Introduction
Although most patients with stage I non-small cell lung cancer (NSCLC) can achieve long-term survival, some still remain at high risk of relapse (1-3). The excellent outcomes and increasing use of stereotactic ablative radiotherapy (SABR) for stage I NSCLC has recently posed a challenge to surgery (4,5). Nevertheless, lobectomy with mediastinal lymph node dissection or sampling has remained the standard of care for operable, stage I NSCLC. Adjuvant chemotherapy should be considered for patients with stage IB (>4 cm) and high-risk NSCLC after surgery (6). Moreover, as a recent randomized phase III trial showed, adjuvant EGFR tyrosine kinase inhibitor (TKI) can significantly prolong disease-free survival (DFS) compared with adjuvant vinorelbine plus cisplatin in patients with completely resected stage II–IIIA (N1–N2) EGFR-mutant NSCLC (7). However, whether adjuvant EGFR-TKI could benefit patients with stage I EGFR-mutant NSCLC remains unknown (8,9).
In addition, oligometastasis is a common relapse patterns after complete resection (10), and the delivery of radiation therapy is increasingly being delivered in these patients to prolong their progression-free survival (PFS) (11). A question that still remains is the impact of local therapy in combination with targeted therapy for patients with oligometastatic disease.
Here, we presented a case of a patient with EGFR-mutant stage IA lung adenocarcinoma clinically benefitting from adjuvant EGFR-TKI. After that, the patient had bone oligometastasis and benefited from the addition of local therapy. During the treatment process, the international multidisciplinary team (iMDT) discussion played a pivotal role in the selection and switch of therapeutic strategies.
Case presentation
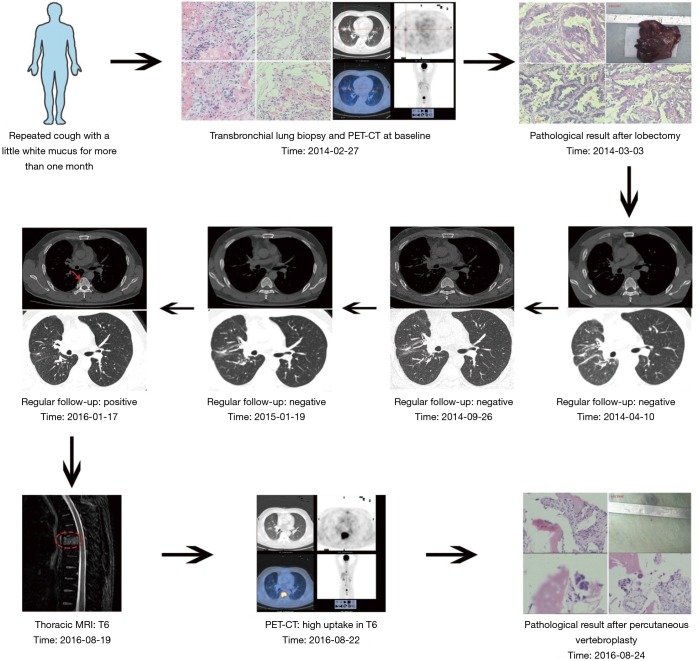
A 44-year-old man presented for medical consultation with persistent cough productive of scant white mucus for more than one month. He was a never-smoker and had no familial history of lung cancer. He received a computed tomography (CT) scan that showed a mass with an approximate diameter of 1.4 cm in the middle right lung. Then, he received a transbronchial lung biopsy, and the resulting pathology was lung adenocarcinoma. To further determine the clinical stage and treatment, he underwent position-emission tomography (PET)-CT and cranial magnetic resonance imaging (MRI) scans. The test results showed no evidence of distant metastases. A complete blood count, comprehensive metabolic panel, blood coagulation test, electrocardiogram, and tumor markers were normal, although hepatic functioning showed a history of hepatitis B (HBsAg, HBeAb and HBcAb test positive). Overall, the preliminary diagnosis was cT1aN0M0 NSCLC.
Without surgical contraindications, he underwent a right middle lobectomy and mediastinal lymph node sampling under epidural anesthesia. The resected tumor specimen by pathological evaluation showed that the lung adenocarcinoma had an acinar pattern, as well as areas of papillary adenocarcinoma with a diameter of 1.2 cm. The tumor did not involve the pleura, and resected lymph nodes were all negative. Immunohistochemistry (IHC) testing showed CK-7(+), TTF-1(+), Napsin A(+), TS(−), CD34(+), ERK2(++), BRCA1(−), ERCC1(+++), β-Tubulin-3(+++), VEGF-c(+++), Stathmin(++), and RRM1(−).The patient asked for the diver gene detection. The result showed an EGFR exon 19 deletion, and no other gene mutations or translocations were found. The patient received adjuvant erlotinib therapy for 6 months and stopped voluntarily. During regular follow-up, he was in stable condition.
After 23 months of erlotinib discontinuation, he presented again to our department due to new-onset back pain. He received an MRI of the lumbar spine, which demonstrated bone metastases in the vertebral bodies (T4 and T6). PET-CT imaging was obtained to assess for additional sites of metastatic spread of disease, which demonstrated lesions with high 18F-FDG uptake in the T6 vertebral body. A percutaneous vertebroplasty and biopsy of the T6 vertebra was performed in an effort to provide symptomatic relief of his back pain. Pathology confirmed metastases from lung adenocarcinoma. IHC testing showed CK-7(+) and TTF-1(+). Diver gene detection was similar to the primary lesion (EGFR exon 19 deletion). The patient requested that an iMDT discussion take place to help guide the treatment approach for his recurrent disease and to potentially improve his prognosis.
iMDT
Expert opinion 1
It appears that he had a very early stage lung cancer that was resected and treated with adjuvant targeted therapy years ago, and now he has pathologic confirmation of spinal vertebral body metastasis status post vertebroplasty. Since a vertebroplasty is not an oncologic procedure and is more designed to stabilize the spinal column and in this case to also make a pathologic diagnosis of recurrence, he likely should receive short course (likely 10 fractions) adjuvant radiation therapy to the involved operated on vertebral disease. Thereafter, he should likely restart systemic therapy. Given that the mutation test was still EGFR exon 19 deletion, first-generation EGFR-TKI instead of cytotoxic chemotherapy or immunotherapy is likely the best option.
Expert opinion 2
The case is quite interesting. Based on documents, it seems that the patient has a solitary metastasis in the bone, with evidence of EGFR mutation. My suggestion is to perform a PET scan to correctly evaluate disease sites, brain MRI to assess if there are any brain metastases, and start an EGFR-TKI (gefitinib or erlotinib or afatinib). It is also important to consider radiation therapy for pain or risk of spinal cord compression.
Expert opinion 3
Based on a very limited history (e.g., no pathological stage nor details of surgery, current symptoms e.g., back pain) and no images to compare from CT 2014–2016 for lung changes and vertebra lesions, I recommend awaiting molecular testing—previous EGFR exon 19 deletion—although not calcified bone can be a difficult template. They should also have a specialist review and recurrence should be confirmed. Then, consider local radiation therapy and systemic treatment depending on the detailed information.
Expert opinion 4
The patient is a 44-year-old man with a relapse of an early lung adenocarcinoma (middle right lobectomy in March, 2014). The resected tumor specimen shows that the lung adenocarcinoma had an acinar pattern, as well as areas of papillary adenocarcinoma. The small resected tumor of 1.2 cm harbored the EGFR exon 19 deletion. The patient received adjuvant erlotinib therapy for 6 months and stopped voluntarily without signs of progression. A technical comment, in preclinical studies of several EGFR mutant NSCLC cell lines, single therapy with EGFR TKIs (either gefitinib or erlotinib) induces tumor cell migration, invasion and metastases. Therefore, from the pre-clinical data, it currently is not advisable to recommend adjuvant oral therapies in early resected NSCLC patients that could harbor EGFR mutations.
The current situation started in August with the finding of bone metastases in vertebras T4 and T6. The patient underwent surgery on the vertebra. The resected specimen demonstrated lung adenocarcinoma with the EGFR exon 19 deletion. He is pending additional molecular results from the plasma tissue by whole exome gene sequencing.
The strong recommendation is to start EGFR TKIs (forgetting the above mentioned technical comments for early resected NSCLC). As you know, there are 1st, 2nd and 3rd generation EGFR-TKIs. Recent data report that osimertinib obtained a very impressive median PFS that was double that obtained with gefitinib or erlotinib. In 2017, osimertinib was approved by both the United States Food and Drug Administration and the European Commission (12).
My primary treatment options: (I) osimertinib as 1st therapy option to a dose of 80 mg/day or (II) combine osimertinib with chemotherapy, such as taxol or doxorubicin, since there is a clear synergism.
Expert opinion 5
It is unclear why the patient stopped erlotinib, and I would restart the erlotinib. As next step, there is no difference in my opinion between gefitinib, erlotinib or afatinib. There is no resistance EGFR T790M mutation. I think the tumor grew after the patient stopped erlotinib.
Treatment and follow-up
According to iMDT discussion, two treatment options were strongly recommended: (I) local therapy, and (II) EGFR-TKI (gefitinib, erlotinib or afatinib) or combination of EGFR-TKI and chemotherapy. After full communication with the patient, he chose single agent erlotinib (150 mg, once a day). Fortunately, he obtained a clinical benefit for more than 6 months and a good quality of life. Long-term follow-up is ongoing (Figure 1).
Figure 1.
Flow of diagnosis and treatment.
Discussion
In this study, we presented the first case with EGFR-mutant stage IA lung adenocarcinoma clinically benefitting from adjuvant EGFR-TKI. Although the treatment approach was successful, there are several questions that should be further considered.
Although surgery has been the historical standard of care, a recent pooled analysis of two randomized trials reported that SABR had a significantly longer three-year survival rate than surgery for operable stage I NSCLC (4). However, definitive conclusions could not be reached given the limited sample of these trials. Therefore, is SABR or surgery the best definitive treatment option for stage I NSCLC?
Expert opinion 1
Without more randomization study, the best option, at this moment, for stage I NSCLC is to individualize treatment based on patients’ performance status, cardiovascular and pulmonary function status, age, tumor size, location and patient’s preference. Typically, patients with great performance status, tumors >3 cm and/or centrally located, and age younger than 65 years, should consider surgery since surgical mortality and morbidity is not high in this population. Otherwise, patients with significant co-morbidities and higher risk for surgical mortality should be considered for SABR, particularly for patients who are older than 70 years and who have peripherally located tumors <3 cm. These decisions should be made through multidisciplinary discussion involving pulmonary physicians, thoracic surgeons, radiation oncologists and imaging experts.
Expert opinion 2
The definitive management of medically operable, early-stage NSCLC (ES-NSCLC) traditionally involves definitive surgery with lobectomy and mediastinal lymph node dissection or sampling. SABR has emerged as an alternative definitive treatment modality for this group of patients, with local failure rates of 4–11% at 3 years, comparable to rates observed after surgical resection (13-16). SBRT is a particularly important option in the setting of medical inoperability, which is a commonly encountered scenario given the frequent presence of risk factors leading to medical comorbidities precluding surgery.
Even in patients who are candidates for definitive oncologic resection with lobectomy and lymph node dissection or sampling, interest in SBRT as a primary treatment modality has increased due to growing evidence of comparable disease control and decreased morbidity achieved with SBRT. This has led to the development of multiple clinical trials studying outcomes of SBRT in medically operable patients with ES-NSCLC.
Expert opinion 3
The pooled analysis by Chang et al. includes two such investigations pertinent to this case, ROSEL and STARS (4). Both were phase 3 trials randomizing patients with clinical T1–T2a (<4 cm) N0 M0 disease (AJCC 7th edition), operable NSCLC to receive SBRT (54 Gy in 3 fractions for peripheral lesions, 50–60 Gy in 4–5 fractions for central lesions) or surgery (generally lobectomy) with mediastinal lymph node dissection or sampling. On combined analysis of the 58 patients randomized on these two trials, the estimated 3-year overall survival was found to be superior in the SBRT group (95% vs. 79%, P=0.037). Local, regional, and distant recurrence-free survivals were not different between the two groups of patients receiving SBRT versus surgery. Treatment-related toxicities occurred more frequently in the surgery arm, with 1 patient dying of complications from surgery and 48% of patients developing grade ≥3 adverse events. In contrast, only 10% of patients receiving SBRT experienced grade 3 toxicities, and no grade 4 or 5 events occurred. These findings are compelling given the demonstration of superior survival with SBRT since SBRT has historically been reserved for inoperable patients with limited survival and life expectancy at baseline compared with those who are surgical candidates. This bias was minimized in the STARS and ROSEL trials as all patients were required to be eligible for surgery, which may have contributed to the demonstrated results. The superior improved toxicity profile of SBRT found in the pooled analysis is also unsurprising. SBRT is a noninvasive treatment without many of the inherent potential complications of surgery. In addition, refinement in the ability to identify the optimal SBRT dose-fractionation based on tumor characteristics such as tumor location and proximity to critical thoracic structures has further improved the therapeutic ratio of SBRT.
Limitations of this study and similar investigations to date include a relatively small number of patients available for analysis and the lack of long term follow-up. The former issue is obviated to some extent by the greater number of patients who have been studied on previous trials, albeit primarily in the inoperable setting. Future attempts to address this question in a randomized fashion will also likely be limited in sample size due to difficulty of accrual as a result of patient preference, provider bias, and institutional experience. The latter limitation requires further attention, as long-term data are critical in achieving more widespread acceptance of SBRT by the oncologic community as a treatment that provides clinical equipoise with lobectomy. Therefore, while SBRT continues to emerge as a modality that can likely provide equivalent disease control and diminished toxicities for ES-NSCLC, the decision of which treatment modality is optimal should still be considered on a highly individualized basis, with input from the multidisciplinary team and after detailed discussion with the patient to weigh the risks and benefits of each approach. SBRT should continue to be strongly considered for patients with limited performance status, borderline operability status, and preference for nonsurgical treatment.
A phase III trial indicated that adjuvant EGFR-TKI could benefit patients with completely resected stage II–IIIA EGFR-mutant NSCLC. However, the survival curves began to converge at around 36 months, meeting by about 48 months (17). Therefore, should we recommend all patients with completely resected stage II-IIIA EGFR-mutant NSCLC receive adjuvant EGFR-TKI? Furthermore, is it reasonable to recommend adjuvant EGFR-TKI for patients with completely resected stage I EGFR-mutant NSCLC?
Expert opinion 1
Lacking the OS benefit of adjuvant EGFR-TKI and issues of cost of EGFR-TKI with potential development of drug-resistant, EGFR-TKI should not be considered as a standard adjuvant treatment for all stage II/IIIA NSCLC EGFR-mutant NSCLC. It may be considered for patients who cannot tolerate adjuvant chemotherapy. There are no robust data for the use of adjuvant EGFR-TKI for patients with completely resected stage I EGFR-mutant NSCLC, and this therapy should not be used in this setting outside of the setting of a clinical trial.
Expert opinion 2
The expected 5-year survival of patients with stage II–IIIa NSCLC remains quite low, ranging from 15–30%. The addition of adjuvant chemotherapy increases survival by around 5%. However, cytotoxic chemotherapy is associated with significant toxicities. Recent findings have emerged demonstrating that patients with NSCLC who harbor EGFR mutations, most commonly a deletion in exon 19 or point mutation in exon 21, have favorable response rates and outcomes, as well as more limited toxicities, when treated with EGFR-TKIs, including afatinib, erlotinib, gefitinib, and osimertinib.
The RADIANT trial was a randomized, double-blinded, placebo-controlled phase 3 trial reported in 2015, in which patients with stage IB to IIIA NSCLC who underwent complete resection surgery followed by adjuvant chemotherapy were then randomized to placebo versus additional therapy with erlotinib for 24 months (8). On subgroup analysis of patients who had EGFR mutation-positive disease, those who received adjuvant erlotinib experienced a significant improvement in median DFS compared with those randomized to placebo (46.4 versus 28.5 months, P=0.039). While these results are promising, survival curves began to converge around the 36 months’ time point and overlapped at 48 months, findings that raise the question of durability of the benefit of erlotinib therapy.
Erlotinib has also demonstrated efficacy after combined adjuvant cytotoxic chemotherapy and radiation therapy. In the SELECT study, 100 patients with stage IA-IIIA NSCLC harboring a TKI-sensitizing EGFR mutation received standard adjuvant chemotherapy and radiation therapy followed by erlotinib for 2 years. Results of the study were promising, revealing a 2-year DFS of 90%. However, the study was also limited by relatively short follow-up and, therefore, limited ability to demonstrate sustained favorable response with erlotinib.
Expert opinion 3
EGFR-TKI has also been shown to have a similar positive effect in this population of patients. In the ADJUVANT study, 222 Chinese patients with stage II–IIIA EGFR-mutant NSCLC were randomized to receive either 4 cycles of adjuvant cisplatin and vinorelbine or gefitinib for 24 months after complete resection surgery (7). DFS favored patients who received gefitinib (28.7 versus 18.0 months, P=0.0054). However, as seen in the RADIANT study, DFS curves also began to converge in the ADJUVANT trial, beginning at 24 months and meeting by 36 months. Toxicity was far more frequently observed in the cisplatin plus vinorelbine arm, with significantly increased incidences of grade 3 or worse hematologic and gastrointestinal toxicities experienced by patients who received cytotoxic chemotherapy. Finally, a recent meta-analysis including 2,223 patients from 7 studies treated with an EGFR-TKI after surgery demonstrated that while all patients receiving adjuvant EGFR-TKI therapy had an absolute benefit in DFS at 3 years, those with EGFR mutant-positive disease experienced an even greater benefit, with an absolute improvement in DFS of 7.1% at 3 years (9).
Available data to date suggest that patients who harbor TKI-sensitizing EGFR mutations should be strongly considered for first-line treatment with one of these agents. DFS outcomes are consistently improved with the addition of EGFR-TKIs to the adjuvant treatment regimen of patients with stage II–IIIA NSCLC, with or without the use of adjuvant chemotherapy. The use of EGFR-TKIs as a substitute for cytotoxic chemotherapy is quickly becoming the primary approach to systemic therapy for these patients given the substantial reduction in treatment morbidity associated with these agents versus traditional chemotherapy drugs. Continued follow-up of these trials and results of ongoing studies will be needed to further clarify the role of EGFR-TKIs in the setting of EGFR-mutated locally advanced NSCLC.
Patients with ES-NSCLC generally experience excellent local control after definitive surgery with lobectomy and mediastinal lymph node sampling or dissection. Overall survival and distant disease control are similarly relatively good in this population; therefore, the addition of a systemic agent is not yet well-defined and may add unnecessary toxicity for little to no added benefit. Patients with tumors that harbor a TKI-sensitizing EGFR mutation and have high-risk features that would be considered for adjuvant chemotherapy may instead be considered for an EGFR-TKI in this scenario. For other patients with stage I NSCLC, the addition of this therapy should be approached with caution, particularly given recent evidence that these therapies may promote cell migration and increase the propensity for drug resistance, effects that would be particularly detrimental in patients who may not have required this treatment at baseline.
Current evidence suggests that local therapy could provide a survival benefit to NSCLC patients with oligometastasis (11,18-20). However, could the addition of local therapy to EGFR-TKIs provide a better survival benefit than TKIs alone in EGFR-mutant NSCLC patients with oligometastasis or oligoprogressive disease?
Expert opinion 1
Based on emerging clinical data, local treatment has been shown to improve PFS for NSCLC with oligometastasis in stage IV NSCLC regardless of EGFR status and treatment. A specific randomized study to evaluate local therapy for patients with stage IV NSCLC with EGFR-mutant who have received EGFR-TKIs treatment is ongoing. I would recommend local therapy for these patients off clinical study at this moment based on the preliminary available data.
Expert opinion 2
In a multicenter, randomized, phase 2 trial reported by Gomez et al., patients with histologically-confirmed stage IV NSCLC with 3 or fewer metastatic lesions who had received first-line systemic therapy (platinum doublet chemotherapy or EGFR or ALK inhibitors if respective mutations or rearrangements were present) were randomized to receive local consolidative therapy (radiotherapy with or without chemotherapy or surgical resection of all lesions, with or without subsequent maintenance therapy) or observation alone (11). At a median follow-up of 12.4 months, PFS was improved in patients who received local consolidative therapy versus those who received observation (11.9 vs. 3.9 months, P=0.0054). Although a subgroup analysis of patients harboring a TKI-sensitizing EGFR mutation was not performed, it is likely that the benefit of adding local consolidation does not exclude this group of patients. A similar tripling of the PFS from 3.5 months in the maintenance chemotherapy-alone arm to 9.7 months in the SABR-plus-maintenance chemotherapy arm in a second phase 2 randomized trial of oligometastatic NSCLC patients with up to 5 sites of metastatic disease recently reported by Iyengar et al. Further study with phase 3 trials are forthcoming and will be needed to provide additional information on the need for consolidative therapy in oligometastatic NSCLC patients, particularly in different molecular subgroups of patients who are candidates for novel targeted agents.
Conclusions
Currently, a rapidly growing number of cancer patients from developing or remote countries are eager to request the expert advice of top physicians worldwide. They rely on this input in hopes of obtaining knowledge of and access to optimal therapeutic strategies with state of the art treatment concepts, techniques and drugs. The importance and advantages of iMDT discussion, which can provide a systematic approach to this type of communication and information-sharing, is reviewed.
First, in general, the management of advanced cancers in developing countries has been more challenging than in developed countries. For some intractable or rare cases, iMDT could help to provide comprehensive, correct and prompt suggestions and allow for modification of therapeutic strategy that could significantly improve therapeutic effectiveness and patient quality of life. This can introduce novel thinking and discussion in the diagnosis and treatment of intractable cases, which may be of significant value for young physicians and doctors from underdeveloped regions of medical technology.
Second, iMDT discussion can effectively save patients’ time and medical cost burden by avoiding the unnecessary inconvenience and discomfort from lengthy travels to seek appropriate medical care. For cancer patients, particularly for severely ill patients, this is an important protective measure.
Finally, iMDT could become a valuable platform for resource sharing. Through iMDT, patients will not only have access to advanced treatment concepts and suggestions from international experts, but they will also have the opportunity to gain up-to-date knowledge of clinical trials, novel drugs, and technological or treatment advances. iMDT also provides physicians with an expanded knowledge base through which they can help more cancer patients, provide more current and evidence-based treatments, and increase access to clinical trials.
These potential benefits of iMDT will have a significant positive impact on medical communication, international collaboration, and the overall progress of clinical cancer research.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. 10.1097/JTO.0b013e318206a221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. 10.1016/j.jtho.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. 10.1016/S1470-2045(15)70168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simone CB, 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015:CD011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 8.Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant Erlotinib Versus Placebo in Patients With Stage IB-IIIA Non-Small-Cell Lung Cancer (RADIANT): A Randomized, Double-Blind, Phase III Trial. J Clin Oncol 2015;33:4007-14. 10.1200/JCO.2015.61.8918 [DOI] [PubMed] [Google Scholar]
- 9.Yuan Y, Huang Q, Gu C, et al. Disease-free survival improved by use of adjuvant EGFR tyrosine kinase inhibitors in resectable non-small cell lung cancer: an updated meta-analysis. J Thorac Dis 2017;9:5314-21. 10.21037/jtd.2017.12.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novoa NM, Varela G, Jimenez MF. Surgical management of oligometastatic non-small cell lung cancer. J Thorac Dis 2016;8:S895-S900. 10.21037/jtd.2016.08.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez DR, Blumenschein GR, Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 13.Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. 10.1093/annonc/mdt026 [DOI] [PubMed] [Google Scholar]
- 14.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. 10.1016/j.jtcvs.2009.12.054 [DOI] [PubMed] [Google Scholar]
- 15.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. 10.1200/JCO.2009.25.0928 [DOI] [PubMed] [Google Scholar]
- 16.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. 10.1016/j.prro.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 17.Ng TL, Camidge DR. Lung cancer’s real adjuvant EGFR targeted therapy questions. Lancet Oncol 2018;19:15-7. 10.1016/S1470-2045(17)30875-6 [DOI] [PubMed] [Google Scholar]
- 18.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. 10.1001/jamaoncol.2017.3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel AN, Simone CB, 2nd, Jabbour SK. Risk factors and management of oligometastatic non-small cell lung cancer. Ther Adv Respir Dis 2016;10:338-48. 10.1177/1753465816642636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Q, Zhou F, Liu H, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol 2018. [Epub ahead of print]. 10.1016/j.jtho.2018.05.019 [DOI] [PubMed] [Google Scholar]