Abstract
Background
Electrocardiography R-wave to Radial artery pulse delay (RRD) represents pulse transit time inclusive of pre-ejection period (PEP) and arterial pulse propagation time. RRD is proposed to largely reflect arterial stiffness when PEP is accounted for (shorter RRD = higher arterial stiffness). Sleep disordered breathing (SDB) causes intermittent hypoxemia and sympathetic activation, which negatively influence vascular function. We aimed to examine the association of measures of SDB with RRD.
Methods
The sample consisted of participants in the Multi-Ethnic Study of Atherosclerosis without prevalent cardiovascular disease who underwent a daytime arterial elasticity exam, cardiac magnetic resonance imaging (MRI), and overnight polysomnography. SDB measures of interest included apnea hypopnea index (AHI) and oxygen desaturation index (ODI) (N = 1173). RRD was regressed on each measure of SDB separately, with adjustment for other cardiovascular risk factors as well as for correlates of the PEP, another component of RRD, by including cardiac MRI measures of contractility and preload.
Results
In multivariate analysis, among measures of SDB, ODI, a marker of intermittent hypoxemia, was inversely associated with RRD (β= −60.2 msec per SD [15.5/hr], p = 0.04). No significant association was found with AHI. In gender stratified analyses, ODI and AHI were predictive of RRD in men only (β= −111.3 msec per SD [15.5/hr], p = 0.01 and β= −100.3 msec per SD [16.1/hr], p=0.02 respectively).
Conclusion
Severity of SDB as measured by ODI was associated with RRD, a marker of arterial stiffness. Association of RRD with measures of SDB appears to be gender-dependent.
Keywords: Arterial stiffness, sleep apnea, sleep disordered breathing, pulse transit time
Introduction
Recent decades have witnessed a rising prevalence of sleep disordered breathing (SDB) and growing evidence of its adverse impact on health. 1, 2 In particular, epidemiological studies have demonstrated an association of SDB with increased cardiovascular (CV) morbidity.3–5 While the mechanisms underlying such association are not fully understood, factors leading to macro- or microvascular abnormalities such as hypertension, 4 endothelial dysfunction,6 systemic inflammation7 and oxidative stress8 have been proposed. In addition, numerous studies have shown independent associations between SDB and various markers of subclinical CV disease (CVD).9–11
One of the functional subclinical markers that have recently emerged as an early independent predictor of CV morbidity and mortality is arterial stiffness.12–14 The majority of the studies, but not all, have shown increased arterial stiffness in SDB patients after considering major confounding factors.15 Furthermore some, but not all studies have demonstrated improvement in the measures of arterial stiffness after treatment of SDB.16–18
Pulse wave velocity (PWV) based on arterial pulse transit time (hereafter “aPTT”) is a well-established and commonly used measures of arterial stiffness but requires two tonometric measurement sites.19 A major determinant of aPTT is the vascular wall property (stiffness), which in turn is heavily influenced by blood pressure (BP) at the time of measurement.20 While aPTT change within an individual is mainly determined by change in BP, aPTT variability across individuals is determined by baseline vascular wall property if BP is accounted for. A proxy for aPTT can be measured from the electrocardiography (ECG) R wave to the pulse arrival time at the selected peripheral site (i.e., PTT). Inherently, this measure also includes the pre-ejection period (PEP), which reflects the time between the ECG R wave peak to the aortic valve opening. Because PTT can be readily and non-invasively measured, it has been evaluated as a surrogate for aPTT or as its own metric.21, 22
The purpose of this study was to determine the relationship between measures of SDB and PTT (in the form of ECG-tonometry-based R-wave to radial artery pulse delay [RRD]) in ethnically diverse community dwelling adults. We hypothesized that severity of SDB is associated with RRD. Given differences in the reported relationships between SDB and CVD in men and women, we also explored gender-specific associations, as well as race-specific associations.
Methods
Study Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-site prospective cohort study of community-dwelling men and women aged 45–84 years without known CVD (history of coronary heart disease, heart failure, or stroke) at enrollment in 2000–2002.23 For this study, we included MESA Exam 5 (2010–2012) participants who underwent radial artery tonometry, cardiac magnetic resonance imaging (MRI) and who participated in a Sleep Exam on a separate day (2010–2013). Those with any prevalent CVD or any receiving continuous positive airway pressure were excluded. The research protocols were approved by the Institutional Review Boards at each participating institution, and all participants gave written informed consent.
Sleep Study
The MESA exam 5 sleep study has been previously described.24 All recordings from overnight in-home polysomnography (Compumedics Ltd., Abbostville, Australia) were centrally scored at the Brigham and Women’s Hospital Sleep Reading Center, by research polysomnologists blinded to other data, with demonstrated high levels of scorer reliability. For this analysis, our primary metrics of SDB were the apnea hypopnea index (AHI) and oxygen (O2) desaturation index (ODI). AHI was defined as the sum of all apneas (either obstructive or central) plus hypopneas with ≥4% O2 desaturation per hour and analyzed as a continuous measure. Apnea was further classified as obstructive or central apneas on the basis of the presence of respiratory effort. Common clinical cutoffs (AHI< 5, 5≤AHI<15/hr, 15≤AHI<30, AHI≥ 30/ hr) were used to classify SDB severity. ODI was defined as the number of desaturation episodes per hour of at least a 4% decrease in O2 saturation (SpO2) from the maximum SpO2 during the event. Nocturnal hypoxemia burden was measured by % time spent with O2 saturation (SpO2) less than 90% (PCT90%) and by mean SpO2 during sleep.
ECG R-wave to Radial Artery Pulse Delay (RRD)
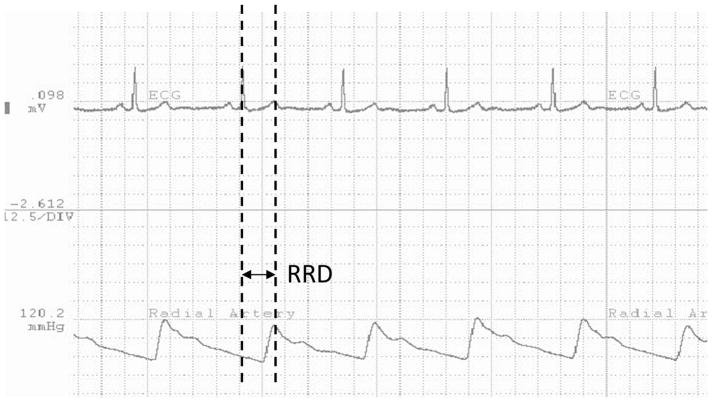
As part of MESA Exam 5, arterial pulse waveform was obtained during wakefulness by placing the tip of the arterial tonometer (Millar Instruments. Houston, TX) on the wrist perpendicular to the plane of the radial artery while the participant was lying supine. A wrist stabilizer was used to stabilize the position of the tonometer, which was held in a stiff-necked holder, and appropriate tonometer pressure was maintained throughout the recording to ensure proper data acquisition. All the measurements were acquired by certified technologists. The signal was then visualized by WinDaq software (DATAQ Inc. Akron, OH). A single ECG lead was recorded simultaneously and was displayed alongside with the arterial pulse waveform with a sampling rate at 250 Hz for both. All recordings were transmitted to a central collection site (University of Washington) and then to a reading site (University of Minnesota). aPTT was estimated from the R-wave peak until the start of the systolic upstroke of the radial artery pulse waveform (RRD). Entire tonometry recording period was 30 seconds except some cases that required additional 30 seconds of recording. For consistency, first 30 second period was used. Since RRD can be influenced by respiratory cycle, median value of multiple RRD readings during this 30 second period was used. (Figure 1). The R wave was identified as the highest peak in each beat, which was checked visually, then additionally by custom-built program (http://www.R-project.org/) to find the R wave peak when the T wave was the maximum. The systolic upslope defined as the next strong upslope in pressure following on the R wave peak was detected both visually and automatically. Any discrepancies between the two methods were adjudicated.
Figure 1.
RRD (ECG R-wave to Radial Artery Pulse Delay) measurement Time interval between dotted lines represent RRD (ECG R-wave to Radial Artery Pulse Delay).
Covariates
Demographic characteristics, body habitus (Body mass index [BMI]) and CV risk factors (hypertension, diabetes) were obtained during the MESA Exam 5 clinic visit. Hypertension was defined as seated systolic BP ≥ 140, diastolic BP ≥ 90, or the combination of anti-hypertensive medication use and a physician diagnosis of hypertension (Sixth Report of the Joint National Committee (1997) criteria). Diabetes was defined as a fasting glucose ≥7.0 mmol/l (126 mg/dl), or use of insulin or oral hypoglycemic medications. Information about use of any antidepressants or antipsychotic medications were obtained. Distance along the radial pulse transit path (i.e., manubrium to wrist distance [RR distance]) was estimated from height, gender and race. This was based on a regression model that was constructed against the measured distance from subaxilla to extended fingertip (which approximated distance from manubrium to radial pulse) in over 1200 consecutive participants at exam 5 (R2 = 0.56). The estimated RR distance was used for all participants in this study. BP was measured immediately before tonometry acquisition. Mean arterial pressure (MAP) was used as a covariate. To account for PEP, we adjusted for MRI measures that can influence this time interval (i.e., left ventricular ejection fraction [LVEF] and LV end-diastolic volume [LVEDV] for contractility and preload respectively). MRI examinations were performed as previously described.25 Imaging data were read using MASS software (version 4.2; Medis, Leiden, The Netherlands) at a single reading center (Johns Hopkins University) by readers trained in the MESA protocol. Functional parameters and left ventricular (LV) mass were determined by volumetric imaging. Ejection fraction (EF) was calculated as stroke volume divided by LV end-diastolic volume.
Statistical Analysis
Characteristics of participants included in the study were described by absence or presence of ‘significant SDB’ using an AHI cut off of 15/hr. Exposure variables included key measures of SDB: AHI, ODI and % PCT90%. The main outcome variable was RRD (msec). All values were expressed as mean (SD) unless specified otherwise. To better characterize the distribution of the measures of SDB in relation to RRD, descriptive statistics were derived based on terciles of RRD. For this cross-sectional analysis, we used multiple linear regression with model 1 adjusting for age, gender, race, BMI, RR distance, LVEF and LVEDV and model 2 adjusting additionally for MAP, hypertension, hypertension medication and diabetes. Estimates of association were provided per 1 unit and also per SD of the exposure of interest. The latter facilitates comparisons across the different exposures of interest. In addition to considering measures of SDB as continuous predictors, AHI clinical severity category was evaluated. Possible effect modification of associations between SDB and RRD by age, gender and race was tested by including cross-product terms in the models. In the presence of significant interaction (p<0.05), subgroup analysis was performed. All analysis was performed using SAS (ver 9.3 SAS Institute Inc., Cary, NC, USA).
Results
Participant Characteristics
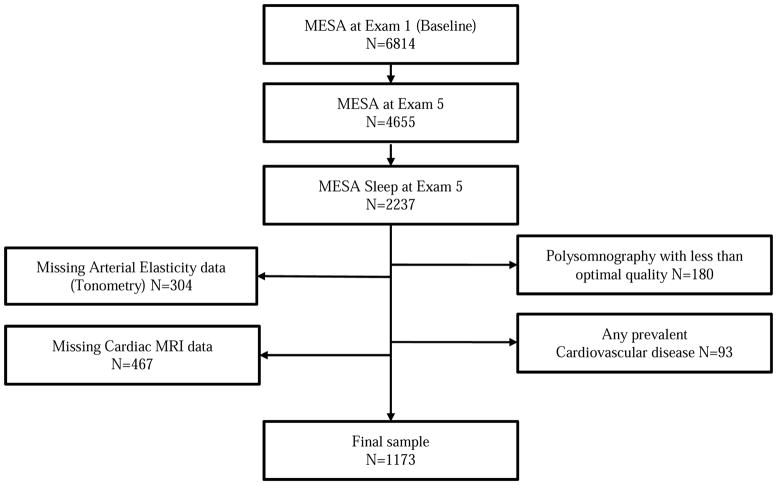
A total of 1073 participants were included in the study (Figure 2). The distribution of participant characteristics is shown in Table 1. The mean age of the participants was 67.8 (8.8), 523 (44.6 %) of 1173 participants were men and nearly about two thirds (62.6%) of the participants were of non-white race/ethnicity. SDB was common with 31% of the participants meeting the criteria for moderate or more severe SDB with mean AHI at 13.8 (Median: 7.5 [IQR: 2.8–17.5])/hr. 36.7%, 32.3%, 17.8% and 13.2% were classified as normal, mild, moderate and severe SDB severity categories, respectively. Hypertension was present in half of the participants. As expected, participants with SDB when compared to those without were more likely to be men, obese, diabetic, and characterized by higher MAP. With regards to cardiac MRI measures, those with SDB had higher LVEDV and lower LVEF (Table 1a). Men were more obese, had higher AHI, MAP and LVEDV, and lower LVEF compared to women (Table 1b). Men had a slightly longer RRD than women but the difference was not statistically significant (Men: 120.2 [1.54] vs. Women: 119.1 [1.5] msec, p = 0.3).
Figure 2.
Study participants MESA, Multi-Ethnic Study of Atherosclerosis; MRI, magnetic resonance imaging
Table 1a.
Baseline characteristics by sleep disordered breathing (N =1173): The Multi-Ethnic Study of Atherosclerosis (2010–2013)
| All | No SDB (AHI < 15) | Moderate or Severe SDB (AHI ≥ 15) | P value | |||
|---|---|---|---|---|---|---|
| N | 1173 | 809 | 364 | |||
| Mean (SD) Median [IQR] |
Mean (SD) Median [IQR] |
Range | Mean (SD) Median [IQR] |
Range | ||
| AHI (/hour) | 13.8 (16.1) 7.9 [2.9–18.0] |
5.4 (4.2) 4.5 [1.9–8.5] |
0.0–14.96 | 32.5(17.1) 26.9 [19.4–40.8] |
15.1–102.9 | |
| RRD (msec) | 119.6 (16.1) | 120.2 (15.9) | 75.2–187.0 | 118.1 (16.8) | 75.4–189.5 | 0.02 |
| RR distance (cm) | 65.3 (3.7) | 65.0 (3.7) | 54–77.1 | 65.9 (3.6) | 56.6–77.3 | <.0001 |
| Age (years) | 67.8 (8.8) | 67.3 (8.8) | 54.0–92.0 | 68.8 (8.8) | 54.0–93.0 | 0.0008 |
| BMI (kg/m2) | 27.4 (4.9) | 26.2 (4.7) | 16.8–47.5 | 29.1 (5.0) | 16.3–49.8 | <.0001 |
| MAP (mm Hg) | 95.4 (11.9) | 94.5(11.7) | 67.0–138.0 | 97.3 (12.1) | 64.3–139.0 | 0.0002 |
| LVEF (%) | 62.0 (7.2) | 62.3 (6.8) | 37.3–82.4 | 61.3 (8.0) | 30.9–82.6 | 0.02 |
| LVEDV (ml) | 119.3 (31.6) | 116.4 (29.6) | 37.4–288.8 | 125.8(34.8) | 55.5–310.2 | <.0001 |
| N (%) | ||||||
| Male | 523 (44.6) | 298 (36.8) | 225 (61.8) | <.0001 | ||
| Race | 0.03 | |||||
| White | 439 (37.4) | 322 (39.8) | 117 (32.1) | |||
| Chinese | 159 (13.6) | 99 (12.2) | 60 (16.5) | |||
| Black | 310 (26.4) | 215 (26.6) | 95 (26.1) | |||
| Hispanic | 265 (22.6) | 173 (21.4) | 92 (25.3) | |||
| Smoking (Current) | 78 (6.7) | 58 (7.2) | 20 (5.5) | 0.5 | ||
| Hypertension | 625 (53.3) | 417(51.6) | 208 (57.1) | 0.08 | ||
| Diabetes | 185 (15.9) | 106 (13.2) | 79 (21.8) | 0.0002 | ||
SDB, sleep disordered breathing; AHI, apnea hypopnea index; BMI, body mass index; MAP, mean arterial pressure; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end diastolic volume; RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec); RR distance, radial pulse transit path distance. P value is based on independent t test and Chi-square test for continuous and categorical variables respectively.
Table 1b.
Baseline characteristics by gender (N =1173)
| All | Men | Women | P value | |||
|---|---|---|---|---|---|---|
| 1173 | 523 (44.6) | 650 (55.4) | ||||
| Mean (SD) Median [IQR] |
Mean (SD) Median [IQR] |
Range | Mean (SD) Median [IQR] |
Range | ||
| AHI (/hour) | 13.8 (16.1) 7.9 [2.9–18.0] |
18.2 (18.4) 12.6 [4.8–24.6] |
0–102.9 | 10.4 (13.0) 5.9 [2.1–13.2] |
0–95.5 | <0.0001 |
| RRD (msec) | 119.6 (16.1) | 120.2 (17.1) | 75.2–187 | 119.1 (15.4) | 75.5–189.5 | 0.3 |
| RR distance (cm) | 65.3 (3.7) | 67.8 (3.1) | 60.7–77.3 | 63.2 (2.7) | 54–72.4 | <0.0001 |
| Age (years) | 67.8 (8.8) | 67.7 (9.1) | 54–92 | 67.8 (8.6) | 54–93 | 0.9 |
| BMI (kg/m2) | 27.4 (4.9) | 27.7 (4.2) | 16.3–39.9 | 28.3 (5.7) | 16.8–49.8 | 0.03 |
| MAP (mm Hg) | 95.4 (11.9) | 97.3 (11.6) | 64–138 | 93.8 (11.9) | 67–139 | <0.0001 |
| LVEF (%) | 62.0 (7.2) | 59.7 (7.2) | 30.9–80.3 | 63.9 (6.6) | 43.5–82.6 | <0.0001 |
| LVEDV (ml) | 119.3 (31.6) | 135.7 (33.4) | 59.2–310.2 | 106.1 (22.8) | 37.4–203.1 | <0.0001 |
| N (%) | ||||||
| Race | 0.3 | |||||
| White | 439 (37.4) | 187 (35.8) | 252 (38.8) | |||
| Chinese | 159 (13.6) | 76 (14.5) | 83 (12.8) | |||
| Black | 310 (26.4) | 131 (25.1) | 179 (27.5) | |||
| Hispanic | 265 (22.6) | 129 (24.7) | 136 (20.9) | |||
| Smoking (Current) | 78 (6.7) | 40 (7.7) | 38 (5.9) | 0.2 | ||
| Hypertension | 625 (53.3) | 360 (55.4) | 265 (50.7) | 0.1 | ||
| Diabetes | 185 (15.9) | 100 (15.5) | 85 (16.3) | 0.7 | ||
SDB, sleep disordered breathing; AHI, apnea hypopnea index; BMI, body mass index; MAP, mean arterial pressure; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end diastolic volume; RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec); RR distance, radial pulse transit path distance. P value is based on independent t test and Chi-square test for continuous and categorical variables respectively.
Distribution of RRD
RRD was inversely correlated with age, MAP and LVEF and positively correlated with LVEDV. No correlation was found between RRD and BMI (Table 2). There was significant difference in SDB measures between the terciles of RRD except mean O2 saturation and PCT90% (Table 2).
Table 2.
Distribution of sleep variables based on RRD tercile categories.
| Sleep parameters | All | RRD (msec) Tercile groups | P value | ||
|---|---|---|---|---|---|
| Median [IQR] | Shortest 75.2–112.2 |
Middle 112.2–125.0 |
Longest 125.0–189.5 |
||
| AHI (/hr) | 7.5 [2.8–17.5] | 9.0 [3.2–24.3] | 7.7 [2.8–16.4] | 7.5 [2.7–15.8] | 0.0002 |
| O2 desaturation index (ODI) (/hr) | 7.5 [2.8–17.5] | 8.1 [2.9–23.2] | 7.0 [2.6–16.0] | 7.4 [2.5–15.3] | 0.0005 |
| Mean O2 saturation (Mean O2 Sat) (%) | 94.8 [93.7–95.7] | 94.9 [93.7–95.5] | 94.8 [93.6–95.7] | 94.7 [93.4–95.8] | 0.6 |
| PCT90% (%) | 0.5 [0.03–2.7] | 0.6 [0.05–3.2] | 0.5 [0.03–2.8] | 0.5 [0.03–2.1] | 0.06 |
RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec); PCT90%, % time spent with O2 saturation (SpO2) less than 90%. P value is based on Kruskal-Wallis test.
Association between Measures of SDB and Nocturnal Hypoxemia and RRD
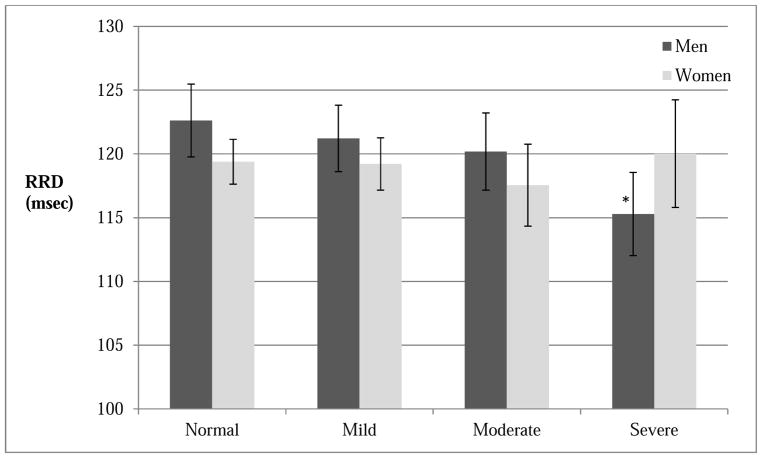
Adjusting for demographics, BMI, radial pulse transit path distance, LVEF and LVEDV, severity of SDB as measured by ODI was significantly associated with shorter RRD time. The associations remained significant when the model was further adjusted for MAP and CVD risk factors (β= −60.2 msec per SD [15.5/hr], p = 0.04) (Table 3). There was a trend but statistical significance was not reached with AHI as a predictor of RRD. Neither subtypes of AHI (i.e., obstructive AHI and central apneas) nor markers of nocturnal hypoxemia (i.e., mean SpO2 and PCT90%) showed significant association with RRD. In this multivariable model, male, older age, shorter RR distance, hypertension, higher MAP and LVEF, and lower LVEDV were found to be associated with shorter RRD. In contrast, no association was found with BMI or race. Significant interactions between key measures of SDB and gender (AHI-gender, pinteraction= 0.01; ODI-gender, pinteraction = 0.01) were present. No interactions were found with either age or race. The subsequent gender specific subgroup analysis showed that the associations of SDB measures with RRD were significant in men (AHI: β= −100.3 msec per SD [16.1/hr], p=0.02; ODI: β= −111.3 msec per SD [15.5/hr], p = 0.01) but not in women (Table 4). There was a trend of decrease in RRD with the increasing clinical SDB severity category in men (Figure 3). RRD in severe SDB group was lower than normal group in men (mean 115.3 msec [112.0–118.6] vs. 122.6 [119.8–125.5], P=0.001) (Figure 3). Adjusting for covariates attenuated the results (Adjusted mean 117.0 msec [113.9–120.2] vs. 121.3 [118.6–124.1], P=0.05). No association of nocturnal hypoxemia measures and RRD was found in either men or women.
Table 3.
Multiple linear regression analysis: Measures of SDB and RRD (N =1173): The Multi-Ethnic Study of Atherosclerosis (2010–2013)
| Unadjusted | Model 1 | Model 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P | |
| AHI (/hr) | 1173 | −0.09 | 0.03 | −89.9 | 0.002 | 1172 | −0.05 | 0.01 | −54.3 | 0.07 | 1165 | −0.05 | 0.03 | −50.3 | 0.09 |
| ODI (/hr) | 1156 | −0.09 | 0.03 | −91.1 | 0.02 | 1155 | −0.06 | 0.03 | −64.3 | 0.03 | 1148 | −0.06 | 0.03 | −60.2 | 0.04 |
| Mean O2 Sat (%) | 1173 | −0.12 | 0.29 | −11.6 | 0.69 | 1171 | −0.02 | 0.29 | −21.5 | 0.5 | 1165 | −0.23 | 0.29 | −22.6 | 0.4 |
| PCT90% (%) | 1173 | −0.11 | 0.06 | −52.7 | 0.07 | 1172 | −0.07 | 0.06 | −32.1 | 0.3 | 1165 | −0.06 | 0.06 | −27.0 | 0.3 |
SDB, sleep disordered breathing; RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec); AHI, apnea hypopnea index; ODI, O2 desaturation index; Mean O2 Sat, mean oxygen saturation; PCT90%, % time less than O2 saturation less than 90%; SE, standard error. Model 1 adjusted for age, gender, race, body mass index, radial pulse transit path distance, left ventricular ejection fraction and left ventricular end diastolic volume. Model 2 adjusted for Model 1 + mean arterial pressure, hypertension, hypertension medication and diabetes. 1 SD= 16.1 (AHI), 15.5 (ODI), 1.6 (Mean O2 Sat) and 7.7 (PCT90%).
Table 4.
Multiple linear regression analysis: Measures of SDB and RRD among men (N=523) and women (N=650): The Multi-Ethnic Study of Atherosclerosis (2010–2013)
| Unadjusted | Model 1 | Model 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men (N=523) | N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P |
| AHI (/hr) | 523 | −0.1 | 0.04 | −166.9 | 0.0001 | 522 | −0.1 | 0.04 | −105.1 | 0.02 | 520 | −0.1 | 0.04 | −100.3 | 0.02 |
| ODI (/hr) | 512 | −0.2 | 0.04 | −167.2 | 0.0001 | 511 | −0.1 | 0.04 | −115.4 | 0.01 | 509 | −0.1 | 0.04 | −111.3 | 0.01 |
| Mean O2 Sat (%) | 523 | 0.07 | 0.5 | 6.3 | 0.9 | 522 | −0.3 | 0.5 | −31.7 | 0.5 | 520 | −0.3 | 0.5 | −30.0 | 0.5 |
| PCT90% (%) | 523 | −0.2 | 0.1 | −99.60 | 0.02 | 522 | −0.1 | 0.09 | −56.3 | 0.2 | 520 | −0.1 | 0.09 | −52.8 | 0.2 |
| Women (N=650) | N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P | N | Estimate per 1 unit | SE | Estimate per SD | P |
| AHI (/hr) | 650 | −0.02 | 0.05 | −17.9 | 0.6 | 650 | 0.03 | 0.05 | 2.7 | 0.5 | 645 | 0.04 | 0.05 | 34.8 | 0.4 |
| ODI (/hr) | 644 | −0.02 | 0.05 | −18.2 | 0.6 | 644 | 0.02 | 0.04 | 17.4 | 0.7 | 639 | 0.03 | 0.05 | 24.9 | 0.5 |
| Mean O2 Sat (%) | 650 | −0.2 | 0.4 | −21.7 | 0.6 | 650 | −0.02 | 0.4 | −22.3 | 0.6 | 645 | −0.2 | 0.37 | −24.9 | 0.5 |
| PCT90% (%) | 650 | −0.03 | 0.08 | −15.8 | 0.7 | 650 | −0.01 | 0.07 | −0.7 | 1.0 | 645 | −0.02 | 0.08 | −9.0 | 0.8 |
SDB, sleep disordered breathing; RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec); PCT90%, % time less than O2 saturation less than 90%; SE, standard error. Model 1 adjusted for age, gender, race, body mass index, radial pulse transit path distance, left ventricular ejection fraction and left ventricular end diastolic volume. Model 2 adjusted for Model 1 + mean arterial pressure, hypertension, hypertension medication and diabetes
Figure 3.
RRD by SDB severity category SDB, sleep disordered breathing; RRD, ECG R-wave to Radial Artery Pulse Delay (unit: msec). Based on unadjusted analysis. Men (total N=523): Normal (N=135), Mild (N=163), Moderate (N=121) and Severe (N=104); Women (total N=650): Normal (N=295), Mild (N=216), Moderate (N=88) and Severe (N=51). Bar represents 95% CI. * indicates statistical significance when compared to reference (Normal) group (p=0.001). P values for linear trend were 0.001 for men and 0.7 for women.
Discussion
RRD is a measure of arterial stiffness that is mostly determined by arterial transit time (shorter transit time values suggesting greater arterial stiffness). In this racially and ethnically diverse, community-based cohort we found that severity of SDB as measured by ODI was associated with RRD. Inverse association shown in our study suggests that higher ODI (more frequent oxygen desaturation events) is associated with shorter RRD taking into account factors that may influence PEP. In addition to ODI, variables such as age, hypertension and BP, each of which is well known to affect arterial stiffness, and thus PWV, were all found to be related to RRD. The association of ODI with RRD accounting for these factors implies that the severity of SDB (i.e., high ODI) may be independently associated with higher arterial stiffness (short RRD).
The results of this study is consistent with findings from a number of studies that have largely demonstrated a higher degree of arterial stiffness in patients with SDB.15 Majority of studies conducting various arterial stiffness measures revealed that SDB (vs. no SDB), severe SDB (vs. milder SDB) and SDB (vs. no SDB) in the setting of hypertension or metabolic syndrome conferred higher arterial stiffness.
A recent meta-analysis of 5 clinic-based studies showed that carotid-femoral PWV was significantly higher in patients with SDB than controls (standardized mean difference 0.45, 95% CI 0.21–0.69, P<0.0001).26 A population based study involving 153 participants from Wisconsin Sleep cohort did not reveal any independent association between measures of SDB and PWV in multivariable analysis.27 In a small study that evaluated augmentation index as a measure of arterial stiffness, an association with SDB was shown in men only.28 In demonstrating this relationship, almost all prior studies have used AHI, which is indeed the most commonly used index in assessing SDB. In our study, as an exploratory strategy we included several related measures of SDB that are routinely reported in clinical sleep study. Although AHI and ODI were highly correlated and provided similar information regarding associations with RRD, the ODI showed more significant association. Moreover no evidence of association was found when the degree of hypoxemia as expressed by mean O2 saturation or PCT90% were considered.
That ODI among various SDB measures had the strongest association with RRD highlights the potential importance of intermittent hypoxemia-reoxygenation in explaining the pathophysiological link between SDB and RRD. Intermittent hypoxemia induces ischemia-reperfusion type of injury, which is a potent trigger to oxidative stress, endothelial dysfunction and increased sympathetic tone.8, 29–31 Cumulative exposure to these stresses can change vascular properties explaining the increased basal arterial stiffness.32 Although most conventional SDB measures reflect severity of intermittent hypoxemia, ODI is a simple and reliable metric that is easily obtainable via pulse oximetry unlike AHI that requires measurement of air flow. Moreover AHI is rather a sensitive metric that is subject to varying definitions of hypopnea.33
Notably, we found a significant role of gender in modifying this relationship as the association was present only in men but not in women. Gender-based analysis prompted by the presence of significant interaction revealed that in men both higher ODI and AHI were associated with shorter RRD. Indeed, there was a trend of shorter RRD across AHI-based SDB severity class. No such pattern was found in women. Many of previous studies investigating SDB and more conventional measures of arterial stiffness included men only. Even in studies including both gender, gender-specific difference in the relationship has not been reported. Mechanisms behind this gender-specific finding of our study is unclear. While marked difference in the prevalence of SDB by gender is well known, gender-related difference in SDB’s CV effects remains elusive.34 However, pathophysiologic response to SDB between men and women may differ. Experimental studies showed more pronounced peripheral vasoconstriction response to arousal in men than women.35 Wadhwa et al. demonstrated sustained alteration in autonomic nervous system activity in response to exposure to intermittent hypoxia in men but not in women.36 Conversely, endothelial dysfunction, which represent different aspects of vascular property than arterial stiffness, appeared to be affected in women with SDB but not in men.37, 38 In the Sleep Heart Health Study, the association of severe SDB with incident coronary heart disease, heart failure and stroke was observed in men but not in women although this finding could have been partly attributed to insufficient power in women and a lower lifetime cumulative exposure to SDB when the women were studied.3, 5
It should be noted that RRD (PTT) in our study represents a single measure of arterial stiffness obtained during wakefulness. The measurement approach is identical to that of conventional pulse wave analysis measurement. This approach is distinct from using dynamic continuous PTT changes during sleep as an autonomic arousal index to quantify SDB severity.39 In this regards, examining continuous measure of RRD (vs. single) as a dynamic arterial stiffness measure in sleep in comparison to existing peripheral arterial tonometer-based sleep diagnostic technology would be of interest in the future.40
Recently, Zhang et al. suggested that radial PTT, equivalent to RRD in our study, is a useful index of arterial stiffness by demonstrating its inverse relationship with age in both men and women.41 Likewise, the significant associations of RRD with age and BP shown in our study confers validity of RRD as an index of arterial stiffness. RRD has the advantage that it can be easily obtained by a single tonometric measurement with simultaneous ECG compared to traditional PWV measure that require two separate tonometric measurements. In the MESA cohort, RRD was associated with BP, metabolic syndrome and coronary artery calcium score.42 Despite this, RRD used in this study encompasses PEP in addition to aPTT (a direct derivative of PWV) and therefore possibly confounding the RRD-arterial stiffness relationship. While we attempted to overcome this by adjusting for LVEDV (loading condition), LVEF (inotropic state) and MAP (afterload) known to be major determinants of PEP, these MRI measures were not obtained at the time of RRD measurement. However, we argue that cardiac MRI measures of LVEDV and LVEF in resting state are relatively stable measures. The RRD incorporates both an elastic segment (ascending aorta and aortic arch) and muscular arterial segment (segments distal to the takeoff of the brachiocephalic trunk or subclavian arteries from the aorta). It is widely accepted that large/elastic artery stiffening/PWV is associated with aging and CV risk, whereas muscular artery stiffening is not.43 In Framingham cohort, in contrast to carotid femoral PWV, carotid radial PWV was not linked to CV outcomes.44 Therefore, we acknowledge the need for further studies to validate RRD as a marker of arterial stiffness or as a useful index of CV risk. Future studies should examine the reproducibility of RRD as well as the relationship between RRD and other conventional measures of arterial stiffness including PWV and pulse wave contour analysis derivatives. Furthermore, the utility of RRD in prediction of CV outcomes is warranted. Finally, cross-sectional and observational nature of the study does not allow us to infer causality. In spite of the limitations, a strength of this study includes objective measurements of SDB and RRD in a large, ethnically diverse, community-based cohort. To the best of our knowledge this study represents the largest population-based study examining markers of SDB in relation to a surrogate of arterial stiffness.
Our findings add to the growing body of evidence that SDB is associated with subclinical markers of CVD and that associations between SDB and vascular property differ in men and women. In conclusion, we found that severity of SDB in men was associated with RRD, a novel subclinical CV marker.
Supplementary Material
ECG R-wave to Radial artery pulse delay (RRD) is a marker of arterial stiffness
Severity of sleep disordered breathing was associated with higher arterial stiffness
This association was found only in men but not in women
Acknowledgments
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHBLI, by grants UL1-TR-000040, UL1-RR-025005 from NCRR, R01HL098433 (MESA Sleep) and T32-HL069764.
Footnotes
Conflict of interest: No relevant conflicts of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. American journal of respiratory and critical care medicine. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. American journal of respiratory and critical care medicine. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 7.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. The Journal of clinical endocrinology and metabolism. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 8.Lavie L. Obstructive sleep apnoea syndrome--an oxidative stress disorder. Sleep medicine reviews. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 9.Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117:2599–607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. American journal of respiratory and critical care medicine. 2004;169:354–60. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 11.Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. Journal of hypertension. 1999;17:61–6. doi: 10.1097/00004872-199917010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Duprez DA, Jacobs DR, Jr, Lutsey PL, et al. Association of small artery elasticity with incident cardiovascular disease in older adults: the multi-ethnic study of atherosclerosis. American journal of epidemiology. 2011;174:528–36. doi: 10.1093/aje/kwr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peralta CA, Adeney KL, Shlipak MG, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2010;171:63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirinos JA, Kips JG, Jacobs DR, Jr, et al. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2012;60:2170–7. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips CL, Butlin M, Wong KK, Avolio AP. Is obstructive sleep apnoea causally related to arterial stiffness? A critical review of the experimental evidence. Sleep medicine reviews. 2013;17:7–18. doi: 10.1016/j.smrv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Litvin AY, Sukmarova ZN, Elfimova EM, et al. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vascular health and risk management. 2013;9:229–35. doi: 10.2147/VHRM.S40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones A, Vennelle M, Connell M, et al. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep medicine. 2013;14:1260–5. doi: 10.1016/j.sleep.2013.08.786. [DOI] [PubMed] [Google Scholar]
- 18.Vlachantoni IT, Dikaiakou E, Antonopoulos CN, Stefanadis C, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep medicine reviews. 2013;17:19–28. doi: 10.1016/j.smrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. American journal of hypertension. 2002;15:426–44. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 20.Geddes LA, Voelz MH, Babbs CF, Bourland JD, Tacker WA. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology. 1981;18:71–4. doi: 10.1111/j.1469-8986.1981.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 21.Pollak MH, Obrist PA. Aortic-radial pulse transit time and ECG Q-wave to radial pulse wave interval as indices of beat-by-beat blood pressure change. Psychophysiology. 1983;20:21–8. doi: 10.1111/j.1469-8986.1983.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang YL, Zheng YY, Ma ZC, Sun YN. Radial pulse transit time is an index of arterial stiffness. Hypertension research : official journal of the Japanese Society of Hypertension. 2011;34:884–7. doi: 10.1038/hr.2011.41. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Kwon Y, Gharib SA, Biggs ML, et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2015;70:873–9. doi: 10.1136/thoraxjnl-2014-206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Yu W, Gao M, et al. Impact of Obstructive Sleep Apnea Syndrome on Endothelial Function, Arterial Stiffening, and Serum Inflammatory Markers: An Updated Meta-analysis and Metaregression of 18 Studies. Journal of the American Heart Association. 2015:4. doi: 10.1161/JAHA.115.002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korcarz CE, Gepner AD, Peppard PE, Young TB, Stein JH. The effects of sleep-disordered breathing on arterial stiffness are modulated by age. Sleep. 2010;33:1081–5. doi: 10.1093/sleep/33.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yim-Yeh S, Rahangdale S, Nguyen AT, et al. Obstructive sleep apnea and aging effects on macrovascular and microcirculatory function. Sleep. 2010;33:1177–83. doi: 10.1093/sleep/33.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. Journal of neurophysiology. 2011;105:3080–91. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gras E, Belaidi E, Briancon-Marjollet A, Pepin JL, Arnaud C, Godin-Ribuot D. Endothelin-1 mediates intermittent hypoxia-induced inflammatory vascular remodeling through HIF-1 activation. Journal of applied physiology. 2016;120:437–43. doi: 10.1152/japplphysiol.00641.2015. [DOI] [PubMed] [Google Scholar]
- 31.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (Auckland, NZ) 2016;4:99–108. doi: 10.2147/HP.S103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32:150–7. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah N, Yaggi H, Redline S. Sex-related differences in cardiometabolic outcomes associated with obstructive sleep apnea: a review. Clinical Pulmonary Medicine. 2013;20:149–54. [Google Scholar]
- 35.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. American journal of respiratory and critical care medicine. 2003;168:1512–9. doi: 10.1164/rccm.200302-150OC. [DOI] [PubMed] [Google Scholar]
- 36.Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? Journal of applied physiology. 2008;104:1625–33. doi: 10.1152/japplphysiol.01273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep. 2004;27:1113–20. doi: 10.1093/sleep/27.6.1113. [DOI] [PubMed] [Google Scholar]
- 38.Randby A, Namtvedt SK, Hrubos-Strom H, Einvik G, Somers VK, Omland T. Sex-dependent impact of OSA on digital vascular function. Chest. 2013;144:915–22. doi: 10.1378/chest.12-2283. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti B, Emegbo S, Craig S, Duffy N, O'Reilly J. Pulse transit time changes in subjects exhibiting sleep disordered breathing. Respiratory medicine. 2017;122:18–22. doi: 10.1016/j.rmed.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123:695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Gao M, Xu D, Olivier NB, Mukkamala R. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure. Journal of applied physiology. 2011;111:1681–6. doi: 10.1152/japplphysiol.00980.2011. [DOI] [PubMed] [Google Scholar]
- 42.Duprez D, Steffen L, Brumback LC, et al. Association of ECG R wave to radial pulse delay with subclinical cardiovascular disease and risk factors: the MESA study. Journal of the American College of Cardiology. 2013:61. [Google Scholar]
- 43.Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.