Abstract
Background
Hypertriglyceridemia is associated with increased remnant-like particle cholesterol (RLP-C) and triglycerides in LDL (LDL-TG). Recent studies have focused on atherogenicity of RLP-C, with few data on LDL-TG.
Objectives
We examined associations of RLP-C and LDL-TG with incident cardiovascular disease (CVD) events and genetic variants in the Atherosclerosis Risk in Communities study.
Methods
Fasting plasma RLP-C and LDL-TG levels were measured in 9334 men and women without prevalent CVD. Participants were followed for incident CVD events (coronary heart disease [CHD] and ischemic stroke) for up to 16 years. Associations between LDL-TG and RLP-C levels and genetic variants were assessed by whole exome sequencing using single-variant analysis for common variants and gene-based burden tests for rare variants; both an unbiased and a candidate gene approach were explored
Results
RLP-C and LDL-TG levels were correlated with triglyceride levels (r=0.85 and r=0.64, p<0.0001). In minimally adjusted analyses, RLP-C and LDL-TG were associated with CVD risk, but in models adjusted for traditional risk factors including lipids, only LDL-TG was associated with incident CHD (hazard ratio [HR] 1.28, 95% confidence interval [CI] 1.10–1.50) and stroke (HR 1.47, 95% CI 1.13–1.92). A common APOE variant, rs7412, had the strongest association with LDL-TG and RLP-C (p<5×10−8).
Conclusions
RLP-C and LDL-TG levels were predictive of CVD and associated with APOE variants. LDL-TG may represent a marker of dysfunctional remnant lipoprotein metabolism associated with increased CVD risk. Further research is needed to determine whether LDL-TG plays a causal role in CVD and may be a target for therapy.
Keywords: coronary heart disease, remnant lipoproteins, risk, stroke, triglyceride-rich lipoproteins
Introduction
Although the association between elevated plasma triglycerides (TGs) and cardiovascular disease (CVD) has been known for decades (1,2), genetic studies provide new evidence that genes associated with triglyceride-rich lipoprotein (TGRL) metabolism are related to development of atherosclerotic CVD (3,4).
Genetic variants associated with TG metabolism indicate the importance of lipases (e.g., lipoprotein lipase [LPL] and hepatic lipase), their activators (e.g., apoCII and apoAV) and inhibitors (e.g., apoCIII and angiopoietin-like protein [ANGPTL]–4), and ligands for cellular receptors involved in clearance of TGRLs (apoB and apoE) in CVD (5). However, these variants affect multiple lipoproteins, complicating investigations into direct pathophysiology. Increased production and delayed catabolism of TGRLs lead to increased TG-enriched remnant lipoproteins, with increased levels of remnant-like particle cholesterol (RLP-C). In hypertriglyceridemia, cholesteryl ester transfer protein–mediated transfer of TGs from chylomicrons and very low density lipoprotein (VLDL) to low-density lipoprotein (LDL) and high-density lipoprotein (HDL) in exchange for cholesteryl esters from LDL and HDL leads to TG-enriched VLDL remnants, intermediate-density lipoprotein (IDL), and LDL, and to small dense LDL. Numerous studies have focused on the atherogenic potential of remnant lipoproteins and RLP-C (6-8). However, few data describe the association between TGs in LDL (LDL-TG) and future CVD risk.
We examined these two lipoprotein measures linked to hypertriglyceridemia—LDL-TG and RLP-C—and their association to CVD in the Atherosclerosis Risk in Communities (ARIC) study. We hypothesized that elevated LDL-TG and RLP-C levels were associated with increased CVD risk. We also used genetic array analysis to investigate associations of genetic variants with LDL-TG and RLP-C levels.
Methods
See online supplement for details.
Study Population
ARIC is a prospective study of CVD in 15,792 middle-aged adults recruited from four U.S. communities in 1987–1989 (9). Figure 1 describes selection and demographics of the 9334 individuals included in this analysis.
Figure 1. Study population.
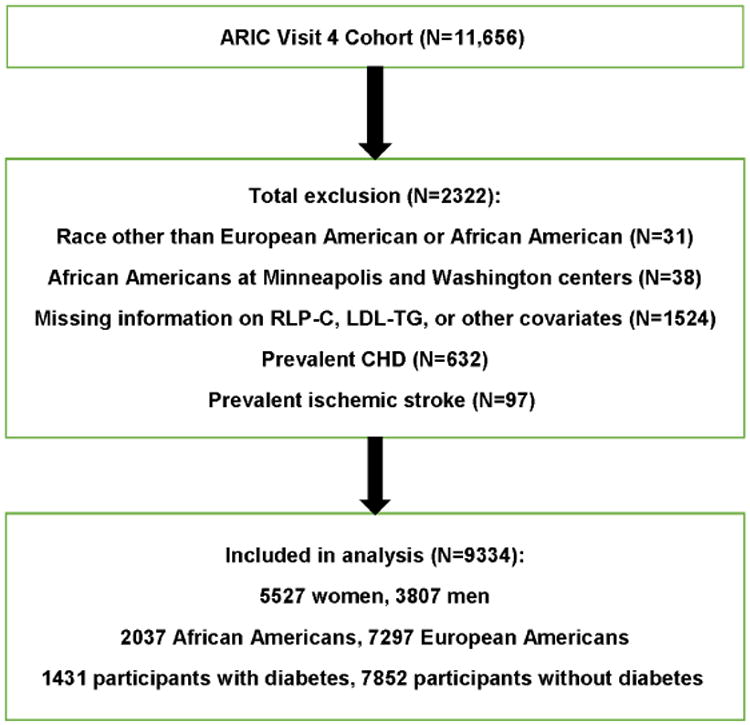
The Atherosclerosis Risk in Communities study (ARIC) is a prospective study of cardiovascular disease (CVD) in 15,792 middle-aged adults recruited from four US communities in 1987–1989. The current study was conducted among participants in ARIC visit 4 (1996–1998). Of 11,656 eligible individuals, we excluded those with self-reported race neither white nor black (n=31) and African American participants at the Minnesota and Washington County field centers (n=38) because of small enrollment numbers, individuals missing data for low-density lipoprotein triglyceride (LDL-TG), remnant-like particle cholesterol (RLP-C), or other covariates (n=1524), and those with prevalent coronary heart disease (CHD) (n=632) or ischemic stroke (n=97) at visit 4. Therefore, 9334 individuals were included in this analysis.
Incident CVD events were a composite of incident coronary heart disease (CHD) and incident ischemic stroke after visit 4 and through December 31, 2013. Methods of assessing incident CHD events and ischemic strokes in ARIC have been described (10,11). Median (25th, 75th percentile) follow-up for CVD, CHD, and ischemic stroke events was 15.6 (10.8, 16.6) years, 15.6 (11.5, 16.6) years, and 15.8 (13.8, 16.7) years, respectively.
Lipoprotein and Lipid Assays
Lipids were measured in 12-hour fasting plasma stored at −70°C with ethylenediaminetetraacetic acid. Total cholesterol, HDL-C, and TGs were measured using enzymatic measures (12). RLP-C (13) and LDL-TG (14) were determined by fully automated detergent-based homogeneous methods (Denka Seiken, Tokyo, Japan).
Statistical Analysis
LDL-TG and RLP-C were modeled as continuous and categorical variables. Associations between exposure variables and outcomes were determined using Cox proportional-hazards modeling. Linear terms representing quartile number were used to obtain p-value for trend. Model 1 was adjusted for age, gender, and race. Model 2 included model 1 plus risk factors in the Pooled Cohort Equation (PCE). Kaplan–Meier survival curves were calculated for each outcome across RLP-C and LDL-TG quartiles.
Genetic Methods and Analysis
In a targeted gene approach, we investigated candidate genes and well-established variants within those genes (LPL, LIPC, LIPG, APOC3, APOA5, ANGPTL3, and ANGPTL4) and APOE haplotypes with respect to LDL-TG and RLP-C.
In an unbiased approach, genotypes were obtained from the Illumina HumanExome BeadChip. Genes with cumulative minor allele count ≥3 in both European Americans and African Americans (13,690 genes) were included.
Whole exome sequencing for 5847 European Americans and 1915 African Americans was completed at Baylor College of Medicine Human Genome Sequencing Center. Exomes were captured using HGSC VCRome 2.1 reagent (15); samples were paired-end sequenced using Illumina GAII or HiSeq instruments. Variant calling was done using Atlas2 (16). Whole exome variants were annotated using ANNOVAR (17) and dbNSFP v2.0 (18).
Both exome chip and whole exome sequencing were available in 5767 European Americans and 1857 African Americans.
Results
In the 9334 participants, RLP-C levels were higher in European Americans than African Americans (median [25th percentile, 75th percentile] 6.7 [3.4, 13.5] mg/dL vs 3.9 [2.2, 7.2] mg/dL; p=0.0001 [Wilcoxon rank-sum test]). Individuals with RLP-C and LDL-TG levels in the highest quartile (Table 1) had proatherogenic lipid profiles, were more likely to have diabetes and hypertension, and had higher body mass index, fasting blood glucose, and plasma levels of the inflammatory markers high-sensitivity C-reactive protein (hs-CRP) and white blood cell count. Statin use was higher in individuals with RLP-C or LDL-TG levels in the third and fourth quartiles.
Table 1A. Baseline characteristics and distribution of CVD risk factors across RLP-C quartiles.
| Characteristic | RLP-C Quartiles | P trend | |||
|---|---|---|---|---|---|
| Q1 (0.4–3.1) | Q2 (3.2–5.9) | Q3 (6.0–12.2) | Q4 (12.3–259.1) | ||
| Age (years) | 62.7 ± 5.8 | 62.6 ± 5.7 | 62.8 ± 5.6 | 62.8 ± 5.6 | 0.275 |
| Female (%) | 63.6 | 62.7 | 57.2 | 53.2 | <0.001 |
| African American (%) | 34.3 | 25.0 | 16.6 | 11.0 | <0.001 |
| BMI (kg/m2) | 27.5 ± 6.0 | 28.7 ± 5.8 | 29.3 ± 5.6 | 29.6 ± 4.9 | <0.001 |
| SBP (mmHg) | 125.9 ± 19.8 | 126.8 ± 19.1 | 127.5 ± 18.3 | 128.9 ± 18.2 | <0.001 |
| Hypertension (%) | 42.0 | 44.2 | 46.3 | 50.5 | <0.001 |
| Hypertensive medication user (%) | 36.4 | 40.4 | 41.6 | 45.5 | <0.001 |
| Diabetes (%) | 10.6 | 13.4 | 15.8 | 22.1 | <0.001 |
| Current smoking (%) | 15.6 | 14.7 | 14.2 | 12.5 | 0.002 |
| HDL-C (mg/dL) | 59.7 ± 17.0 | 54.0 ± 16.7 | 48.2 ± 14.5 | 41.2 ± 12.3 | <0.001 |
| LDL-C (mg/dL) | 115.5 ± 30.8 | 124.8 ± 32.7 | 126.6 ± 32.7 | 124.9 ± 35.9 | <0.001 |
| Total cholesterol (mg/dL) | 190.6 ± 32.5 | 200.6 ± 35.0 | 203.8 ± 34.9 | 211.9 ± 40.8 | <0.001 |
| Triglycerides (mg/dL) | 74 (61, 90) | 107 (91, 125) | 141 (119, 167) | 216 (176, 275) | <0.001 |
| Fasting glucose (mg/dL) | 102.3 ± 24.9 | 104.9 ± 25.3 | 107.6 ± 30.1 | 114.6 ± 38.8 | <0.001 |
| Statin user (%) | 6.2 | 8.9 | 9.9 | 12.8 | <0.001 |
| Cholesterol lowering medication user (%) | 7.9 | 10.7 | 12.7 | 17.5 | <0.001 |
| WBC | 5.7 (4.9, 6.9) | 5.9 (5.0, 7.2) | 6.3 (5.3, 7.4) | 6.4 (5.5, 7.6) | <0.001 |
| hs-CRP (mg/L) | 1.94 (0.87, 5.11) | 2.32 (1.04, 5.44) | 2.63 (1.20, 5.50) | 2.78 (1.29, 5.67) | <0.001 |
Data presented as means ± SD, median (25th percentile, 75th percentile), or percentages.
Association of RLP-C and LDL-TG with Other Lipids
As expected, RLP-C and LDL-TG showed strong positive correlations with TGs (r=0.85 and r=0.65, respectively; p<0.0001) (Table 2). RLP-C and LDL-TG were also positively associated with the cholesterol in small dense LDL (sdLDL-C) and with non-HDL-C, and were negatively correlated with HDL-C. RLP-C and LDL-TG were also correlated with each other (r=0.5108; p<0.0001).
Table 2. Spearman's correlation coefficients, including regression coefficients and intercepts, among CVD risk factors.
| RLP-C (mg/dL) | LDL-TG (mg/dL) | |||||
|---|---|---|---|---|---|---|
| Spearman's R (P-value) | Regression coefficient (95% CI) | Intercept (95% CI) | Spearman's R (P-value) | Regression coefficient (95% CI) | Intercept (95% CI) | |
| Triglycerides (mg/dL) | 0.8535 (<0.0001) | 0.103 (0.101, 0.105) | -4.860 (-5.137, -4.583) | 0.6425 (<0.0001) | 0.065 (0.063, 0.067) | 15.14 (14.79, 15.48) |
| Small dense LDL-C (mg/dL) | 0.5879 (<0.0001) | 0.256 (0.246, 0.266) | -1.303 (-1.783, -0.824) | 0.6968 (<0.0001) | 0.341 (0.333, 0.348) | 9.632 (9.262, 10.003) |
| Total cholesterol (mg/dL) | 0.2055 (<0.0001) | 0.067 (0.061, 0.074) | -3.798 (-5.064, -2.532) | 0.3947 (<0.0001) | 0.120 (0.114, 0.125) | 0.267 (-0.817, 1.352) |
| LDL-C (mg/dL) | 0.1083 (<0.0001) | 0.015 (0.010, 0.021) | 7.228 (6.478, 7.977) | 0.3491 (<0.0001) | 0.107 (0.101, 0.113) | 11.04 (10.29, 11.78) |
| HDL-C (mg/dL) | -0.4429 (<0.0001) | -0.227 (-0.240, -0.214) | 21.31 (20.61, 22.01) | -0.3117 (<0.0001) | -0.178 (-0.191, -0.166) | 33.44 (32.79, 34.10) |
| Non-HDL-C (mg/dL) | 0.3957 (<0.0001) | 0.108 (0.103, 0.114) | -6.536 (-7.429, -5.644) | 0.5316 (<0.0001) | 0.148 (0.143, 0.153) | 2.060 (1.316, 2.805) |
| Lp(a) (mg/dL) | -0.2231 (<0.0001) | -0.052 (-0.059, -0.044) | 11.11 (10.81, 11.42) | -0.0290 (0.0057) | -0.001 (-0.008, 0.006) | 24.41 (24.13, 24.69) |
Association of RLP-C and LDL-TG with Incident CVD
In quartile analyses (Figure 2), RLP-C showed a graded association with incident CVD, but no association with incident ischemic stroke. LDL-TG also showed a graded association with incident CVD, but its association with incident ischemic stroke was largely driven by LDL-TG levels in the highest quartile.
Figure 2. Kaplan–Meier survival analyses.
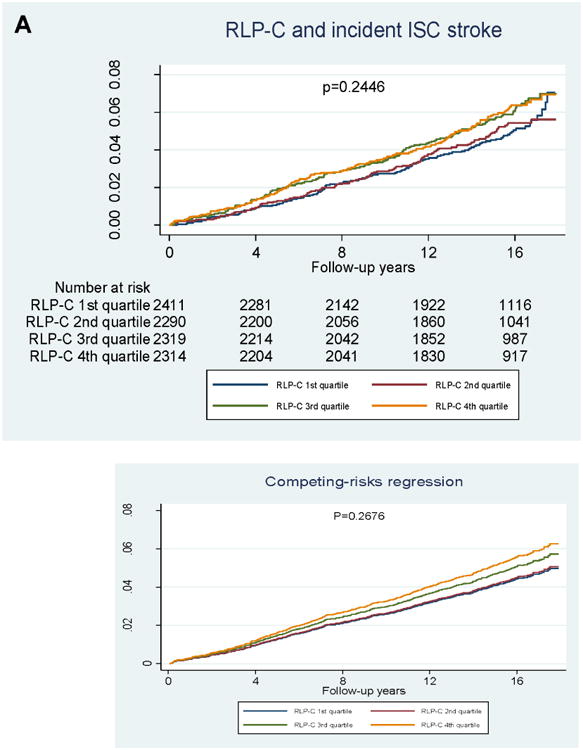
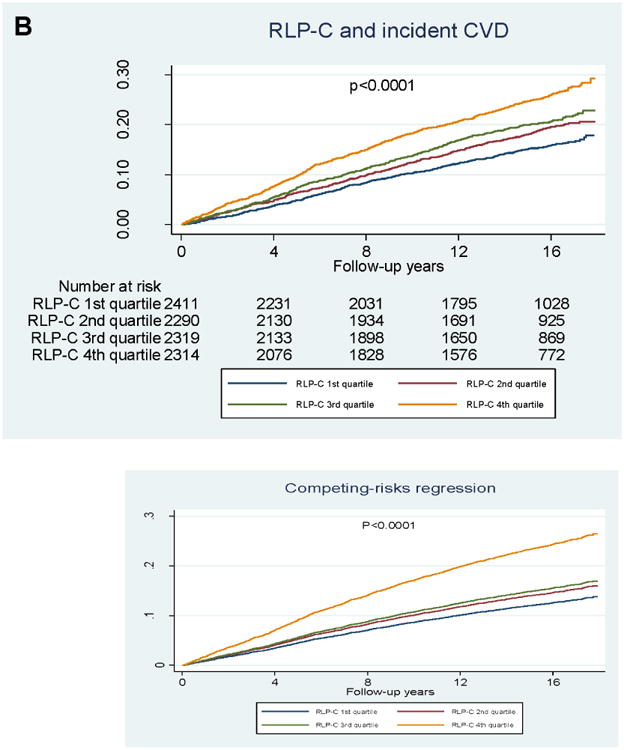
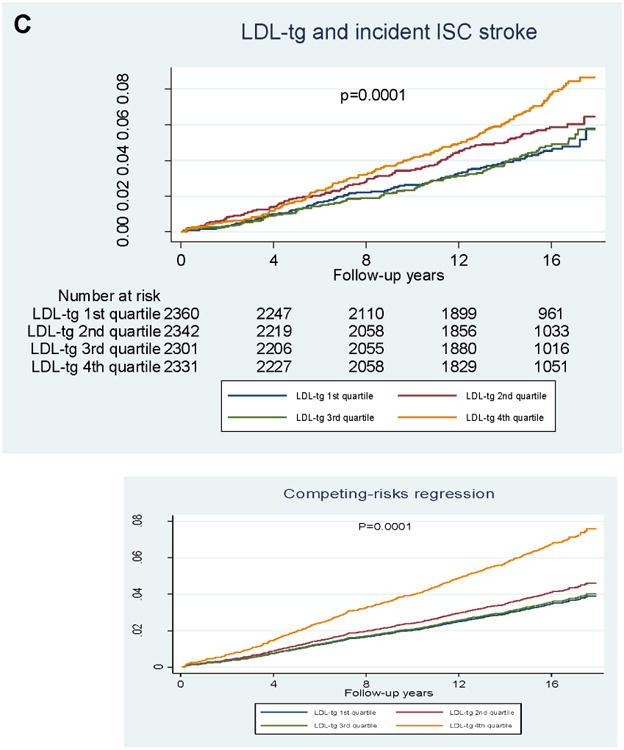
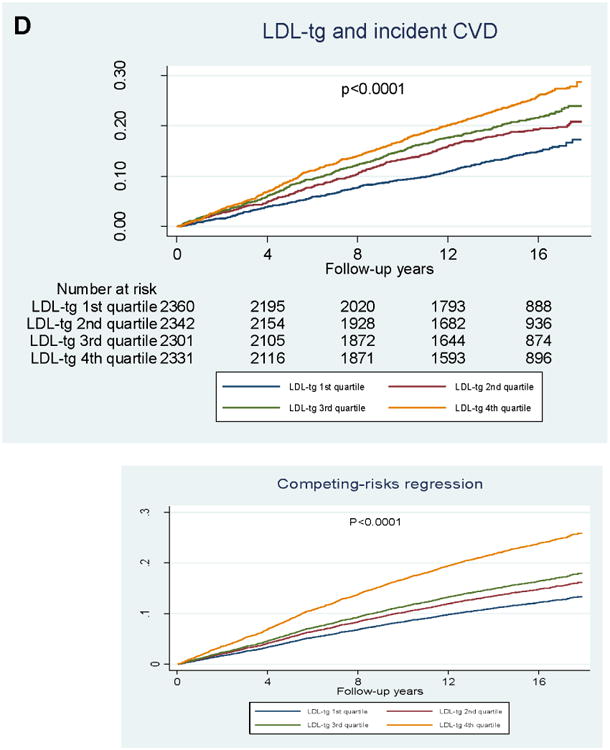
A) Incident ischemic stroke and RLP-C; B) incident CVD and RLP-C; C) incident ischemic stroke and LDL-TG; D) incident CVD and LDL-TG. P-values from logrank tests.
In the categorical analysis of RLP-C, risk for CHD, ischemic stroke, and CVD was significantly higher across increasing quartiles of RLP-C in model 1, but not after adjustment for PCE risk factors in model 2 (Table 3A). Similarly, RLP-C analyzed as a continuous variable was significantly associated with incident CHD (hazard ratio [HR] 1.26, 95% confidence interval [CI] 1.19–1.34; p<0.001) and ischemic stroke (HR 1.18, 95% CI 1.07–1.30; p<0.001) in model 1, but not with any outcome after adjustment for PCE risk factors (Table 3C). Additional adjustment for log-TGs (model 3) resulted in an inverse association of RLP-C with CVD risk (Table 3C). However, given the extremely high correlation between TG and RLP-C levels (Spearman r=0.8535), our risk prediction modeling was most likely impacted by multicollinearity.
Table 3A. Association of CHD, ischemic stroke, and CVD events competing with nonevent death across quartiles of RLP-C.
| Incident event | Model | RLP-C (mg/dL) | P trend of linearity | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| (0.4–3.1) N=2411 | (3.2–5.9) N=2290 | (6.0–12.2) N=2319 | (12.3–259.1) N=2314 | |||
| CHD | # CVD (%) | 267 (11.07) | 335 (14.63) | 357 (15.39) | 475 (20.53) | <0.001 |
| # Non-CVD death | 526 (21.82) | 469 (20.48) | 498 (21.47) | 447 (19.32) | NA | |
| Model 1 | Reference | 1.35 (1.15-1.59) | 1.38 (1.17-1.62) | 1.86 (1.60-2.17) | <0.0001 | |
| Model 2 | Reference | 1.10 (0.94-1.30) | 0.98 (0.82-1.16) | 1.06 (0.88-1.27) | 0.41 | |
| Ischemic stroke | # Ischemic stroke (%) | 113 (4.69) | 110 (4.80) | 130 (5.61) | 130 (5.62) | 0.31 |
| # Nonischemic stroke death (%) | 599 (24.84) | 544 (23.76) | 580 (25.01) | 600 (25.93) | NA | |
| Model 1 | Reference | 1.12 (0.86-1.46) | 1.38 (1.07-1.79) | 1.43 (1.10-1.86) | 0.02 | |
| Model 2 | Reference | 0.96 (0.73-1.26) | 1.16 (0.88-1.53) | 1.07 (0.78-1.45) | 0.54 | |
| CVD | # CVD (%) | 355 (14.72) | 414 (18.08) | 450 (19.40) | 566 (24.46) | <0.001 |
| # Non-CVD death | 482 (19.99) | 426 (18.60) | 451 (19.45) | 408 (17.63) | NA | |
| Model 1 | Reference | 1.28 (1.11-1.47) | 1.36 (1.18-1.57) | 1.77 (1.55-2.03) | <0.0001 | |
| Model 2 | Reference | 1.05 (0.91-1.22) | 0.99 (0.85-1.16) | 1.05 (0.89-1.23) | 0.77 | |
Data presented as hazard ratio (95% confidence interval) using the first (lowest) quartile as the referent. Model 1 was adjusted by age, gender, and race; model 2 (Pooled Cohort Equation model) was model 1 plus total cholesterol, HDL-C, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetic status.
Table 3C. Association of CHD, ischemic stroke, and CVD events with RLP-C and LDL-TG as continuous variables.
| Incident Event | Model | RLP-C | LDL-TG | ||
|---|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | P | Hazard ratio (95% confidence interval) | P | ||
| CHD | Model 1 | 1.26 (1.19–1.34) | <0.001 | 1.97 (1.73–2.24) | <0.001 |
| Model 2 | 0.99 (0.92–1.06) | 0.73 | 1.28 (1.10–1.50) | 0.002 | |
| Model 3 | 0.85 (0.76–0.96) | 0.008 | 1.27 (1.07–1.50) | 0.006 | |
| Ischemic stroke | Model 1 | 1.18 (1.07–1.30) | 0.001 | 1.64 (1.32–2.04) | <0.001 |
| Model 2 | 1.05 (0.93–1.18) | 0.46 | 1.47 (1.13–1.92) | 0.005 | |
| Model 3 | 0.82 (0.68–1.01) | 0.058 | 1.36 (1.01–1.82) | 0.040 | |
| CVD | Model 1 | 1.25 (1.19–1.32) | <0.001 | 1.94 (1.73–2.17) | <0.001 |
| Model 2 | 1.00 (0.94–1.06) | 0.97 | 1.35 (1.17–1.55) | <0.001 | |
| Model 3 | 0.84 (0.76–0.93) | 0.001 | 1.31 (1.13–1.53) | <0.001 | |
Data are presented as hazard ratio (per Ln unit increase for RLP-C and LDL-TG) and 95% confidence interval. Exposure values assessed as continuous variables. Model 1 was adjusted by age, gender, and race; model 2 (Pooled Cohort Equation model) was model 1 plus total cholesterol, HDL-C, systolic blood pressure, antihypertensive medication use, current smoking, and diabetes mellitus; model 3 was model 2 plus log-triglycerides.
For LDL-TG, risk for CHD, ischemic stroke, and CVD was significantly higher across increasing quartiles of LDL-TG in the categorical analysis, and the associations with ischemic stroke and CVD risk persisted after adjustment for PCE risk factors (Table 3B). In the continuous analysis, even after adjustment for PCE risk factors, LDL-TG was significantly associated with all outcomes: CHD (HR 1.28, 95% CI 1.10–1.50; p<0.002), ischemic stroke (HR 1.47, 95% CI 1.13–1.92; p<0.005), and CVD (HR 1.35, 95% CI 1.17–1.55; p<0.001) (Table 3c). Further adjustment for log-TGs (model 3) did not have a significant impact on the association of LDL-TG with CVD outcomes (Table 3C).
Table 3B. Association of CHD, ischemic stroke, and CVD events competing with nonevent death across quartiles of LDL-TG.
| Model | LDL-TG (mg/dL) | P trend of linearity | ||||
|---|---|---|---|---|---|---|
| Q1 (0.7–17) | Q2 (17.1–22.6) | Q3 (22.7–29.6) | Q4 (29.7–104) | |||
| CHD | n/N (%) | 257/2360 (10.89) | 326/2342 (13.92) | 403/2301 (17.51) | 448/2331 (19.22) | <0.001 |
| Model 1 | Reference | 1.30 (1.10-1.53) | 1.69 (1.45-1.98) | 2.02 (1.73-2.36) | <0.0001 | |
| Model 2 | Reference | 1.08 (0.91-1.28) | 1.22 (1.03-1.45) | 1.23 (1.02-1.47) | 0.07 | |
| Ischemic stroke | n/N (%) | 98/2360 (4.15) | 123/2342 (5.25) | 101/2301 (4.39) | 161/2331 (6.91) | <0.001 |
| Model 1 | Reference | 1.34 (1.03-1.75) | 1.13 (0.85-1.49) | 1.85 (1.43-2.38) | <0.0001 | |
| Model 2 | Reference | 1.28 (0.97-1.69) | 1.02 (0.75-1.38) | 1.58 (1.17-2.15) | 0.002 | |
| CVD | n/N (%) | 327/2360 (13.86) | 420/2342 (17.93) | 472/2301 (20.51) | 566/2331 (24.28) | <0.001 |
| Model 1 | Reference | 1.35 (1.17-1.56) | 1.59 (1.38-1.83) | 2.04 (1.78-2.34) | <0.0001 | |
| Model 2 | Reference | 1.16 (1.00-1.35) | 1.20 (1.03-1.40) | 1.33 (1.13-1.57) | 0.007 | |
Data presented as hazard ratio (95% confidence interval) using the first (lowest) quartile as the referent. Model 1 was adjusted by age, gender, and race; model 2 (Pooled Cohort Equation model) was model 1 plus total cholesterol, HDL-C, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetic status.
Table 4A. Log(RLP-C), single-variant meta-analysis of candidate genes.
| Gene | Name | rs | Meta-analysis | African Americans | European Americans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | Beta | SE | MAC | p | Beta | SE | maf | MAC | p | Beta | SE | maf | MAC | |||
| ANGPTL3 | 1:63063472:G:A | NA | 3.08E-02 | 1.985 | 0.919 | 1 | NA | NA | NA | NA | NA | 3.08E-02 | 1.985 | 0.919 | 0.0001 | 1 |
| ANGPTL3 | 1:63064415:G:A | rs144284900 | 3.68E-02 | -1.257 | 0.602 | 2 | 3.68E-02 | -1.257 | 0.602 | 0.0005 | 2 | NA | NA | NA | NA | NA |
| APOA5 | 11:116662407:G:C | rs3135506 | 6.73E-19 | 0.267 | 0.030 | 963 | 3.45E-03 | 0.180 | 0.062 | 0.0555 | 208 | 1.34E-17 | 0.295 | 0.035 | 0.0659 | 755 |
| APOA5 | 11:116661392:C:A | rs2075291 | 1.79E-04 | 0.837 | 0.223 | 15 | 7.29E-04 | 0.832 | 0.246 | 0.0032 | 12 | 1.05E-01 | 0.862 | 0.531 | 0.0003 | 3 |
| APOA5 | 11:116661001:G:A | rs143292359 | 1.52E-02 | 0.845 | 0.348 | 7 | NA | NA | NA | NA | NA | 1.52E-02 | 0.845 | 0.348 | 0.0006 | 7 |
| APOA5 | 11:116661346:C:T | rs780433260 | 4.35E-02 | -1.717 | 0.850 | 1 | 4.35E-02 | -1.717 | 0.850 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| APOA5 | 11:116661656:G:A | rs201079485 | 4.55E-02 | 1.300 | 0.650 | 2 | NA | NA | NA | NA | NA | 4.55E-02 | 1.300 | 0.650 | 0.0002 | 2 |
| APOC3 | 11:116701354:G:A | rs138326449 | 1.76E-10 | -1.164 | 0.182 | 25 | 1.28E-01 | -0.747 | 0.491 | 0.0008 | 3 | 3.76E-10 | -1.230 | 0.196 | 0.0019 | 22 |
| APOC3 | 11:116701353:C:T | rs76353203 | 3.29E-03 | -0.998 | 0.340 | 7 | 2.96E-01 | -0.629 | 0.602 | 0.0005 | 2 | 4.44E-03 | -1.170 | 0.411 | 0.0004 | 5 |
| APOC3 | 11:116701613:G:T | rs140621530 | 1.41E-02 | -0.863 | 0.352 | 6 | 1.25E-02 | -0.951 | 0.381 | 0.0013 | 5 | 7.04E-01 | -0.349 | 0.920 | 0.0001 | 1 |
| APOC3 | 11:116701608:G:T | NA | 4.77E-02 | -1.820 | 0.919 | 1 | NA | NA | NA | NA | NA | 4.77E-02 | -1.820 | 0.919 | 0.0001 | 1 |
| LIPC | 15:58723939:G:A | rs2070895 | 0.0732 | -0.033 | 0.018 | 4765. 336 | 0.0297 | -0.068 | 0.031 | 0.52 | 1788. 486 | 0.0608 | -0.036 | 0.019 | 0.216 | 2976 .85 |
| LIPC | 15:58855760:A:C | rs142036980 | 4.79E-03 | 0.735 | 0.261 | 11 | 8.55E-03 | 0.747 | 0.284 | 0.0024 | 9 | 3.05E-01 | 0.670 | 0.653 | 0.0002 | 2 |
| LIPC | 15:58833993:G:A | rs6078 | 1.77E-02 | -0.089 | 0.038 | 578 | 1.05E-01 | -0.089 | 0.055 | 0.0664 | 250 | 8.32E-02 | -0.089 | 0.052 | 0.0289 | 328 |
| LIPG | 18:47091689:A:G | NA | 2.45E-02 | 2.068 | 0.920 | 1 | NA | NA | NA | NA | NA | 2.45E-02 | 2.068 | 0.920 | 0.0001 | 1 |
| LIPG | 18:47110110:C:T | rs777816384 | 3.22E-02 | 1.970 | 0.920 | 1 | NA | NA | NA | NA | NA | 3.22E-02 | 1.970 | 0.920 | 0.0001 | 1 |
| LIPG | 18:47113195:G:A | NA | 3.98E-02 | 1.237 | 0.602 | 2 | 3.98E-02 | 1.237 | 0.602 | 0.0005 | 2 | NA | NA | NA | NA | NA |
| LIPG | 18:47109933:C:T | rs144717284 | 4.06E-02 | 1.232 | 0.602 | 2 | 4.06E-02 | 1.232 | 0.602 | 0.0005 | 2 | NA | NA | NA | NA | NA |
| LPL | 8:19819724:C:G | rs328 | 7.16E-16 | -0.206 | 0.026 | 1392 | 9.47E-03 | -0.141 | 0.054 | 0.0712 | 270 | 8.38E-15 | -0.225 | 0.029 | 0.0989 | 1122 |
| LPL | 8:19811733:G:A | rs118204057 | 4.35E-04 | 1.447 | 0.411 | 5 | NA | NA | NA | NA | NA | 4.35E-04 | 1.447 | 0.411 | 0.0004 | 5 |
| LPL | 8:19818441:C:T | rs141502542 | 8.14E-04 | 2.851 | 0.852 | 1 | 8.14E-04 | 2.851 | 0.852 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LPL | 8:19805708:G:A | rs1801177 | 1.96E-03 | 0.144 | 0.046 | 372 | 7.65E-02 | 0.115 | 0.065 | 0.0482 | 183 | 8.91E-03 | 0.173 | 0.066 | 0.0165 | 189 |
| LPL | 8:19809322:G:A | rs145657341 | 1.51E-02 | 2.235 | 0.920 | 1 | NA | NA | NA | NA | NA | 1.51E-02 | 2.235 | 0.920 | 0.0001 | 1 |
Meta-analysis p ≤0.05.
To assess the extent to which LDL-TG provides incremental value in the prediction of future CVD risk beyond circulating TG and apo B levels we performed AUC/NRI/IDI analyses (Online Table 4). Although improvements of the C-statistics are generally modest for each lipid trait added separately, LDL-TG does show greater improvement in the AUC (with significant effects on continuous NRI and IDI) compared to apoB and TGs. Furthermore, addition of LDL-TG to a PCE model including both apoB and TGs resulted in further improvement in the AUC for CVD risk prediction. The overall modest improvement in C-statistics of each of these lipid measures is not surprising given the traditional CVD lipid risk factors already included in the PCE model and the well-described phenomena of pleiotropy affecting various lipid traits.
Exome Analysis: Unbiased Approach
Using an unbiased approach, we assessed the association of nonsynonymous common variants by race (MAF >1%) and performed a meta-analysis. In the meta-analysis, 11 detected single variant–trait associations with RLP-C and LDL-TG reached predefined significance (p<2.5×10−8; Online Table 1), all in genes previously associated with other lipid traits, including sdLDL-C (19). Genetic variants associated with both RLP-C and LDL-TG tended to have the same direction of effect on both traits, except rs7412 in APOE.
We also assessed the association of nonsynonymous rare variants by race (MAF <1%) and performed a meta-analysis. A total of 13,690 genes contained ≥1 annotated nonsynonymous variant (MAF ≤1%) and cumulative minor allele count ≥3 in each race. Two aggregate gene-based tests, APOC3 for RLP-C and TARM1 for LDL-TG, reached predefined significance in the meta-analysis (p≤2.5×10−6; Online Table 2). The association with APOC3 was in a consistent direction in both races, with 3 nonsynonymous variants in APOC3 leading the association in the meta-analysis (p<0.05; Online Table 3). The single nonsynonymous variant (rs2361558) which was monomorphic in African Americans led to the association between the aggregated rare variants in TARM1 and LDL-TG levels (Online Table 4). The association of LDL-TG with genetic variants in TARM1 (20) may be important because of the potential link between remnant lipoproteins and the inflammatory response in the etiology of atherosclerotic CVD.
Exome Analysis: Candidate Gene Approach
Associations between RLP-C and LDL-TG levels and coding nonsynonymous and splicing common variants belonging to 7 candidate genes (LPL, LIPC, LIPG, APOC3, APOA5, ANGPTL3, ANGPTL4) were evaluated using single-variant analysis of whole exome sequencing data (Tables 4a and 4b). These candidate genes were selected because lipases and their activators and inhibitors play a key role in remnant lipoprotein metabolism. Not surprisingly, multiethnic meta-analysis showed significant associations between 2 common variants—rs3135506 (APOA5) and rs328 (LPL)—and both RLP-C and LDL-TG levels, in a consistent direction in both races (p<0.05 in both races), as well as between RLP-C and LDL-TG. Multiethnic meta-analysis showed relatively weak associations between a common LIPC variant (rs6078) and both RLP-C and LDL-TG levels, in a consistent direction in both races but different directions for RLP-C and LDL-TG. Since it was previously reported that rs2070895 in the promotor region of the hepatic lipase gene was the lead SNP associated with decreased hepatic lipase activity, we imputed rs2070895 in ARIC participants using the 1000 Genomes Project reference panel (21). Multiethnic meta-analysis showed a strong association between rs2070895 and higher LDL-TG levels in both races but no significant association between rs2070895 and RLP-C levels (Tables 4A and 4B).
Table 4B. Log(LDL-TG), single-variant meta-analysis of candidate genes.
| Gene | Name | rs | Meta-analysis | African Americans | European Americans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | Beta | SE | MAC | p | Beta | SE | maf | MAC | p | Beta | SE | maf | MAC | |||
| ANGPTL3 | 1:63067964:T:C | rs776441268 | 4.61E-03 | -1.168 | 0.412 | 1 | NA | NA | NA | NA | NA | 4.61E-03 | -1.168 | 0.412 | 0.0001 | 1 |
| ANGPTL4 | 19:8438715:C:T | rs769769905 | 2.26E-02 | 1.009 | 0.442 | 1 | 2.26E-02 | 1.009 | 0.442 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| APOA5 | 11:116662407:G:C | rs3135506 | 1.86E-07 | 0.073 | 0.014 | 960 | 3.84E-02 | 0.067 | 0.033 | 0.0550 | 201 | 1.69E-06 | 0.074 | 0.015 | 0.0663 | 759 |
| APOA5 | 11:116661656:G:A | rs201079485 | 5.60E-03 | 0.808 | 0.292 | 2 | NA | NA | NA | NA | NA | 5.60E-03 | 0.808 | 0.292 | 0.0002 | 2 |
| APOA5 | 11:116660870:C:T | rs774294731 | 3.70E-02 | 0.923 | 0.443 | 1 | 3.70E-02 | 0.923 | 0.443 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| APOA5 | 11:116661338:C:G | NA | 4.10E-02 | -0.640 | 0.313 | 2 | 4.10E-02 | -0.640 | 0.313 | 0.0006 | 2 | NA | NA | NA | NA | NA |
| APOC3 | 11:116701354:G:A | rs138326449 | 7.96E-04 | -0.279 | 0.083 | 25 | 7.85E-02 | -0.450 | 0.256 | 0.0008 | 3 | 3.27E-03 | -0.259 | 0.088 | 0.0019 | 22 |
| APOC3 | 11:116701613:G:T | rs140621530 | 3.62E-03 | -0.520 | 0.179 | 6 | 4.00E-03 | -0.570 | 0.198 | 0.0014 | 5 | 4.67E-01 | -0.300 | 0.413 | 0.0001 | 1 |
| APOC3 | 11:116701353:C:T | rs76353203 | 3.16E-02 | -0.342 | 0.159 | 7 | 6.59E-02 | -0.576 | 0.313 | 0.0005 | 2 | 1.58E-01 | -0.260 | 0.184 | 0.0004 | 5 |
| APOC3 | 11:116703578:CA:C | rs750185333 | 3.25E-02 | -0.946 | 0.443 | 1 | 3.25E-02 | -0.946 | 0.443 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LIPC | 15:58723939:G:A | rs2070895 | 3.48E-08 | 0.047 | 0.009 | 4704. 913 | 0.0665 | 0.029 | 0.016 | 0.518 | 1744. 309 | 1.84E-08 | 0.048 | 0.009 | 0.215 | 2960. 604 |
| LIPC | 15:58837989:G:A | rs200684324 | 5.52E-04 | 0.822 | 0.238 | 3 | NA | NA | NA | NA | NA | 5.52E-04 | 0.822 | 0.238 | 0.0003 | 3 |
| LIPC | 15:58860927:T:A | rs374799133 | 1.62E-03 | 1.300 | 0.412 | 1 | NA | NA | NA | NA | NA | 1.62E-03 | 1.300 | 0.412 | 0.0001 | 1 |
| LIPC | 15:58840562:G:A | rs200613217 | 9.60E-03 | 1.149 | 0.444 | 1 | 9.60E-03 | 1.149 | 0.444 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LIPC | 15:58833993:G:A | rs6078 | 2.10E-02 | 0.042 | 0.018 | 570 | 3.36E-02 | 0.061 | 0.029 | 0.0668 | 245 | 2.11E-01 | 0.029 | 0.023 | 0.0287 | 325 |
| LIPC | 15:58853143:G:A | rs746042863 | 3.04E-02 | 0.893 | 0.412 | 1 | NA | NA | NA | NA | NA | 3.04E-02 | 0.893 | 0.412 | 0.0001 | 1 |
| LIPC | 15:58855778:T:C | rs761668960 | 4.95E-02 | 0.811 | 0.413 | 1 | NA | NA | NA | NA | NA | 4.95E-02 | 0.811 | 0.413 | 0.0001 | 1 |
| LIPC | 15:58861013:G:A | NA | 4.95E-02 | -0.810 | 0.412 | 1 | NA | NA | NA | NA | NA | 4.95E-02 | -0.810 | 0.412 | 0.0001 | 1 |
| LPL | 8:19819724:C:G | rs328 | 1.44E-06 | -0.057 | 0.012 | 1378 | 3.92E-03 | -0.082 | 0.028 | 0.0720 | 266 | 6.92E-05 | -0.052 | 0.013 | 0.0982 | 1112 |
| LPL | 8:19818441:C:T | rs141502542 | 6.15E-04 | 1.518 | 0.443 | 1 | 6.15E-04 | 1.518 | 0.443 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LPL | 8:19805708:G:A | rs1801177 | 3.02E-03 | 0.067 | 0.022 | 367 | 2.03E-01 | 0.044 | 0.035 | 0.0473 | 175 | 4.93E-03 | 0.083 | 0.029 | 0.0168 | 192 |
| LPL | 8:19811796:GA:G | NA | 1.09E-02 | 1.050 | 0.412 | 1 | NA | NA | NA | NA | NA | 1.09E-02 | 1.050 | 0.412 | 0.0001 | 1 |
| LPL | 8:19811733:G:A | rs118204057 | 1.64E-02 | 0.443 | 0.184 | 5 | NA | NA | NA | NA | NA | 1.64E-02 | 0.443 | 0.184 | 0.0004 | 5 |
| LPL | 8:19805745:A:T | rs367924602 | 2.57E-02 | -0.460 | 0.206 | 4 | NA | NA | NA | NA | NA | 2.57E-02 | -0.460 | 0.206 | 0.0003 | 4 |
| LPL | 8:19819645:G:A | rs149089920 | 3.70E-02 | 0.923 | 0.443 | 1 | 3.70E-02 | 0.923 | 0.443 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LPL | 8:19797000:A:G | rs756418111 | 3.93E-02 | -0.914 | 0.444 | 1 | 3.93E-02 | -0.914 | 0.444 | 0.0003 | 1 | NA | NA | NA | NA | NA |
| LPL | 8:19816887:A:G | rs300 | 4.80E-02 | 0.151 | 0.076 | 34 | 4.78E-02 | 0.154 | 0.078 | 0.0091 | 33 | 8.59E-01 | 0.073 | 0.412 | 0.0001 | 1 |
Meta-analysis p ≤0.05.
Association of RLP-C and LDL-TG with Genetic Variants of APOE
Our unbiased approach showed the most significant associations with genetic variants at the APOE locus, particularly rs7412. ApoE has high affinity for the LDL (apoB/E) receptor as well as other hepatic receptors and plays an important role in clearance of remnant lipoproteins from the circulation (22). The three common allelic variants of APOE (APOE ε2, APOE ε3, and APOE ε4) have genotype-specific effects on TG and total cholesterol levels (23).
Since rs7412 defines APOE ε2 allele status, we assessed APOE haplotypes and found that APOE ε2/2 was associated with reduced LDL-TG and increased RLP-C (p<0.0001 vs any other haplotype; Figure 3). Furthermore, rs7412 was significantly associated with increased TG and HDL-C levels, and with decreased LDL-TG, LDL-C, total cholesterol, non-HDL-C, and Lp(a) levels (Table 5).
Figure 3. Associations of apoE haplotypes with log LDL-TG (left) and RLP-C (right).
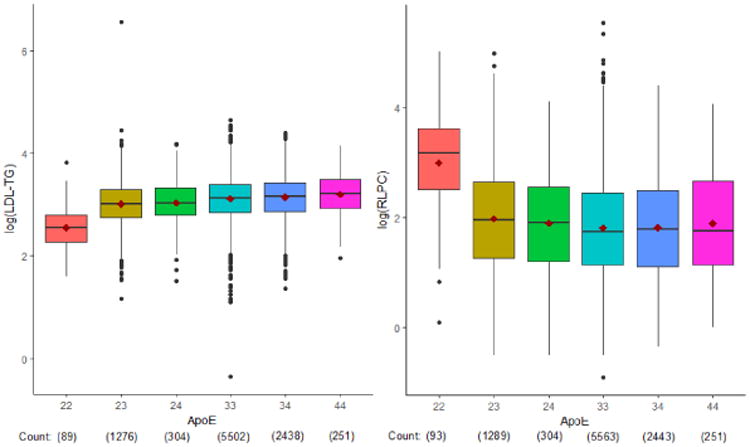
Associations between LDL-TG and RLP-C levels and genetic variants were assessed by whole exome sequencing. A common APOE variant, rs7412, had the strongest association with LDL-TG and RLP-C (p<5×10−8). Since rs7412 defines APOE e2 allele status, we assessed APOE haplotypes and found that APOE e2/2 was associated with reduced LDL-TG and increased RLP-C (p<0.0001 vs any other haplotype). The different relationships of RLP-C and LDL-TG with APOE ε2/2 may be partly explained by the low affinity of apoE2 for the LDL (apoB/E) receptor, potentially leading to delayed clearance of remnant particles. This delayed clearance may lead to increased RLP-C levels, while simultaneously lowering LDL and LDL-TG levels via upregulation of cellular LDL receptors in these individuals.
Table 5. Associations between rs7412 (reference/alternative alleles: C/T, MAF=0.08 in European Americans, MAF=0.11 in African Americans) and lipids.
| Trait | Meta-Analysis | European Americans | African Americans | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | p | Beta | SE | p | Beta | SE | p | |
| Log RLPC | 0.267 | 0.02 | 2.64×10-32 | 0.264 | 0.027 | 6.01×10-23 | 0.275 | 0.042 | 6.10×10-11 |
| Log TG | 0.062 | 0.01 | 3.27×10-09 | 0.080 | 0.01 | 3.88×10-10 | 0.025 | 0.018 | 0.177279 |
| HDL-C | 1.170 | 0.32 | 0.0003 | 0.827 | 0.38 | 0.0273 | 2.193 | 0.647 | 7.07×10-4 |
| HDL-2 | 0.044 | 0.01 | 0.0003 | 0.032 | 0.02 | 0.00901 | 0.070 | 0.022 | 9.00×10-05 |
| HDL-3 | 0.463 | 0.22 | 0.0337 | 0.315 | 0.25 | 0.2113 | 0.909 | 0.436 | 0.0372 |
| Log LDL-TG | -0.139 | 0.01 | 5.68×10-39 | -0.138 | 0.012 | 3.39×10-30 | -0.140 | 0.022 | 2.35×10-10 |
| LDL-C | -16.581 | 0.817 | 1.20×10-91 | -16.170 | 0.94 | 5.78×10-66 | -17.815 | 1.64 | 1.22×10-27 |
| TC | -0.327 | 0.02 | 5.39×10-49 | -0.304 | 0.03 | 3.22×10-32 | -0.393 | 0.04 | 4.07×10-19 |
| Non-HDL-C | -0.358 | 0.02 | 1.83×10-52 | -0.326 | 0.03 | 1.22×10-32 | -0.447 | 0.05 | 1.15×10-22 |
| Lp(a) | -0.139 | 0.03 | 3.40×10-10 | -0.12 | 0.03 | 3.66×10-05 | -0.165 | 0.03 | 1.32×10-06 |
Discussion
Although both RLP-C and LDL-TG were strongly associated with TGs, as expected, they had different associations with incident CVD events in up to 16 years of follow-up in the ARIC study. Both RLP-C and LDL-TG were associated with incident CVD events in minimally adjusted models, but only LDL-TG remained significantly associated with incident CHD and ischemic stroke in models adjusted for traditional PCE risk factors. With further adjustment for TGs and hs-CRP, LDL-TG remained significantly associated with CVD events (HR 1.26, 95% CI 1.08–1.47; p=0.003). In the genetic analyses, a common APOE variant had the strongest association with both RLP-C and LDL-TG, but individuals with ε2/2 had decreased LDL-TG and increased RLP-C.
RLP-C and CVD
Unlike in previous studies, RLP-C was quantified directly using a fully automated detergent-based homogenous assay. Numerous studies suggest that high RLP-C concentrations increase risk for atherosclerosis and CHD (24,25).
As in prior studies (24,27,28), in ARIC RLP-C was significantly correlated with elevated TGs and diabetes at baseline. While RLP-C was also significantly associated with incident CVD in a basic model adjusted for age, gender, and race, after adjustment for traditional CVD risk factors including total cholesterol, HDL-C, diabetes, and antihypertensive medication use, RLP-C was not significantly associated with incident CVD events.
In an analysis of genetic variants affecting single lipoprotein classes, including nonfasting remnants, HDL-C, and LDL-C, the causal odds ratio for ischemic heart disease was 2.8 per 39-mg/dL increase in nonfasting remnant cholesterol levels (7). However, in contrast to our study, remnant cholesterol was calculated, as total cholesterol minus HDL-C and directly measured LDL-C.
In a biracial cohort from the Jackson Heart Study and Framingham Offspring Cohort Study, RLP-C was positively associated with incident CHD in unadjusted models (8). Lipoproteins were classified by ultracentrifugation, and RLP-C was determined by the sum of cholesterol in the densest VLDL subfraction (VLDL3-C) and IDL-C. After adjustment for HDL-C and LDL-C levels, the association of RLP-C with CHD was not significant, similar to in our study, which directly quantified fasting RLP-C.
LDL-TG and CVD
To our knowledge, our study is the first to report significant associations of LDL-TG with both ischemic stroke and CHD. Few data are available on clinical utility of LDL-TG levels in CVD risk prediction, possibly because of the complexity of measuring LDL-TG (27). In a cross-sectional study of cases with stable CHD, in which LDL-TG was measured after fractionation of LDL by equilibrium density-gradient centrifugation, altered LDL metabolism characterized by high LDL-TG was correlated with prevalent CHD and systemic low-grade inflammation independent of LDL-C (29). Our results corroborate and extend these findings in a large population without clinical CHD and demonstrate that high LDL-TG levels measured by a validated automated assay (14) are associated with incident stroke and CHD after adjustment for traditional risk factors including total cholesterol and HDL-C. Furthermore, in our study we found that individuals with elevated LDL-TG and RLP-C levels also had increased levels of inflammatory markers hs-CRP and white blood cell count.
In a secondary analysis of the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial (27), LDL-TG failed to predict CVD events including stroke. AIM-HIGH was a secondary-prevention trial in 3094 patients on statin therapy, predominantly white men, with a mean 3-year follow-up. By comparison, the ARIC cohort is larger and biracial, with a longer follow-up of up to 16 years.
Prior studies evaluating the relationship between lipids and stroke risk have shown varied associations (30-32). Interestingly, data now suggest that TG level is independently associated with the stroke risk, and that this association is stronger in women than men (33). Although men have higher TG levels than women, we observed that women had higher LDL-TG levels than men, which may be one reason high TG level had a stronger association with stroke in women than men.
Arterial disease may differ among vascular beds, particularly smaller arteries and arterioles (34). Plaque composition in the smaller cerebral arteries suggests a more fibrotic process than in the coronary arteries, which have more lipid-rich cores and typical atheromatous lesions (35). In addition, arteriolar lesions are characterized by hyalinosis instead of lipid. The associations of higher LDL-TG level with increased hs-CRP level and white blood cell count may reflect an adverse impact on inflammation, which may lead to more cerebrovascular disease.
Exome Analysis: Unbiased Approach
Our exome chip survey showed 11 common (MAF >1%) nonsynonymous variant–trait associations, all detected variants of genes previously associated with lipid CVD risk factors, including TG, total cholesterol, HDL-C, LDL-C, and sdLDL-C. In exome analysis of rare variants, aggregated variants of TARM1 and APOC3 were also associated with decreased levels of LDL-TG and RLP-C, respectively. The association of LDL-TG levels with genetic variants in TARM1 has not been previously reported. TARM1 encodes a novel costimulator of proinflammatory cytokine secretion by macrophages and neutrophils (20) and may provide a link between LDL-TG and chronic low-grade inflammation underlying CVD progression. ApoCIII inhibits lipolysis by LPL and can delay clearance of atherogenic lipoproteins (36). APOC3 loss-of function variants are associated with lower TG and sdLDL-C levels, higher HDL-C levels, reduced postprandial lipemia, and reduced CHD risk (37). Our findings that APOC3 loss-of-function variants are associated with decreased RLP-C and LDL-TG levels support these previous reports. Notably, a gain-of-function variant of LPL (rs328), identified by both the unbiased approach and the candidate gene approach, was strongly associated with lower RLP-C and LDL-TG levels in our study. This well-known missense variant has been associated with lower TG and increased HDL-C levels (38) and reduced CHD (39).
Exome Analysis: Candidate Gene Approach
Our candidate gene approach showed significant associations between common variants in APOA5 and LPL and circulating RLP-C and LDL-TG levels. ApoAV is postulated to regulate plasma TG levels by enhancing TGRL catabolism by LPL (40) or by inhibiting VLDL synthesis (41). The highly statistically significant variant–trait associations for LPL variants in both unbiased and candidate gene approaches may indicate the importance of LPL as the rate-limiting enzyme for hydrolysis of circulating TGs. We found weaker associations between a common LIPC variant (rs6078) and RLP-C and LDL-TG levels. However, a strong association was found between rs2070895 and LDL-TG levels. rs2070895 is located in the promotor region of the hepatic lipase gene and was previously found to be associated with decreased hepatic lipase activity (42). Hepatic lipase plays an important role in the lipolytic conversion of VLDL to LDL, a process modulated by HDL composition (43). Mutations in the hepatic lipase gene were associated with increased ischemic heart disease risk in the Copenhagen City Heart Study (44). Complete deficiency of hepatic lipase has also been linked with impaired catabolism and accumulation of remnant particle RLPC as well as increased TG content of LDL (42).
ApoE and CVD
A novel aspect of this study is the identification of genetic variants associated with RLP-C and LDL-TG, including the APOE variant rs7412. A review of epidemiologic studies of APOE polymorphism and CHD estimated that ∼6% of the variation in CHD risk in North Americans is attributable to this locus (45). Most genotyping assays used in population studies did not include rs7412, but the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium recently demonstrated that APOE ε2 was associated with reduced subclinical atherosclerosis assessed by carotid intima–media thickness and coronary calcium scores and also with clinical CHD (46). Previous studies also suggest a protective effect of APOE ε2 on atherosclerosis (47,48), despite the association between apoE2/2 and type III hyperlipoproteinemia (47), which is characterized by accumulated remnant lipoproteins with resulting increased blood TG and cholesterol levels.
In our cohort, the APOE variant rs7412 was significantly associated with LDL-TG and RLP-C in both races. Further, APOE ε2/2 was associated with higher RLP-C and TG levels, but lower LDL-TG levels. The different relationships of RLP-C and LDL-TG with APOE ε2/2 may be explained in part by the low affinity of apoE2 for the LDL (apoB/E) receptor, potentially leading to delayed clearance of VLDL and chylomicron remnants (49). The slower removal of remnant particles may lead to increased RLP-C levels in the circulation, while the reduced uptake of RLP-C via the LDL receptor may simultaneously upregulate cellular LDL receptors, leading to increased removal of LDL and thus lower LDL-TG in these individuals. We propose a model in which defective TGRL catabolism, with subsequent increased TGRL remnants, in the presence of delayed LDL catabolism leads to increased LDL-TG levels through interaction with cholesteryl ester transfer protein. Although RLP-C and LDL-TG may both be considered markers of remnant lipoprotein metabolism (Central Illustration), our data suggest that LDL-TG may be a more important marker of atherogenic altered remnant/LDL metabolism not detected by a routine lipid profile. Indeed, although most circulating TGs are in chylomicron and VLDL remnants, the relatively short half-life of these particles compared with that of LDL may render remnant particles (or measures of their lipid content, such as cholesterol or TGs) less useful as cardiovascular risk markers. Alternatively, LDL-TG may represent a lipoprotein subfraction with specific proatherogenic properties. Therapies that lower LDL-C by enhanced LDL receptor-mediated clearance (e.g., statins, ezetimibe, proprotein convertase subtilisin/kexin type 9 inhibitors) would also be expected to lower LDL-TG levels. An alternative approach suggested by the genetic observations is to use therapies that inhibit apoCIII or activate LPL to clear TGRLs more rapidly, which would also be expected to lower LDL-TG levels. Future studies are needed to determine whether the relationship between LDL-TG and cardiovascular outcomes is causal, and if so, which therapies may be most effective.
Central Illustration. Remnant lipoprotein metabolism.
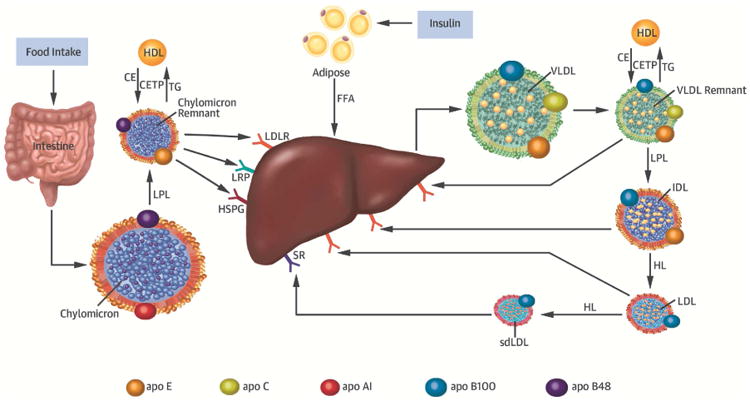
Chylomicrons secreted from the intestine and very low density lipoprotein (VLDL) secreted from the liver are lipolyzed by lipoprotein lipase (LPL), leading to triglyceride-rich lipoprotein (TGRL) remnants. Chylomicron secretion is largely regulated by food intake, whereas VLDL secretion is controlled by insulin. Remnant particles undergo remodeling via the enzymatic action of cholesteryl ester transfer protein (CETP) with high-density lipoprotein (HDL), hepatic lipase (HL), and the exchange of soluble apolipoproteins such as E, C-I, C-II, and C-III. TGRL remnants are cleared from the circulation via receptor-mediated uptake involving the low-density lipoprotein (LDL) receptor (LDLR), LDL receptor–like protein (LRP), and heparan sulfate proteoglycans (HSPG). Chylomicron remnants and VLDL remnants compete for the same lipolytic pathway, a process mediated by apoE. While chylomicron remnant clearance may be mediated by LDLR-, LRP-, or HSPG, VLDL remnants are believed to be predominantly cleared via LDLR. Individuals with apoE2 isoforms have reduced remnant clearance and are postulated to have compensatory upregulation of cellular LDLR expression that may lead to decreased LDL-TG and LDL-C levels. The purported role of HL in the lipolytic conversion of IDL to LDL may at least partly explain why individuals with decreased HL activity due to genetic variation in the LIPC gene (e.g., rs 2070895) have elevated LDL-TG levels.
Strengths and Limitations
Strengths of the current study include a large, well-characterized, biracial population followed for up to 16 years in a study designed to examine CVD incidence and risk factors, and the use of a homogenous assay to measure RLP-C and LDL-TG directly. A limitation is measurement at only one time point using frozen plasma samples. Also, despite adjustments, residual confounding is possible and the relationships are, at best, associations.
Conclusions
Although elevated TGs were associated with increased RLP-C and LDL-TG, only LDL-TG predicted CVD risk in models adjusted for traditional risk factors. APOE variants were associated with RLP-C and LDL-TG, but individuals with ε2/2 had decreased LDL-TG and increased RLP-C. Further research is needed to determine whether LDL-TG plays a causal role in CVD and may be a target for therapy.
Supplementary Material
Online Table 1. Common variants (MAF >1%) significantly associated in meta-analysis (p ≤ 2.5E-8)
Online Table 2. T1 results (MAF ≤1%, MAC ≥3 in both AA and EA) significantly associated in meta-analysis (p ≤1.83E-6)
Online Table 3. Rare nonsynonymous and splicing exonic variants in TARM1 and APOC3.
Online Table 4. Comparisons of PCE model and PCE plus apoB, triglycerides, or LDL-TG with differences in AUC, NRI, and IDI for risk prediction of CVD
Table 1B. Baseline characteristics and distribution of CVD risk factors across LDL-TG quartiles.
| Characteristic | LDL-TG Quartiles | P trend | |||
|---|---|---|---|---|---|
| Q1 (0.7-17.0) | Q2 (17.1-22.6) | Q3 (22.7-29.6) | Q4 (29.7-104.0) | ||
| Age (years) | 62.5 ± 5.7 | 62.6 ± 5.8 | 62.8 ± 5.6 | 63.0 ± 5.6 | 0.001 |
| Female (%) | 57.1 | 56.4 | 56.9 | 66.4 | <0.001 |
| African American (%) | 31.7 | 22.3 | 17.9 | 15.2 | <0.001 |
| BMI (kg/m2) | 27.8 ± 5.8 | 28.6 ± 5.7 | 28.9 ± 5.5 | 29.6 ± 5.4 | <0.001 |
| SBP (mmHg) | 126.0 ± 19.6 | 126.4 ± 19.0 | 127.1 ± 18.2 | 129.5 ± 18.6 | <0.001 |
| Hypertension (%) | 42.4 | 44.3 | 46.2 | 50.2 | <0.001 |
| Hypertensive medication user (%) | 36.4 | 39.6 | 42.3 | 45.4 | <0.001 |
| Diabetes (%) | 11.6 | 14.5 | 15.1 | 20.6 | <0.001 |
| Current smoking (%) | 11.7 | 15.2 | 16.2 | 14.0 | 0.014 |
| HDL-C (mg/dL) | 58.7 ± 18.2 | 52.2 ± 16.5 | 47.4 ± 15.1 | 45.0 ± 13.4 | <0.001 |
| LDL-C (mg/dL) | 108.0 ± 29.7 | 119.0 ± 29.7 | 126.9 ± 31.2 | 138.4 ± 34.8 | <0.001 |
| Total cholesterol (mg/dL) | 184.6 ± 32.7 | 194.7 ± 32.0 | 204.6 ± 33.0 | 222.9 ± 37.3 | <0.001 |
| Triglycerides (mg/dL) | 79 (62, 105) | 105 (83, 137) | 134 (108, 177) | 182 (142, 240) | <0.001 |
| Fasting glucose (mg/dL) | 102.5 ± 24.5 | 106.6 ± 30.6 | 108.1 ± 29.9 | 111.9 ± 35.8 | <0.001 |
| Statin user (%) | 6.4 | 7.8 | 10.6 | 12.9 | <0.001 |
| Cholesterol-lowering medication user (%) | 8.2 | 9.8 | 13.8 | 16.9 | <0.001 |
| WBC | 5.7 (4.8, 6.8) | 6.0 (5.1, 7.2) | 6.3 (5.3, 7.4) | 6.5 (5.4, 7.7) | <0.001 |
| hs-CRP (mg/L) | 1.67 (0.79, 4.44) | 2.10 (1.04, 4.97) | 2.58 (1.20, 5.45) | 3.53 (1.56, 6.74) | <0.001 |
Data presented as means ± SD, median (25th percentile, 75th percentile), or percentages.
Clinical Perpectives.
Competencies in Medical Knowledge
Although both remnant-like particle cholesterol (RLP-C) and low-density lipoprotein triglyceride (LDL-TG) levels correlate with triglyceride (TG) levels and incident cardiovascular events, after adjusting for traditional risk factors only LDL-TG predicts incident coronary heart disease and ischemic stroke. Hence, for risk assessment in a primary-prevention setting, measurement of LDL-TG provides additional information beyond traditional risk factors and lipid levels.
Translational Outlook
Prospective clinical trials should examine whether pharmacotherapies that reduce LDL-TG reduce ischemic events.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Funding support for “Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium” was provided by the NIH through the American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2HL102419). Sequencing was carried out at the Baylor College of Medicine Human Genome Sequencing Center (U54 HG003273).
Abbreviations
- apo
apolipoprotein
- ARIC
Atherosclerosis Risk in Communities
- CHD
coronary heart disease
- CVD
cardiovascular disease
- hs-CRP
high-sensitivity C-reactive protein
- LDL-TG
low-density lipoprotein triglycerides
- MAF
minor allele frequency
- RLP-C
remnant-like particle cholesterol
- TG
triglyceride
- TGRL
triglyceride-rich lipoprotein
Footnotes
Disclosures: Dr. Hoogeveen has received a research grant from Denka Seiken. Denka Seiken had no role in the design, analysis, or data interpretation of this study. The other authors have declared no relationship with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81:7b–12b. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 3.Boullart AC, de Graaf J, Stalenhoef AF. Serum triglycerides and risk of cardiovascular disease. Biochim Biophys Acta. 2012;1821:867–75. doi: 10.1016/j.bbalip.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Irvin MR, Zhi D, Joehanes R, et al. Epigenome-wide association study of fasting blood lipids in the Genetics of Lipid-lowering Drugs and Diet Network study. Circulation. 2014;130:565–72. doi: 10.1161/CIRCULATIONAHA.114.009158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res. 2016;118:579–85. doi: 10.1161/CIRCRESAHA.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varbo A, Nordestgaard BG. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2016;36:2133–2135. doi: 10.1161/ATVBAHA.116.308305. [DOI] [PubMed] [Google Scholar]
- 7.Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–36. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 8.Joshi PH, Khokhar AA, Massaro JM, et al. Remnant lipoprotein cholesterol and incident coronary heart disease: The Jackson Heart and Framingham Offspring Cohort Studies. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.Rosamond WD, Chambless LE, Folsom AR, et al. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N Engl J Med. 1998;339:861–7. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–43. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 12.Sharrett AR, Patsch W, Sorlie PD, Heiss G, Bond MG, Davis CE. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study Arterioscler Thromb. 1994;14:1098–104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Hirao Y. Development of a novel homogeneous assay for remnant like particle cholesterol (abstract 484) Atheroscler Suppl. 2011;12:103. [Google Scholar]
- 14.Ito Y, Ohta M, Ikezaki H, et al. Development and population results of a fully automated homogeneous assay for LDL triglyceride. J Appl Lab Med. 2018;2:746–756. doi: 10.1373/jalm.2017.024554. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge MN, Wang M, Wu Y, et al. Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol. 2011;12:R68. doi: 10.1186/gb-2011-12-7-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challis D, Yu J, Evani US, et al. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinformatics. 2012;13:8. doi: 10.1186/1471-2105-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Jian X, Boerwinkle E. dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum Mutat. 2013;34:E2393–402. doi: 10.1002/humu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoogeveen RC, Gaubatz JW, Sun W, et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014;34:1069–77. doi: 10.1161/ATVBAHA.114.303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radjabova V, Mastroeni P, Skjodt K, et al. TARM1 is a novel leukocyte receptor complex-encoded ITAM receptor that costimulates proinflammatory cytokine secretion by macrophages and neutrophils. J Immunol. 2015;195:3149–59. doi: 10.4049/jimmunol.1401847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.1000 Genomes Project Consortium. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117:94–8. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maxwell TJ, Ballantyne CM, Cheverud JM, Guild CS, Ndumele CE, Boerwinkle E. APOE modulates the correlation between triglycerides, cholesterol, and CHD through pleiotropy, and gene-by-gene interactions. Genetics. 2013;195:1397–405. doi: 10.1534/genetics.113.157719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara JR, Shah PK, Nakajima K, et al. Remnant-like particle (RLP) cholesterol is an independent cardiovascular disease risk factor in women: results from the Framingham Heart Study. Atherosclerosis. 2001;154:229–36. doi: 10.1016/s0021-9150(00)00484-6. [DOI] [PubMed] [Google Scholar]
- 25.Devaraj S, Vega G, Lange R, Grundy SM, Jialal I. Remnant-like particle cholesterol levels in patients with dysbetalipoproteinemia or coronary artery disease. Am J Med. 1998;104:445–50. doi: 10.1016/s0002-9343(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 26.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547–63. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 27.Albers JJ, Slee A, Fleg JL, O'Brien KD, Marcovina SM. Relationship of baseline HDL subclasses, small dense LDL and LDL triglyceride to cardiovascular events in the AIM-HIGH clinical trial. Atherosclerosis. 2016;251:454–9. doi: 10.1016/j.atherosclerosis.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaefer EJ, McNamara JR, Shah PK, et al. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25:989–94. doi: 10.2337/diacare.25.6.989. [DOI] [PubMed] [Google Scholar]
- 29.Marz W, Scharnagl H, Winkler K, et al. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2004;110:3068–74. doi: 10.1161/01.CIR.0000146898.06923.80. [DOI] [PubMed] [Google Scholar]
- 30.Prospective studies collaboration. Cholesterol, diastolic blood pressure, and stroke: 13,000 strokes in 450,000 people in 45 prospective cohorts. Lancet. 1995;346:1647–53. [PubMed] [Google Scholar]
- 31.Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ. 1994;309:11–5. doi: 10.1136/bmj.309.6946.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–62. doi: 10.1212/01.wnl.0000254472.41810.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 34.Shahar E, Chambless LE, Rosamond WD, et al. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–31. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 35.Puddu P, Puddu GM, Bastagli L, Massarelli G, Muscari A. Coronary and cerebrovascular atherosclerosis: two aspects of the same disease or two different pathologies? Arch Gerontol Geriatr. 1995;20:15–22. doi: 10.1016/0167-4943(94)00600-c. [DOI] [PubMed] [Google Scholar]
- 36.Pollin TI, Damcott CM, Shen H, et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng C, Khoo C, Furtado J, Sacks FM. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation. 2010;121:1722–34. doi: 10.1161/CIRCULATIONAHA.109.875807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentley AR, Chen G, Shriner D, et al. Gene-based sequencing identifies lipid-influencing variants with ethnicity-specific effects in African Americans. PLoS Genet. 2014;10:e1004190. doi: 10.1371/journal.pgen.1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khera AV, Won HH, Peloso GM, et al. Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317:937–946. doi: 10.1001/jama.2017.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkel M, Loeffler B, Kluger M, et al. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280:21553–60. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 41.Schaap FG, Rensen PC, Voshol PJ, et al. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J Biol Chem. 2004;279:27941–7. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 42.Deeb SS, Zambon A, Carr MC, Ayyobi AF, Brunzell JD. Hepatic lipase and dyslipidemia: interactions among genetic variants, obesity, gender, and diet. J Lipid Res. 2003;44:1279–86. doi: 10.1194/jlr.R200017-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee C, Sparks DL. Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am J Pathol. 2011;178:1429–33. doi: 10.1016/j.ajpath.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen RV, Wittrup HH, Tybjaerg-Hansen A, Steffensen R, Schnohr P, Nordestgaard BG. Hepatic lipase mutations,elevated high-density lipoprotein cholesterol, and increased risk of ischemic heart disease: the Copenhagen City Heart Study. J Am Coll Cardiol. 2003;41:1972–82. doi: 10.1016/s0735-1097(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 45.Sing CF, Moll PP. Genetics of variability of CHD risk. Int J Epidemiol. 1989;18:S183–95. [PubMed] [Google Scholar]
- 46.Natarajan P, Bis JC, Bielak LF, et al. Multiethnic exome-wide association study of subclinical atherosclerosis. Circ Cardiovasc Genet. 2016;9:511–520. doi: 10.1161/CIRCGENETICS.116.001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Pablos-Mendez A, Mayeux R, Ngai C, Shea S, Berglund L. Association of apo E polymorphism with plasma lipid levels in a multiethnic elderly population. Arterioscler Thromb Vasc Biol. 1997;17:3534–41. doi: 10.1161/01.atv.17.12.3534. [DOI] [PubMed] [Google Scholar]
- 49.Hatters DM, Peters-Libeu CA, Weisgraber KH. Apolipoprotein E structure: insights into function. Trends Biochem Sci. 2006;31:445–54. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1. Common variants (MAF >1%) significantly associated in meta-analysis (p ≤ 2.5E-8)
Online Table 2. T1 results (MAF ≤1%, MAC ≥3 in both AA and EA) significantly associated in meta-analysis (p ≤1.83E-6)
Online Table 3. Rare nonsynonymous and splicing exonic variants in TARM1 and APOC3.
Online Table 4. Comparisons of PCE model and PCE plus apoB, triglycerides, or LDL-TG with differences in AUC, NRI, and IDI for risk prediction of CVD


