Abstract
Background
It has not been determined if adjuvant chemotherapy would be helpful for completely resected early-stage lung adenocarcinoma even with unfavorable genetic markers. As the positive anaplastic lymphoma kinase (ALK) rearrangement is associated with aggressive clinical feature in lung adenocarcinoma, we evaluated the treatment outcomes of completely resected stage IA lung adenocarcinoma according to initial ALK status.
Methods
This is a retrospective cohort study including 309 patients with surgically resected stage IA lung adenocarcinoma from February 2010 to December 2013. Patients were screened for ALK rearrangement using immunohistochemistry. A positive ALK status was defined as an immunohistochemistry score of 2+ or more. Both disease-free survival (DFS) and the initial recurrence pattern were analyzed according to ALK status.
Results
Twenty-three (7.4%) patients had ALK-positive adenocarcinoma. During the median follow-up of 35.8 months, recurrence developed in 34 (11.0%) patients. The patients with ALK-positive tumor had significantly lower 5-year DFS rate (62.4%) compared to those with ALK-negative tumor (86.5%; P=0.038). The multivariable analysis showed that ALK rearrangement was associated with a higher risk of disease recurrence (adjusted hazard ratio =2.64; 95% confidence interval, 1.08–6.44). In addition, patient with ALK-positive tumor showed more frequent recurrence in regional lymph nodes compared with those with ALK-negative tumor (83.3% vs. 28.6%; P=0.031).
Conclusions
In patients with completely resected stage IA lung adenocarcinoma, ALK rearrangement was associated with unfavorable DFS and more frequent regional lymph node metastasis. Therefore, careful surveillance for recurrence should be performed in this subset of patients.
Keywords: Anaplastic lymphoma kinase (ALK), non-small cell lung cancer (NSCLC), stage IA lung adenocarcinoma, surgery, prognosis
Introduction
The anaplastic lymphoma kinase (ALK) gene is an oncogenic driver. Its rearrangement results in activated ALK protein kinase, which is a validated therapeutic target in advanced-stage non-small cell lung cancer (NSCLC) (1-3). Depending on the study population and detection method, NSCLC harboring ALK gene rearrangement (ALK-positive) represents approximately 2–7% of all NSCLC patients in both Caucasian and Asian populations, accounting for approximately 60,000 new cases worldwide each year (2,4-7). Of these, most are histologically adenocarcinoma.
ALK-positive lung adenocarcinoma patients reportedly have demonstrated several distinct clinical features. Compared with patients with ALK-negative lung adenocarcinoma, those with an ALK-positive lung adenocarcinoma are younger and never or light smokers (2,4,7,8). It has also been found that ALK-positive adenocarcinomas are likely to have advanced lymph node, lymphangitic, and pleural or pericardial metastases (8,9). In positron emission tomography, ALK-positive adenocarcinoma patients present with a higher glucose metabolism of tumor and show a tendency of more rapid metastases to lymph nodes or to distant sites compared with those with the epidermal growth factor receptor mutation or with wild type, suggesting more aggressive features of the tumor (10). In several morphologic studies, patients with ALK rearrangement demonstrated more solid tumors with little proportion of ground-glass opacity component than those without ALK rearrangement, thus representing histopathologically micropapillary or solid subtypes of tumor (11,12).
Despite these reports of aggressive clinical features, the prognostic value of ALK rearrangement remains controversial in surgically resectable ALK-positive lung adenocarcinoma (11,13-18). Two previous studies showed that positive ALK rearrangement was associated with poor disease-free survival (DFS) (15,17), whereas ALK rearrangement was not associated with DFS in other studies (11,13,14,16,18). In contrast, positive ALK rearrangement was suggested to be a predictor of better overall survival in a recent study (18). One reason for these opposite results might be that a substantial number of patients enrolled in the previous studies had locally advanced-stage lung adenocarcinoma and eventually received adjuvant therapy. Thus, included patients were inhomogeneous in terms of tumor stage and size. Therefore, to minimize the influence of such confounding factors, a homogeneous study population achieved by confining patients to those having stage IA lung adenocarcinoma, might help evaluate the natural course of surgically resected lung adenocarcinomas.
With our experiences and the results of previous studies, we hypothesized that patients with positive ALK rearrangement would show poorer prognosis than those with negative ALK rearrangement. Therefore, the purpose of the present study was to evaluate the prognostic implication of ALK rearrangement on tumor recurrence in patients with stage IA lung adenocarcinoma who underwent curative surgical resection.
Methods
Study population and data collection
Of the 1,246 patients with stage IA lung adenocarcinoma who underwent curative surgical resection at Samsung Medical Center (a tertiary referral hospital in Seoul, Korea) between February 2010 and December 2013, 372 were evaluated for the presence of ALK rearrangement with immunohistochemistry (IHC) staining at the time of pathologic evaluation of surgical specimens of the primary tumors. Patients with a previous history of lung cancer (n=4), second primary lung cancer of different histology or that of unknown or different ALK status (n=34), active malignancy in extrathoracic organs (n=7), or who did not undergo mediastinal lymph node dissection (n=18) were excluded. Some data of these patients were used in previous reports (8,16). The Institutional Review Board of Samsung Medical Center approved this retrospective study, and informed consent was waived (IRB no. SMC 2016-05-096-002).
From the review of the electronic medical records and cancer registry at Samsung Medical Center, information was collected regarding age, sex, smoking history, body mass index, past medical history, type of surgery, pathologic stage, recurrence, death, and initial recurrence sites. Pathologic stages of tumors were determined according to the 7th edition of the American Joint Committee on Cancer. DFS was defined as the duration from the day of surgery to first relapse (radiologically or biopsy-confirmed) or death from any cause. To compare the pattern of initial recurrence, loco-regional recurrence was defined when the recurrence was in the lungs or mediastinal or supraclavicular lymph nodes, and distant metastasis was defined when the recurrence was in the pleura, pericardium, or extra-thoracic site (16).
IHC
In this study, ALK rearrangement was screened using IHC, which remains a reliable and cost-effective screening tool for ALK rearrangement with relatively high sensitivity (90%) and specificity (95%) compared with the fluorescence in situ hybridization (FISH) test (the current standard for ALK rearrangement) (19,20). In brief, for IHC analysis, the formalin-fixed, paraffin-embedded and resected primary tumor was sliced at a thickness of 4 µm. An antibody against ALK (clone 5A4; Novocastra, Newcastle, UK) was diluted at a ratio of 1:30, and sections were incubated with this diluted antibody for 2 h at 42 °C. An IHC score was assigned as follows: 3+ (intense, granular cytoplasmic staining), 2+ (moderate, smooth cytoplasmic staining), 1+ (faint cytoplasmic staining in ≥10% of tumor cells), and 0 (no staining). A positive ALK status was defined as an IHC score of 2+ or 3+, according to the findings of previous studies showing high correlation of ALK FISH and ALK IHC score of 2+ or 3+ (18,21). Thus, patients were categorized into two groups according ALK status: ALK-positive and ALK-negative.
Statistical analysis
Data are reported as number (%) for categorical variables and as median (interquartile range, IQR) for continuous variables. We compared categorical variables using a chi-square test or Fisher’s exact test and continuous variables using Mann-Whitney U test. DFS rate was estimated using Kaplan-Meier method, and differences of DFS curves were compared using a log-rank test. A Cox’s proportional hazard model was used to determine whether ALK status was associated with tumor recurrence. Initial clinical variables entered into the model were age, sex, body mass index, smoking history, T stage (T1a or T1b), and surgery type. A stepwise backward selection method, with elimination of variables exhibiting P value >0.1 in univariate analyses, was used to identify clinical variables for inclusion in the final model. We used univariate logistic regression to compare recurrence sites between two groups. All tests were two-sided, and a P value <0.05 was considered as significant. We used Stata (version 13.0; Stata Corporation, College Station, TX, USA) and R (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria) for analysis.
Results
The baseline characteristics and surgical procedure results of 309 patients with a stage IA lung adenocarcinoma are presented in Table 1. Of the total patients, 23 (7.4%) had ALK-positive adenocarcinoma. There were no significant differences in sex, smoking history, history of malignancy other than lung cancers, or tumor (T) stage or location according to ALK status. However, compared with the ALK-negative group, the ALK-positive group was younger (median age, 52.5 vs. 61.1 years; P<0.001). With regard to treatment, no significant difference in surgery type or surgical approach was found between the two groups, and no patient received neoadjuvant or adjuvant treatment.
Table 1. Baseline characteristics of 309 patients with surgically resected stage IA lung adenocarcinoma according to ALK IHC status.
| Characteristics | Total (n=309) | ALK IHC status* | P value | |
|---|---|---|---|---|
| Negative (n=286) | Positive (n=23) | |||
| Age, years | 60.7 (54.0–68.0) | 61.1 (55.0–68.0) | 52.5 (45.0–58.0) | <0.001 |
| Sex, male | 139 (45.0) | 128 (44.8) | 11 (47.8) | 0.776 |
| Smoking history | 0.569 | |||
| Never-smoker | 198 (64.1) | 182 (63.6) | 16 (69.6) | |
| Current or former smoker | 111 (35.9) | 104 (36.4) | 7 (30.4) | |
| BMI (kg/m2) | 23.9 (22.6–25.6) | 23.9 (22.4–25.7) | 24.1 (22.7–25.3) | 0.744 |
| Previous history of other malignancy | 55 (17.8) | 53 (18.5) | 2 (8.7) | 0.393 |
| Tumor size (mm) | 18 (13–23) | 18 (13–23) | 18 (15–21) | 0.981 |
| T stage | 0.802 | |||
| T1a | 194 (62.8) | 179 (62.6) | 15 (65.2) | |
| T1b | 115 (37.2) | 107 (37.4) | 8 (34.8) | |
| Tumor location | 0.089 | |||
| RUL | 95 (30.7) | 92 (32.2) | 3 (13.0) | |
| RML | 22 (7.1) | 18 (6.3) | 4 (17.4) | |
| RLL | 68 (22.0) | 61 (21.3) | 7 (30.4) | |
| LUL | 70 (22.7) | 66 (23.1) | 4 (17.4) | |
| LLL | 54 (17.5) | 49 (17.1) | 5 (21.7) | |
| Surgery type | 0.272 | |||
| Lobectomy | 251 (81.2) | 230 (80.4)† | 21 (91.3) | |
| Segmentectomy or wedge resection | 58 (18.8) | 56 (19.6) | 2 (8.7) | |
| Surgical approach | 0.488 | |||
| VATS | 277 (89.6) | 255 (89.2) | 22 (95.7) | |
| Open thoracotomy | 32 (10.4) | 31 (10.8) | 1 (4.3) | |
| Follow-up duration after surgery, months | 35.8 (32.1–39.3) | 35.8 (32.2–38.7) | 36.3 (24.1–44.0) | 0.870 |
| Time interval between surgery and recurrence, months | 16.0 (8.1–24.5) | 16.0 (8.0–25.0) | 15.5 (10.2–24.1) | 0.738 |
Results are presented as number (%) or median (interquartile range). *, ALK-positive was defined as an IHC score ≥2; †, one patient underwent right lower bi-lobectomy. ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry; BMI, body mass index; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; VATS, video-assisted thoracoscopic surgery.
During the median follow-up period of 35.8 months (IQR, 32.1–39.3 months), 34 (11.0%) of 309 patients experienced tumor recurrence. Among the 34 patients who recurred, 21 were confirmed by biopsy of a recurred lesion(s). Otherwise, recurrence was diagnosed by definite clinical and radiologic evidence of recurrence and was treated accordingly (i.e., palliative chemotherapy and/or radiotherapy). As shown in Table 2, the risk of tumor recurrence was 2.5 times higher in patients with an ALK-positive tumor compared with those with an ALK-negative tumor [crude hazard ratio (HR), 2.55; 95% confidence interval (CI), 1.05–6.19]. After adjustment for the selected variables, the risk of tumor recurrence remained significant (adjusted HR, 2.64; 95% CI, 1.08–6.44). The DFS differed significantly by ALK status (Figure 1; log-rank test, P=0.031). The 5-year DFS rate was significantly lower in the ALK-positive group (62.4%) compared with the ALK-negative group (86.5%; P=0.038).
Table 2. Unadjusted and adjusted hazard ratios of positive ALK IHC status for tumor recurrence in patients with stage IA lung adenocarcinoma who underwent surgical resection.
| Variables | ALK IHC status* | P value | |
|---|---|---|---|
| Negative (n=286) | Positive (n=23) | ||
| Unadjusted | Ref | 2.55 (1.05, 6.19) | 0.038 |
| Adjusted† | Ref | 2.64 (1.08, 6.44) | 0.033 |
Values represent the hazard ratio (95% confidence interval). *, ALK-positive was defined as an IHC score ≥2; †, initial clinical variables entered into the Cox’s proportional hazard model were age, sex, body mass index, smoking history, T stage (T1a or T1b), and surgery type. Using a stepwise backward selection method, with elimination of variables exhibiting P value >0.1 in univariate analyses, sex, T stage, and surgery type were included in the final model. ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry.
Figure 1.
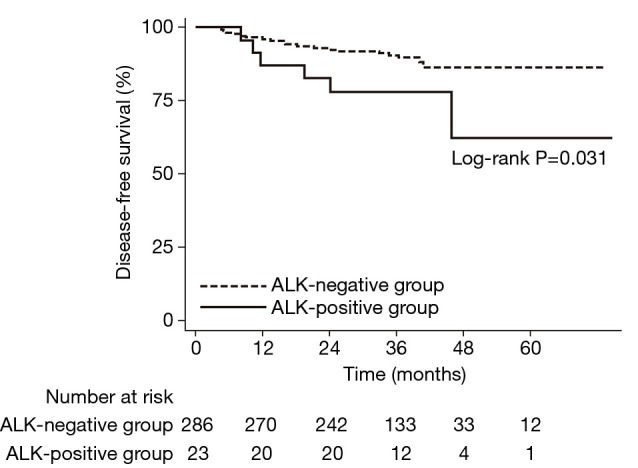
Disease-free survival curve according to ALK IHC status. ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry.
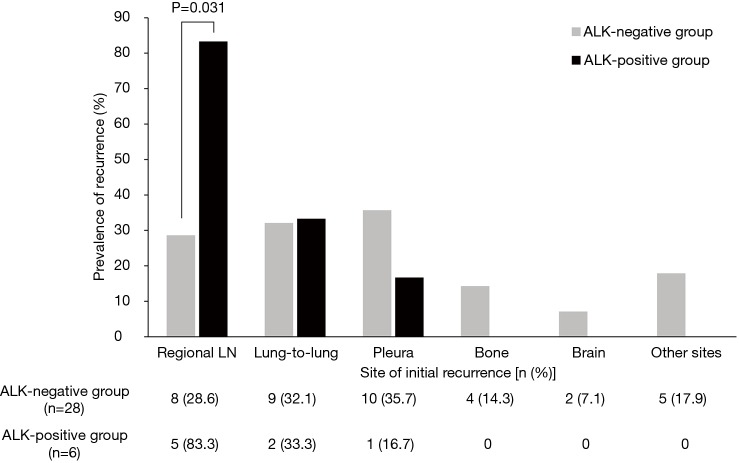
As shown in Figure 2, among the 34 patients with recurrent tumor, those in the ALK-positive group tended to have initial recurrence in regional lymph nodes compared with those in the ALK-negative group [83.3% (5/6) vs. 28.6% (8/28); P=0.031]. There was no difference in recurred lymph node stations between ALK-positive and ALK-negative groups. When sites of recurrence were dichotomized into loco-regional and distant metastases, the ALK-positive group was more likely to have loco-regional recurrence than the ALK-negative group [83.3% (5/6) vs. 32.1% (9/28); P=0.044].
Figure 2.
Initial recurrence site according to ALK IHC status among 34 patients with tumor recurrence (some patients had more than one initial recurrence site). ALK, anaplastic lymphoma kinase; IHC, immunohistochemistry.
Discussion
In the present study, we investigated the prognostic implications of ALK rearrangement in patients with stage IA adenocarcinoma who underwent complete surgical resection. During a median follow-up period of 35.8 months, patients with ALK-positive adenocarcinoma were approximately 2.5 times more likely to experience disease recurrence than those with ALK-negative adenocarcinoma. ALK rearrangement was also associated with more frequent recurrence in regional lymph nodes than other patterns of recurrence. To our knowledge, this is the first study to show the usefulness of testing ALK rearrangement in patients with early-stage lung adenocarcinoma who underwent complete surgical resection.
The past decade has seen marked advances in the treatment of a subset of NSCLCs with ALK rearrangement. With a dramatic response to the ALK inhibitor, the current guideline recommends that ALK rearrangement testing be conducted at the time of diagnosis for patients presenting with stage IV NSCLC or at the time of recurrence or progression in patients who originally presented with early-stage disease (22). However, it is left to local clinicians to decide whether to test all patients with early-stage disease because the result cannot be used to select therapy in patients who do not experience relapse.
Beyond the use as a therapeutic target in advanced-stage lung adenocarcinoma, the role of ALK rearrangement has been addressed in several retrospective studies of surgically resected NSCLC or adenocarcinoma (11,13,15-18). Two studies showed that ALK rearrangement was associated with a poor prognosis. In a Chinese study that evaluated NSCLC patients, ALK rearrangement was suggested as a poor predictor for DFS in a subgroup of stage IIIA patients (15). In another study that investigated tumors with ALK or c-ros oncogene 1 rearrangement, patients with fusion-positive lung adenocarcinoma had poorer DFS, especially those with stage IB to IIB (17). However, in contrast to the findings of these studies, ALK status was not associated with DFS in other studies (11,13,14,16,18). Indeed, a recent European multinational cohort study showed that positive ALK rearrangement was related to better overall survival (18).
There are several reasons for these different results. First, previous studies included a heterogeneous study population with a relatively small number of patients in each tumor stage. Second, neo- or adjuvant treatment might have affected disease recurrence patterns. Third, considering locally progressive features with less potential for distant metastasis of ALK-positive lung adenocarcinoma in our results, tumors with ALK rearrangement might behave more aggressively only in the early stages. Overcoming these limitations by confining our study to stage IA patients who underwent surgical resection, we addressed the natural features of surgically resected ALK-positive lung adenocarcinoma.
Echoing the findings of a previous study showing that these tumors are more likely to advance locally rather than metastasize to distant organs (9), our study showed that these tumors are associated with more frequent recurrence pattern of local invasiveness to lymph nodes. The results of our study suggest that routine testing for ALK rearrangement is needed in early-stage lung adenocarcinoma (especially for patients under the age of 60), and close surveillance of loco-regional recurrence following complete surgical resection should be performed in patients with ALK-positive lung adenocarcinoma. Our findings also support the rationale for an ongoing randomized placebo-controlled trial of adjuvant crizotinib with completely resected ALK-positive NSCLC following standard therapy (NCT02201992) (23).
There are several limitations to this study. First, it was conducted in a single referral center. Second, not all patients with stage IA lung adenocarcinoma were screened for ALK rearrangement, especially during the earlier study period. In our institution, ALK rearrangement test for early-stage lung cancer is performed according to the discretion of attending physicians. Thus, several factors might have influenced this decision such as age, gender, and smoking history. However, there was no significant difference in age, gender, or smoking history between the patients who were tested for ALK IHC and those who were not. Furthermore, we excluded patients who were tested for ALK status after surgery with a suspicion of recurrence to minimize the selection bias. Therefore, the authors think that our study design does not carry a significant selection bias. Third, the number of recurred patients was too small, especially in the ALK-positive group, to draw a firm conclusion regarding the pattern of recurrence. Fourth, we defined a positive ALK status using IHC rather than FISH, which had been regarded as the reference standard for ALK rearrangement (22). This is due to the compulsory universal health insurance system in Korea, which covers ALK FISH testing only when it is used to determine the selection of tyrosine kinase inhibitor therapy. Given that increased cellular protein levels in IHC might not always accompany ALK rearrangement in FISH, we might have underestimated the number of patients with ALK rearrangement. However, data from our institution showed high correlation of ALK IHC of 2+ or 3+ with ALK FISH, with 91.3–100% sensitivity (24), although the performance of the antibody used in this study (clone 5A4) was less accurate than the recently approved ALK (D5F3) CDx Assay (25). In addition, considering cost-effectiveness, we carefully suggest that ALK IHC is more reasonable than ALK FISH to screen high-risk patients who are more likely to have loco-regional recurrence (the cost for ALK IHC and ALK FISH in our institution is 48 and 304 dollars, respectively). Lastly, although we identified distinct characteristics in the recurrence pattern of surgically resected stage IA ALK-positive lung adenocarcinoma, the mechanisms remain largely unknown. Therefore, more studies are needed to elucidate further the nature of lung adenocarcinoma with ALK rearrangement at its early stage.
Conclusions
Our study showed that ALK rearrangement was associated with more frequent recurrence in patients with surgically resected stage IA lung adenocarcinoma. When recurred, regional lymph node metastases are the predominant recurrence pattern. Routine screening for ALK rearrangement with IHC might be useful in the triage of patients necessitating vigilant surveillance for loco-regional recurrence and for potentially determining the need for adjuvant treatment in patients with early-stage lung adenocarcinoma.
Acknowledgements
None.
Ethical Statement: The Institutional Review Board of Samsung Medical Center approved this retrospective study, and informed consent was waived (IRB no. SMC 2016-05-096-002).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 2.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. 10.1056/NEJMoa1006448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. 10.1200/JCO.2009.22.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. 10.1158/1078-0432.CCR-08-0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol 2013;31:1105-11. 10.1200/JCO.2012.44.5353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. 10.1002/cncr.24181 [DOI] [PubMed] [Google Scholar]
- 8.Lee B, Lee T, Lee SH, et al. Clinicopathologic characteristics of EGFR, KRAS, and ALK alterations in 6,595 lung cancers. Oncotarget 2016;7:23874-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi CM, Kim MY, Hwang HJ, et al. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015;275:272-9. 10.1148/radiol.14140848 [DOI] [PubMed] [Google Scholar]
- 10.Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013;79:242-7. 10.1016/j.lungcan.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 11.Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012;77:319-25. 10.1016/j.lungcan.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Kim TJ, Lee CT, Jheon SH, et al. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann Thorac Surg 2016;101:473-80. 10.1016/j.athoracsur.2015.07.062 [DOI] [PubMed] [Google Scholar]
- 13.Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. 10.1016/j.lungcan.2011.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with nonsmall cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 2012;118:729-39. 10.1002/cncr.26311 [DOI] [PubMed] [Google Scholar]
- 15.Zhou JX, Yang H, Deng Q, et al. Oncogenic driver mutations in patients with non-small-cell lung cancer at various clinical stages. Ann Oncol 2013;24:1319-25. 10.1093/annonc/mds626 [DOI] [PubMed] [Google Scholar]
- 16.Sun JM, Lira M, Pandya K, et al. Clinical characteristics associated with ALK rearrangements in never-smokers with pulmonary adenocarcinoma. Lung Cancer 2014;83:259-64. 10.1016/j.lungcan.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 17.Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014;83:389-95. 10.1016/j.lungcan.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 18.Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and clinical outcomes for patients with ALK-positive resected stage I to III adenocarcinoma: results from the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780-7. 10.1200/JCO.2013.54.5921 [DOI] [PubMed] [Google Scholar]
- 19.Conklin CM, Craddock KJ, Have C, et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol 2013;8:45-51. 10.1097/JTO.0b013e318274a83e [DOI] [PubMed] [Google Scholar]
- 20.Marchetti A, Di Lorito A, Pace MV, et al. ALK protein analysis by IHC staining after recent regulatory changes: a comparison of two widely used approaches, revision of the literature, and a new testing algorithm. J Thorac Oncol 2016;11:487-95. 10.1016/j.jtho.2015.12.111 [DOI] [PubMed] [Google Scholar]
- 21.Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65. 10.1097/JTO.0b013e318209edb9 [DOI] [PubMed] [Google Scholar]
- 22.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823-59. 10.1097/JTO.0b013e318290868f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST Trials: a golden opportunity to transform outcomes in early-stage non-small cell lung cancer. Clin Cancer Res 2015;21:5439-44. 10.1158/1078-0432.CCR-15-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi IH, Kim DW, Ha SY, et al. Analysis of histologic features suspecting anaplastic lymphoma kinase (ALK)-expressing pulmonary adenocarcinoma. J Pathol Transl Med 2015;49:310-7. 10.4132/jptm.2015.05.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne-Nuzzo T, Williams C, Catallini A, et al. A sensitive ALK immunohistochemistry companion diagnostic test identifies patients eligible for treatment with crizotinib. J Thorac Oncol 2017;12:804-13. 10.1016/j.jtho.2017.01.020 [DOI] [PubMed] [Google Scholar]