, Dillon C, Georgiou G, Hunt C. Selective attentional biases for physical threat in physical anxiety sensitivity. Journal of Anxiety Disorders. 2001;15(4):299–315. doi: 10.1016/S0887-6185(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI) Pain. 1985;23(4):345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- Kool MB, Van Middendorp H, Lumley M, Bijlsma JW, Geenen R. Social support and invalidation by others contribute uniquely to the understanding of physical and mental health of patients with rheumatic diseases. Journal of Health Psychology. 2012;18(1):86–95. doi: 10.1177/1359105312436438. [DOI] [PubMed] [Google Scholar]
- Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, … Nieuwkerk PT. Predictors and correlates of adherence to combination antiretro-viral therapy (ART) for chronic HIV infection: A meta-analysis. BMC Medicine. 2014;12(1):142. doi: 10.1186/s12916-014-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson E, Sabin C, Perry N, Richardson D, Gilleece Y, Churchill D, … Walker-Bone K. Is HIV painful? an epidemiologic study of the prevalence and risk factors for pain in HIV-infected patients. The Clinical Journal of Pain. 2015;31(9):813–819. doi: 10.1097/AJP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, … Dunbar GC. The Mini International Neuropsychiatric Interview (MINI): A short diagnostic structured interview: Reliability and validity according to the CIDI. European Psychiatry. 1997;12(5):224–231. doi: 10.1016/S0924-9338(97)83296-8. [DOI] [Google Scholar]
- Martin C, Pehrsson P, Österberg A, Sönnerborg A, Hansson P. Pain in ambulatory HIV-infected patients with and without intravenous drug use. European Journal of Pain. 1999;3(2):157–164. doi: 10.1053/eujp.1999.0111. [DOI] [PubMed] [Google Scholar]
- Meints SM, Miller MM, Hirsh AT. Differences in pain coping between black and white Americans: A meta-analysis. The Journal of Pain. 2016;17(6):642–653. doi: 10.1016/j.jpain.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Zinski A, Norton WE, Ritchie CS, Saag MS, Mugavero MJ, Hooten WM. A conceptual framework for understanding chronic pain in patients with HIV. Pain Practice. 2014;14(3):207–216. doi: 10.1111/papr.12052. [DOI] [PubMed] [Google Scholar]
- Ocañez KL, Kathryn McHugh R, Otto MW. A meta-analytic review of the association between anxiety sensitivity and pain. Depression and Anxiety. 2010;27(8):760–767. doi: 10.1002/da.20681. [DOI] [PubMed] [Google Scholar]
- Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: A systematic review. Journal of the International AIDS Society. 2014;17(1):18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Reiss S, McNally RJ. Expectancy model of fear. In: Bootzin SRRR, editor. Theoretical issues in behavior therapy. San Diego, CA: Academic Press; 1985. pp. 107–121. [Google Scholar]
- Richardson JL, Heikes B, Karim R, Weber K, Anastos K, Young M. Experience of pain among women with advanced HIV disease. AIDS patient care and STDs. 2009;23(7):503–511. doi: 10.1089/apc.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs J, Peters ML, McCracken L, Vlaeyen JW. The pain vigilance and awareness questionnaire (PVAQ): Further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101(3):299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]
- Sharpe L, Ianiello M, Dear BF, Perry KN, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain. 2012;153(3):722–731. doi: 10.1016/j.pain.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Skelton JA. How negative are attitudes toward persons with AIDS? Examining the AIDS–leukemia paradigm. Basic and Applied Social Psychology. 2006;28(3):251–261. doi: 10.1207/s15324834basp2803_4. [DOI] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, … Cardenas SJ. Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Uysal A, Lu Q. Is self-concealment associated with acute and chronic pain? Health Psychology. 2011;30(5):604–614. doi: 10.1037/a0024287. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS and Behavior. 2006;10(5):473–482. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil JM, Rowell LN, Lutz C. Gender expression, sexual orientation and pain sensitivity in women. Pain Research and Management. 2014;19(2):87–92. doi: 10.1155/2014/297060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Waugh OC, Byrne DG, Nicholas MK. Internalized stigma in people living with chronic pain. The Journal of Pain. 2014;15(5):550–e551. doi: 10.1016/j.jpain.2014.02.001. [DOI] [PubMed] [Google Scholar]
- West SG, Aiken LS. Toward understanding individual effects in multicompo-nent prevention programs: Design and analysis strategies. In: Bryant KJ, Windle M, West SG, editors. The science of prevention: Methodological advances from alcohol and substance abuse research. Washington, DC: American Psychological Association; 1997. [Google Scholar]
- Wicksell RK, Lekander M, Sorjonen K, Olsson GL. The Psychological Inflexibility in Pain Scale (PIPS)––Statistical properties and model fit of an instrument to assess change processes in pain related disability. European Journal of Pain. 2010;14(7):771e–771.e14. doi: 10.1016/j.ejpain.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Wicksell RK, Renöfält J, Olsson GL, Bond FW, Melin L. Avoidance and cognitive fusion—Central components in pain related disability? Development and preliminary validation of the Psychological Inflexibility in Pain Scale (PIPS) European Journal of Pain. 2008;12(4):491–500. doi: 10.1016/j.ejpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Wong WS, Lam HMJ, Chow YF, Chen PP, Lim HS, Wong S, Fielding R. The effects of anxiety sensitivity, pain hypervigilance, and pain catastrophizing on quality of life outcomes of patients with chronic pain: A preliminary, cross-sectional analysis. Quality of Life Research. 2014;23(8):2333–2341. doi: 10.1007/s11136-014-0683-y. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Bakhshaie J, Paulus DJ, Langdon KJ, Garza M, Valdivieso J, Manning K. Exploring the mechanism underlying the association between pain intensity and mental health among Latinos. Journal of Nervous and Mental Disease. 2017;205(4):300–307. doi: 10.1097/nmd.0000000000000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Forsyth JP. Anxiety sensitivity dimensions in the prediction of body vigilance and emotional avoidance. Cognitive Therapy and Research. 2002;26(4):449–460. doi: 10.1023/A:1016223716132. [DOI] [Google Scholar]