Abstract
Background
This study aims to report our experience with robot assisted esophagectomy (RAE) for the treatment of resectable esophageal squamous cell carcinoma (ESCC).
Methods
A series of 249 consecutive patients diagnosed with ESCC who underwent RAE from November 2015 to December 2017 at Shanghai Chest Hospital were evaluated, and their clinical data were reviewed retrospectively. One hundred patients were equally divided into four groups according to the surgery order, and the short-term outcomes in each group were analyzed.
Results
Overall, 249 patients (201 males and 48 females) with a mean age of 63.4±7.3 years who underwent RAE were analyzed. The thoracic procedure was successfully performed with the assistance of a robot. The mean total duration was 250.6±58.4 mins, and the estimated blood loss was 215.5±87.6 mL. R0 resection was performed in 232 (93.2%) patients with a mean total number of dissected lymph nodes of 18.5±9.1 and mean yield of lymph nodes along the recurrent laryngeal nerve (RLN) of 4.4±3.2. The median postoperative hospital stay was 11 days, and no 90-day mortality was observed. Forty-five (18.1%) patients experienced pulmonary complications, and the recurrent laryngeal nerve injury were observed in 38 (15.3%) patients. A significant reduction in thoracic duration was observed after the initial 25 cases (P<0.001). After 50 cases, the dissection of total lymph nodes, mediastinum lymph nodes and lymph nodes along the RLN were significantly improved (P<0.001, P<0.001, P=0.001, respectively) with a shorter postoperative hospital stay (P=0.005).
Conclusions
RAE is a safe and feasible alternative surgical approach for resectable esophageal carcinoma and is associated with a large yield of lymph nodes, especially along the RLN. The surgeon will reach a plateau of operative duration after 25 cases and a plateau of lymphadenectomy after 50 cases.
Keywords: Robot surgery, esophagectomy, learning curve, esophageal cancer
Introduction
Minimally invasive esophagectomy (MIE) has developed rapidly over the last two decades with the purpose of reducing postoperative complications and surgical-related mortality (1-3). Recent studies have demonstrated that MIE is comparable to other procedures even prior to open esophagectomy with regard to short-term surgical outcomes and long-term oncological survival (4,5).
Robot assisted esophagectomy (RAE) has been introduced to overcome the limitations of MIE and uses steady robotic arms and a three-dimensional view. RAE has advantages in terms of better three-dimensional images, hand-eye consistency and a flexible endowrist. Although recent studies of RAE have shown its safety and advantages in lymph node dissection, results from large scale samples are lacking (6-8).
The learning curve of MIE has been demonstrated to be 40–60 cases (9,10). However, reports on the learning curve of RAE are lacking, probably due to the slow promotion of this new surgery, which was introduced in 2004 (11). A previous small series on the learning curve of RAE had results of a learning curve of 6–20 cases, but the results remain controversial due to simplifying subgroup patients into two groups and defining the learning curve only according to surgical time.
This study aimed to present our short-term outcomes of RAE for ESCC to evaluate the safety and feasibility of RAE and to define the precise learning curve of RAE.
Methods
This is a retrospective study of 249 patients who underwent RAE at Shanghai Chest Hospital from November 2015 to December 2017. The inclusion criteria for RAE were as follows: (I) histologically diagnosed with ESCC; (II) tumor clinical stage T1–4a, N0–2, M0 according to the 8th edition of the American Joint Committee on Cancer tumor-node-metastasis (TNM) classification; (III) generally good physical shape to tolerate open esophagectomy; and (IV) allowed to have a McKeown esophagectomy. The exclusion criteria were: (I) a tumor clinical stage of T4b or M1; (II) American Society of Anesthesiologists grade greater than IV.
All patients underwent upper endoscopy and acquired a pathological diagnosis prior to surgery. Clinical staging was based on the findings of imaging examinations, including enhanced computed tomography of the chest and abdomen, endoscopic ultrasonography, and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET). A bronchoscope was used in patients with upper esophageal cancer. Cranial magnetic resonance imaging was performed selectively.
The clinical data were collected in the Esophageal Surgery Section of Shanghai Chest Hospital database. To define the learning curve for RAE, the initial 100 patients were divided into four periods according to surgical consequence (25 in each group).
Approval for the study was obtained from the Ethics Committee of Shanghai Chest Hospital [ID of the ethic approval: KS(Y)1657]. Written informed consent was obtained from each patient or his/her legal representative.
Operation
Thoracic stage
Patients were induced with one-lung ventilation with a single lumen bronchial blocker and were then placed in a semi-prone position. Four ports were placed as follows: the camera port (12 mm trocar) was placed at the 6th intercostal place along the anterior of latissimus dorsi; the robotic left arm (10 mm trocar) was placed at the 8th intercostal place along the scapular line; the robotic right arm (10 mm trocar) was placed at the 4th intercostal place between the posterior axillary line and middle axillary line; and the accessory port (10 mm trocar) was placed at the 5th intercostal place along the middle axillary line. Moreover, to help retract the esophagus, a purse-string needle was passed into the thorax through the 4th intercostal place along the inner border of the scapula (Figure 1). The da Vinci robotic cart (Intuitive Surgical, Mountain View, CA, USA) was docked from the right rear of the patient.
Figure 1.

The robotic port placement for the thoracic procedure. C, camera port; L, left robotic arm port; R, right robotic arm port; A, assistant port; H, helping purse-string needle.
The mediastinal pleura above the arch of the azygos vein was first divided, and the right recurrent laryngeal nerve (RLN) was identified after it was anatomically exposed along the right vagus nerve. Lymph nodes and fatty tissues along the right-RLN and the superior esophagus were dissected en bloc. The arch of azygos vein was divided, and the middle esophagus with surrounding tissue was en bloc dissected. Dissection continued from the right main bronchus up to the plane between the esophagus and the trachea membrane. The upper esophagus was retracted by the purse-string, and the left-RLN was pulled away from the tracheoesophageal groove. A lymphadenectomy was performed along the left-RLN from the thoracic outlet to the aortic-pulmonary window (Figure 2). The subcarinal lymph nodes were dissected after this step. Finally, the lower esophagus was dissected to the hiatus and the whole progress of thoracic esophagectomy was complete. The pulmonary branches of the vagus nerve were preserved, but the bronchial arteries were regularly transected to help with a rigid lymphadenectomy. The chest tube and a mediastinal drainage were inserted regularly.
Figure 2.

The “skeletonized” right recurrent laryngeal nerve and complete lymph node dissection. Rt RLN, right recurrent laryngeal nerve.
Abdominal stage
The patient was placed in the reverse Trendelenburg position, and the robotic cart was docked from the head side of the patient. Five ports were used in the abdominal stage, including one camera port, two robotic arm port and two assistant port (Figure 3). The greater curve was dissected first until the short gastric artery. Then, the celiac area was dissected and the left gastric artery was cut off (Figure 4). The operation converted to the hepatogastric ligament and the right crus was dissected. The last procedure with the robot assisted laparoscope was dissection of the left crus and fundus. A small incision was made at the sub-xiphoid. A narrow gastric tube (3–4 cm) was made from the abdomen. The anastomosis was completed at the neck. The patient was transported to the ICU with a nasogastric tube and nasoduodenal nutrition tube.
Figure 3.
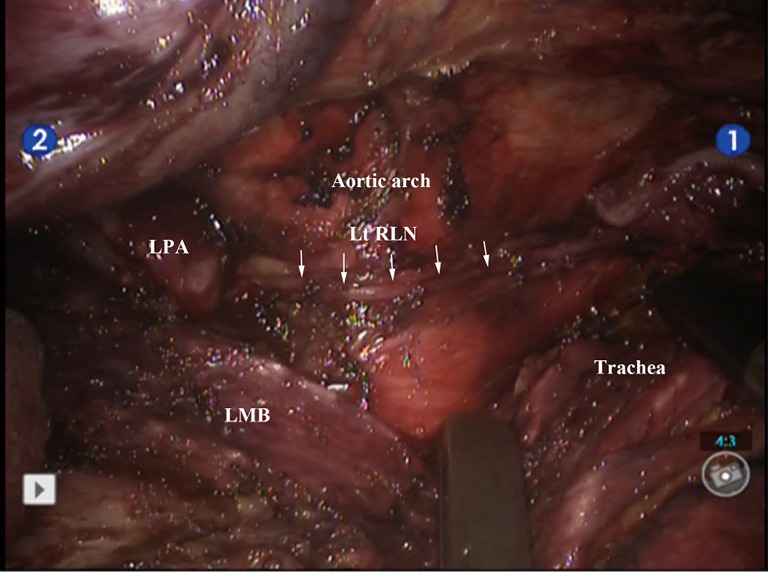
The “skeletonized” left recurrent laryngeal nerve and complete lymph node dissection. Lt RLN, left recurrent laryngeal nerve; LMB, left main bronchus; LPA, left pulmonary artery.
Figure 4.

The robotic port placement for the abdominal procedure. C, camera port; L, left robotic arm port; R, right robotic arm port; A1, assistant port 1; A2, assistant port 2.
The durations of the two operative stages were recorded; the total operative duration was defined as the amount of time from the first incision at the thoracic stage to the closure of the abdominal incision. The thoracic operative duration was defined as the amount of time from the first skin incision to the closure of the thoracic incision. Blood loss was estimated based on the suctioned volume and gauze pieces with blood during surgery.
Postoperative care
Patients were transferred to the intensive care unit (ICU) 2–3 h after the operation, and extubation was performed after an assessment of the patients’ respiratory function.
Statistical analysis was performed with SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The mean, median, and standard deviation were calculated for continuous variables. Student’s t-test, the chi-square test, and Fisher’s exact test were used to compare categorical variables between the two groups. One-way analysis of variance (ANOVA) was used to compare the quantitative variables between the four groups. A P value of <0.05 was considered statistically significant.
Results
The patient demographics and pathological data are listed in Table 1. From November 2015 to December 2017, a total of 249 patients diagnosed with ESCC received RAE. There were 201 (80.7%) males and 48 (19.3%) females with a mean age of 63.4±7.3 years. Most patients (62.7%) had a tumor located at the middle esophagus. The majority of patients (71.1%) were clinically classified as stage II or higher. Twenty patients (8.0%) underwent RAE subsequent to neoadjuvant concurrent chemoradiotherapy (CCRT).
Table 1. Patient demographics.
| Variables | n (%) |
|---|---|
| Gender | |
| Male | 201 (80.7) |
| Female | 48 (19.3) |
| Age (y) | 63.4±7.3 |
| BMI (kg/m2) | 23.1±2.9 |
| ASA | |
| I | 7 (2.8) |
| II | 222 (89.2) |
| III | 20 (8.0) |
| Tumor location | |
| Upper | 33 (13.3) |
| Middle | 156 (62.7) |
| Lower | 60 (24.0) |
| Clinical stage | |
| I | 72 (28.9) |
| II | 112 (45.0) |
| III | 43 (17.3) |
| IV | 22 (8.8) |
| Neoadjuvant therapy | |
| Yes | 20 (8.0) |
| No | 229 (92.0) |
BMI, body mass index; ASA, American Society of Anesthesiologists.
The short-term outcomes are summarized in Table 2. The thoracic procedures were successfully performed in all patients with the assistance of the robot, except for 2 conversions to open thoracotomy due to extensive pleural adhesion. In all, 74 (29.7%) patients received laparotomy, 174 (69.9%) patients underwent abdominal procedures performed with the assistance of the robot, and 8 patients developed conversions due to abdominal adhesions. One male patient failed to have a complete McKeown esophagectomy causing of intraoperative acute myocardial infarction. The cervical esophagus was excluded and the tumor was removed. The mean total operative duration was 250.6±58.4 min, and the mean operative duration of the thoracic procedure was 88.0±28.4 min. The estimated blood loss was 215.5±87.6 mL.
Table 2. Short-term outcomes.
| Variables | n (%) |
|---|---|
| Duration (minutes) | |
| Total | 250.6±58.4 |
| Thoracic procedure | 88.0±28.4 |
| Estimated blood loss (mL) | 215.5±87.6 |
| Conversion | 10 (4.0) |
| Number of yield lymph nodes | |
| Total | 18.5±9.1 |
| Mediastinum | 11.8±6.4 |
| Along RLN chain | 4.4±3.2 |
| R0 resection | 232 (93.2) |
| Pathological stage | |
| I | 61 (24.5) |
| II | 71 (28.5) |
| III | 96 (38.6) |
| IV | 21 (8.4) |
| Reoperation | 6 (2.4) |
| Postoperative hospital stay (days), median [range] | 11 [7–81] |
| ICU stay (d), median [range] | 2 [1–15] |
| 90-day mortality | 0 |
RLN, recurrent laryngeal nerve; ICU, intensive care unit.
The mean total number of lymph nodes was 18.5±9.1, and the mean number of lymph nodes dissected along the RLN was 4.4±3.2. R0 resection was successfully performed in 232 (93.2%) patients. The pathological stages were stage I for 61 (24.5%) patients, stage II for 71 (28.5%) patients, stage III for 96 (38.6%) patients and stage IV for 21 (8.4%) patients. There was no 90-day mortality. Six patients required reoperation, one underwent conduit resection due to conduit necrosis and three required tracheotomy due to severe respiratory failure and the other two underwent jejunostomy due to anastomic leakage. The median ICU stay was 2 (range, 1–15) days, and the median postoperative hospital stay was 11 (range, 7–81) days. Postoperative complications were observed in 91 (36.5%) patients, and forty-five (18.1%) patients experienced pulmonary complications; recurrent laryngeal nerve injury were observed in 38 (15.3%) patients. Thirty-two (12.9%) patients had anastomotic leakage within 3–10 days after surgery 1 (0.4%). Table 3 shows the postoperative complications.
Table 3. Complication data.
| Variables | n (%) |
|---|---|
| Total complications | 91 (36.5) |
| Pulmonary | 45 (18.1) |
| Pneumonia | 25 (10.0) |
| Pleural effusion requiring drainage procedure | 18 (7.2) |
| Pneumothorax requiring treatment | 4 (1.6) |
| Respiratory failure | 5 (2.0) |
| Empyema | 9 (3.6) |
| Cardiac | |
| Atrial arrhythmias | 9 (3.6) |
| Myocardial infarction | 1 (0.4) |
| Gastrointestinal | 32 (12.9) |
| Anastomotic leakage | 32 (12.9) |
| Conduit necrosis | 1 (0.4) |
| Tracheoesophageal fistula | 1 (0.4) |
| Deep venous thrombosis | 1 (0.4) |
| Recurrent laryngeal nerve injury | 38 (15.3) |
| Wound infection | 2 (0.8) |
| Chyle leak | 3 (1.2) |
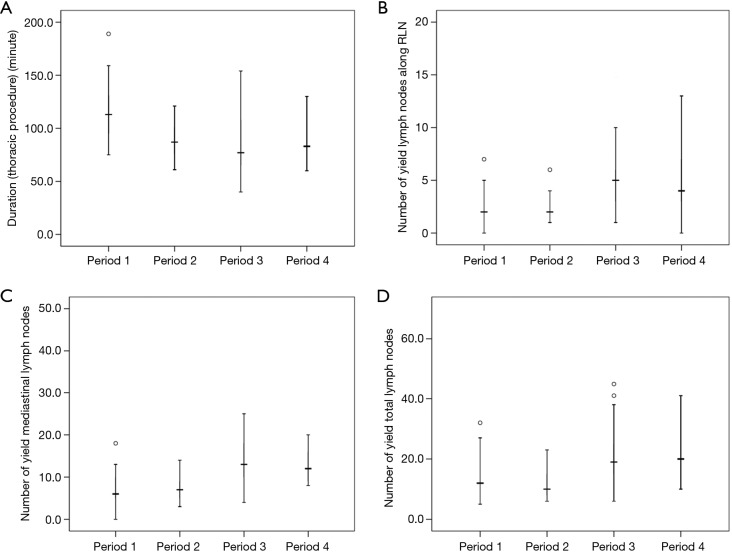
To identify the learning curve for RAE, we performed period to period comparisons of the short-term outcomes for the initial 100 patients (Table 4). The mean thoracic operative duration was 115.1±26.4 min in the first period, and the duration significantly decreased after the initial 25 cases and remained unchanged in the next 3 periods (115.1±26.4 vs. 88.4±16.0, 83.4±27.8, 87.0±18.3, P<0.001) (Figure 5A). Figure 5B,C,D shows that after the surgeon performed 50 RAE procedures, the number of lymph nodes (total, mediastinum, along RLN) was significantly increased (P<0.001, P<0.001, P=0.001, respectively) with a shorter postoperative hospital stay (P=0.005). However, no reduction was observed between the four periods with regard to the surgical related complications of anastomotic leakage and recurrent laryngeal nerve injury.
Table 4. Period to period comparisons of the short-term outcomes.
| Variables | Period 1 (n=25) | Period 2 (n=25) | Period 3 (n=25) | Period 4 (n=25) | P |
|---|---|---|---|---|---|
| Duration (thoracic procedure) | 115.1±26.4 | 88.4±16.0 | 83.4±27.8 | 87.0±18.3 | <0.001 |
| Estimated blood loss | 204.0±67.6 | 236.0±70.0 | 252.0±77.0 | 231.5±81.1 | 0.137 |
| Number of yield lymph nodes | |||||
| Total | 13.5±7.1 | 13.0±8.6 | 23.0±14.0 | 21.0±7.0 | <0.001 |
| Mediastinum | 7.8±5.2 | 7.6±3.0 | 12.7±3.8 | 15.5±9.6 | <0.001 |
| Along RLN | 2.5±1.9 | 2.9±1.9 | 5.3±3.7 | 5.1±3.8 | 0.001 |
| Vocal cord palsy | 4 (16.0%) | 5 (20.0%) | 4 (16.0%) | 3 (12.0%) | 0.859 |
| Anastomotic leakage | 5 (20.0%) | 4 (16.0%) | 3 (12.0%) | 3 (12.0%) | 0.901 |
| Postoperative hospital stay | 21.0±13.0 | 20.2±15.7 | 13.6±8.1 | 11.6±3.3 | 0.005 |
RLN, recurrent laryngeal nerve.
Figure 5.
The period-to-period results according to the sequence of operation. (A) The duration of thoracic procedure was significantly reduced after period 1 (25 cases); (B,C,D) the number of yield lymph nodes (along RLN, mediastinum, total) were significantly improved after period 2 (50 cases). RLN, recurrent laryngeal nerve.
Discussion
In this study, we confirmed that RAE was a safe and feasible surgical treatment for esophageal carcinoma. No 90-day mortality was observed. Additionally, complications were observed in 91 (36.5%) patients with a median postoperative hospital stay of 11 days. The short-term oncologic outcomes (including R0 resection and number of resected lymph nodes) were comparable with recent reports of traditional open, thoraco-laparoscopic and robotic esophagectomy (3,12,13). The results of the period to period comparisons showed that the learning curve of RAE was 25–50 cases.
Kernstine and colleagues first introduced transthoracic RAE in 2004, and the use of RAE has expanded over the last decade (11). At our institution, a McKeown esophagectomy with two-field lymphadenectomy is the standard surgery for the treatment of intrathoracic esophageal carcinoma. We demonstrated the safety of RAE in our study, no 90-day mortality was observed and the rate of R0 resection was 93.2%. Numerous experts have described their experience with RAE, and most studies focus on safety, feasibility and short-term surgical outcomes. Chiu and colleagues (14) described their experience with 20 RAE procedures with a mean operative time of 499.5±70 min and blood loss of 355.7±329.6 mL. Boone et al. (15) conducted 47 RAE procedures with 3 incisions. The rate of R0 resection was 76.6% (36/47), and 48.6% of patients were in the pathological IVa stage. The rate of R0 resection in our study was 93.2%, which was comparable with conventional minimally invasive thoraco-laparoscopic esophagectomy (2-4). In a series of 114 consecutive RAE procedures, Park and colleagues (16) reported a mean number of total retrieved lymph nodes of 43.5±1.4, while in our study, the mean number of dissected lymph nodes was 18.5±9.1. This difference may be due to the different principles of pathologists who examined the lymph nodes. In our institution, a pathologic examination was performed for lymph nodes that were 5 mm and larger. A total of 256 patients underwent RAE within two years, and we assume that the statistical power was adequate due to the sufficient number and consistency of the patients enrolled.
Esophagectomy was associated with many complications (17). In a randomized controlled trial of MIE versus traditional open esophagectomy, Biere et al. (3) reported a higher rate of recurrent laryngeal nerve injury (14%) in open esophagectomy and 12% anastomotic leakage in MIE. In this study, the overall rate of complications was 36.5%, and recurrent laryngeal nerve injury was the most common complication (18.1%). Anastomotic leakage was observed in 32 (12.9%) patients, which was higher but comparable to the recent RAE studies. Park et al. (16) described 114 RAE procedures with extensive mediastinal lymphadenectomy (ML) for intrathoracic esophageal cancer, and 30 (26.3%) cases of recurrent laryngeal nerve injury and 17 (14.9%) cases of anastomotic leakage were observed. van der Sluis and colleagues (18) described 108 RAE procedures with a high rate of recurrent laryngeal nerve injury (9%) and anastomotic leakage (19%). We attribute the high rate of recurrent laryngeal nerve injury to the radical lymphadenectomy along the RLN and RLN “skeletonized” technique. Better visualization and a flexible but steady robot arm allowed the surgeon to precisely dissect the lymph nodes along the RLN where the narrow place cannot be easily mobilized under the long rigid thoracoscopic instrument. The surgeon preferred to skeletonize the RLN chain, and the tissue, including the lymph nodes, was thoroughly dissected, which may lead to transient recurrent laryngeal nerve injury. All 45 patients in our study recovered within 3 months after surgery. However, we failed to show a decline in the rate of recurrent laryngeal nerve injury or anastomotic leakage with period-to-period analyses. The high rates of recurrent laryngeal nerve injury and anastomotic leakage were assumed to remain in future RAE cases.
RAE was assumed to have a shorter learning curve than traditional minimally invasive thoraco-laparoscopic esophagectomy due to better 3D images and hand-eye consistency. In a study of 100 cases of esophageal cancer, Oshikiri et al. (19) described their experience with thoracoscopic esophagectomy. After 33 cases, the rate of lymphadenectomy in the chest reached a plateau, and less operative time and fewer nerve injuries were observed after period 2 (66 cases). The learning curve was assumed to be 30–60 cases. Hernandez et al. (6) reported on 52 patients with esophageal cancer who underwent RAE; the operative time was significantly decreased after 20 cases, and the complication rates remained low across the successive 10-patient cohorts. Surgeon-specific and team-related factors were assumed to be the main contributors to the decreased operative time. Kim et al. (7) reported in their series of twenty-one patients who received RAE that the mean robot console time of the thoracic phase was 108.8±46.3 min and reached a plateau after 6 cases. The two RAE reports were in accordance with the point that a surgeon can easily manipulate the robotic system, and the steep learning curve had been described as 8–20 cases. However, the learning curve was mainly defined according to the overall operative time and length of the postoperative hospital stay, and the studies lacked an adequate number of patients. To define the precise learning curve of the thoracic procedure, we recruited the initial 100 patients and divided the patients into 4 periods (25 each) according to the sequence of operation. All of the factors that were assumed to be associated with the surgical technique of the surgeon were recorded, including the duration of the thoracic operation, lymphadenectomy, length of hospital stay, duration of ICU stay and number of surgical-related complications, including anastomotic leakage and recurrent laryngeal nerve injury.
In this study, the thoracic procedures were performed with the assistance of the Da Vinci robotic system. There was a significant reduction in the mean operative duration of the thoracic procedure (P<0.001) after the initial 25 patients. We attribute this reduction to the surgeon’s proficiency with the robotic system and the surgical teamwork. Although we did not record the robotic docking time, the nurses and assistants had become more familiar with the robotic system and the operative procedure of the RAE after the initial learning curve. The number of dissected lymph nodes (total, mediastinum, along the RLN) was significantly increased (P<0.001, P<0.001, P=0.001, respectively). The surgeon was cautious with lymphadenectomy in the initial cases and was concerned about the safety of the anatomy under the robot system and the lack of haptic feedback from the robot console. With more experience, the surgeon became familiar with the RAE and the efficiency of lymphadenectomy was improved. In our experience, the surgeon reached a plateau of operative duration after 25 cases, which was fewer than traditional MIE. However, considering the surgical technique of lymphadenectomy and postoperative recovery, the precise learning curve for RAE was assumed to be 50 cases.
There are a few limitations of this study. First, this is a single center retrospective study, and the results of the learning curve may be affected by the surgeon’s experience with traditional thoracoscopic esophagectomy. Second, we did not record the robot docking time, which was an important factor in the total operative duration. Third, although the early oncological results showed that RAE was at least comparable to open and thoraco-laparoscopic esophagectomy, long-term follow-up is needed to clarify the long-term survival of patients treated with RAE.
Conclusions
In conclusion, RAE was a safe and feasible surgical approach for patients with esophageal cancer. The short-term outcomes were not inferior to open and thoracoscopic esophagectomy. After 25–50 cases, RAE reached a plateau with beneficial outcomes. Multi-center randomized controlled clinical trials of RAE will be needed to clarify the advantages and disadvantages of traditional thoraco-laparoscopic esophagectomy.
Acknowledgements
Funding: This study was supported by Three years of clinical innovation action plan (16CR1035B).
Ethical Statement: The study was approved by the Ethics Committee of Shanghai Chest Hospital [No. KS(Y)1657] and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb 1992;37:7-11. [PubMed] [Google Scholar]
- 2.Osugi H, Takemura M, Higashino M, et al. A comparison of video-assisted thoracoscopic oesophagectomy and radical lymph node dissection for squamous cell cancer of the oesophagus with open operation. Br J Surg 2003;90:108-13. 10.1002/bjs.4022 [DOI] [PubMed] [Google Scholar]
- 3.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. 10.1016/S0140-6736(12)60516-9 [DOI] [PubMed] [Google Scholar]
- 4.Ye B, Zhong CX, Yang Y, et al. Lymph node dissection in esophageal carcinoma: Minimally invasive esophagectomy versus open surgery. World J Gastroenterol 2016;22:4750-6. 10.3748/wjg.v22.i19.4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. 10.1097/SLA.0b013e3182590603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophago gastrectomy. J Gastrointest Surg 2013;17:1346-51. 10.1007/s11605-013-2225-2 [DOI] [PubMed] [Google Scholar]
- 7.Kim DJ, Hyung WJ, Lee CY, et al. Thoracoscopic esophagectomy for esophageal cancer: Feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg 2010;139:53-9.e1. 10.1016/j.jtcvs.2009.05.030 [DOI] [PubMed] [Google Scholar]
- 8.Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: Technical report and short-term outcomes. World J Surg 2012;36:1608-16. 10.1007/s00268-012-1538-8 [DOI] [PubMed] [Google Scholar]
- 9.Guo W, Zou YB, Ma Z, et al. One surgeon’s learning curve for video-assisted thoracoscopic esophagectomy for esophageal cancer with the patient in lateral position: how many cases are needed to reach competence? Surg Endosc 2013;27:1346-52. 10.1007/s00464-012-2614-8 [DOI] [PubMed] [Google Scholar]
- 10.Oshikiri T, Yasuda T, Hasegawa H, et al. Short-term outcomes and one surgeon’s learning curve for thoracoscopic esophagectomy performed with the patient in the prone position Surg Today 2017;47:313. 10.1007/s00595-016-1378-5 [DOI] [PubMed] [Google Scholar]
- 11.Kernstine KH, De Armond DT, Karimi M, et al. The robotic, 2-stage, 3-field esophago lymphadenectomy. J Thorac Cardiovasc Surg 2004;127:1847-9. 10.1016/j.jtcvs.2004.02.014 [DOI] [PubMed] [Google Scholar]
- 12.Akaishi T, Kaneda I, Higuchi N, et al. Thoracoscopic en bloc total esophagectomy with radical mediastinal lymphadenectomy. J Thorac Cardiovasc Surg 1996;112:1533-40; discussion 1540-1. [DOI] [PubMed] [Google Scholar]
- 13.Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. 10.1111/j.1442-2050.2011.01246.x [DOI] [PubMed] [Google Scholar]
- 14.Chiu PW, Teoh AY, Wong VW, et al. Robotic-assisted minimally invasive esophagectomy for treatment of esophageal carcinoma. J Robot Surg 2017;11:193-9. 10.1007/s11701-016-0644-2 [DOI] [PubMed] [Google Scholar]
- 15.Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic esophagectomy for cancer. Br J Surg 2009;96:878-86. 10.1002/bjs.6647 [DOI] [PubMed] [Google Scholar]
- 16.Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. 10.1111/dote.12335 [DOI] [PubMed] [Google Scholar]
- 17.Dolan JP, Kaur T, Diggs BS, et al. Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surg Endosc 2013;27:4094-103. 10.1007/s00464-013-3066-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. 10.1245/s10434-015-4544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshikiri T, Yasuda T, Hasegawa H. Short-term outcomes and one surgeon's learning curve for thoracoscopic esophagectomy performed with the patient in the prone position. Surg Today 2017;47:313-9. 10.1007/s00595-016-1378-5 [DOI] [PubMed] [Google Scholar]