Abstract
Purpose
Psychomotor slowing is a common but understudied cognitive impairment in epilepsy. Here we test the hypothesis that psychomotor slowing is associated with alterations in brain status reflected through analysis of large scale structural networks. We test the hypothesis that children with epilepsy with cognitive slowing at diagnosis will exhibit a cross-sectional and prospective pattern of altered brain development.
Methods
A total of 78 children (age 8–18) with new/recent onset idiopathic epilepsies underwent 1.5 T MRI with network analysis of cortical, subcortical and cerebellar volumes. Children with epilepsy were divided into slow and fast psychomotor speed groups (adjusted for age, intelligence and epilepsy syndrome).
Results
At baseline, slow-speed performers (SSP) presented lower modularity, lower global efficiency, higher transitivity, and lower number of hubs than fast-speed performers (FSP). Community structure in SSP exhibited poor association between cortical regions and both subcortical structures and the cerebellum while FSP presented well-defined communities. Prospectively, SSP displayed lower modularity but higher global efficiency and transitivity compared to FSP. Modules in FSP showed higher integration between and within themselves compared to SSP. SSP showed hubs mainly from frontal and temporal regions while in FSP were spread among frontal, temporal, parietal, subcortical areas and the left cerebellum.
Implications
Results suggest the presence of widespread alterations in large scale networks between fast- and slow-speed children with recent onset epilepsies both at baseline and 2 years later. Slower processing speed appears to be a marker of abnormal brain development antecedent to epilepsy onset as well as brain development over the 2 years following diagnosis.
Highlights
-
•
Baseline: slow-speed performers (SSP) showed lower modularity and global efficiency
-
•
They also showed higher transitivity but fewer hubs than fast-speed performers (FSP)
-
•
Prospective: SSP showed lower modularity, harmonic mean and higher transitivity
-
•
Regional volume changes seem to be occurring as one in SSP, but more modular in FSP
-
•
SSP showed hubs mainly from frontal and temporal while FSP showed them widespread
1. Introduction
Commonly appreciated neuropsychological complications of epilepsy include anomalies in memory, language, and executive function (Dodrill, 2004; Elger et al., 2004; Lin et al., 2012). Cognitive and psychomotor slowing is a less frequent focus of research as a core complication of epilepsy, but it appears as an abnormality in the cognitive status of adults with chronic epilepsy (Piazzini et al., 2006) as well as children with established cryptogenic localization-related (van Mil et al., 2010) and uncomplicated epilepsies (Boelen et al., 2005), as well in children and adults with new onset epilepsies prior to initiation of medications (Oostrom et al., 2003; Prevey et al., 1998; Taylor et al., 2010). Furthermore, especially in adults, psychomotor slowing worsens over time and can exceed the rate of change in other cognitive abilities (Baker et al., 2011; Hermann et al., 2006a). Cognitive and psychomotor slowing is a known complication of seizure medications (Vermeulen and Aldenkamp, 1995; Eddy et al., 2011; Loring et al., 2007; Park and Kwon, 2008), representing an impact that may be added to the intrinsic slowing observed antecedent to medication initiation in pediatric and adult new onset epilepsies (Oostrom et al., 2003; Taylor et al., 2010). With epilepsy remission and cessation of treatment with epilepsy medication there can be improvement in psychomotor speed in both children (Aldenkamp et al., 1993, Aldenkamp et al., 1998) and adults (Lossius et al., 2008), as well as following successful pediatric epilepsy surgery with reduction in medications (van Schooneveld et al., 2013). However, even with terminal remission of epilepsy a signal of persisting slowing has been reported (Aldenkamp et al., 1993; Berg et al., 2008).
While there is a long and extensive history of interest in processing speed and its relationship to intelligence and other cognitive abilities (O'Brien and Tulsky, 2008), the amount of research dedicated to characterizing the underlying neurobiological correlates of cognitive/psychomotor slowing in epilepsy in particular has been modest and varied. Dow et al. (2004) examined mental scanning speed in adults with temporal lobe epilepsy and found performance related to global white matter volume. Alexander et al. (2014) reported that increased FA of the left fornix was related to faster processing speed in temporal lobe epilepsy patients without hippocampal sclerosis. Van Veenendaal et al. (2017) examined the relationship between central information processing speed and rs-fMRI network efficiency and found no relationship between speed and network analysis in 55 patients with localization-related epilepsy. Normal aging in the general population is known to be associated with slowing of psychomotor speed (Era et al., 2011; Salthouse, 1996; Salthouse and Madden, 2008), an effect that has been attributed to age-related disruption of both cerebral white (Lu et al., 2011) and gray matter (see Seidler et al., 2010 for review). Clearly, much remains to be learned regarding the underlying neurobiology of psychomotor slowing as well as its impact on the efficiency of other cognitive processes in patients with epilepsy.
Complicating the situation in epilepsy, as well as general processing speed research in general, is the fact that cognitive and psychomotor speed has been assessed through a diversity of measures including simple and complex reaction time, finger tapping, mental scanning, motor assembly tasks, and other methods (Grevers et al., 2016; Martin and Bush, 2008) and the generalizability of findings across these diverse metrics is a persisting concern. At its most basic level, processing speed can be defined as the time required to complete a cognitive task or the amount of work that can be completed in a finite amount of time (DeLuca, 2008). One commonly used metric of “processing speed”, examined in epilepsy as well as across various clinical disorders, is the digit symbol substitution or number symbol substitution test, with applications to examine speeded performance in schizophrenia (Dickinson et al., 2007; Dickinson and Gold, 2008; Knowles et al., 2010; Morrens et al., 2007), bipolar disorder (Morsel et al., 2015), multiple sclerosis (Batista et al., 2012), chronic fatigue (Demaree et al., 2008), and other clinical groups (c.f., DeLuca and Kalmar, 2008, Dickinson and Gold, 2008), as well as normal aging (Salthouse, 1978, Salthouse, 2000). In a confirmatory factor analysis of an extensive neuropsychological test battery in 233 youth with epilepsy and normally developing controls (age 8–18), we identified a speed-based cognitive factor of which digit symbol was a composite test (Hermann et al., 2016), indicating its direct relevance to epilepsy as well.
The origin of the digit substitution test dates to the early 1900s (for review see Boake, 2002) which were initially thought to represent an assessment of incidental learning. However, careful deconstruction of the task has demonstrated that it is driven in part by speed-dependent processes (graphomotor speed, perceptual speed) with secondary contributions of visual scanning efficiency, learning/memory and executive function (Joy et al., 2003; Ashendorf and Reynolds, 2013). This easily administered and scored procedure has been included in all versions of the child and adult Wechsler intelligence scales since their inception (Dickinson and Gold, 2008). Overall, the digit symbol substitution test is an appropriate but not pure measure of processing speed, but one that has been used and investigated in diverse clinical disorders including epilepsy as well as normal and abnormal aging as well as diverse clinical groups including epilepsy.
Given that human cognitive processes are dependent on distributed neural networks, we first test the hypothesis that psychomotor slowing will be found to be associated with alterations in brain organization reflected through analysis of large scale structural networks. In addition, as baseline psychomotor slowing in children with new onset epilepsy has been shown to be predictive of behavior problems 3 years later (Austin et al., 2011), behaviors that have been demonstrated to be associated anomalies in brain structure (Dabbs et al., 2013), we hypothesize that those children with cognitive slowing at baseline will exhibit a pattern of altered prospective brain development as well. Hence psychomotor slowing may serve as a marker of potential anomalies in current as well as prospective brain structure, organization, and development. We test these hypotheses regarding morphological networks based on regional brain volumes in children with idiopathic epilepsies with fast and slow psychomotor speed performances tested close in time to their diagnosis of epilepsy and two years later.
2. Methods
2.1. Participants
Study participants included 78 children with idiopathic epilepsies who were recruited from pediatric neurology clinics at three Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic) and met the following inclusion criteria: (i) diagnosis of epilepsy within the past 12 months; (ii) no other developmental disabilities (e.g. intellectual impairment, autism); (iii) no other neurological disorder, and (iv) normal clinical MRI. All children entered the study with active epilepsy diagnosed by their treating pediatric neurologists and confirmed by medical record review of the research study pediatric neurologist. Each child's epilepsy syndrome (Idiopathic Generalized or Localization Related Epilepsy) was defined in a research consensus meeting by the research pediatric neurologist who reviewed all available clinical data (e.g., seizure description and phenomenology, EEG, clinical imaging, neurodevelopmental history) while blinded to all research cognitive, behavioral, and neuroimaging data. All participants completed two waves of MRI and neuropsychological evaluations including baseline and 2-year follow-up assessments. Baseline evaluation was performed within 12 months of epilepsy diagnosis. At baseline, all participants attended regular schools (see Hermann et al., 2006b for further details).
2.2. Neuropsychological assessment
At baseline and 2-year follow-up, all participants were administered a neuropsychological test battery that included assessment of intelligence (Wechsler Abbreviated Scale of Intelligence [WASI-4) and psychomotor speed assessed by the digit symbol substitution test (Wechsler Intelligence Scale for Children III, Digit Symbol-Coding) (Wechsler, 1991). The Digit Symbol test was independent of the derivation of the WASI-4 intelligence quotient.
2.3. Psychomotor speed groups
In order to discern brain morphological differences associated with psychomotor speed performance, the epilepsy participants were separated into fast (n = 29) and slow psychomotor speed performers (n = 47) based on a median split (median = 8) of baseline age-adjusted WISC-III Digit Symbol performance (Wechsler, 1991), with those participants falling below the median value considered to be slow-speed performers. The number of participants is not identical in each group as many subjects exhibited the same median scale score and were considered slow-speed performers.
A summary of the demographic and clinical characteristics of the participants is provided in Table 1. The fast and slow processing speed groups both had full scale IQ scores that fell in the average range but with a significant difference in processing speed performance (13th percentile in the slow group and 58th percentile in the fast group). There were no differences between the groups in age, sex, SES, or medication number. The project protocol was reviewed and approved by the Institutional Review Board of the University of Wisconsin School of Medicine and Public Health. Families and children gave written informed consent or assent, respectively, on the day of the study. All but 14 children were treated via monotherapy, 2 children were on 2 medications, 2 children on 3 medications, and 10 on no medications.
Table 1.
Demographic and Neuropsychological Characteristics.
| Slow-speed Performers (n = 47) | Fast-speed Performers (n = 29) | |
|---|---|---|
| Age (mean ± SD) | 11.3 ± 2.9 | 11.8 ± 2.6 |
| Sex (F/M) | 20/27 | 14/15 |
| Grade (mean ± SD) | 5.7 ± 3.0 | 6.2 ± 2.8 |
| SES (mean ± SD) | 4.5 ± 1.7 | 4.0 ± 2.1 |
| Epilepsy syndrome (IGE/ILRE) | 21/26 | 13/16 |
| Age of epilepsy onset (mean ± SD) | 10.9 ± 2.99 | 11.4 ± 2.77 |
| Seizures within 12 months of follow-up (0/≤4/>5/unknown) | 22/15/0/10 | 12/9/1/7 |
| On AEDs at follow-up (y/n/never/unknown) | 21/12/4/10 | 12/6/4/7 |
| WISC-III Coding (mean ± SD)a | 6.8 ± 1.4 | 10.5 ± 1.7 |
| WASI-2 FSIQ (mean ± SD) | 97.9 ± 13.1 | 105.3 ± 16.8 |
Significantly different at a p < 0.05. SES: socioeconomic status based on the mother's education; FSIQ: Full-scale intelligence coefficient; AED: antiepileptic drug.
3. MRI acquisition and volumetric calculations
MR images were obtained on a 1.5-T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, U.S.A.). T1-weighted images were acquired using a three-dimensional (3D) spoiled gradient recall (SPGR). The imaging parameters were: echo time (TE): 5 ms, repetition time (TR): 24 ms, flip angle: 40°, slices: 124, slice thickness: 1.5 mm, plane: coronal, field of view (FOV): 200 mm, matrix: 256 × 256.
MR Images were processed with the FreeSurfer image analysis suite (version 5.1). The T1-weighted MR images were used for cortical reconstruction and volumetric segmentation. The technical details of these procedures include motion correction and averaging, removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (e.g., hippocampus, amygdala, caudate, putamen), intensity normalization, tessellation of the gray matter–white matter boundary, automated topology correction, and surface deformation following intensity gradients to effectively place the boundaries between brain tissue (CSF, WM, and GM) (Segonne et al., 2004; Talairach and Tournoux, 1988; Fischl et al., 2001, Fischl et al., 2004; Fischl and Dale, 2000).
All data were processed with the longitudinal stream in FreeSurfer (Reuter and Fischl, 2011). This stream is designed to be unbiased with respect to any time point by creating a within-subject template volume (base image). In this way, reliability and statistical power are significantly increased (Reuter and Fischl, 2011). All data were visually inspected for quality assurance.
3.1. Network analysis
Investigated were both the regional volume status at baseline (within 12 months of epilepsy onset) and prospectively two years later between epilepsy slow- and fast-speed performers, this done by taking the difference between time points and normalizing it to the baseline evaluation . Subsequently, a morphometric network based on the correlation coefficient representing the co-variation in volumetric changes across subjects was constructed for each group rendering symmetric weighted adjacency matrices of 87 nodes containing cortical, cerebellar, and subcortical structures. The nodes used can be found in Supplementary File 1.
In order to be able to discern group differences, matrices were proportionally thresholded; a way to ensure that only the strongest (highest weighted) percentage of links form the graph. However, given that graphs may at times fail to fully connect at sparse thresholds (relatively low number of links) we combined the use of proportional thresholding with the Minimum Spanning Tree (MST) as its backbone (see Garcia-Ramos et al., 2015a for details). In short, the MST is a subgraph of the network that connects all nodes in the graph while using the strongest weights between them without forming cycles or loops. Adding the proportional threshold would then build up on the rest of the connections between nodes. For the remainder of this manuscript, each graph threshold represents a combination of MST and proportional thresholding, indexed by the density level. For example, a density level of 15% would be the MST plus a proportional threshold of 15%. It might also be referenced as a hybrid threshold.
After graph thresholding, we ensured that no graph contained negative links in order to maintain straightforward interpretations. In this study, global metrics were calculated over a range of graph connectivity densities from 5 to 30% in 5% increments, and local measures were calculated at a density level of 15% given that this level was the closest to the average of full graph connectedness in both groups. The Force Atlas algorithm of the open source software Gephi (http://gephi.github.io/) was used for the 2D visualization of community structure on each group (attraction strength = 10, repulsion strength = 1000, gravity = 30).
3.2. Graph theory measures
Graph theoretical measures were obtained using the Matlab-based Brain Connectivity Toolbox v.2016 (BCT, http://www.brain-connectivity-toolbox.net/) (Rubinov and Sporns, 2010). Weighted-undirected adjacency matrices were created to obtain different graph theory metrics. In order to discern statistically significant group differences at the global level, each group matrix was resampled by replacement (i.e. bootstrapped) a total of 500 times. Since results from graph theory measures can occur by chance alone, each graph measure was calculated on 500 random matrices with the same number of nodes and degree distribution as the pertinent graphs. In this way, the null hypothesis could be tested. P-values were corrected for multiple comparisons for each of the global and local measures. Graph theory measures were then obtained from each resampled matrix over a range of thresholds and averages were used for evaluations. Global measures (harmonic mean and transitivity), local measures (betweenness centrality and local clustering coefficient), and mesoscale organization measures (modularity calculations) were calculated and are described next:
3.2.1. Harmonic mean
Harmonic mean is a measure of integration in a network. It is defined as the inverse of the global efficiency, which is based on the shortest paths or more direct connections between nodes (Wang et al., 2010). Low harmonic mean reflects higher graph integration reflecting a more efficient network.
3.2.2. Transitivity and clustering coefficient (CC)
The CC of a node is a measure that reflects how clustered the neighbors of a node are. A value close to 1 represents a node with highly connected neighbors while a value close to 0 reflects the opposite. The transitivity is an alternative measure of the average CC. It reflects how clustered a network is as a whole, and it is calculated as the ratio of “triangles” (closed connection between three nodes) to “triplets” (connections between three nodes) in the network (Newman, 2003; Humphries and Gurney, 2008). Averaging the CC of all the nodes in the network in order to find the global CC would be biased toward nodes with low degree, which is why transitivity was used instead. Low transitivity values (close to 0) indicate low clustering and values close to 1 indicate a highly clustered network.
3.2.3. Modularity index and community structure
The modularity or community structure of a network is the subdivision of a network into segregated communities that contribute to the same processes. Unlike the other measures, modular/community structure is statistically estimated instead of computed exactly (Blondel et al., 2008). Since the number of communities could be variable with different iterations, community structure was calculated 1000 times for each group matrix and the number with the highest likelihood was chosen. The modularity index is a measure of the goodness of the subdivision of the graph into communities; the higher its value, the stronger the modular structures in the graph (Boccaletti et al., 2006). Modular structure in a network is of great importance because it allows for different processes to take place, therefore representing specialization in the network.
3.2.4. Betweenness centrality (BC) and network hubs
BC is a measure of the importance of a node, for the communication between other nodes in a network (Boccaletti et al., 2006) It is calculated based on the node degree and its closeness, which is just the inverse of the average distance from the other nodes. Nodes with high BC facilitate global integrative processes given that they serve as “highways” to facilitate “traffic” flow of the network (Sporns et al., 2007). The hubs of a network are those nodes with high influence on the network topology. Those can be calculated based on different graph measures. In this study, nodes with high BC (higher than the mean and one standard deviation) were identified as the hubs of the network.
These metrics were computed for each of the adjacency matrices of each group. The identical subjects were represented in baseline and follow-up assessments, thus rendering a clear picture of prospective change over time. As such, the combination of information from these metrics would inform the nature of the development of the cognitive networks of both groups over a two-year period. Statistical testing for the developmental change between slow- and fast-speed performing epilepsy participants was performed, correcting for multiple comparisons using Bonferroni correction. There was a difference in epilepsy syndrome distribution; therefore, the results were adjusted for epilepsy syndrome (IGE, ILRE) and IQ. Given that regional brain volume might be influenced by subject-specific intracranial volume (ICV), the results controlled for this variable as well.
4. Results
4.1. Baseline adjacency matrices
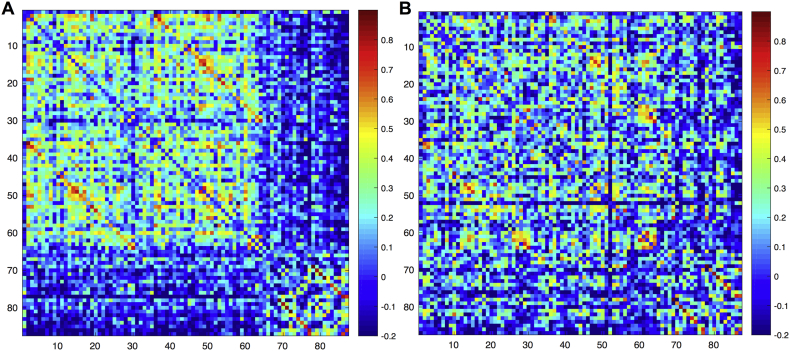
Fig. 1 shows the adjacency matrices for both groups of children with epilepsy. The slow-speed group exhibited highly correlated cortical and subcortical regions and those regions were weakly correlated between themselves. The fast-speed performers showed an undifferentiated pattern of correlations in which both cortical and subcortical regions seem to be equally correlated within and between each other.
Fig. 1.
Adjacency matrices for the (A) slow-speed performers and (B) fast-speed performers epilepsy groups at baseline evaluation. Regions are in the same order as in the table of the Supplementary File 1.
Adjacency matrices for the (A) slow-speed performers and (B) fast-speed performers epilepsy groups at baseline evaluation. Regions are in the same order as in the table of the Supplementary File 1.
4.2. Baseline modularity and hubs
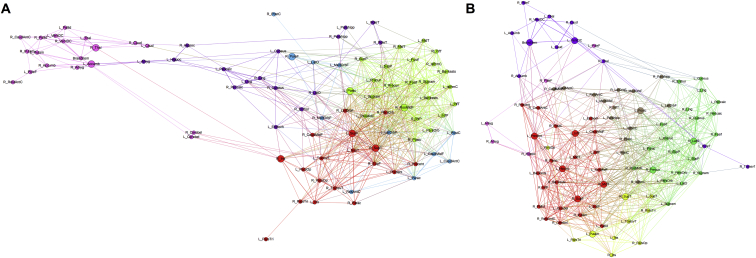
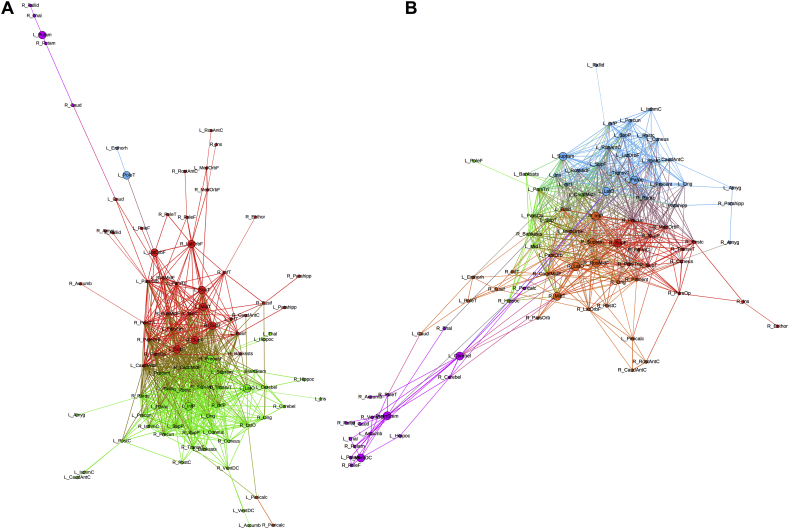
Fig. 2 confirms the observations from Fig. 1 in which the slow-speed performers present their subcortical structures weakly correlated to the rest of the network and mostly pertaining to only one module. However, in the fast-speed performers the subcortical regions are highly correlated with different modules instead of presenting the segregation observed in the slow-speed group.
Fig. 2.
Modular configuration of baseline morphological brain networks in children with epilepsy with (A) slow-speed, and (B) fast-speed performance. Different colors represent different modules. Bigger nodes represent the hubs of the networks as calculated using betweenness centrality. Node abbreviations can be found in the table from the Supplementary File 1.
Modular configuration of baseline morphological brain networks in children with epilepsy with (A) slow-speed, and (B) fast-speed performance. Different colors represent different modules. Bigger nodes represent the hubs of the networks as calculated using betweenness centrality. Node abbreviations can be found in the table from the Supplementary File 1.
Regarding the network hubs as calculated using BC, the slow-speed performers showed 8 hubs while the fast-speed performers showed 12. In the former, although hubs were highly spread across lobes of the brain, the majority belonged to frontal (n = 3) and subcortical (n = 2) regions. In the fast-speed performers, the majority of hubs belong to temporal (n = 3) and subcortical (n = 4) areas. Therefore, even though the baseline networks of both groups appear highly different from each other, both are presenting subcortical areas as key regions in the configuration of their networks. Nevertheless, only two hubs were similar between groups: the left postcentral gyrus and the right superior temporal gyrus.
4.3. Baseline global measures
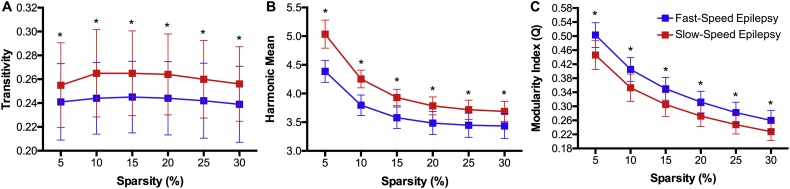
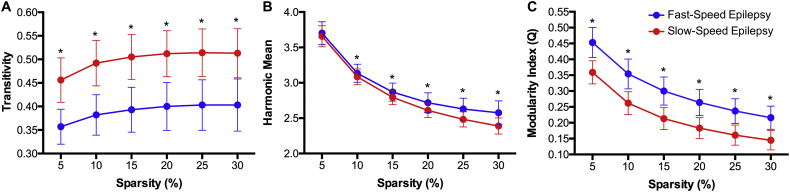
The global measures of the baseline volumetric networks showed higher transitivity and harmonic mean but a lower modularity index in the slow-speed performers compared to fast-speed performers (Fig. 3). Therefore, slow-speed performers are presenting a more clustered morphological network, but lower in integration between nodes. The lower modularity index in slow-speed performers confirms the poorer community structure observed in that group (Fig. 2). The groups were significantly different from each other at each density level.
Fig. 3.
(A) Global clustering, (B) Harmonic mean, and (C) modularity index in pediatric epilepsy patients showing fast- (blue) and slow-speed (red) performances at baseline evaluation. Each value is statistically significant against the null hypothesis for each group. *Statistically significant between groups after correction for multiple comparisons.
4.4. Prospective adjacency matrices
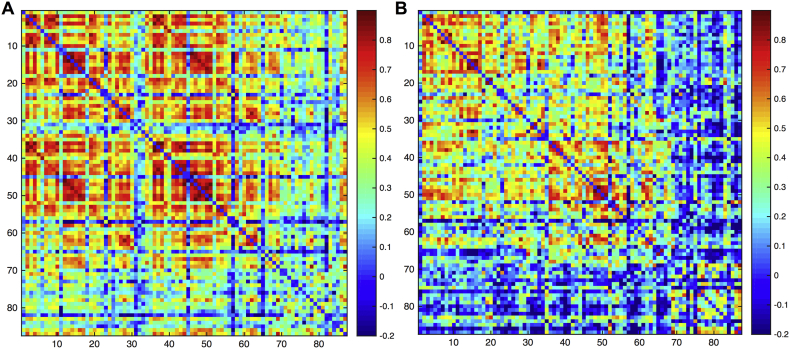
Adjacency matrices for the prospective changes in brain volumes rendered highly different results for both groups (Fig. 4). The fast-speed performers presented high disassociation (more negative correlations) between cortical and subcortical structures. Regarding the slow-speed performers, the left pericalcarine, left rostral anterior cingulate, left caudal anterior cingulate, and left isthmus cingulate (regions 30–33) seem weakly associated with the rest of cortical regions which is not the case in the right hemisphere; with the exception of the right rostral anterior cingulate (region 65).
Fig. 4.
Adjacency matrices for the (A) slow-speed performers and (B) fast-speed performers epilepsy groups. Regions are in the same order as in the Supplementary File 1.
Adjacency matrices for the (A) slow-speed performers and (B) fast-speed performers epilepsy groups. Regions are in the same order as in the Supplementary File 1.
4.5. Prospective modularity and hubs
Regarding community structure, both groups presented five modules, however, three of the five modules in the slow-speed performers were composed of <6 nodes each, while modules in the fast-speed group were more densely constituted. Therefore, modular arrangement of nodes is more evident in the fast-speed performers (Fig. 5B) while the slow-speed performers (Fig. 5A) have many peripheral nodes –nodes that are weakly associated with their own modules. The latter is also showing a peripheral module –one that is not tightly connected with the rest of the network– consisting of 5 subcortical regions. The fast-speed performers also showed a dense module composed mainly of subcortical regions that are not as disassociated with the rest of the network than the slow-speed group. This indicates a low prospective modular arrangement in slow-speed performers, indicating brain development based on volumetric changes that do not differ much between different brain regions.
Fig. 5.
Modular configuration of prospective morphological brain networks in children with epilepsy with (A) slow-speed and (B) fast-speed performance. Different colors represent different modules. Bigger nodes represent the hubs of the networks as calculated using betweenness centrality. Node abbreviations can be found in the Supplementary File 1.
Modular configuration of prospective morphological brain networks in children with epilepsy with (A) slow-speed and (B) fast-speed performance. Different colors represent different modules. Bigger nodes represent the hubs of the networks as calculated using betweenness centrality. Node abbreviations can be found in the Supplementary File 1.
Hubs of the prospective networks were highly different for each group; however, both groups showed the same number (12 each). The fast-speed performers presented the majority of hubs from frontal and parietal regions while the slow-speed performers showed mostly frontal and temporal areas as hubs. Even though both groups had the left lateral occipital and right superior frontal gyrus as hubs, those were the only pair similar between them. Of note is that the fast-speed performers showed the left cerebellum as a hub, which was not the case in the slow-speed group.
4.6. Prospective Global Measures
Global measures were acquired over a range of graph densities (5 to 30%) and were compared to the same measures obtained from random matrices in order to test for the null hypothesis at each level and for each group. Regarding global graph clustering or transitivity, slow-speed performers presented significantly higher values than the fast-speed performers; the former also showing higher graph integration than the latter as represented by the inverse of the harmonic mean (Fig. 6B). However, slow-speed performers presented significantly lower modularity index than fast-speed performers at each density level, indicating higher organization in the latter regarding their morphological development.
Fig. 6.
(A) Global clustering, (B) Harmonic mean, and (C) modularity index in pediatric epilepsy patients showing fast (blue) and slow (red) speed performances. Each value is statistically significant against the null hypothesis for each group. *Statistically significant between groups after correction for multiple comparisons.
4.7. Prospective regional measures
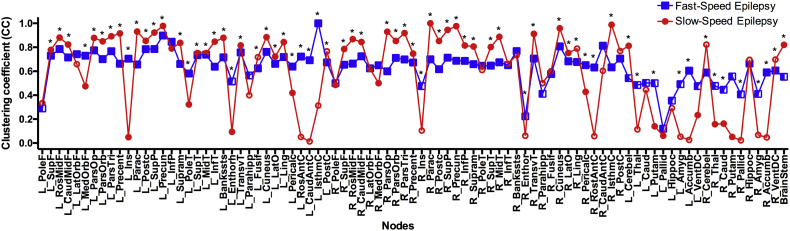
Regional clustering coefficient (CC) was calculated for each of the permuted matrices and averages were used for group comparisons (Fig. 7). As can be seen, fast-speed performers (red) showed mostly constant CC values across nodes while there are several regions showing close-to-zero values in slow-speed performers; the latter representing most of the peripheral nodes previously observed in this group (Fig. 5A). Hence, even though CC values are on average higher in slow-speed performers than fast-speed performers, a higher number of nodes are presenting very little clustering, therefore lower correlation between neighbors in the slow-speed group.
Fig. 7.
Local clustering coefficient in pediatric epilepsy patient having fast (blue) and slow (red) speed performances. Nodes are in the same order as in the Supplementary File 1. Filled nodes are those showing statistical significance against zero. *Statistically significant between groups after correction for multiple comparisons. Calculated at a hybrid threshold of 15%.
Local clustering coefficient in pediatric epilepsy patient having fast (blue) and slow (red) speed performances. Nodes are in the same order as in the Supplementary File 1. Filled nodes are those showing statistical significance against zero. *Statistically significant between groups after correction for multiple comparisons. Calculated at a hybrid threshold of 15%.
5. Discussion
We examined the large scale networks associated with performance on a common task of psychomotor speed (digit symbol substitution) through examination of cortical, subcortical and cerebellar volumes. This issue was examined in both a cross-sectional (near the time of diagnosis of epilepsy) as well as prospective fashion (2 years later) in the same cohort of subjects to determine whether children with epilepsy who varied in age-adjusted psychomotor speed performance exhibited different baseline and prospective developmental brain trajectories. The focus of this investigation was children with “uncomplicated” idiopathic epilepsies, attending regular schools, with documented average intelligence the results also adjusted for epilepsy syndrome. Despite their documented average intelligence, the children with epilepsy examined here exhibited variable processing speed and subgroups of children with epilepsy with slow or intact/fast processing speed were identified. The main result of this study is that psychomotor slowing appears to serve as a “behavioral biomarker” that is reflective of anomalies in both cross-sectional and prospective brain development in children with idiopathic epilepsies.
It is widely known that there are subtle morphometric brain differences between normally developing children and youth with idiopathic epilepsies when examining traditional metrics of brain volume, thickness, surface area, and other morphometry measures (Bin et al., 2017; Garcia-Ramos et al., 2015b; Tondelli et al., 2016). However, little is known regarding brain and network differences in epilepsy patients who differ in psychomotor speed performance—a prevalent cognitive abnormality in children and adults with epilepsy identified even prior to the administration of epilepsy medications (Oostrom et al., 2003; Prevey et al., 1998; Taylor et al., 2010), and a trait that persists following epilepsy remission (Aldenkamp et al., 1993; Berg et al., 2008).
In this study, epilepsy participants exhibiting baseline psychomotor slowing presented with lower modularity, lower global efficiency (as indicated by the inverse of the harmonic mean), and higher global clustering than higher speed performers, while also showing a lower number of hubs at/near the onset of epilepsy. The baseline brain morphometry of slow-speed performers also exhibited poorer integration/association between cortical regions and both subcortical structures and the cerebellum. Fast-speed performers, however, presented well-defined communities with a superior level of integration between themselves, with subcortical structures forming their own module, but integrated to the rest of the network as well as with bilateral cerebellum integrated to the rest of the graph instead of separated as in slower-speed performers. Therefore, a major baseline finding is that children with depressed psychomotor speed exhibit volumes of subcortical areas and cerebellum that seem not to be correlating with the rest of cortical regions.
Another interesting finding is the difference in the number of hubs between both groups. Slow-speed performers demonstrated 33% fewer hubs than fast-speed performers which could indicate a network of low inter-correlation between nodes, therefore, without the need of major highways to connect them. Furthermore, slow-speed performers exhibited hubs spread mainly across lobes with frontal areas comprising the majority, while fast-speed performers had a majority of hubs belonging to subcortical structures. This again indicates noticeable differences between fast and slow-speed children with epilepsy and the regions that appear to play a key role in the network topology of psychomotor speed. Once again, fast and slow-speed groups showed marked differences regarding not just the general network configuration but also their regional composition.
The prospective implications of slowed baseline processing speed for brain development has never been explored in epilepsy. In an intriguing report, Austin et al. (2011) found baseline psychomotor slowing in children with new onset epilepsy to be predictive of behavior problems 3 years later (Austin et al., 2011), behaviors that we previously demonstrated were associated with anomalies in brain structure (Dabbs et al., 2013). Therefore, we hypothesized that baseline psychomotor slowing would be predictive of atypical brain development.
Prospectively, the slow-speed group displayed the same lower modularity as in the baseline evaluation but higher global efficiency and clustering in their brain development compared to higher speed performers. Specifically, slow-speed performers showed two main modules as a core with three more modules in the periphery of the network; therefore, exhibiting low developmental correlation with the rest of the regions. That was not the case in fast-speed performers where there was evidence of more compartmentalized development with segregated modules that at the same time exhibited integration with the rest of the network. Therefore, regional volume changes seem to be occurring as one in slow-speed performers, but occurring in a more modular fashion in fast-speed performers. Regarding network hubs, slow-speed performers showed nodes mainly from frontal and temporal regions while fast-speed performers showed hubs spread more widely among frontal, temporal, parietal, and subcortical areas including the left cerebellum. Therefore, it seems that, developmentally, slow-speed performance at baseline tends to be associated with higher correlation between frontal and temporal regions, while fast-speed performers seem to be presenting a more equitable brain regional development.
Prospectively, we also investigated local CC across every node in the networks of both groups, the results demonstrating that the local story was very different than globally. A high number of nodes in slow-speed performers presented low clustering, indicating low developmental correlation between regions that should be developing together. This was even more evident across subcortical regions.
Overall, psychomotor-speed differences, adjusted for multiple factors (age, IQ, epilepsy syndrome, ICV) appear to be associated with alterations in brain structure in children with epilepsy. It is well-known that AED treatment alters aspects of cognitive ability in children with epilepsy, with psychomotor speed being one of the most affected domains (Meador, 2002). But in this group of children with “uncomplicated” idiopathic epilepsies, the vast majority on monotherapy in both fast and slow speed groups, very few were on polytherapy, several were taking no medications, and a very small number taking high cognitive risk medications (e.g., topiramate) (van Veenendaal et al., 2017), equally represented here in the slow and fast groups, suggesting that the widespread cross-sectional and prospective morphometric differences are not likely attributable to variations in AED treatment.
There may be other factors contributing to the observed morphometric network differences between slow and fast-speed performers, for example, maturation of white matter tracts. Maturation of white matter tracks are associated with increases in information processing speed in children (e.g., Scantlebury et al., 2014), and pediatric disease and/or treatment- related factors (e.g., radiation) can impair white matter maturation and processing speed (Scantlebury et al., 2016). As white matter abnormalities are appreciated in the epilepsies represented in this study (Kim et al., 2014; Ciumas et al., 2014; Widjaja et al., 2013; Amarreh et al., 2013; Hutchinson et al., 2013), this is a relevant consideration. Other studies have shown that functional imaging evidence of neural efficiency are associated with improved processing speed in both healthy controls (Rypma et al., 2006) and MS patients (Fittipaldi-Márquez et al., 2017) and this represents a direction for future research.
6. Conclusions
High modularity and well-defined modules appear to be associated with, and advantageous to, faster psychomotor speed performance reflected in the reported cross-sectional and longitudinal assessments of brain volumes in children with new onset epilepsies. The results suggest that there are indeed widespread baseline and prospective alterations in large scale networks between fast and low psychomotor speed children with new onset epilepsies. Interestingly, slower processing speed appears to be a marker of abnormal brain development antecedent to epilepsy onset as well as brain development over the next 2 years following diagnosis.
The following is the supplementary data related to this article.
Acknowledgements
All phases of this study were supported by the National Institute of Neurological Disorders and Stroke (NINDS) 3RO1-44351 (BPH), 3R01NS044351- 09S1 (CGR), T32 (T32 CA 009206) grant from the National Cancer Institute (CGR), by the Foundation of ASNR 2014 Award (VP), and by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. We thank Raj Sheth MD, Monica Koehn MD, and Jason Dozier MD for study participation and recruitment of participants. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
References
- Aldenkamp A.P., Alpherts W.C., Blennow G., Elmqvist D., Heijbel J., Nilsson H.L., Sandstedt P., Tonnby B., Wåhlander L., Wosse E. Withdrawal of antiepileptic medication in children–effects on cognitive function: the multicenter Holmfrid study. Neurology. 1993 Jan;43(1):41–50. doi: 10.1212/wnl.43.1_part_1.41. [DOI] [PubMed] [Google Scholar]
- Aldenkamp A.P., Alpherts W.C., Sandstedt P., Blennow G., Elmqvist D., Heijbel J., Nilsson H.L., Tonnby B., Wåhlander L., Wosse E. Antiepileptic drug-related cognitive complaints in seizure-free children with epilepsy before and after drug discontinuation. Epilepsia. 1998 Oct;39(10):1070–1074. doi: 10.1111/j.1528-1157.1998.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Amarreh I., Dabbs K., Jackson D.C., Jones J.E., Meyerand M.E., Stafstrom C.E., Hsu D.A., Seidenberg M., Hermann B.P. Cerebral white matter integrity in children with active versus remitted epilepsy 5 years after diagnosis. Epilepsy Res. 2013 Dec;107(3):263–271. doi: 10.1016/j.eplepsyres.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashendorf L., Reynolds E. Process analysis of the digit symbol task. In: Ashendorf l Awenson R., Libon D., editors. The Boston Approach to Neuropsychological Assessment. Oxford University Press; New York: 2013. [Google Scholar]
- Austin J.K., Perkins S.M., Johnson C.S., Fastenau P.S., Byars A.W., deGrauw T.J., Dunn D.W. Behavior problems in children at time of first recognized seizure and changes over the following 3 years. Epilepsy Behav. 2011;21(4):373–381. doi: 10.1016/j.yebeh.2011.05.028. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander R.P., Concha L., Snyder T.J., Beaulieu C., Gross D.W. Correlations between limbic white matter and cognitive function in temporal-lobe epilepsy, preliminary findings. Front. Aging Neurosci. 2014;6:142. doi: 10.3389/fnagi.2014.00142. Jun 30. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin G., Wang T., Zeng H., He X., Li F., Zhang J., Huang B. Patterns of gray matter abnormalities in idiopathic generalized epilepsy: a meta-analysis of voxel-based morphology studies. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169076. e0169076. Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G.A., Taylor J., Aldenkamp A.P. SANAD group. Newly diagnosed epilepsy: cognitive outcome after 12 months. Epilepsia. 2011 Jun;52(6):1084–1091. doi: 10.1111/j.1528-1167.2011.03043.x. doi: 10.1111/j.1528-1167.2011.03043.x. [DOI] [PubMed] [Google Scholar]
- Batista S., Zivadinov R., Hoogs M., Bergsland N., Heininen-Brown M., Dwyer M.G., Weinstock-Guttman B., Benedict R.H. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 2012 Jan;259(1):139–146. doi: 10.1007/s00415-011-6147-1. [DOI] [PubMed] [Google Scholar]
- Berg A.T., Langfitt J.T., Testa F.M., Levy S.R., DiMario F., Westerveld M., Kulas J. Residual cognitive effects of uncomplicated idiopathic and cryptogenic epilepsy. Epilepsy Behav. 2008 Nov;13(4):614–619. doi: 10.1016/j.yebeh.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. 2008;(10):10008–10019. [Google Scholar]
- Boake C. From the Binet-Simon to the Wechsler-Bellevue: tracing the history of intelligence testing. J. Clin. Exp. Neuropsychol. 2002 May;24(3):383–405. doi: 10.1076/jcen.24.3.383.981. [DOI] [PubMed] [Google Scholar]
- Boccaletti S., Latora V., Moreno Y., Chavez M., Hwang D.-U. Complex networks: structure and dynamics. Phys. Rep. 2006;424(4–5):175–308. [Google Scholar]
- Boelen S., Nieuwenhuis S., Steenbeek L., Veldwijk H., van de Ven-Verest M., Tan I.Y., Aldenkamp A.P. Effect of epilepsy on psychomotor function in children with uncomplicated epilepsy. Dev. Med. Child Neurol. 2005 Aug;47(8):546–550. doi: 10.1017/s0012162205001064. [DOI] [PubMed] [Google Scholar]
- Ciumas C., Saignavongs M., Ilski F., Herbillon V., Laurent A., Lothe A., Heckemann R.A., de Bellescize J., Panagiotakaki E., Hannoun S., Marinier D.S., Montavont A., Ostrowsky-Coste K., Bedoin N., Ryvlin P. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain. 2014 Apr;137(Pt 4):1095–1106. doi: 10.1093/brain/awu039. doi: 10.1093/brain/awu039. [DOI] [PubMed] [Google Scholar]
- Dabbs K., Jones J.E., Jackson D.C., Seidenberg M., Hermann B.P. Patterns of cortical thickness and the child behavior checklist in childhood epilepsy. Epilepsy Behav. 2013 Oct;29(1):198–204. doi: 10.1016/j.yebeh.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca J. Information processing speed: How fast, how slow, how Come? In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 265–273. [Google Scholar]
- DeLuca J., Kalmar J.H. Taylor & Francis; 2008. Information Processing Speed in Clinical Populations. [Google Scholar]
- Demaree H.A., Frazier T.W., Johnson C.E. Information processing speed: measurement issues and its relationships with other neuropsychological constructs. In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 54–77. [Google Scholar]
- Dickinson D., Gold J.M. Processing speed and the Digit Symbol Substitution Test in schizophrenia. In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 125–152. [Google Scholar]
- Dickinson D., Ramsey M.E., Gold J.M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry. 2007 May;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dodrill C.B. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl. 1):S21–4. doi: 10.1016/j.yebeh.2003.11.004. Feb. [DOI] [PubMed] [Google Scholar]
- Dow C., Seidenberg M., Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav. 2004 Dec;5(6):919–925. doi: 10.1016/j.yebeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Eddy C.M., Rickards H.E., Cavanna A.E. The cognitive impact of antiepileptic drugs. Ther. Adv. Neurol. Disord. 2011 Nov;4(6):385–407. doi: 10.1177/1756285611417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004 Nov;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Era P., Sainio P., Koskinen S., Ohlgren J., Härkänen T., Aromaa A. Psychomotor speed in a random sample of 7,979 subjects aged 30 years and over. Aging Clin. Exp. Res. 2011 Apr;23(2):135–144. doi: 10.1007/BF03351077. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., van der Kouwe A., Destrieux C. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fittipaldi-Márquez M.S., Cruz-Gómez Á.J., Sanchis-Segura C., Belenguer A., Ávila C., Forn C. Exploring neural efficiency in multiple sclerosis patients during the symbol digit modalities test: a functional magnetic resonance imaging study. Neurodegener. Dis. 2017;17(4–5):199–207. doi: 10.1159/000460252. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos C., Lin J.J., Prabhakaran V., Hermann B.P. Developmental reorganization of the cognitive network in pediatric epilepsy. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C., Jackson D.C., Lin J.J., Dabbs K., Jones J.E., Hsu D.A., Stafstrom C.E., Zawadzki L., Seidenberg M., Prabhakaran V., Hermann B.P. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56(10):1615–1622. doi: 10.1111/epi.13125. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevers E., Breuer L.E., IJff D.M., Aldenkamp A.P. Mental slowing in relation to epilepsy and antiepileptic medication. Acta Neurol. Scand. 2016;134(2):116–122. doi: 10.1111/ane.12517. Aug. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Seidenberg M., Dow C., Jones J., Rutecki P., Bhattacharya A., Bell B. Cognitive prognosis in chronic temporal lobe epilepsy. Ann. Neurol. 2006 Jul;60(1):80–87. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- Hermann B., Jones J., Sheth R., Dow C., Koehn M., Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006 Oct;129(Pt 10):2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Zhao Q., Jackson D.C., Jones J.E., Dabbs K., Almane D., Hsu D.A., Stafstrom C.E., Koehn M.A., Seidenberg M., Rathouz P.J. Cognitive phenotypes in childhood idiopathic epilepsies. Epilepsy Behav. 2016 Aug;61:269–274. doi: 10.1016/j.yebeh.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M.D., Gurney K. Network ‘small-world-ness’: a quantitative method for determining canonical network equivalence. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E., Pulsipher D., Dabbs K., Myers y Gutierrez A., Sheth R., Jones J., Seidenberg M., Meyerand E., Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2013;107(3):263–271. doi: 10.1016/j.eplepsyres.2013.09.012. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy S., Fein D., Kaplan E. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003 Mar;10(1):56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Lee J.H., Chung H.K., Lim S.M., Lee H.W. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur. J. Neurol. 2014;21(5):708–717. doi: 10.1111/ene.12301. May. [DOI] [PubMed] [Google Scholar]
- Knowles E.E., David A.S., Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am. J. Psychiatry. 2010 Jul;167(7):828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- Lin J.J., Mula M., Hermann B.P. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012 Sep 29;380(9848):1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loring D.W., Marino S., Meador K.J. Neuropsychological and behavioral effects of antiepilepsy drugs. Neuropsychol. Rev. 2007 Dec;17(4):413–425. doi: 10.1007/s11065-007-9043-9. [DOI] [PubMed] [Google Scholar]
- Lossius M.I., Hessen E., Mowinckel P., Stavem K., Erikssen J., Gulbrandsen P., Gjerstad L. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus study) Epilepsia. 2008 Mar;49(3):455–463. doi: 10.1111/j.1528-1167.2007.01323.x. [DOI] [PubMed] [Google Scholar]
- Lu P.H., Lee G.J., Raven E.P., Tingus K., Khoo T., Thompson P.M., Bartzokis G. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J. Clin. Exp. Neuropsychol. 2011 Dec;33(10):1059–1068. doi: 10.1080/13803395.2011.595397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T.A., Bush S.S. Assessment tools and research methods for human information processing speed. In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 29–51. [Google Scholar]
- Meador K.J. Cognitive outcomes and predictive factors in epilepsy. Neurology. 2002 Apr 23;58(8 Suppl 5):S21–6. doi: 10.1212/wnl.58.8_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- van Mil S.G., de la Parra N.M., Reijs R.P., van Hall M.H., Aldenkamp A.P. Psychomotor and motor functioning in children with cryptogenic localization related epilepsy. NeuroRehabilitation. 2010;26(4):291–297. doi: 10.3233/NRE-2010-0565. [DOI] [PubMed] [Google Scholar]
- Morrens M., Hulstijn W., Sabbe B. Psychomotor slowing in schizophrenia. Schizophr. Bull. 2007 Jul;33(4):1038–1053. doi: 10.1093/schbul/sbl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsel A.M., Temmerman A., Sabbe B., Hulstijn W., Morrens M. Unraveling psychomotor slowing in bipolar disorder. Neuropsychobiology. 2015;71(4):234–240. doi: 10.1159/000431153. doi: 10.1159/000431153. [DOI] [PubMed] [Google Scholar]
- Newman M.E.J. The structure and function of complex networks. SIAM Rev. 2003;45:167–256. [Google Scholar]
- O'Brien A.R., Tulsky D.S. The history of processing speed and its relationship to intelligence. In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 1–28. [Google Scholar]
- Oostrom K.J., Smeets-Schouten A., Kruitwagen C.L., Peters A.C., Jennekens-Schinkel A. Dutch study group of epilepsy in childhood. Not only a matter of epilepsy: early problems of cognition and behavior in children with "epilepsy only"–a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112(6 Pt 1):1338–1344. doi: 10.1542/peds.112.6.1338. Dec. [DOI] [PubMed] [Google Scholar]
- Park S.P., Kwon S.H. Cognitive effects of antiepileptic drugs. J. Clin. Neurol. 2008 Sep;4(3):99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazzini A., Turner K., Chifari R., Morabito A., Canger R., Canevini M.P. Attention and psychomotor speed decline in patients with temporal lobe epilepsy: a longitudinal study. Epilepsy Res. 2006 Dec;72(2–3):89–96. doi: 10.1016/j.eplepsyres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Prevey M.L., Delaney R.C., Cramer J.A., Mattson R.H. Complex partial and secondarily generalized seizure patients: cognitive functioning prior to treatment with antiepileptic medication. VA epilepsy cooperative study 264 group. Epilepsy Res. 1998 Mar;30(1):1–9. doi: 10.1016/s0920-1211(97)00091-0. [DOI] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rypma B., Berger J.S., Prabhakaran V., Bly B.M., Kimberg D.Y., Biswal B.B., D'Esposito M. Neural correlates of cognitive efficiency. NeuroImage. 2006;33(3):969–979. doi: 10.1016/j.neuroimage.2006.05.065. Nov 15. [DOI] [PubMed] [Google Scholar]
- Scantlebury N., Cunningham T., Dockstader C., Laughlin S., Gaetz W., Rockel C., Dickson J., Mabbott D. Relations between white matter maturation and reaction time in childhood. J. Int. Neuropsychol. Soc. 2014 Jan;20(1):99–112. doi: 10.1017/S1355617713001148. [DOI] [PubMed] [Google Scholar]
- Scantlebury N., Bouffet E., Laughlin S., Strother D., McConnell D., Hukin J., Fryer C., Laperriere N., Montour-Proulx I., Keene D., Fleming A., Jabado N., Liu F., Riggs L., Law N., Mabbott D.J. White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology. 2016 May;30(4):425–438. doi: 10.1037/neu0000258. [DOI] [PubMed] [Google Scholar]
- van Schooneveld M.M., van Erp N., Boshuisen K., Meekes J., Braun K.P. Withdrawal of antiepileptic drugs improves psychomotor speed after childhood epilepsy surgery. Epilepsy Res. 2013;107(1–2):200–203. doi: 10.1016/j.eplepsyres.2013.08.004. Nov. [DOI] [PubMed] [Google Scholar]
- Segonne F., Dale A.M., Busa E. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O., Honey C.J., Kötter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2(10) doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A. The role of memory in the age decline in digit-symbol substitution performance. J. Gerontol. 1978 Mar;33(2):232–238. doi: 10.1093/geronj/33.2.232. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A. The processing speed theory of adult age differences in cognition. Psychol. Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A. Aging and measures of processing speed. Biol. Psychol. 2000 Oct;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse T.A., Madden D.J. Information processing speed and aging. In: DeLuca J., Kalmar J.H., editors. Information Processing Speed in Clinical Populations. Taylor & Francis; 2008. pp. 221–241. [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; New York, NY: 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-dimensional Proportional System–an Approach to Cerebral Imaging. [Google Scholar]
- Taylor J., Kolamunnage-Dona R., Marson A.G., Smith P.E., Aldenkamp A.P., Baker G.A. SANAD study group. Patients with epilepsy: cognitively compromised before the start of antiepileptic drug treatment? Epilepsia. 2010 Jan;51(1):48–56. doi: 10.1111/j.1528-1167.2009.02195.x. doi: 10.1111/j.1528-1167.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Tondelli M., Vaudano A.E., Ruggieri A., Meletti S. Cortical and subcortical brain alterations in juvenile absence epilepsy. Neuroimage Clin. 2016 Jul 18;12:306–311. doi: 10.1016/j.nicl.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veenendaal T.M., IJff D.M., Aldenkamp A.P., Lazeron R.H.C., Hofman P.A.M., de Louw A.J.A., Backes W.H., Jansen J.F.A. Chronic antiepileptic drug use and functional network efficiency: a functional magnetic resonance imaging study. World J. Radiol. 2017 Jun 28;9(6):287–294. doi: 10.4329/wjr.v9.i6.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen J., Aldenkamp A.P. Cognitive side-effects of chronic antiepileptic drug treatment: a review of 25 years of research. Epilepsy Res. 1995 Oct;22(2):65–95. doi: 10.1016/0920-1211(95)00047-x. [DOI] [PubMed] [Google Scholar]
- Wang J., Zuo X., He Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00016. 20589099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Third edition. The Psychological Corporation; San Antonio, TX: 1991. The Wechsler Intelligence Scale for Children. [Google Scholar]
- Widjaja E., Kis A., Go C., Raybaud C., Snead O.C., Smith M.L. Abnormal white matter on diffusion tensor imaging in children with new-onset seizures. Epilepsy Res. 2013 Mar;104(1–2):105–111. doi: 10.1016/j.eplepsyres.2012.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.